Abstract
Background:
Increased demand and escalating costs necessitate innovation in health care. The challenge is to implement complex innovations—those that require coordinated use across the adopting organization to have the intended benefits.
Purpose:
We wanted to understand why and how two of five similar hospitals associated with the same health care authority made more progress with implementing a complex inpatient discharge innovation whereas the other three experienced more difficulties in doing so.
Methodology:
We conducted a qualitative comparative case study of the implementation process at five comparable urban hospitals adopting the same inpatient discharge innovation mandated by their health care authority. We analyzed documents and conducted 39 interviews of the health care authority and hospital executives and frontline managers across the five sites over a 1-year period while the implementation was ongoing.
Findings:
In two and a half years, two of the participating hospitals had made significant progress with implementing the innovation and had begun to realize benefits; they exemplified an integrated implementation mode. Three sites had made minimal progress, following a fragmented implementation mode. In the former mode, a semiautonomous health care organization developed a clear overall purpose and chose one umbrella initiative to implement it. The integrative initiative subsumed the rest and guided resource allocation and the practices of hospital executives, frontline managers, and staff who had bought into it. In contrast, in the fragmented implementation mode, the health care authority had several overlapping, competing innovations that overwhelmed the sites and impeded their implementation.
Practice Implications:
Implementing a complex innovation across hospital sites required (a) early prioritization of one initiative as integrative, (b) the commitment of additional (traded off or new) human resources, (c) deliberate upfront planning and continual support for and evaluation of implementation, and (d) allowance for local customization within the general principles of standardization.
Key words: Acute health care, complex innovation, implementation, integration
Framing the Research Problem
Public health care systems are facing increased demand due to demographic and lifestyle changes. At the same time, governments attempt to constrain escalating health care costs. The challenge is to find and implement innovative solutions to meet the government and the public expectation for standardized care across different settings: urban versus rural, large versus small sites, with different patient and staff demographics (Hinings et al., 2003).
Existing research emphasizes diffusion of innovations or their adoption by individual users such as physicians (Jacobs et al., 2015). Less is known about implementing complex innovations, those that require “active coordinated use by multiple members to achieve organizational benefits” (Helfrich, Weiner, McKinney, & Minasian, 2007, p. 281) within health systems. Existing research on health care innovation has uncovered factors critical for successful implementation—such as leadership commitment, staff engagement, and goal and resource alignment (Chou, Yano, McCoy, Willis, & Doebbeling, 2008; Grove, Meredith, MacIntyre, Angelis, & Neialy, 2010; Lukas et al., 2007). However, few studies have examined the actual implementation process and practices for complex innovations that constitute or affect the above factors in health care organizations (McAlearney, Robbins, Garman, & Song, 2013; Reay, Goodrick, Casebeer, & Hinings, 2013). Yet, 30%–90% of complex innovation implementations in health care are estimated to fail (Jacobs et al., 2015).
A comprehensive model of implementing complex innovations was developed in a manufacturing context by Klein and Sorra (1996). Helfrich et al. (2007) adapted it to a health care setting (cancer research programs) and define innovation as “ideas, practices, or technology that are perceived as new by the adopter” (p. 281). Following Klein and Sorra (1996) and Rogers (2003), they define implementation as “the transition period, following a decision to adopt an innovation, during which intended users bring the innovation into sustained use” (Helfrich et al., 2007, p. 281). Jacobs et al. (2015) statistically validated the Klein and Sorra model, albeit in cancer research programs (versus in patient care) by individual physician users (as opposed to multiple, coordinated users).
Klein and Sorra (1996) identify two determinants of implementation effectiveness defined as “the consistency and quality of the targeted organizational members’ use of the innovation” (p. 1055): (a) the fit between the innovation and the users’ values and (b) implementation climate. Implementation climate refers to organizational members’ shared perception that “innovation implementation is a major organizational priority—promoted, supported, and rewarded by the organization” (Klein, Conn, & Sorra, 2001, p. 813). Implementation climate is shaped by an organization’s management support, resource availability, and implementation policies and practices. On the basis of a multiple case study, Helfrich et al. (2007) suggest that the innovation–values fit affects implementation climate and propose a new determinant for implementation climate particularly in health care settings: innovation champions. McAlearney et al. (2013) also affirm the Klein and Sorra model at five exemplary hospitals that had voluntarily adopted high-performance work practices (as opposed to a specific innovation). These authors do not extend the model but elaborate on implementation practices in their study’s context. Namely, that successful implementations often hinge upon (a) having clear definitions of goals and constructs for all parties involved; (b) building up a commitment, a willingness to work through implementation’s challenges; and (c) ensuring a measure of consistency in how the actual work practices are applied.
Our study makes two new contributions to understanding implementation of complex innovations in health care. First, we induced a new, integrative element for the model: the implementation mode. Second, we identified implementation practices associated with two archetypical modes—integrated and fragmented—at the health care organization, hospital, and hospital unit level. Figure 1 visualizes the Klein and Sorra model, with Helfrich et al. and our extensions.
Figure 1.
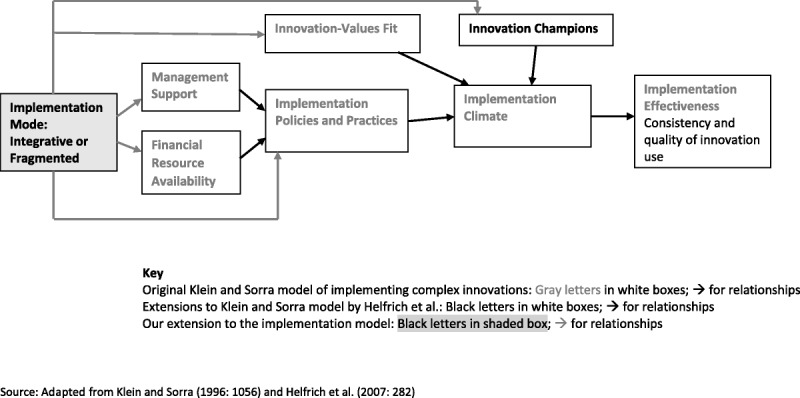
Models of implementing complex innovations.
Research Context
In February 2012, prompted by the ongoing shortage of acute care beds in a Canadian province, its Minister of Health mandated the provincial health care authority (PHA) to design and implement a standardized inpatient discharge model (SDM)—a complex innovation—across several major urban hospitals. A design for the SDM was expected within 6 months. The implementation at the five volunteering pilot hospitals was to be completed by 2015.
The PHA’s project design team, tasked with developing the provincial standards for inpatient discharge, was guided by a steering committee representing different geographical health care districts across the province. The design team worked at a rapid pace with the steering committee and an American consultant, seeking input from all professional groups affecting inpatient flow. Frontline staff and managers of the participating hospitals attended meetings to develop service level agreements with different professional groups, from physicians, nurses, and diagnostic imaging and lab technicians to porters and social workers. These service level agreements were to serve as SDM implementation guidelines.
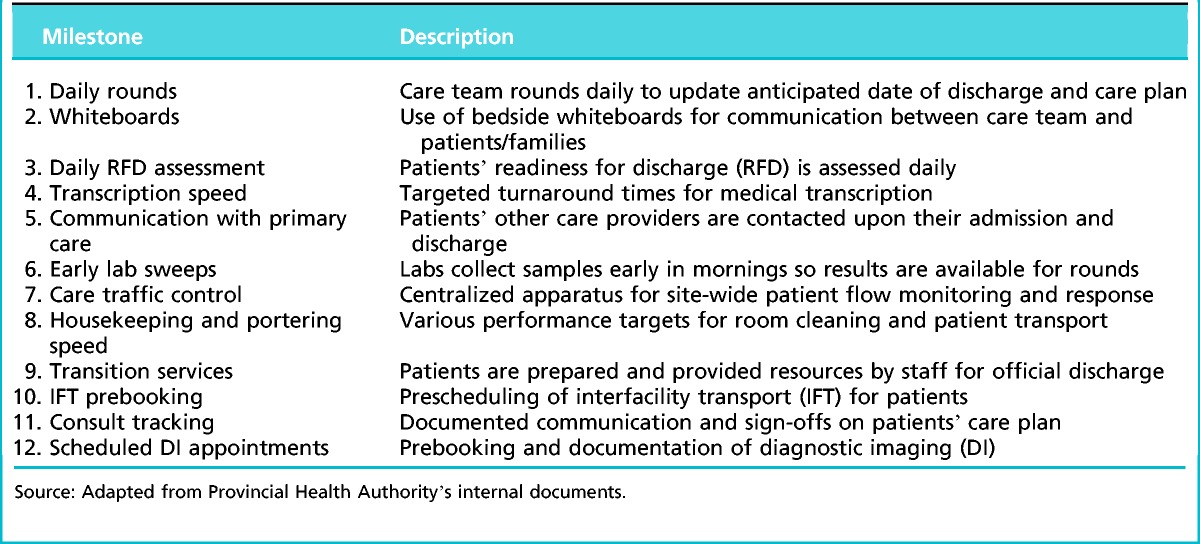
In March 2013, the PHA top executives decided to reassess the project in light of a number of other parallel innovation initiatives that overlapped with the SDM. This led to withdrawal of the provincial funding for the SDM and reassessment of the ongoing initiatives. However, the five pilot hospitals continued implementation with their operating budgets at a reduced scope or speed. The original service level agreements for the SDM were decreased to 12 milestones (Table 1).
Table 1.
The 12 milestones of the standard discharge model
Research Methods
A comparative case study approach was chosen, as we wanted to understand why and how the five hospitals operating in the same provincial health care context differed in their implementation of this innovation (Eisenhardt, 1989; Eriksson & Kovalainen, 2008; Yin, 2009). To understand their implementation processes in context, we collected data at three organizational levels: (a) macro-organization (PHA), (b) organization (hospital site and regional health care district), and (c) subunit (hospital units and ancillary services where the innovation was implemented). We focused on the individuals’ implementation practices at each level (Bresman, 2013).
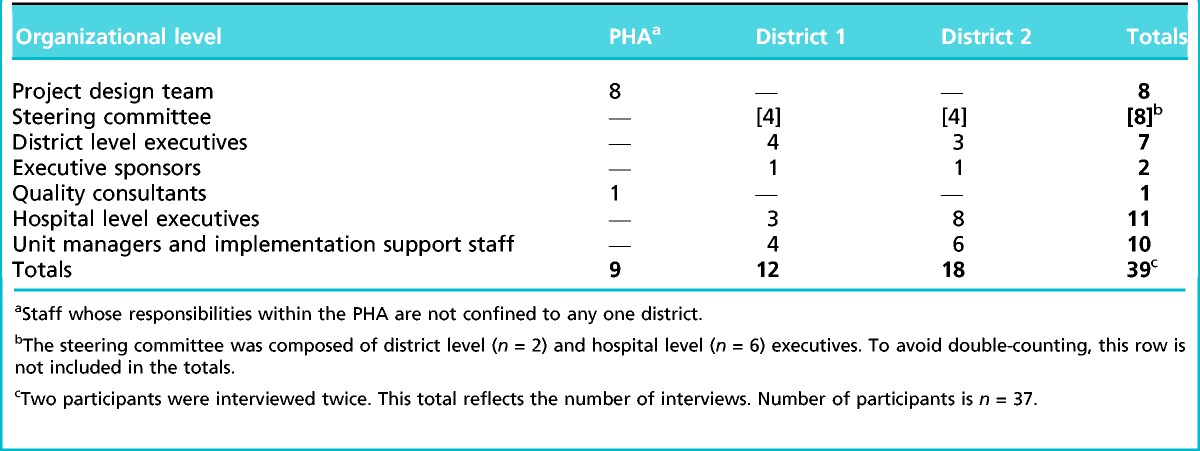
Our primary data sources were interviews, from the provincial health organization executives and provincial project design team and steering committee members to hospital leadership and frontline managers. To protect anonymity, we cannot give identifying information about the interviewees. Table 2 presents their distribution across different organizational levels and districts. We conducted the interviews in about a year, starting in the fall of 2013, to avoid recall problems. We interviewed the PHA executives first, followed by the provincial project design team and the steering committee members, and then moved to interview unit and other frontline managers at the hospital sites. In most cases, implementation was still ongoing at the time of the interviews, and some evidence of implementation effectiveness was available. We conducted 39 interviews in total, including four to six at each hospital. Interviewing multiple subjects at each level helped avoid single-source bias.
Table 2.
Distribution of interviews by organizational level and district within the Provincial Health Authority (PHA)
The semistructured interviews were conducted by one or two researchers, either on site or via telephone, and were guided by a flexible interview protocol designed to complement the various positions participants held. We asked each interviewee to tell their experience of the SDM (how they got involved, their role, the process), including lessons learned; any additional questions were primarily for elaboration or clarification. Everyone was asked about their background (educational, work experience, implementing other innovations) and was requested to add any comments. All interviews were recorded and transcribed. The transcripts were first read and checked for accuracy by another researcher and uploaded into Atlas.ti (version 6.1), a qualitative data analysis software. The transcripts were read a number of times and coded with Atlas.ti, using 44 codes induced from interviews and deduced from literature. To enhance reliability of our findings, two researchers independently developed case histories of the SDM project. The two sets of case histories were very similar, giving us confidence in the accuracy and trustworthiness of our observations and interpretations. A draft research report was sent to the study participants for validation. Their comments improved accuracy and were incorporated.
Various documents were used to triangulate the interview transcripts: the provincial health authority’s documents about the SDM initiative, the provincial SDM project manager’s educational slide show, the implementation plan and supporting materials used by two of the hospitals, and one hospital’s website. Our data were complemented by insights from research team members who are physicians, executives in the hospitals implementing the SDM project, or its steering committee members.
We analyzed the coded transcripts and the documentary data in parallel, informed by the literature on implementing innovation (Ferlie, Fitzgerald, Wood, & Hawkins, 2005; Helfrich et al., 2007; Klein & Sorra, 1996) and organizational change (Bresman, 2013; Kotter, 1995; Thatchenkery & Firbida, 2013) in the health care context in particular (Chou et al., 2008; Grove et al., 2010; Lukas et al., 2007; McAlearney et al., 2013; Reay et al., 2013). Following an interpretive approach (Eriksson & Kovalainen, 2008) and iterating between our data and the literature, we identified two archetypical patterns of implementing complex innovations: an integrated implementation mode and a fragmented implementation mode.
Findings
The five hospitals that volunteered to pilot the SDM were similar in their size (number of beds and staff), resources, services provided, number of innovation initiatives, and urban settings. All provided acute care and received their funding from the Ministry of Health through the PHA. Two hospitals were located in a different geographical district and belonged to a smaller provincial health care organization (RO). Although the RO had a semiautonomous relationship to the PHA, it depended on the PHA for funding and participated in the SDM initiative. To protect participant anonymity, only generic case descriptions are provided in Table 3.
Table 3.
Description of standard discharge model implementation at the five hospitals
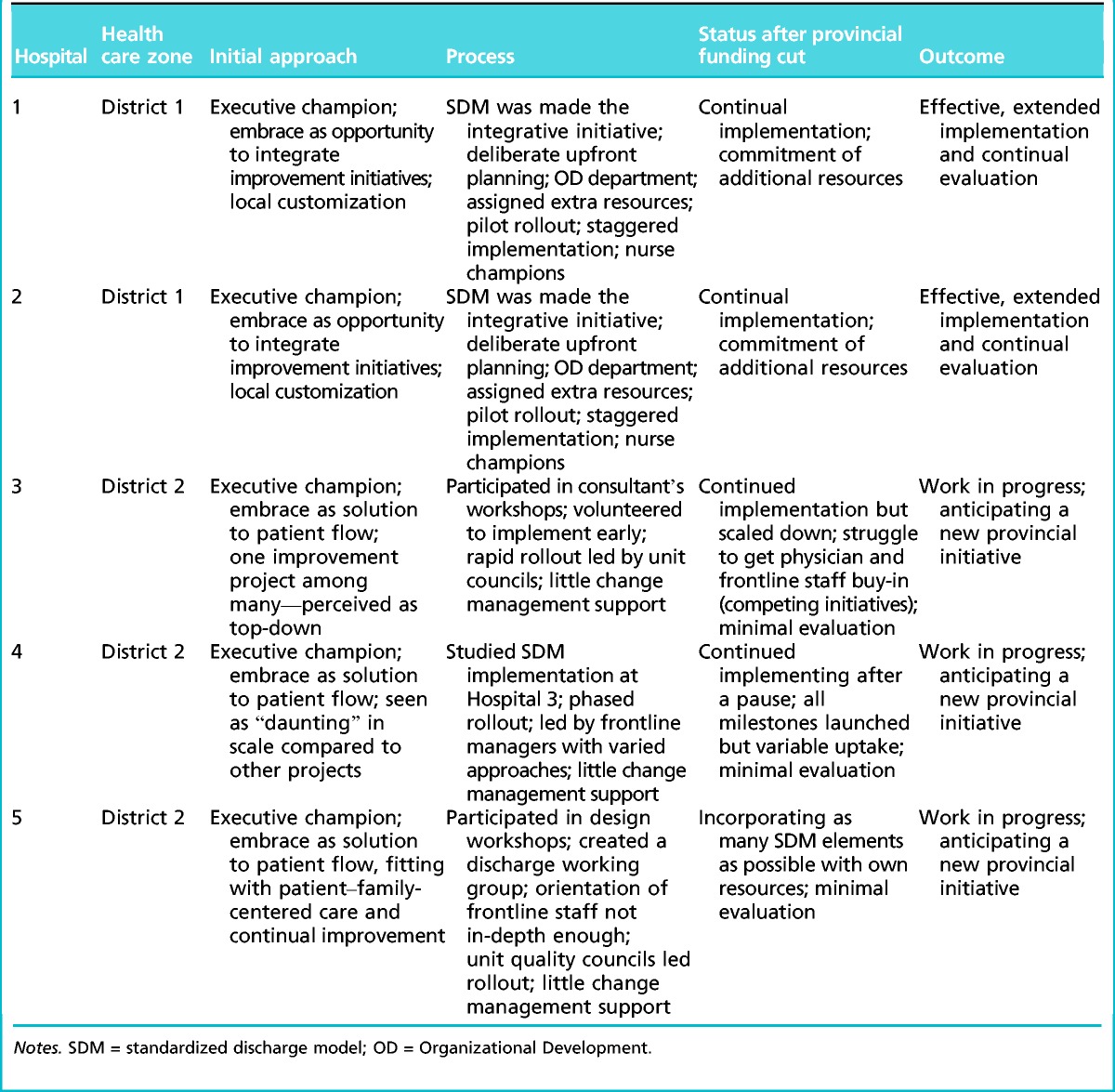
Two and a half years after the Ministerial Directive, two of the hospitals (Hospital 1 and Hospital 2) in District 1 had made noticeable progress with the SDM and were expanding it beyond acute care to other units. We classify these cases as fitting the integrated implementation mode. Belonging to the same health care organization (the RO), they embraced the SDM. They used it to integrate other ongoing innovations, traded off resources to it, planned it carefully, and implemented it systematically, continually evaluating progress and making adjustments based on staff feedback. Accordingly, we labeled Hospital 1 and Hospital 2 as “effective implementation” sites.
Three of the hospitals (Hospital 3, Hospital 4, and Hospital 5) located in District 2 had at least one executive champion enthusiastic about the prospect of improved patient flow, and all had implemented at least some elements of the SDM. However, its uptake was highly variable across different hospital units. Because the SDM was added to several other initiatives without attempting to integrate them, we identified these sites as following a fragmented implementation mode. The interviewees characterized the implementation with terms such as “work in progress,” “a struggle,” and “daunting.” The withdrawal of the provincial funding was crippling, and the SDM was reduced in scope. We labeled these three sites as “work in progress.”
Integrated and Fragmented Implementation Modes
The two implementation modes—integrated and fragmented—are archetypes in the sense that the actual practices we observed at the three levels of analysis exemplify those modes approximately, as is the case with volitional human actors. We summarize the two implementation modes, with interview quotations, in Table 4 and elaborate on them below.
Table 4.
Integrated and fragmented implementation modes
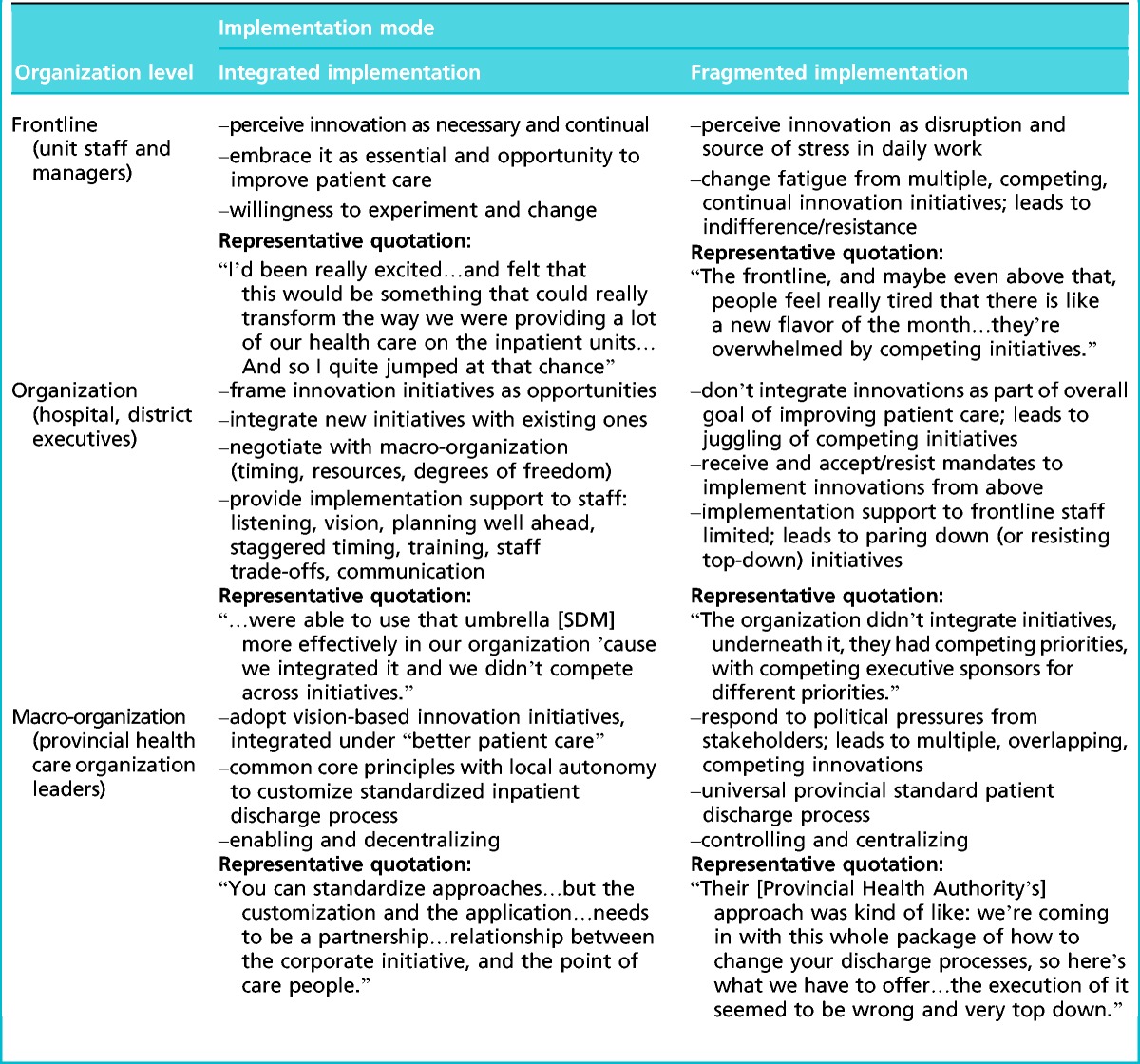
In the integrated implementation mode, actors from frontline staff to executives at the highest levels of the health care organization tend to frame innovation positively and to perceive it as necessary and continual. They embrace it as essential and as an opportunity to improve patient care. They are motivated to incorporate innovation in their daily work and open to changing their practices.
At the organizational level (the hospital or the geographic health care district), the integrated innovation mode manifests in the leaders’ framing and communicating innovation initiatives as opportunities. The leaders seek to integrate them with existing projects so that they make sense to the staff. When new innovations are externally mandated by a higher authority, the organization’s leadership negotiates with the authority. This is to ensure that timing, resources, and degrees of freedom facilitate local implementation and the new innovation initiative can be integrated with those ongoing. The leaders support the frontline staff, for example, by inviting and incorporating staff’s input in the implementation, providing a clear vision for integrating initiatives, training, and additional support (such as trade-off resources).
At the macro-organization level, integrated implementation means developing an overall philosophy for health care delivery based on a common vision, such as patient-centered care. Such a philosophy’s core principles serve as an integrating framework that guide health care districts and hospitals in implementation. A framework of basic principles with local autonomy to implement is necessary, because sites and districts vary significantly (in size, urban vs. rural setting, technologies available, etc.). This kind of integrated hierarchy—from an overall vision to guiding principles, to resource trade-offs and training and changed frontline practices—is critical for effective implementation.
The fragmented implementation mode looks quite different. In it, actors tend to perceive new initiatives as disruptive and a source of stress. They do see innovations as a continual stream, but not as integrated with a cohesive purpose and with each other. Therefore, actors experience “change fatigue” or “initiative burnout” and either resist or react with indifference to innovation initiatives.
At the organizational (hospital or district) level, leaders tend to have a similar collective response as the frontline staff. Rather than embracing new innovation initiatives as opportunities, they see their organizations as receiving burdensome mandates that add to the multiple ongoing initiatives. The leaders’ approach to these initiatives colors the frontline’s response as well. Organizations typically do not have sufficient resources to implement many initiatives simultaneously. Without integration and prioritization, resources are strained. As a consequence, many innovations become diluted or stall, and their outcomes are not evaluated. In some cases, the fragmented implementation mode manifests as resistance to top–down initiatives and as pursuit of own initiatives instead.
At the level of the provincial health care organization, the fragmented innovations approach manifests as responding to pressures from different stakeholders (such as the Ministry of Health). Overlapping or conflicting innovations are developed in response to the different pressure groups. Many of these innovations are intended as integrative, such as the standardized discharge process we studied, yet they often fail at integration. Reactive responses to external pressures hamper the development of a truly integrated implementation framework with a central purpose and an allowance for local customization.
Integrated Versus Fragmented Implementation Practices
We observed integrated implementation practices—adopting the SDM as an integrative innovation, assigning resources to it from elsewhere, planning, sequencing, evaluating the implementation carefully, and allowing local customization—primarily at the effective implementation sites. The work-in-progress sites made some attempts at integration; however, they were less effective and systematic across different levels. At these sites, the leaders saw the SDM more narrowly as a solution to capacity problems and not integrated with other innovations, did not make resource trade-offs to it, did not provide much implementation support beyond the initial planning and orientation, and did not particularly facilitate local customization. Therefore, we report integrated implementation practices at the effective implementation sites in their broader organizational context, with contrast to the more fragmented implementation practices at the work-in-progress sites.
Early commitment by senior leadership
Effective implementation sites: A vision of an integrative initiative. Both effective implementation sites were involved in the design phase of the project. Early on, this innovation project resonated with the RO’s leaders and hospital executives; they perceived it to fit well with other initiatives. The SDM was adopted as a top priority, and both Hospital 1 and Hospital 2 offered to pilot the initiative. One leader commented: “[I was] trying to look at where was the synergy…It took me a few months to really understand how this is all going to connect. Once I did, and felt that it was the right thing to do…[I] was quite successful in suggesting that all our energies needed to be focused on this initiative.”
Another interviewee also emphasized how the SDM became the top priority and a vehicle for integration: “We integrated several initiatives under this umbrella, so that it would actually be more successful.” She1 explained that it would not be possible to implement just the SDM without integrating the full scope of practice with it: “this [SDM] really redesigns care…in order to do that you actually have to do all the other stuff, too.”
The commitment to the SDM was not limited to executives; they continually communicated about it and sought the staff’s input to engage them with the project. A main communication venue was site level meetings, and communication was reinforced through daily interactions and a staff newsletter. For example, physicians were engaged early by inviting the physician leaders to site leadership meetings and by incorporating their input. The overarching idea of integrated patient care appealed to physicians and other staff: “[SDM] had a lot more play value [than other initiatives] with our physicians, and it had a lot more play value with our care teams, ‘cause they really want people [patients] to go home.”
Continual communication happened also through project evaluations, where feedback was sought not only from nurses and physicians but also from patients. These evaluations were shared at regular feedback meetings at the hospital units and through the internal newsletter to reinforce the project’s uptake.
Work-in-progress sites: A solution to capacity and inpatient flow. At the work-in-progress sites, at least one executive committed to the SDM early. They saw the value of the project in solving the constant capacity problem through improving inpatient flow. Many commented that the SDM was necessary to address the increasing demand for hospital beds. But the innovation was still perceived as one among many, and many executives felt that the SDM was handed to their sites by the PHA without much consultation. The leaders’ attitudes were also reflected by their staff. For example: “It was one of the more frustrating experiences I’ve been through as a leader in the PHA…we felt like we had lots of people trying to tell us what to do and how to do it, in a way it didn’t make sense to us…so the execution of it seemed to be wrong and very top down.” The leader’s comments were echoed by a frontline manager: “The problem tends to be: Here is a project—get it done.”
Although the executive champions at the work-in-progress sites were supported by their peers, getting “buy-in” from the other staff seemed more difficult. Many commented about a year after the SDM’s launch that there was “a fair ways to go before we get full buy-in” and that they struggled with getting the physicians engaged. The lack of engagement may reflect the executives’ initial communication about the project. Although they shared their own enthusiasm, the main communication vehicles were the project orientation sessions, facilitated by a project leader from the PHA. These sessions were considered helpful but not sufficient by site leaders: “The orientation wasn’t in-depth enough; we didn’t do as good of a job as we should have in explaining the ‘why’.” After the initial orientations, communication depended primarily on frontline managers. One of them commented: “Just trying to reinforce it as time permitted.”
Resource allocation to facilitate implementation
Effective implementation sites: “Skin in the game.” After prioritizing the SDM as an integrative innovation, leaders at the RO promptly assigned internal resources to complement the PHA’s funding. For example, an executive position was created: “I hired a Corporate Director of Access and I didn’t give her an operational portfolio; her first eight months was actually get our strategy, around [SDM] organized.”
In addition, a clinical operations lead position was created at each site, by seconding staff from elsewhere. She had clinical, LEAN process and management experience and understood the role of each health discipline. The clinical operations leaders worked with the project manager and hospital unit managers to implement the SDM. They followed the SDM rollout, staying for its duration at each unit (6 weeks to 2 weeks; shorter as lessons from the pilot experiences were applied). One interviewee emphasized that the two operations leads were a resource trade-off as opposed to an addition: “the work elsewhere was not going to get done” while the SDM was being implemented. She said that without such “skin in the game,” the implementation would fail.
The RO had its own small Organizational Development (OD) department that facilitated innovation initiatives. The two-person OD staff worked closely with the clinical operations leads to create detailed implementation plans for each work shift at all hospital units. Critical to these plans was the role of a consistent charge nurse: “Probably the biggest, most difficult shift…has been going back to a consistent charge nurse. And that has to be there, or the whole model falls apart…that charge nurse is really the quarterback for all the processes associated with [SDM].”
The OD staff also developed a coaching model for the SDM implementation. At Hospital 2 and Hospital 1, a keen nurse was identified at the first units. She was then allowed to move with the SDM rollout to subsequent units for the duration of the implementation. At each new unit, such a coach helped with implementation in the next unit.
Despite the RO and its hospitals’ ability to creatively stretch their resources through trade-offs, they were affected by the SDM funding cancelation by the PHA. For example, as part of that funding, they had been promised upgraded information technology, such as scheduling software for staff and patient appointments. They were left with their outdated information technology, perceived to hinder the SDM implementation due to the heavy administrative paperwork.
Work-in-progress sites: Lacking implementation resources. Although resources were scarce also at the effective implementation sites, the work-in-progress sites felt the resource shortage more acutely and were less able to make trade-offs. The PHA’s funding cancellation was more devastating. An interviewee said that “uptake of such a massive project was daunting; we didn’t know how to continue after [the provincially funded project leader] left.” At another site, they “kept going fast after the PHA funding was pulled, but cut many SDM modules.” At a third site, they were “incorporating as many as possible SDM elements with our own resources” and created a discharge working group; however, the implementation mainly depended on the unit quality councils. A frontline manager commented that there was no time to measure the SDM outcomes, and another said: “We also don’t have those supports to really help move that [SDM] along. It’s really staff themselves and management…groups to break away from routines, that’s harder to do without somebody kind of leading that for you.”
Upfront planning and implementation support
Effective implementation sites: Deliberate upfront planning and support for implementation. The SDM’s implementation at the RO’s effective implementation sites also depended on deliberate planning and careful sequencing. Planning started at the executive level as development of an overall strategy for the SDM as the RO’s integrative innovation, primarily by the Corporate Director of Access with the OD department’s help. Implementation was staggered, moving from medicine units, eventually, to mental health and geriatric units. The implementation sequence in each area depended on the readiness-for-change assessment. The frontline staff, including the hospital unit supervisors, were educated about the principles of the SDM and consulted on local customization: “Supervisors were really heavily involved…in the design, so lots of engagement that way, and I gave them as much choice as we possibly could, so we’d identify up front like, ‘here’s the non-negotiables.’”
The OD staff with the clinical operations leads designed detailed implementation plans for each shift, promoted the role of the constant charge nurse, and developed the coaching model.
Work-in-progress sites: Deliberate initial orientation—scarce implementation support. The interprofessional design teams for the SDM developed the service level agreements for each profession affecting patient flow and the project orientation/education modules. The PHA-funded project manager facilitated the implementation initially, rotating to each hospital for the rollout. However, this happened only for a few months before the provincial funding was canceled. Afterwards, a District Level Quality Improvement consultant offered some facilitation, but the implementation was left mostly for unit quality councils, which varied depending on the unit leadership and frontline managers. There were no detailed, shift-by-shift implementation plans, coaches, or much other facilitation.
Effectiveness of SDM Implementation
No independent analysis about the SDM implementation outcomes is available, but according to the interviewees, there had been a 1.5 day reduction in average length of stay at the two RO sites that had started to evaluate the implementation. White boards were widely used. Patient complaints had also decreased. These sites conducted regular monthly project evaluations, besides intermittent surveys of nursing staff, patients, and physicians, and made adjustments as needed. According to one leader: “It can take more than a year to sustain the [innovation].” The RO’s Operations group also evaluated the implementation semimonthly, and its Integrated Access Advisory Committee did the same every quarter. The feedback from the evaluations was mostly positive: “Qualitatively, people really like it; like even one unit that…essentially went into it kicking and screaming. And [now]…that unit is probably some of our biggest supporters; they’re like, ‘it works so well, everybody knows what’s happening.’”
The effective implementation sites also reported increased physician engagement with the SDM as they started to see the value of rapid rounds (Table 1) and the consistent charge nurse. Most were establishing the anticipated date of discharge (see Table 1) upon admission, although there was no 100% compliance yet.
Another indication of the implementation effectiveness was external recognition. The word about the success of the SDM implementation at the RO hospitals had spread: Their leaders had many invitations to give presentations about it at national health care conferences.
At the work-in-progress sites, the SDM implementation was assessed less positively. Interviewees reported success about white boards in patient rooms (Table 1). All three sites had made the white boards’ use a contest between units, with good results. However, there were challenges with getting physicians to do anticipated dates of discharge and with the multidisciplinary ward rounds (Table 1). One manager reported that the SDM had done nothing to change the length of stay at her site, although she acknowledged that there was no time to measure its impact. She also said, “on the whole, I think we’ve become a little more efficient in what we are doing.” The assessment of the SDM at the work-in-progress sites was also hindered by the discontinuation of provincial funding and the PHA’s order to stop using the SDM name and terminology, as yet another new initiative was anticipated.
Discussion
We studied implementation of a complex innovation: a system-wide SDM at five acute care hospitals within a broader health system context. The goal was to investigate why two of the hospitals were more effective in implementation whereas three others struggled more, despite the hospitals’ many similarities.
Our research design, utilizing multiple levels of analysis and focusing on actors’ practices and cross-level effects, contrasted more effective and less effective implementations and yielded new contributions. Our findings affirmed the Helfrich et al. (2007) model and Klein and Sorra’s (1996) original implementation model for complex innovations. Management support (in our study: early commitment by leadership and the practices it entailed) and resource availability (in our study: management allocating resources to implementation through stretching and trade-offs) were clearly observable in our data.
Our study also makes new contributions that extend the Helfrich et al. (2007) model. First, the two implementation modes we induced (integrative and fragmented) exemplify two different kinds of fit (or lack of fit) between the innovation and the users’ values and between two innovation climates (Helfrich et al., 2007). Second, employing multiple levels of analysis allowed a more fine-grained description of implementation practices.
The most important contribution of our study is the observation that integration of innovation initiatives (as opposed to having multiple competing initiatives vying for resources and staff’s attention and time) was the key driver of effective implementation. The significance of integrating innovations is that it allows the drivers of complex, sustainable innovations in health care settings to be organized in a hierarchy.
Whereas previous research (Helfrich et al., 2007; Klein & Sorra, 1996; McAlearney et al., 2013) has identified many determinants of effective implementation, our findings suggest that integration—in the sense of identifying one broad innovation initiative as primary to which all the others are subordinated—was the fundamental differentiator between more and less effective implementation. Integration required the health care organization’s leaders to identify the most suitable initiative as the umbrella and to sell it to the hospital executives. This enabled the hospital executives to serve one integrated innovation initiative to their project-weary frontline managers and staff as relief from the continual, new “flavor-of-the-month” initiatives. They also used the integrated innovation to coordinate other patient care improvements. Once the integrative innovation was selected, resource allocation, planning, and implementation practices followed the hierarchy of implementation requirements.
Limitations
Although we were fortunate to access participants in an ongoing innovation implementation and identified contrasting cases for comparing more and less effective implementation practices, the usual limitations of a qualitative case study in a single context apply (Jacobs et al., 2015). We highlight two of them here. First, we studied one mandated (as opposed to voluntary) innovation about inpatient flow in acute care hospitals in one Canadian provincial health system only. Therefore, any generalizations to different types of innovations and health care settings should be made cautiously. Second, although we collected data over a 1-year period and captured the implementation processes through the interviewers’ narratives as well as documentary evidence, we did not capture the entire trajectory of the innovation’s implementation. Future studies should be conducted longitudinally, where data are collected before, during, and after implementation.
Practical Implications
Our study provided insights that might be useful to executives and managers at health care organizations and hospitals facing implementation of complex innovations. First, it is important to integrate various innovation initiatives by selecting one major innovation as the umbrella under which others can be subsumed or to which they can be related. On the basis of our evidence, this is the fundamental driver of effective implementation and helps prioritize resources, sequence implementation, and engage staff. Without such integration, staff will be overwhelmed by the many overlapping initiatives that compete for their time and may react to them with resistance or indifference.
Second, as others have found in primary care settings (Reay et al., 2013), without dedicated human resources, implementations are likely to fail (volunteer innovation champions are not enough). This may require putting “skin in the game,” by stretching the existing resources, trading them off from elsewhere in the organization (such as the clinical operations leads and coach nurses in the effective implementation sites), or creating permanent positions (such as OD staff).
Third, the need for deliberate upfront planning of the implementation, including sequencing, staffing, and the shift level new practices, seems self-evident. Yet, such careful planning has been observed to be a challenge in other studies (Grove et al., 2010). Indeed, it differentiated the more and less effective implementation in our study and was clearly dependent on having the dedicated human resources available to both help with the planning and carrying out of the plans. To ensure that a complex innovation also “sticks,” regular evaluation and adjustment of the implementation are also necessary.
Finally, when standardization of a complex innovation across different sites is mandated, local customization within a framework of general guiding principles is important. Change in health care systems is difficult, particularly because of the professional staff’s ability to resist it and stick to established routines (Ferlie et al., 2005; Reay et al. 2013). Mandating standardized innovation across very different contexts is likely to trigger resistance; allowing local customization may help alleviate it and increase the fit between the innovation and the users’ values (Klein & Sorra, 1996; Helfrich et al., 2007).
Conclusion
Implementation of complex innovations in health care is challenging, yet necessary for health systems to meet the dual challenges of cost containment and effective patient care. Our study suggests that the key to the implementation of complex innovations is an integrated implementation mode and practices: early commitment by leadership to integrating innovations, stretching and trading off resources to facilitate implementation, upfront planning and ongoing implementation support and evaluation, and allowance for local customization. We suggest that these practices, as well as the more fine-grained practices at the hospital unit level we have identified, might help implement and establish complex innovations.
Acknowledgments
The authors thank all the anonymous participants for their time, input, and consideration. Special thanks to Diane Bischak and Bob Hinings for helpful comments on earlier drafts of the paper.
1We use the female gender to refer to all interviewees to further protect their anonymity.
The study was funded by an operating grant (177709) from the Canadian Institutes of Health Research and approved by the University of Calgary’s Conjoint Health Research Ethics Board (Study #REB13-0674).
Sachin Pendharkar was a member of the Steering Committee and Evaluation Lead for the initiative under study until approximately 6 months before this study began. For the remaining authors, none are declared.
References
- Bresman H. (2013). Changing routines: A process model of vicarious group learning in pharmaceutical R&D. Academy of Management Journal, 56(1), 36–61. [Google Scholar]
- Chou A. F., Yano E. M., McCoy K. D., Willis D. R., Doebbeling B. N. (2008). Structural and process factors affecting the implementation of antimicrobial resistance prevention and control strategies in U.S. hospitals. Health Care Management Review, 33(4), 308–322. [DOI] [PubMed] [Google Scholar]
- Eisenhardt K. (1989). Theory building from case study research. Academy of Management Review, 14(4), 532–550. [Google Scholar]
- Eriksson P., Kovalainen A. (2008). Qualitative methods for business research. Thousand Oaks, CA: Sage. [Google Scholar]
- Ferlie E., Fitzgerald L., Wood M., Hawkins C. (2005). The (non)diffusion of innovations: The mediating role of professional groups. Academy of Management Journal, 48(1), 117–134. [Google Scholar]
- Grove A., Meredith J., MacIntyre M., Angelis J., Neialy K. (2010). UK health visiting: challenges faced during lean implementation. Leadership in Health Services, 23(3), 2014–218. [Google Scholar]
- Helfrich C. D., Weiner B. J., McKinney M. M., Minasian L. (2007). Determinants of implementation effectiveness: Adapting a framework for complex innovations. Medical Care Research and Review, 64(3), 279–303. [DOI] [PubMed] [Google Scholar]
- Hinings C., Casebeer A., Reay T., Golden-Biddle K., Pablo A., Greenwood R. (2003). Regionalizing healthcare in Alberta: Legislated change, uncertainty and loose coupling. British Journal of Management, S15–S30. [Google Scholar]
- Jacobs S. R., Weiner B. J., Reeve B. B., Hofmann D. A., Christian M., Weinberger M. (2015). Determining the predictors of innovation implementation in healthcare: A quantitative analysis of implementation effectiveness. BMC Health Services Research, 15, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K. J., Conn A. B., Sorra J. S. (2001). Implementing computerized technology: An organizational analysis. Journal of Applied Psychology, 86(5), 811–24. [DOI] [PubMed] [Google Scholar]
- Klein K., Sorra J. (1996). The challenge of innovation implementation. Academy of Management Review, 21(40), 1055–1080. [Google Scholar]
- Kotter J. (1995). Leading change: Why transformation efforts fail. Harvard Business Review, 73(2), 59–68. [Google Scholar]
- Lukas C. V., Holmes S. K., Cohen A. B., Restuccia J., Cramer I. E., Schwartz M., Charns M. P. (2007). Transformational change in health care systems: An organizational model. Health Care Management Review, 32(4), 309–320. [DOI] [PubMed] [Google Scholar]
- McAlearney A. S., Robbins J., Garman A. N., Song P. H. (2013). Implementing high-performance work practices in healthcare organizations: Qualitative and conceptual evidence. Journal of Healthcare Management, 58(6), 446–464. [PubMed] [Google Scholar]
- Reay T., Goodrick E., Casebeer A., Hinings C. R. (2013). Legitimizing new practices in primary health care. Health Care Management Review, 38(1), 9–19. [DOI] [PubMed] [Google Scholar]
- Rogers E. M. (2003) Diffusion of innovations. New York, NY: Free Press. [Google Scholar]
- Thatchenkery T., Firbida I. (2013). Appreciative intelligence and generativity: A case study of Rocky Flats nuclear weapons facility cleanup. Cooperrider D., Zandee D., Godwin L., Avital M., Boland B., (Eds.). Advances in Appreciative Inquiry, 4, 409–432. [Google Scholar]
- Yin R. (2009). Case study research: Design and methods. Thousand Oaks, CA: Sage. [Google Scholar]


