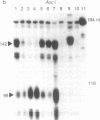
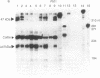
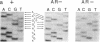Abstract
Chronic granulomatous disease (CGD) is a rare inherited condition rendering neutrophils incapable of killing invading pathogens. This condition is due to the failure of a multicomponent microbicidal oxidase that normally yields a low-midpoint-potential b cytochrome (cytochrome b245). Although defects in the X chromosome-linked cytochrome account for the majority of CGD patients, as many as 30% of CGD cases are due to an autosomal recessive disease. Of these, greater than 90% have been shown to be defective in the synthesis of a 47-kDa cytosolic component of the oxidase. We demonstrate here in three unrelated cases of autosomal recessive CGD that the identical underlying molecular lesion is a dinucleotide deletion at a GTGT tandem repeat, corresponding to the acceptor site of the first intron-exon junction. Slippage of the DNA duplex at this site may contribute to the high frequency of defects in this gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley D. L., Groudine M. Novel promoter upstream of the human c-myc gene and regulation of c-myc expression in B-cell lymphomas. Mol Cell Biol. 1986 Oct;6(10):3481–3489. doi: 10.1128/mcb.6.10.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Malech H. L., Gallin J. I., Nunoi H., Volpp B. D., Pearson D. W., Nauseef W. M., Curnutte J. T. Genetic variants of chronic granulomatous disease: prevalence of deficiencies of two cytosolic components of the NADPH oxidase system. N Engl J Med. 1989 Sep 7;321(10):647–652. doi: 10.1056/NEJM198909073211005. [DOI] [PubMed] [Google Scholar]
- Dinauer M. C., Pierce E. A., Bruns G. A., Curnutte J. T., Orkin S. H. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990 Nov;86(5):1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding G. B., Glickman B. W. Evidence for local DNA influences on patterns of substitutions in the human alpha-interferon gene family. Can J Genet Cytol. 1986 Aug;28(4):483–496. doi: 10.1139/g86-072. [DOI] [PubMed] [Google Scholar]
- Jorde L. B., Lathrop G. M. A test of the heterozygote-advantage hypothesis in cystic fibrosis carriers. Am J Hum Genet. 1988 Jun;42(6):808–815. [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Markham A. F., Chapman C. R., Youssoufian H., Waber P. G. Quantification of the close association between DNA haplotypes and specific beta-thalassaemia mutations in Mediterraneans. Nature. 1984 Jul 12;310(5973):152–154. doi: 10.1038/310152a0. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax K. J., Leto T. L., Nunoi H., Gallin J. I., Malech H. L. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science. 1989 Jul 28;245(4916):409–412. doi: 10.1126/science.2547247. [DOI] [PubMed] [Google Scholar]
- Maly F. E., Cross A. R., Jones O. T., Wolf-Vorbeck G., Walker C., Dahinden C. A., De Weck A. L. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J Immunol. 1988 Apr 1;140(7):2334–2339. [PubMed] [Google Scholar]
- Maly F. E., Nakamura M., Gauchat J. F., Urwyler A., Walker C., Dahinden C. A., Cross A. R., Jones O. T., de Weck A. L. Superoxide-dependent nitroblue tetrazolium reduction and expression of cytochrome b-245 components by human tonsillar B lymphocytes and B cell lines. J Immunol. 1989 Feb 15;142(4):1260–1267. [PubMed] [Google Scholar]
- Matsuzaki F., Matsumoto S., Yahara I., Yonezawa N., Nishida E., Sakai H. Cloning and characterization of porcine brain cofilin cDNA. Cofilin contains the nuclear transport signal sequence. J Biol Chem. 1988 Aug 15;263(23):11564–11568. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoi H., Rotrosen D., Gallin J. I., Malech H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988 Dec 2;242(4883):1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- Rodaway A. R., Teahan C. G., Casimir C. M., Segal A. W., Bentley D. L. Characterization of the 47-kilodalton autosomal chronic granulomatous disease protein: tissue-specific expression and transcriptional control by retinoic acid. Mol Cell Biol. 1990 Oct;10(10):5388–5396. doi: 10.1128/mcb.10.10.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaya S., Rieder R. F. Dysfunctional alpha-globin gene in hemoglobin H disease in blacks. A dinucleotide deletion produces a frameshift and a termination codon. J Biol Chem. 1988 Mar 25;263(9):4328–4332. [PubMed] [Google Scholar]
- Segal A. W. Cytochrome b-245 and its involvement in the molecular pathology of chronic granulomatous disease. Hematol Oncol Clin North Am. 1988 Jun;2(2):213–223. [PubMed] [Google Scholar]
- Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985 Aug 8;316(6028):547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W. The electron transport chain of the microbicidal oxidase of phagocytic cells and its involvement in the molecular pathology of chronic granulomatous disease. J Clin Invest. 1989 Jun;83(6):1785–1793. doi: 10.1172/JCI114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifers R. N., Brashears-Macatee S., Kidd V. J., Muensch H., Woo S. L. A frameshift mutation results in a truncated alpha 1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem. 1988 May 25;263(15):7330–7335. [PubMed] [Google Scholar]
- Teahan C. G., Totty N., Casimir C. M., Segal A. W. Purification of the 47 kDa phosphoprotein associated with the NADPH oxidase of human neutrophils. Biochem J. 1990 Apr 15;267(2):485–489. doi: 10.1042/bj2670485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teahan C., Rowe P., Parker P., Totty N., Segal A. W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. 1987 Jun 25-Jul 1Nature. 327(6124):720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Clark R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988 Dec 2;242(4883):1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Donelson J. E., Moser D. R., Clark R. A. Cloning of the cDNA and functional expression of the 47-kilodalton cytosolic component of human neutrophil respiratory burst oxidase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7195–7199. doi: 10.1073/pnas.86.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. B., Amos J., Hsu J. M., Gerrard B., Finn P., Dean M. A frame-shift mutation in the cystic fibrosis gene. Nature. 1990 Apr 12;344(6267):665–667. doi: 10.1038/344665a0. [DOI] [PubMed] [Google Scholar]