Abstract
The resuscitation of traumatic hemorrhagic shock has undergone a paradigm shift in the last 20 years with the advent of damage control resuscitation (DCR). Major principles of DCR include minimization of crystalloid, permissive hypotension, transfusion of a balanced ratio of blood products, and goal-directed correction of coagulopathy. In particular, plasma has replaced crystalloid as the primary means for volume expansion for traumatic hemorrhagic shock. Predicting which patient will require DCR by prompt and accurate activation of a massive transfusion protocol, however, remains a challenge.
Keywords: massive transfusion protocol, hemorrhagic shock, resuscitation
Introduction
Hemorrhage is one of the top causes of death after injury and is the leading cause of potentially preventable trauma deaths.1,2,3 In contrast to other causes of trauma death – such as traumatic brain injury (TBI), multiple organ dysfunction syndrome (MODS), and sepsis – exsanguination occurs rapidly with a median time to death of 2–3 hours after presentation.4,5 Advances in the treatment of hemorrhagic shock have historically been made during times of armed conflict: major milestones include the first blood banks during World War I, the development of dried plasma during World War II, recognition of a close association between shock and coagulopathy during the Vietnam War,6 and the advent of damage control resuscitation (DCR) during the recent wars in Afghanistan and Iraq. DCR was a paradigm shift in the management of traumatic hemorrhagic shock and will be a major focus of this chapter.
As the management of trauma evolves over time, we are discovering more and more that resuscitation of the severely injured patient – the normalization of deranged physiology and correction of the shock state – has as much impact on patient outcome as surgical treatment of the injured tissues. Development of an optimal resuscitation strategy with attention to the type, quantity, and timing of fluid therapy is of paramount importance to any clinician caring for trauma patients presenting with hemorrhagic shock.
Patient Evaluation Overview
A small minority (1–3%) of patients presenting to a major urban trauma center will require substantial blood product transfusion following injury, typically referred to as “massive transfusion” (MT). Traditionally, MT had been arbitrarily defined as transfusion of at least 10 units of packed red blood cells (PRBC) within 24 hours. Early identification of the trauma patient who will require MT is difficult, but nonetheless essential since early activation of a predefined massive transfusion protocol (MTP) is associated with decreased blood product waste7 as well as reduced incidence of organ failure and other complications.8
Several scoring systems have been proposed to predict need for MT, but early iterations such as the Trauma Associated Severe Haemorrhage (TASH) score9 and the McLaughlin score10 relied on laboratory values which are not available until some time after presentation. In 2010, Cotton et al published a multicenter validation study of the Assessment of Blood Consumption (ABC) score, which gives one point for each of the following: penetrating mechanism, systolic blood pressure <90 mmHg, heart rate >120 beats per minute, and positive focused abdominal sonography in trauma (FAST) exam (Box 1) (Table).11
Box 1. Assessment of blood consumption (ABC) score. Score of ≥2 points predicted need for MT within 24 hours with sensitivity 75–90%, specificity 67–86%, and overall accuracy 84–86%.
|
MT, massive transfusion; FAST, focused assessment with sonography in trauma.
Table Criteria to trigger MTP activation (1 or more):
|
| From: |
| ACS TQIP Massive Transfusion in Trauma Guidelines |
| https://www.facs.org/~/media/files/quality%20programs/trauma/tqip/massive%20transfusion%20in%20trauma%20guildelines.ashx |
A score of ≥2 was predictive of MT with a sensitivity of 75% to 90%, specificity of 67% to 88%, and overall accuracy of 84 to 87% for all trauma patients. Importantly, the ABC score requires no laboratory data, can be determined within minutes of patient arrival, and can be easily recalculated over time. The American College of Surgeons Trauma Quality Improvement Program (TQIP) Massive Transfusion in Trauma Guidelines now recommend use of the ABC score of 2 or more for MTP activation.
Pommerening et al examined the reliability of physician gestalt in predicting need for MT by performing a secondary analysis on patients from the Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study.12 Of note, entry into this study required transfusion of at least 1 PRBC unit within the first 6 hours, and patients who died within 30 minutes of presentation were excluded. Therefore, included patients were at overall intermediate risk for requiring MT, while patients at the two extremes were excluded. In this patient group, investigators found that physician gestalt and several scoring systems including ABC performed relatively poorly, achieving modest sensitivities and specificities of 65–70%. Independent predictors of a false negative physician gestalt were bleeding in the pelvis and relatively normal blood gas parameters.
Viscoelastic assays of coagulation (thromboelastography [TEG] and rotational thromboelastometry [ROTEM]) can be used to diagnose and monitor trauma-induced coagulopathy and is covered in detail elsewhere in this series. Since clinically meaningful results can be available within minutes of trauma patient arrival, TEG and ROTEM have been proposed as predictors of need for MT. Prospective observational studies by Cotton et al13 and Davenport et al14 found that admission TEG and ROTEM parameters available within 5 minutes of assay initiation more accurately predicted MT compared to the slower conventional coagulation assays.
One difficulty in conducting MT research is that the traditional definition upon which much of the literature is based (≥10 PRBC units within 24 hours) is woefully inadequate: it does not capture the intensity of resuscitative efforts and is prone to survivor bias.15 In particular, rapidly hemorrhaging patients are excluded when they exsanguinate before reaching the threshold, while less critically ill patients are included when they accrue units steadily over 24 hours. In response, newer definitions of MT which delineate use of blood products within a narrower timeframe have gained acceptance. For example, the concept of the critical administration threshold (CAT), defined as the transfusion of at least 3 PRBC units within any 1 hour time window within the first 24 hours,16 has been prospectively validated in trauma patients and found to be a more sensitive predictor of mortality than the traditional MT definition.17 An advantage of CAT is that it can be obtained prospectively – studies have shown that patients who reach CAT soon after presentation or who reach CAT multiple times have increased mortality,16,17 and CAT may serve as an indicator to proceed with abbreviated instead of definitive laparotomy.18
Pharmacologic Treatment Options
The term “damage control” originated in the United States Navy to describe the protocol used to save a ship which has suffered catastrophic structural damage from sinking, placing a heavy emphasis on the limitation and containment of fires and flooding.19 This term was adopted by trauma surgeons to describe the use of abbreviated surgeries to rapidly temporize life threatening injuries (namely, hemorrhage) with delay of definitive repair until after adequate resuscitation. Damage control surgery is associated with improved survival in severely injured trauma patients presenting with profound physiologic derangement,20,21 but is associated with several complications including ventral hernias, surgical site infections, and increased ventilator dependence.22
Damage control resuscitation (DCR) describes the resuscitation strategy that evolved in conjunction with damage control surgery. It represents a major paradigm shift away from the old days of resuscitation with isotonic crystalloids, followed sequentially by PRBCs, plasma, and finally platelets. Instead, DCR emphasizes the early use of plasma to not only restore volume, but also correct major physiologic derangements including hypoperfusion, shock, and coagulopathy. Major principles of DCR in the management of hemorrhagic shock include minimization of isotonic crystalloids, permissive hypotension, transfusion of a balanced ratio of blood products, and goal-directed correction of coagulopathy (Box 2).
Box 2. Components of damage control resuscitation (DCR).
|
Minimization of crystalloid
Isotonic crystalloids
Before the advent of DCR, isotonic crystalloids were a major component of fluid therapy for patients presenting with traumatic hemorrhagic shock. In the 1980s, Shoemaker et al reported associations between increased cardiac output, oxygen delivery, and survival in critically ill surgical patients.23,24 The replication of this finding in a prospective randomized trial25 helped popularize the strategy of “supra-normal” resuscitation, which utilized large boluses of isotonic crystalloids to drive cardiac output and oxygen delivery to supra-normal levels. At about this time, damage control laparotomy was first described and also began gaining widespread use.20,21 The combination of these two treatment modalities in severely injured trauma patients resulted in the inability to close the abdominal wall due to massive intestinal and retroperitoneal edema,26 which often persisted well after hemorrhagic shock was corrected. In 2003, Balogh et al demonstrated an association between supra-normal resuscitation and increased incidences of abdominal compartment syndrome, multiple organ failure, and mortality.27
Afterward, investigators began taking a closer look at the consequences of aggressive fluid therapy in surgical patients.28 For example, intracellular edema secondary to aggressive crystalloid infusion was found to disrupt many vital biochemical processes including pancreatic insulin synthesis and secretion,29 hepatocyte glucose metabolism,30 and cardiac myocyte excitability.31 A subsequent randomized trial of supra-normal resuscitation failed to replicate Shoemaker’s initial success.32 Still other trials reported that patients randomized to “restricted” intravenous fluid resuscitation after elective surgery experienced less post-operative ileus33 and fewer cardiopulmonary and wound complications34 compared to those who underwent “standard” fluid resuscitation. Additional observational studies demonstrated significant associations between large volumes of crystalloid and adverse outcomes such as increased incidence of dilutional coagulopathy, acute respiratory distress syndrome (ARDS), MODS, extremity and abdominal compartment syndromes, and mortality in severely injured trauma patients.35,36 Analysis of the PROMMTT dataset found that increasing crystalloid use was independently associated with moderate or severe hypoxemia.37
Although clinicians now recognize the significant complications associated with infusing large volumes of crystalloid in severely injured trauma patients, the safety of relatively small crystalloid volumes has also been questioned. A retrospective study of over 3,000 trauma patients by Ley et al found that infusion of ≥1.5 liters of crystalloid in the emergency department was independently associated with increased mortality, although the authors did not report or include in their analysis the use of blood products.38 A retrospective study of over 1,200 blunt trauma patients dichotomized patients into high (>500 ml) and low (≤500 ml) groups based on infusion of prehospital crystalloid and stratified patients by presence of prehospital hypotension.39 After propensity adjustment including PRBC units transfused, the investigators reported that there was no difference in mortality in patients with prehospital hypotension, but >500 ml prehospital crystalloid was associated with increased mortality (hazard ratio 2.5, 95% confidence interval [CI] 1.3 – 4.9) and increased coagulopathy by admission international normalized ratio (INR) (odds ratio [OR] 2.2, 95% CI 1.0 – 4.9) in patients without prehospital hypotension. While it is clear that liberal infusion of crystalloid in trauma patients is harmful, it is interesting that any volume of crystalloid may cause harm – or, at least, offer no benefit.
Hypertonic saline
The trauma community has long been interested in hypertonic saline (HTS) as an initial resuscitative fluid after traumatic hemorrhage. Several in vitro and animal studies have demonstrated benefits of initial HTS resuscitation including brisk increases in mean arterial pressure and cardiac output,40 improved microciculatory flow by decreasing endothelial cell edema,41 and favorable immunomodulatory effects.42,43 In small randomized trials of trauma patients presenting with hemorrhagic shock, initial resuscitation with HTS resulted in decreased pro-inflammatory and increased anti-inflammatory markers.44,45 The Resuscitation Outcomes Consortium (ROC) performed a multicenter randomized control trial investigating prehospital resuscitation with normal saline, HTS, or hypertonic saline dextran (HSD) for patients with hemorrhagic shock.46 After enrollment of 853 patients, interim analysis demonstrated no mortality difference between groups, and the trial was stopped early due to futility. Further analysis demonstrated that the hypertonic solutions, particularly HSD, resulted in worse coagulopathy on admission compared to prehospital resuscitation with normal saline.47
Enthusiasm for HTS has waned since publication of the ROC trial results. Based on the above data, crystalloids including HTS have little role in the initial resuscitation of traumatic hemorrhagic shock. As will be explained below, plasma has become the preferred means of volume expansion in this patient population.
Permissive hypotension
While caring for combat casualties during World War I, W.B. Cannon recognized that
“Injection of a fluid that will increase blood pressure has dangers in itself. Hemorrhage in a case of shock may not have occurred to a marked degree because blood pressure has been too low and the flow too scant to overcome the obstacle offered by the clot.”48
The goal of permissive hypotension is to maintain only the minimal blood pressure necessary to perfuse the vital organs. The rationale, which Cannon recognized a century ago, is that elevations in blood pressure before surgical hemostasis is achieved may compromise a tenuous clot and exacerbate blood loss. Much of the evidence for this practice comes from animal studies. In a swine model of uncontrolled hemorrhage, Sondeen et al demonstrated that there was a reproducible mean arterial blood pressure of 64 ± 2 mmHg where the clot was “popped” and rebleeding occurred.49 A meta-analysis identified 9 animal studies which investigated hypotensive resuscitation after hemorrhage, all of which reported decreased mortality with a pooled relative risk of 0.37 (95% CI 0.33 – 0.71) in animals undergoing hypotensive fluid resuscitation compared to those undergoing normotensive resuscitation.50
Comparatively, there are fewer such studies in patients. Bickell et al published the first such study in 1994, which randomized 289 patients after penetrating torso injury to standard fluid resuscitation which was begun prehospital or delayed fluid resuscitation which was begun in the operating room.51 The authors reported significantly improved survival in the delayed resuscitation group (70% versus 62%). However, subsequent studies have reported mixed results. Dutton et al randomized 110 patients with hemorrhagic shock in the emergency department to initial fluid resuscitation with high (100 mmHg) and low (70 mmHg) systolic blood pressure (SBP) goals and reported no mortality difference between groups in 2002.52 A multicenter pilot trial by ROC randomized 192 prehospital hypotensive trauma patients to high (110 mmHg) and low (70 mmHg) SBP goals and reported improved 24-hour survival in the low SBP group after blunt trauma (97% versus 82%), but no difference after penetrating trauma (81% versus 81%).53 Significantly, all of the randomized trials cited above excluded patients with significant head injury.
The ideal target blood pressure for the initial resuscitation of hemorrhagic shock before definitive hemorrhage control remains unclear. There are currently no concrete recommendations from any of the leading trauma organizations. An open question is how low and for how long the blood pressure can be kept before the harm outweighs the benefit. Another consideration is the head-injured patient, where even a single episode of hypotension may substantially increase TBI-related morbidity and mortality.54 Finally, previous studies utilized crystalloid to achieve blood pressure goals, and there is currently little data regarding hypotensive resuscitation with blood products such as plasma. However, the available data suggests permissive hypotension is probably safe for short periods of time (in the absence of TBI) until definitive hemorrhage control can be achieved.
Optimal transfusion of blood products
In the mid-17th century, Richard Lower performed the first successful animal-to-animal blood transfusion when he demonstrated that a dog hemorrhaged nearly to the point of death could be “completely restored” by the transfusion of another dog’s blood.55 In the early 19th century, English obstetrician James Blundell performed the first successful human-to-human blood transfusion to save the life of a woman with postpartum hemorrhage.56 However, several barriers including infections secondary to lack of sterile technique, no understanding of the different blood types, and catheter thrombosis due to deficient knowledge of anticoagulants made blood transfusion prohibitively dangerous and difficult. It was not until the early 20th century when these barriers were overcome that blood transfusions could become routine. For the next 50 years, transfusion of whole blood was the norm. By the 1970s, however, component therapy had replaced whole blood transfusions in order to maximize the efficient utilization of donated blood and to limit the spread of blood-borne pathogens.
Plasma
Early definitive hemorrhage control and MT with DCR are the preferred treatment for the trauma patient in severe hemorrhagic shock. The actual composition of a MTP has changed drastically over the last 20 years. Prior to the advent of DCR, a trauma patient undergoing MT would have received stepwise resuscitation with crystalloid, artificial colloids, and PRBCs. Not until 1–2 blood volumes have already been replaced would plasma and platelets be given.57 This all changed after the shift to DCR, which began in earnest with the landmark study by Borgman et al in 2007, a retrospective study of 246 massively transfused military trauma patients treated at a US combat support hospital in Iraq.58 The authors separated patients into 3 groups by ratio of plasma to PRBC: low (median ratio 1:8), medium (median ratio 1:2.5), and high (median ratio 1:1.4). The all-cause mortality for the 3 groups were 65%, 34%, and 19% respectively, while the mortality due to hemorrhage were 93%, 78%, and 37% respectively. Every 1 unit increase in the ratio of plasma to PRBC was associated with an odds ratio of 8.6 (95% CI 2.1 – 35.2) improved likelihood of survival. The same year, Johansson et al demonstrated that early transfusion of high ratios of plasma and platelets in patients who underwent repair of a ruptured abdominal aortic aneurysm had significantly improved 30 day survival (66% versus 44%) compared to historical controls.59
Further observational data describing the benefit of early high plasma ratios followed for civilian trauma patients.60,61,62,63 In particular, the Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study was a multicenter prospective observational trial which analyzed 905 bleeding trauma patients who received at least 1 PRBC unit within 6 hours, at least 3 PRBCs units within 24 hours, and survived for at least 30 minutes after arrival.4 PROMMTT demonstrated that early utilization of higher ratios of plasma and platelets to PRBCs was associated with decreased in-hospital mortality. Specifically, every unit increase in the plasma to PRBC and platelet to PRBC ratios within the first 6 hours (when hemorrhage was the primary cause of death) was associated with an adjusted hazard ratio of 0.31 (95% CI 0.16 – 0.58) and 0.55 (95% CI 0.31 – 0.98) of in-hospital mortality, respectively. After the first 24 hours when other causes of death increased in incidence, ratios of plasma and platelets to PRBCs were no longer significantly associated with mortality.
The first randomized control trial investigating the optimal ratio of blood products was the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) study, a multicenter study which randomized 680 severely injured, bleeding trauma patients to resuscitation with a 1:1:1 or 1:1:2 ratio of plasma, platelets, and PRBC.5 The investigators found no differences in 24-hour or 30-day mortality, but patients in the higher plasma and platelets ratio (1:1:1) group had significantly increased achievement of hemostasis (86% vs 78%) and decreased death due to bleeding (9% versus 15%) compared to the low ratio (1:1:2) group. Other studies have demonstrated improved patient outcomes after implementation of DCR principles. Shrestha et al have shown increased likelihood of successful non-operative management and survival in civilian patients with high grade liver injuries after blunt trauma.64 In the military setting, soldiers injured in combat are also surviving with more severe injuries after implementation of DCR.65 Based on these studies, the ACS TQIP Massive Transfusion in Trauma Guidelines recommend DCR in patients who meet MTP triggers (see comment 1) (Table 2)
Table 2.
If MTP Triggers are met, initiate DCR:
|
From:
ACS TQIP Massive Transfusion in Trauma Guidelines
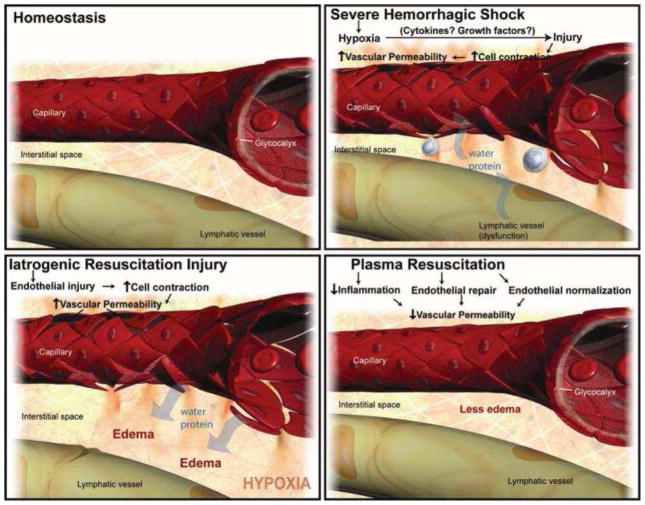
The underlying mechanism behind these benefits, however, is unclear. Restoration of intravascular volume and correction of coagulopathy are clearly important aspects. In animal models of hemorrhagic shock, plasma-based resuscitation mitigated hyperfibrinolysis66 and platelet dysfunction67 compared to crystalloid resuscitation. However, proteins involved with coagulation represent only a small fraction of the human plasma proteome. Besides restoration of intravascular volume and clotting factors, another benefit is likely repair of endothelial injury. Severe trauma68 as well as several other inflammatory conditions including diabetes,69 sepsis,70 and ischemia-reperfusion,71 are known to result in injury to the endothelium with loss of microvascular integrity, resulting in extravasation of intravascular fluid into the interstitial space. Liberal resuscitation with crystalloids and artificial colloids increases hydrostatic pressure without repairing the endothelial injury, resulting in edema and the edema-related complications which were common in the pre-DCR era (Figure 1). In contrast, in vitro72,73,74 and animal models75,76 of hemorrhagic shock demonstrate that plasma restores microvascular integrity, in part by repair of the endothelial glycocalyx layer (EGL). In a large animal model of concomitant hemorrhagic shock and TBI, resuscitation with FFP resulted in less secondary brain injury compared to resuscitation with crystalloid or artificial colloid, likely secondary to restoration of cerebral endothelium.77 In trauma patients, there are strong correlations between increasing circulating levels of glycocalyx components (a marker for EGL injury) and trauma severity, coagulopathy, and mortality,78,79,80 although it remains unclear if these relationships are causative or merely associative.
Figure 1.
Proposed effect of hemorrhagic shock and crystalloid versus plasma resuscitation on the microvasculature. Panel A. Homeostasis prior to injury. Panel B. Hemorrhagic shock results in shedding of EGL components, resulting in endothelial injury, microvascular permeability, and leakage of fluid into the interstitial space. Panel C. Crystalloids increase hydrostatic pressure in the presence of persistent endothelial injury, resulting in edema. Panel D. Plasma restores intravascular volume while restoring the EGL and repairing endothelial injury, limiting edema. EGL, endothelial glycocalyx layer
From Pati S, Matijevic N, Doursout MF. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma 2010;69(Suppl 1):S55–63. Used with permission.
Clinicians have long recognized that time is against the bleeding trauma patient and that faster initiation of lifesaving interventions improves outcomes. In light of this, key logistical hurdles must be overcome in order to expedite the delivery of plasma. Blood banks stock fresh frozen plasma (FFP), which has a shelf of life of up to one year at −18°C but requires 20–30 minutes of thaw time prior to use, limiting immediate availability. Options to make plasma readily available for emergency use include stocking thawed plasma and liquid plasma. After FFP is thawed, the most labile clotting factors (V and VIII) maintain 65% of their activity at the end of its 5 day shelf life.81 Liquid plasma, on the other hand, is never frozen and includes a preservative to maintain stability of most clotting factors for up to 26 days. Toward the end of its shelf life, most clotting factors maintain 88% activity, and in vitro studies demonstrate that never-frozen liquid plasma has a better coagulation profile than thawed plasma.82
Another logistical hurdle affecting speed of delivery is storage location. For example, a before-and-after study demonstrated that moving 4 units of universal-donor thawed plasma from the blood bank to the emergency department was independently associated with decreased time to first plasma transfusion, decreased usage of PRBCs (coefficient −2.9, 95% CI −5.7 to −0.2) and plasma (coefficient −2.7, 95% CI −5.4 to −0.1) within the first 24 hours, and decreased mortality (OR 0.43, 95% CI 0.19 – 0.96).83 The multicenter PROPPR study demonstrated the feasibility of high-level trauma centers to rapidly deliver universal donor plasma to hemorrhaging trauma patients quickly and consistently with minimal wastage.84
In the last several years, many have proposed transitioning hospital interventions for trauma patients to the prehospital phase of care, including plasma. A retrospective study which analyzed 1,677 severely injured trauma patients who were transported by helicopter found that in-flight plasma transfusion was associated with improved acid-base status on arrival and decreased overall transfusion of blood products within the first 24 hours.85 Although there was no mortality benefit in all patients, prehospital plasma transfusion was associated with decreased mortality in the most critically ill patients (those admitted directly to the ICU, IR, OR, or morgue). Randomized trials to study the impact of prehospital plasma in ground86 and aeromedical transport87 are on-going.
A final logistic consideration is that individuals with the universal plasma donor AB blood type comprise only 4% of the population of the United States.88 The scarcity of AB plasma has been further exacerbated by the widespread adoption of DCR principles. To circumvent this shortage, the use of type A plasma has been proposed as an alternative. The rationale is that 1) 85% of individuals in the US have either type A or type O blood,88 making type A plasma compatible with the vast majority of potential recipients, 2) the plasma transfused with type O apheresis platelets is routinely given to type-incompatible recipients with hemolytic reactions occurring very rarely,89,90 3) laboratory examination of male type A plasma units found predominantly low titers of anti-B,91 and 4) the risk of transfusion-related acute lung injury (TRALI) is currently much higher with type AB plasma than type A.92 Based on limited retrospective data, the emergency use of type A plasma appears safe.93,94,95 As this practice becomes widespread,96 more accurate data about the safety profile of this practice will soon become available.
Previous studies had raised concerns regarding the safety of increased plasma use, citing potentially increased risk of developing inflammatory complications such as TRALI, ARDS, and MODS.97,98,99 Analysis of the prospectively-acquired PROMMTT dataset did not demonstrate an independent association between blood product use and moderate-to-severe hypoxemia.37 Indeed, the PROPPR randomized trial found no difference in inflammatory or transfusion-related complications between the high and low ratio groups.5
Platelets
The inclusion of platelets with a balanced MTP approach is intuitive in order to more accurately mimic the whole blood that was lost. Retrospective studies60,100,101 report increased survival in massively transfused patients who received high ratios of platelets to PRBCs. PROMMTT provided prospective observational data demonstrating that every unit increase in the platelet to PRBC ratio decreased the hazard ratio of mortality within the first 6 hours by 0.55 (95% CI 0.31 – 0.98).4 As was the case with plasma, this relationship parallels the risk of death from hemorrhage and became weaker with time such that the platelet to PRBC ratio after 24 hours was no longer significantly associated with mortality. In the PROPPR randomized trial,5 patients in the high plasma and platelets ratio group had lower death due to hemorrhage, although the independent effects of plasma and platelets cannot be disentangled in this study. The most recent guidelines from the American College of Surgeons Committee on Trauma recommend transfusing one unit of platelets (what was previously known as a “six-pack” of platelets) for every 6 PRBC units.102
Fresh whole blood
Fresh whole blood (FWB) can be stored at room temperature for up to 8 hours and ≤6°C for 24–48 hours depending on institutional guidelines.103 Although economic and other considerations have all but eliminated the civilian use of FWB, several factors make FWB an attractive option for the resuscitation of hemorrhagic shock. First, the diluting effect secondary to the anticoagulants and additives in each blood product component reduces the hematocrit, factor activity, and platelet count of a 1:1:1 ratio of component therapy compared to a unit of FWB (Table 1).104 An in vitro study compared the parameters of FWB and reconstituted whole blood (1:1:1 ratio component therapy) and reported findings similar to the theoretical calculations.105 Second, the use of fresh whole blood would preclude the loss of quality of blood product components due to storage time.106,107,108 Indeed, use of blood products with increased storage duration is associated with increased mortality and morbidity.109,110,111 Third, use of FWB would reduce the number of donors to which the recipient is exposed and may reduce the risk of blood-borne pathogens. Finally, transfusion of one whole blood unit is logistically simpler than transfusion of multiple components and may reduce harm from administrative errors.
Table 1.
Calculated parameters of fresh whole blood versus reconstituted whole blood with 1 PRBC unit (335 mL with hematocrit of 55%), 1 platelet unit (5.5 × 1010 platelets in 50 mL), and 1 FFP unit (80% coagulation factor activity).
| Fresh whole blood | 1:1:1 component therapy | |
|---|---|---|
| Hematocrit (%) | 38–50 | 29 |
| Platelets (x 109/L) | 150–400 | 88 |
| Coagulation factor activity (%) | 100 | 65 |
The limited availability of refrigeration and potential shortages of blood product components means that FWB will always have a niche in the austere environment. Military experience with the transfusion of FWB is extensive, dating as far back as World War I, and includes the transfusion of over 9,000 whole blood units during the recent military conflicts in Afghanistan and Iraq.112 Two retrospective analyses of combat casualties (treated at a combat support hospital113 and by forward surgical teams114) reported improved survival in patients who received FWB compared to component therapy (pRBC and FFP only – no platelets due to a shortage). Lingering questions regarding the use of FWB include warm versus cold storage, shelf life, and the ability to rapidly screen FWB units for infectious agents and type compatability,103 although some have advocated the use of low anti-A/B titer type O whole blood as an alternative to type specificity.115
Establishing the ability to rapidly and safely transfuse FWB for trauma patients requires changes to donor blood processing at regional blood banks. Donor whole blood could be theoretically held for its shelf life (24–48 hours) and subsequently processed into components if not transfused as FWB. Our group recently performed a pilot study which demonstrated the feasibility of modified whole blood therapy in a civilian trauma setting;116 however, the whole blood units used in the study were leukoreduced and platelet-poor, meaning patients still required transfusions of apheresis platelets.
Goal-directed correction of coagulopathy
Perturbations of different aspects of TEG and ROTEM tracings point to deficiencies in different components of coagulation, and this creates the possibility of using blood products or other adjuncts to correct these deficiencies in a targeted manner. The use of TEG and ROTEM in liver transplantation117 and cardiac surgery118 has been shown to reduce blood product use with similar or improved patient outcomes. In the case of the relatively stable trauma patient, where surgical hemorrhage control is achieved and MTP is deactivated, the use of TEG and ROTEM to guide further blood product utilization is intuitive and an important component of DCR.57 A recent single-institution RCT (n=111) reported that utilization of a goal-directed, TEG-guided MTP compared to MTP guided by conventional coagulation assays (CCA) to resuscitate severely injured patients was associated with improved survival (11/56 deaths TEG vs. 20/55 deaths CCA, p=0.049). Data regarding the use of blood products and other therapeutics in response to abnormalities of different TEG/ROTEM parameters are presented elsewhere in this series.
However, the role of coagulation assays to guide transfusion of blood products during MTP in lieu of fixed ratios is unclear and controversial.119,120 Although it was not designed to answer this question, analysis of data from the PROPPR randomized trial demonstrated patients randomized to the low (1:1:2) plasma and platelet ratio group were transfused additional units of plasma and platelets in a laboratory-driven, goal-directed fashion after MTP was deactivated such that the cumulative ratio of blood products used approached 1:1:1 by 24 hours,5 suggesting that the optimal ratio may be close to 1:1:1 regardless of how one arrives at it. Direct comparisons between fixed ratio versus goal-directed MTP are lacking in the literature,121,122 although others argue that the two strategies are not mutually exclusive.123
Treatment resistance and complications
Cryoprecipitate and fibrinogen concentrate
Cryoprecipitate, collected as the precipitate of plasma after a freeze-thaw cycle, is enriched in factors VIII and XIII, von-Willibrand factor, fibronectin, and fibrinogen. These factors are theoretically replaced at physiologic levels by plasma during the course of MT, and discussion regarding the use of cryoprecipitate focuses on the need for additional boluses of these components, particularly fibrinogen. Although previous data suggested a critical fibrinogen threshold of 100 mg/dL (1.0 g/L), more recent studies found significant bleeding at this level,124,125 indicating the need for a higher cutoff. Currently, the American College of Surgeons Committee on Trauma recommends transfusing cryoprecipitate to maintain fibrinogen ≥180 mg/dL,102 while European guidelines describe a minimum cutoff of 150–200 mg/dL.126
An analysis of blood samples from 52 massively transfused patients found that fibrinogen was commonly the first factor to reach critically low levels,127 and a review of 1,332 massively transfused combat casualties found that use of cryoprecipitate within the first 24h was independently associated with improved survival.128 These data suggest a potential benefit with early delivery of fibrinogen, either by fibrinogen concentrate (off-label use in the United States) or cryoprecipitate. A randomized controlled trial to evaluate the use of prehospital fibrinogen concentrate (Fibrinogen in Trauma-induced coagulopathy; FIinTIC)129 is ongoing.
Continued hemorrhage
Continued hemorrhage despite adequate surgical control and damage control resuscitation is secondary to worsening trauma-induced coagulopathy. Although DCR is designed to treat coagulopathy directly, adjunctive measures may also be employed. We defer discussion of these to elsewhere in this series.
Transfusion-related acute lung injury (TRALI)
The most notable complication resulting from blood product transfusion is transfusion-related acute lung injury (TRALI), characterized by inflammatory-mediated pulmonary edema resulting in hypoxia within hours of transfusion.130 Although any blood product may precipitate TRALI, the risk was historically highest with plasma.92 Recognizing that a significant source of TRALI cases were precipitated by plasma donated by multiparous women who likely developed alloantibodies after becoming sensitized during pregnancy, initiatives were introduced in 2006 to reduce the incidence of TRALI.92 This included screening programs and preferentially using plasma from male donors for transfusion. After implementation of these initiatives, the American Red Cross reported that the incidence of TRALI decreased from 18.6 cases per million plasma units transfused to 4.2 cases per million, no different from the TRALI risk associated with PRBC. 92 An exception is type AB plasma; due to its scarcity, a substantial proportion of transfused AB plasma continues to come from female donors. As a result, the incidence of TRALI associated with AB plasma has remained constant. Currently, the risk of TRALI after transfusion of AB plasma carries an odds ratio of 14.5 (95% CI 6.8 – 30.9) compared to other plasma types,92 which has been cited as a rationale for the transition to type A plasma for emergency use.
Discussion/Summary
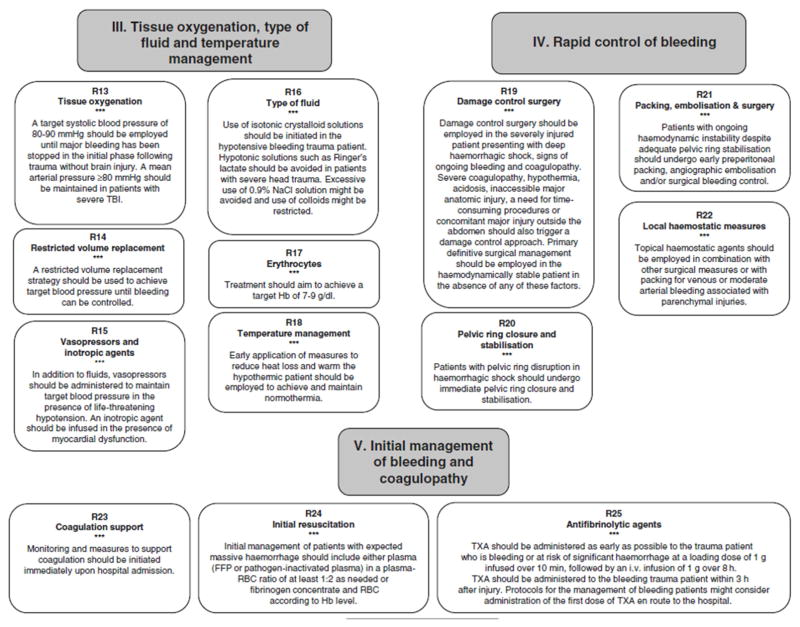
One of the most important lessons of the last 20 years is that the choice, timing, and volume of resuscitation fluid for the trauma patient has as much impact on outcome as operative treatment of the tissue injuries. The advent of DCR was a breakthrough in trauma care. Crystalloids now have little role in the initial resuscitation of traumatic hemorrhagic shock, and plasma is the preferred means of volume expansion after traumatic hemorrhagic shock. The American College of Surgeons Committee on Trauma (Table 2) 102 and European Task Force for Advanced Bleeding Care in Trauma (Figure 2)126 recommend using a ratio of plasma to PRBC of at least 1:2 for the initial resuscitation of hemorrhaging trauma patients, while the National Institute for Health and Care Excellence in the United Kingdom recommends a 1:1 ratio.131 At this stage, the use of plasma is quite low risk compared to its potential benefits. We emphasize again that replacement of volume and clotting factors is likely only part of the story of plasma, and research efforts are on-going to identify the specific mechanisms underlying its benefits.
Figure 2. Summary of treatment modalities for the bleeding trauma patient.
From: Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fourth edition. Critical Care (2016):20:100.
Although we have come a long way since the pre-DCR days, many questions remain. What are the roles of whole blood and prehospital transfusion, as well as the safety of uncross matched type A plasma for emergency use? Rather than the use of fixed transfusion ratios, can improvements in diagnostics and therapeutics enable precise, targeted correction of coagulopathy and hypoperfusion during massive transfusion? Improvements in trauma care have historically arisen out of armed conflict, but randomized trials run by multicenter research consortiums have enabled a new generation of physician-scientists to produce high-level evidence outside of the theater of war in an effort to answer these questions. With increasing collaboration across multiple centers and specialties, we will continue to further our understanding in this challenging area of medicine.
Key Points.
Hemorrhage is the leading cause of preventable trauma deaths and occurs rapidly (median 2–3 hours after presentation).
Early activation of a predefined massive transfusion protocol improves outcomes for the patient with exsanguinating hemorrhage, although accurate identification remains a challenge.
Large infusions of crystalloid are dangerous for patients with traumatic hemorrhagic shock, and even relatively small volumes of crystalloid may be harmful.
Plasma should be used as the primary means of volume expansion for resuscitation of trauma patients with hemorrhagic shock.
Although the exact mechanisms underlying the benefits of plasma are unclear, it is likely more than simple replacement of volume and clotting factors.
Footnotes
No relevant financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tien HC, Spencer F, Tremblay LN, et al. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. 2007;62:142–146. doi: 10.1097/01.ta.0000251558.38388.47. [DOI] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Malone D, Holcomb JB. Early predictors of transfusion and mortality after injury: A review of the data-based literature. J Trauma. 2006;60(6 Suppl):S20–S25. doi: 10.1097/01.ta.0000199544.63879.5d. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons RL, Collins JA, Heisterkamp CA, Mills DE, Andren R, Phillips LL. Coagulation disorders in combat casualties. Ann Surg. 1969;169(4):455–482. doi: 10.1097/00000658-196904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan S, Allard S, Weaver A, et al. A major haemorrhage protocol improves the delivery of blood component therapy and reduces waste in trauma massive transfusion. Injury. 2013;44(5):587–592. doi: 10.1016/j.injury.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Cotton BA, Au BK, Nunez TC, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66(1):41–48. doi: 10.1097/TA.0b013e31819313bb. [DOI] [PubMed] [Google Scholar]
- 9.Yücel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006;60(6):1228–1236. doi: 10.1097/01.ta.0000220386.84012.bf. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin DF, Niles SE, Salinas J, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):S57–S63. doi: 10.1097/TA.0b013e318160a566. [DOI] [PubMed] [Google Scholar]
- 11.Cotton BA, Dossett LA, Haut ER, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69(Suppl 1):S33–S39. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]
- 12.Pommerening MJ, Goodman MD, Holcomb JB, et al. Clinical gestalt and the prediction of massive transfusion after trauma. Injury. 2015;46(5):807–813. doi: 10.1016/j.injury.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71(2):407–414. doi: 10.1097/TA.0b013e31821e1bf0. [DOI] [PubMed] [Google Scholar]
- 14.Davenport R, Manson J, De’Ath H, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–2658. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra B, Cameron PA, Gruen RL, et al. The definition of massive transfusion in trauma: a critical variable in examining evidence for resuscitation. Eur J Emerg Med. 2011;18:137–142. doi: 10.1097/MEJ.0b013e328342310e. [DOI] [PubMed] [Google Scholar]
- 16.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74(2):396–400. doi: 10.1097/TA.0b013e31827a3639. [DOI] [PubMed] [Google Scholar]
- 17.Savage SA, Sumislawski JJ, Zarzaur BL, Dutton WP, Croce MA, Fabian TC. The new metric to define large-volume hemorrhage: results of a prospective study of the critical administration threshold. J Trauma Acute Care Surg. 2015;78(2):224–229. doi: 10.1097/TA.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 18.Savage SA, Sumislawski JJ, Croce MA, Zarzaur BL. Using critical administration thresholds to predict abbreviated laparotomy. J Trauma Acute Care Surg. 2014;77(4):599–603. doi: 10.1097/TA.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Navy. Surface Ship Survivability. United States: Naval War Publications; 1996. [Google Scholar]
- 20.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197(5):532–535. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35(3):375–382. [PubMed] [Google Scholar]
- 22.Miller RS, Morris JA, Jr, Diaz JJ., Jr Complications after 344 damage-control open celiotomies. J Trauma. 2005;59(6):1365–1371. doi: 10.1097/01.ta.0000196004.49422.af. [DOI] [PubMed] [Google Scholar]
- 23.Shoemaker WC, Montgomery ES, Kaplan E, et al. Physiologic patterns in surviving and nonsurviving shock patients: use of sequential cardiorespiratory variables in defining criteria for therapeutic goals and early warning of death. Arch Surg. 1973;106(5):630–636. doi: 10.1001/archsurg.1973.01350170004003. [DOI] [PubMed] [Google Scholar]
- 24.Shoemaker WC, Appel P, Bland R. Use of physiologic monitoring to predict outcome and to assist in clinical decisions in critically ill postoperative patients. Am J Surg. 1983;146(1):43–50. doi: 10.1016/0002-9610(83)90257-x. [DOI] [PubMed] [Google Scholar]
- 25.Shoemaker WC, Appel PL, Kram HB, et al. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 26.Shah SK, Uray KS, Stewart RH, et al. Resuscitation-induced intestinal edema and related dysfunction: state of the science. J Surg Res. 2011;166(1):120–130. doi: 10.1016/j.jss.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balogh Z, McKinley BA, Cocanour CS, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138(6):637–642. doi: 10.1001/archsurg.138.6.637. [DOI] [PubMed] [Google Scholar]
- 28.Cotton BA, Guy JS, Morris JA, Jr, et al. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26(2):115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 29.Lang F, Busch GL, Ritter M, et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78(1):247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 30.Häussinger D, Schliess F, Warskulat U, et al. Liver cell hydration. Cell Biol Toxicol. 1997;13(4–5):275–287. doi: 10.1023/a:1007483324138. [DOI] [PubMed] [Google Scholar]
- 31.Tseng GN. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol. 1992;262(4 Pt 1):C1056–1068. doi: 10.1152/ajpcell.1992.262.4.C1056. [DOI] [PubMed] [Google Scholar]
- 32.Velmahos GC, Demetriades D, Shoemaker WC, et al. Endpoints of resuscitation of critically injured patients: normal or supranormal? A prospective randomized trial. Ann Surg. 2000;232(3):409–418. doi: 10.1097/00000658-200009000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359(9320):1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 34.Brandstrup B, Tønnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biffl WL, Moore EE, Burch JM, et al. Secondary abdominal compartment syndrome is a highly lethal event. Am J Surg. 2001;182(6):645–648. doi: 10.1016/s0002-9610(01)00814-5. [DOI] [PubMed] [Google Scholar]
- 36.Kasotakis G, Sideris A, Yang Y, et al. Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J Trauma Acute Care Surg. 2013;74(5):1215–1222. doi: 10.1097/TA.0b013e3182826e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson BR, Cotton BA, Pritts TA, et al. Application of the Berlin definition in PROMMTT patients: the impact of resuscitation on the incidence of hypoxemia. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S61–S67. doi: 10.1097/TA.0b013e31828fa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ley EJ, Clond MA, Srour MK, et al. Emergency department crystalloid resuscitation of 1. 5 L or more is associated with increased mortality in elderly and nonelderly trauma patients. J Trauma. 2011;70(2):398–400. doi: 10.1097/TA.0b013e318208f99b. [DOI] [PubMed] [Google Scholar]
- 39.Brown JB, Cohen MJ, Minei JP, et al. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013;74(5):1207–1212. doi: 10.1097/TA.0b013e31828c44fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velasco IT, Pontieri V, Rocha e Silva M, Jr, Lopes OU. Hyperosmotic NaCl and severe hemorrhagic shock. Am J Physiol. 1980;239(5):H664–H673. doi: 10.1152/ajpheart.1980.239.5.H664. [DOI] [PubMed] [Google Scholar]
- 41.Mazzoni MC, Borgstrom P, Arfors KE, Intaglietta M. Dynamic fluid redistribution in hyperosmotic resuscitation of hypovolemic hemorrhage. Am J Physiol. 1988;255(3 Pt 2):H629–H637. doi: 10.1152/ajpheart.1988.255.3.H629. [DOI] [PubMed] [Google Scholar]
- 42.Junger WG, Rhind SG, Rizoli SB, et al. Resuscitation of traumatic hemorrhagic shock patients with hypertonic saline-without dextran-inhibits neutrophil and endothelial cell activation. Shock. 2012;38(4):341–350. doi: 10.1097/SHK.0b013e3182635aca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascual JL, Ferri LE, Seely AJ, et al. Hypertonic Saline Resuscitation of Hemorrhagic Shock Diminishes Neutrophil Rolling and Adherence to Endothelium and Reduces In Vivo Vascular Leakage. Ann Surg. 2002;236(5):634–642. doi: 10.1097/00000658-200211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizoli SB, Rhind SG, Shek PN, et al. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Ann Surg. 2006;243(1):47–57. doi: 10.1097/01.sla.0000193608.93127.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulger EM, Cuschieri J, Warner K, Maier RV. Hypertonic resuscitation modulates the inflammatory response in patients with traumatic hemorrhagic shock. Ann Surg. 2007;245(4):635–641. doi: 10.1097/01.sla.0000251367.44890.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulger EM, May S, Kerby JD, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011;253(3):431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delano MJ, Rizoli SB, Rhind SG, et al. Prehospital Resuscitation of Traumatic Hemorrhagic Shock with Hypertonic Solutions Worsens Hypocoagulation and Hyperfibrinolysis. Shock. 2015;44(1):25–31. doi: 10.1097/SHK.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannon WB, Fraser J, Cowell EB. The preventive treatment of wound shock. JAMA. 1918;70(9):618–621. [Google Scholar]
- 49.Sondeen JL, Coppes VG, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in a swine with aortic injury. J Trauma. 2003;54(5 Suppl):S110–S117. doi: 10.1097/01.TA.0000047220.81795.3D. [DOI] [PubMed] [Google Scholar]
- 50.Mapstone J, Roberts I, Evans P. Fluid resuscitation strategies: a systematic review of animal trials. J Trauma. 2003;55(3):571–589. doi: 10.1097/01.TA.0000062968.69867.6F. [DOI] [PubMed] [Google Scholar]
- 51.Bickell WH, Wall MJ, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 52.Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52(6):1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 53.Schreiber MA, Meier EN, Tisherman SA, et al. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687–695. doi: 10.1097/TA.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Learoyd P. The history of blood transfusion prior to the 20th century--part 1. Transfus Med. 2012;22(5):308–314. doi: 10.1111/j.1365-3148.2012.01180.x. [DOI] [PubMed] [Google Scholar]
- 56.Learoyd P. The history of blood transfusion prior to the 20th century--part 2. Transfus Med. 2012;22(6):372–376. doi: 10.1111/j.1365-3148.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- 57.Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–3058. doi: 10.1182/blood-2014-05-575340. [DOI] [PubMed] [Google Scholar]
- 58.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 59.Johansson PI, Stensballe J, Rosenberg I, et al. Proactive administration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: evaluating a change in transfusion practice. Transfusion. 2007;47(4):593–598. doi: 10.1111/j.1537-2995.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 60.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 61.Teixeira PG, Inaba K, Shulman I, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66(3):693–697. doi: 10.1097/TA.0b013e31817e5c77. [DOI] [PubMed] [Google Scholar]
- 62.Mitra B, Mori A, Cameron PA, et al. Fresh frozen plasma (FFP) use during massive blood transfusion in trauma resuscitation. Injury. 2010;41(1):35–39. doi: 10.1016/j.injury.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 63.Peiniger S, Nienaber U, Lefering R, et al. Balanced massive transfusion ratios in multiple injury patients with traumatic brain injury. Crit Care. 2011;15(1):R68. doi: 10.1186/cc10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shrestha B, Holcomb JB, Camp EA, et al. Damage-control resuscitation increases successful nonoperative management rates and survival after severe blunt liver injury. J Trauma Acute Care Surg. 2015;78(2):336–341. doi: 10.1097/TA.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 65.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg. 2014;149(9):904–912. doi: 10.1001/jamasurg.2014.940. [DOI] [PubMed] [Google Scholar]
- 66.Moore HB, Moore EE, Morton AP, et al. Shock-induced systemic hyperfibrinolysis is attenuated by plasma-first resuscitation. J Trauma Acute Care Surg. 2015;79(6):897–903. doi: 10.1097/TA.0000000000000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sillesen M, Johansson PI, Rasmussen LS, et al. Fresh frozen plasma resuscitation attenuates platelet dysfunction compared with normal saline in a large animal model of multisystem trauma. J Trauma Acute Care Surg. 2014;76(4):998–1007. doi: 10.1097/TA.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 68.Rahbar E, Cardenas JC, Baimukanova G, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13:117. doi: 10.1186/s12967-015-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nieuwdorp M, Mooij HL, Kroon J, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55(4):1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 70.Liang Y, Li X, Zhang X, et al. Elevated levels of plasma TNF-α are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-κB and p38 mitogen-activated protein kinase in endothelial cells. Shock. 2014;41(4):275–281. doi: 10.1097/SHK.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 71.Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116(17):1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 72.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wataha K, Menge T, Deng X, et al. Spray-dried plasma and fresh frozen plasma modulate permeability and inflammation in vitro in vascular endothelial cells. Transfusion. 2013;53(Suppl 1):80S–90S. doi: 10.1111/trf.12040. [DOI] [PubMed] [Google Scholar]
- 74.Peng Z, Pati S, Potter D, et al. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Potter DR, Baimukanova G, Keating SM, et al. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S7–S17. doi: 10.1097/TA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 77.Jin G, DeMoya MA, Duggan M, et al. Traumatic brain injury and hemorrhagic shock: evaluation of different resuscitation strategies in a large animal model of combined insults. Shock. 2012;38(1):49–56. doi: 10.1097/SHK.0b013e3182574778. [DOI] [PubMed] [Google Scholar]
- 78.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 79.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73(1):60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 80.Rahbar E, Cardenas JC, Baimukanova G, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13:117. doi: 10.1186/s12967-015-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Downes KA, Wilson E, Yomtovian R, Sarode R. Serial measurement of clotting factors in thawed plasma stored for 5 days. Transfusion. 2001;41(4):570. doi: 10.1046/j.1537-2995.2001.41040570.x. [DOI] [PubMed] [Google Scholar]
- 82.Matijevic N, Wang YW, Cotton BA, et al. Better hemostatic profiles of never-frozen liquid plasma compared with thawed fresh frozen plasma. J Trauma Acute Care Surg. 2013;74(1):84–90. doi: 10.1097/TA.0b013e3182788e32. [DOI] [PubMed] [Google Scholar]
- 83.Radwan ZA, Bai Y, Matijevic N, et al. An emergency department thawed plasma protocol for severely injured patients. JAMA Surg. 2013;148(2):170–175. doi: 10.1001/jamasurgery.2013.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Novak DJ, Bai Y, Cooke RK, et al. Making thawed universal donor plasma available rapidly for massively bleeding trauma patients: experience from the Pragmatic, Randomized Optimal Platelets and Plasma Ratios (PROPPR) trial. Transfusion. 2015;55(6):1331–1339. doi: 10.1111/trf.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holcomb JB, Donathan DP, Cotton BA, et al. Prehospital Transfusion of Plasma and Red Blood Cells in Trauma Patients. Prehosp Emerg Care. 2015;19(1):1–9. doi: 10.3109/10903127.2014.923077. [DOI] [PubMed] [Google Scholar]
- 86.Moore EE, Chin TL, Chapman MC, et al. Plasma first in the field for postinjury hemorrhagic shock. Shock. 2014;41(Suppl 1):35–38. doi: 10.1097/SHK.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown JB, Guyette FX, Neal MD, et al. Taking the Blood Bank to the Field: The Design and Rationale of the Prehospital Air Medical Plasma (PAMPer) Trial. Prehosp Emerg Care. 2015;19(3):343–350. doi: 10.3109/10903127.2014.995851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.American Red Cross. [Accessed April 14, 2016];Blood Types. 2010 Available at http://givebloodgivelife.org/education/bloodtypes.php.
- 89.Mair B, Benson K. Evaluation of changes in hemoglobin levels associated with ABO-incompatible plasma in apheresis platelets. Transfusion. 1998;38:51–55. doi: 10.1046/j.1537-2995.1998.38198141498.x. [DOI] [PubMed] [Google Scholar]
- 90.Karafin MS, Blagg L, Tobian AAR, et al. ABO antibody titers are not predictive of hemolytic reactions due to plasma-incompatible platelet transfusions. Transfusion. 2012;48:2087–2093. doi: 10.1111/j.1537-2995.2012.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stubbs JR, Zielinski MD, Berns KS, et al. How we provide thawed plasma for trauma patients. Transfusion. 2015;55(8):1830–1837. doi: 10.1111/trf.13156. [DOI] [PubMed] [Google Scholar]
- 92.Eder AF, Dy BA, Perez JM, et al. The residual risk of transfusion-related acute lung injury at the American Red Cross (2008–2011): limitations of a predominantly male-donor plasma mitigation strategy. Transfusion. 2013;53(7):1442–1449. doi: 10.1111/j.1537-2995.2012.03935.x. [DOI] [PubMed] [Google Scholar]
- 93.Zielinski MD, Johnson PM, Jenkins D, et al. Emergency use of prethawed Group A plasma in trauma patients. J Trauma Acute Care Surg. 2013;74(1):69–74. doi: 10.1097/TA.0b013e3182788f8e. [DOI] [PubMed] [Google Scholar]
- 94.Zielinski MD, Schrager JJ, Johnson P, et al. Multicenter comparison of emergency release group A versus AB plasma in blunt-injured trauma patients. Clin Transl Sci. 2015;8(1):43–47. doi: 10.1111/cts.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chhibber V, Green M, Vauthrin M, et al. Is group A plasma suitable as the first option for emergency release transfusion? Transfusion. 2014;54:1751–1755. doi: 10.1111/trf.12537. [DOI] [PubMed] [Google Scholar]
- 96.Dunbar NM, Yazer MH Biomedical Excellence for Safer Transfusion (BEST) Collaborative. A possible new paradigm? A survey-based assessment of the use of thawed group A plasma for trauma resuscitation in the United States. Transfusion. 2016;56(1):125–129. doi: 10.1111/trf.13266. [DOI] [PubMed] [Google Scholar]
- 97.Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221–217. doi: 10.1097/TA.0b013e3181ad5957. [DOI] [PubMed] [Google Scholar]
- 98.Johnson JL, Moore EE, Kashuk JL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145(10):973–977. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 99.Inaba K, Branco BC, Rhee P, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg. 2010;210(6):957–965. doi: 10.1016/j.jamcollsurg.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 100.Gunter OL, Au BK, Isbell JM, et al. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65(3):527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 101.Holcomb JB, Zarzabal LA, Michalek JE, et al. Increased platelet: RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–328. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 102.Committee on Trauma of the American College of Surgeons. ACS TQIP Massive Transfusion in Trauma Guidelines. Chicago, IL: American College of Surgeons; 2015. [Accessed April 13, 2016]. https://www.facs.org/~/media/files/quality%20programs/trauma/tqip/massive%20transfusion%20in%20trauma%20guildelines.ashx. [Google Scholar]
- 103.Spinella PC, Reddy HL, Jaffe JS, et al. Fresh whole blood use for hemorrhagic shock: preserving benefit while avoiding complications. Anesth Analg. 2012;115(4):751–758. doi: 10.1213/ANE.0b013e318261f40e. [DOI] [PubMed] [Google Scholar]
- 104.Kauvar DS, Holcomb JB, Norris GC, Hess JR. Fresh whole blood transfusion: a controversial military practice. J Trauma. 2006;61(1):181–184. doi: 10.1097/01.ta.0000222671.84335.64. [DOI] [PubMed] [Google Scholar]
- 105.Ponschab M, Schöchl H, Gabriel C, et al. Haemostatic profile of reconstituted blood in a proposed 1:1:1 ratio of packed red blood cells, platelet concentrate and four different plasma preparations. Anaesthesia. 2015;70(5):528–536. doi: 10.1111/anae.13067. [DOI] [PubMed] [Google Scholar]
- 106.Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67(1):29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 107.Arslan E, Sierko E, Waters JH, Siemionow M. Microcirculatory hemodynamics after acute blood loss followed by fresh and banked blood transfusion. Am J Surg. 2005;190(3):456–462. doi: 10.1016/j.amjsurg.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 108.Lozano ML, Rivera J, Gonzalez-Conejero R, et al. Loss of high-affinity thrombin receptors during platelet concentrate storage impairs the reactivity of platelets to thrombin. Transfusion. 1997;37(4):368–375. doi: 10.1046/j.1537-2995.1997.37497265336.x. [DOI] [PubMed] [Google Scholar]
- 109.Inaba K, Lustenberger T, Rhee P, et al. The impact of platelet transfusion in massively transfused trauma patients. J Am Coll Surg. 2010;211(5):573–579. doi: 10.1016/j.jamcollsurg.2010.06.392. [DOI] [PubMed] [Google Scholar]
- 110.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in-hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weinberg JA, McGwin G, Jr, Vandromme MJ, et al. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69(6):1427–1431. doi: 10.1097/TA.0b013e3181fa0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chandler MH, Roberts M, Sawyer M, Myers G. The US military experience with fresh whole blood during the conflicts in Iraq and Afghanistan. Semin Cardiothorac Vasc Anesth. 2012;16(3):153–159. doi: 10.1177/1089253212452344. [DOI] [PubMed] [Google Scholar]
- 113.Spinella PC, Perkins JG, Grathwohl KW, et al. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66(4 Suppl):S69–S76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nessen SC, Eastridge BJ, Cronk D, et al. Fresh whole blood use by forward surgical teams in Afghanistan is associated with improved survival compared to component therapy without platelets. Transfusion. 2013;53(Suppl 1):107S–113S. doi: 10.1111/trf.12044. [DOI] [PubMed] [Google Scholar]
- 115.Strandenes G, Berséus O, Cap AP, et al. Low titer group O whole blood in emergency situations. Shock. 2014;41(Suppl 1):70–75. doi: 10.1097/SHK.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 116.Cotton BA, Podbielski J, Camp E, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013;258(4):527–532. doi: 10.1097/SLA.0b013e3182a4ffa0. [DOI] [PubMed] [Google Scholar]
- 117.Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42(7):2590–2593. doi: 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 118.Weber CF, Gorlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117(3):531–547. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 119.Kelly JM, Callum JL, Rizoli SB. 1:1:1-warranted or wasteful? even where appropriate, high ratio transfusion protocols are costly: early transition to individualized care benefits patients and transfusion services. Expert Rev Hematol. 2013;6(6):631–633. doi: 10.1586/17474086.2013.859520. [DOI] [PubMed] [Google Scholar]
- 120.Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251(4):604–614. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 121.Nascimento B, Callum J, Tien H, et al. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ. 2013;185(12):583–589. doi: 10.1503/cmaj.121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tapia NM, Chang A, Norman M, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378–385. doi: 10.1097/TA.0b013e31827e20e0. [DOI] [PubMed] [Google Scholar]
- 123.Ho AM, Holcomb JB2, Ng CS3, Zamora JE4, Karmakar MK5, Dion PW. The traditional vs “1:1:1” approach debate on massive transfusion in trauma should not be treated as a dichotomy. Am J Emerg Med. 2015;33(10):1501–1504. doi: 10.1016/j.ajem.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 124.Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5(2):266–273. doi: 10.1111/j.1538-7836.2007.02297.x. [DOI] [PubMed] [Google Scholar]
- 125.Karlsson M, Ternstrom L, Hyllner M, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost. 2009;102(1):137–144. doi: 10.1160/TH08-09-0587. [DOI] [PubMed] [Google Scholar]
- 126.Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20(1):100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chambers LA, Chow SJ, Shaffer LE. Frequency and characteristics of coagulopathy in trauma patients treated with a low- or high-plasma-content massive transfusion protocol. Am J Clin Pathol. 2011;136(3):364–370. doi: 10.1309/AJCPH16YXJEFSHEO. [DOI] [PubMed] [Google Scholar]
- 128.Morrison JJ, Ross JD, Dubose JJ, Jansen JO, Midwinter MJ, Rasmussen TE. Association of cryoprecipitate and tranexamic acid with improved survival following wartime injury: findings from the MATTERs II Study. JAMA Surg. 2013;148(3):218–225. doi: 10.1001/jamasurg.2013.764. [DOI] [PubMed] [Google Scholar]
- 129.Maegele M, Zinser M, Schlimp C, Schöchl H, Fries D. Injectable hemostatic adjuncts in trauma: Fibrinogen and the FIinTIC study. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S76–S82. doi: 10.1097/TA.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 130.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007;136(6):788–799. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 131.National Institute for Health and Care Excellence. Major trauma: Assessment and Initial Management, NICE Guideline: Short Version, Draft for Consultation, August 2015. London, UK: 2015. [Google Scholar]