Abstract
Primary optic nerve gliomas are most commonly benign pilocytic astrocytomas (World Health Organization [WHO] Grade I) occurring in childhood and following an indolent course. Malignant optic gliomas occur in adulthood and follow an extremely aggressive course, with rapid infiltration of the chiasm, blindness, and death typically within months. A third category of optic glioma, occurring in adulthood, histopathologically benign (WHO Grade I–II) but following an aggressive course, has been rarely reported. The authors describe clinical and histopathologic features of clinically aggressive but histopathologically benign optic nerve gliomas of adulthood. Retrospective review of cases of biopsy-proven optic nerve glioma in the neuro-ophthalmology division of the Jules Stein Eye Institute from 1990 to 2011 was carried out. Cases following an aggressive course were selected for review of clinical, neuroradiologic, and histopathologic features. Three cases were selected for detailed study. Ages ranged from 31 to 45 years. All were initially diagnosed with optic nerve inflammation or benign neoplasm based on clinical and neuroradiologic features, but all suffered neuroradiologic extension and rapid deterioration of vision in the affected eye to no light perception over 3–8 weeks. Optic nerve biopsies were undertaken for the suspicion of malignancy. Features ranged from WHO Grade I (pilocytic astrocytoma, ganglioglioma) in two cases, to WHO Grade II in one case (diffuse astrocytoma, histopathologically benign, but associated with aggressive features such as high p53 [13–21%] and Ki-67 [40%]). The diffuse astrocytoma case subsequently developed extensive intracranial extension suspicious for malignant transformation. These findings indicate that benign optic nerve glioma in adults may be initially misdiagnosed as inflammation, be clinically aggressive, and require excision to prevent further intracranial involvement.
Keywords: Adult optic glioma, ganglioglioma, pilocytic astrocytoma, WHO Grade I–II
Introduction
Primary optic nerve gliomas are most commonly benign pilocytic astrocytomas (World Health Organization [WHO] Grade I) occurring in childhood and following an indolent course.1 Malignant optic gliomas (WHO Grade III–IV occur in adulthood and follow an extremely aggressive course, with rapid infiltration of the chiasm, blindness, and death typically within months.2,3 A third category of optic glioma, occurring in adulthood, histopathologically benign (WHO Grade I–II) but following an aggressive course, has been rarely reported.4–8
Methods
We performed a retrospective review of cases of biopsy-proven optic nerve glioma in the neuro-ophthalmology division of the Jules Stein Eye Institute from 1990 to 2011. The study was approved by the University of California Los Angeles (UCLA) Institutional Review Board (IRB) (Study No. 12-001343, 9-12-12, “Aggressive Low Grade Optic Nerve Glioma”). Three cases following an aggressive course were selected for review of clinical, neuroradiologic, and histopathologic, and immunopathologic features.
Case 1
A 45-year-old woman reported a 3-month history of vague periorbital pain and decreased vision left eye, without systemic illness. Initial visual acuity was 20/20 in each eye, with equivocal left relative afferent pupillary defect (RAPD), optic disc oedema, and normal perimetry. Magnetic resonance imaging (MRI) showed mild to moderate enhancement of the left intraorbital optic nerve (Figure 1); no white matter lesions were seen. Complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), angiotensin-converting enzyme (ACE), and rapid plasma reagin (RPR) were normal. A course of intravenous and oral steroids produced no change in findings. One month later, visual fields showed an inferior arcuate defect left eye (Figure 2).
FIGURE 1.
Case 1. Axial MRI, orbits, T1-weighted with fat suppression and contrast. There is diffuse enhancement of the left optic nerve from globe to the proximal intracranial segment.
FIGURE 2.
Case 1. Automated perimetry, showing inferior arcuate defect left eye.
Two months later, visual acuity was 20/200 left eye, with diffuse depression of visual field (Figure 3), persistent left RAPD, and disc oedema. Additional ancillary testing included negative chest X-ray and whole-body positron emission tomography (PET) scan. Lumbar puncture revealed 1 white blood cell/high-power field (WBC/hpf), cytospin negative for malignant cells, venereal disease research laboratory (VDRL) test negative, protein 36, and multiple sclerosis panel negative. Repeat MRI demonstrated continued enhancement of the optic nerve extending to the pre-chiasmatic segment (Figure 4). Two months later, after being lost to follow-up, visual acuity was no light perception left eye. Eight months after initial presentation, she underwent optic nerve biopsy.
FIGURE 3.
Case 1. Automated perimetry, showing generalised depression left eye.
FIGURE 4.
Case 1. Axial MRI, orbits, T1-weighted with fat suppression and contrast. Enhancement of the left optic nerve has increased in intensity and extent, now to the distal intracranial segment. The diameter of the nerve has also increased.
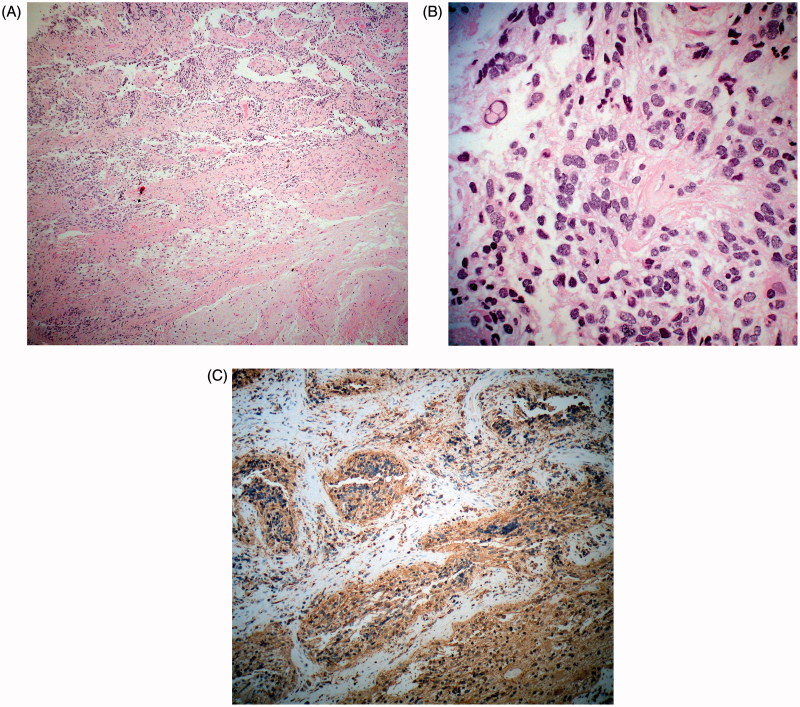
Sections showed infiltration of the optic nerve by tumour with a disorganised hypercellular glial architecture (Figure 5A). Cells had both ovoid and spindle characteristics, with marked pleomorphism and nuclear atypia, including regions of microcystic degeneration and apoptosis. No mitotic figures, endothelial proliferation, or necrosis were seen (Figure 5B). Immunohistochemistry showed strongly positive glial fibrillary acidic protein (GFAP) (Figure 5C), with Ki-67 reactivity in approximately 13–21% of the cells (Figure 6A), and p53 reactivity in 40% (Figure 6B). The tumour was classified as glioma WHO Grade II by strict morphologic criteria, with aggressive characteristics
FIGURE 5.
Case 1. (A) Low-magnification view of the tumour shows an infiltrative glial neoplasm (haematoxylin-eosin; ×50). (B) The tumour is composed of moderately atypical glial cells (haematoxylin-eosin; ×300). (C) Tumour is prominently GFAP immunoreactive (GFAP; ×100).
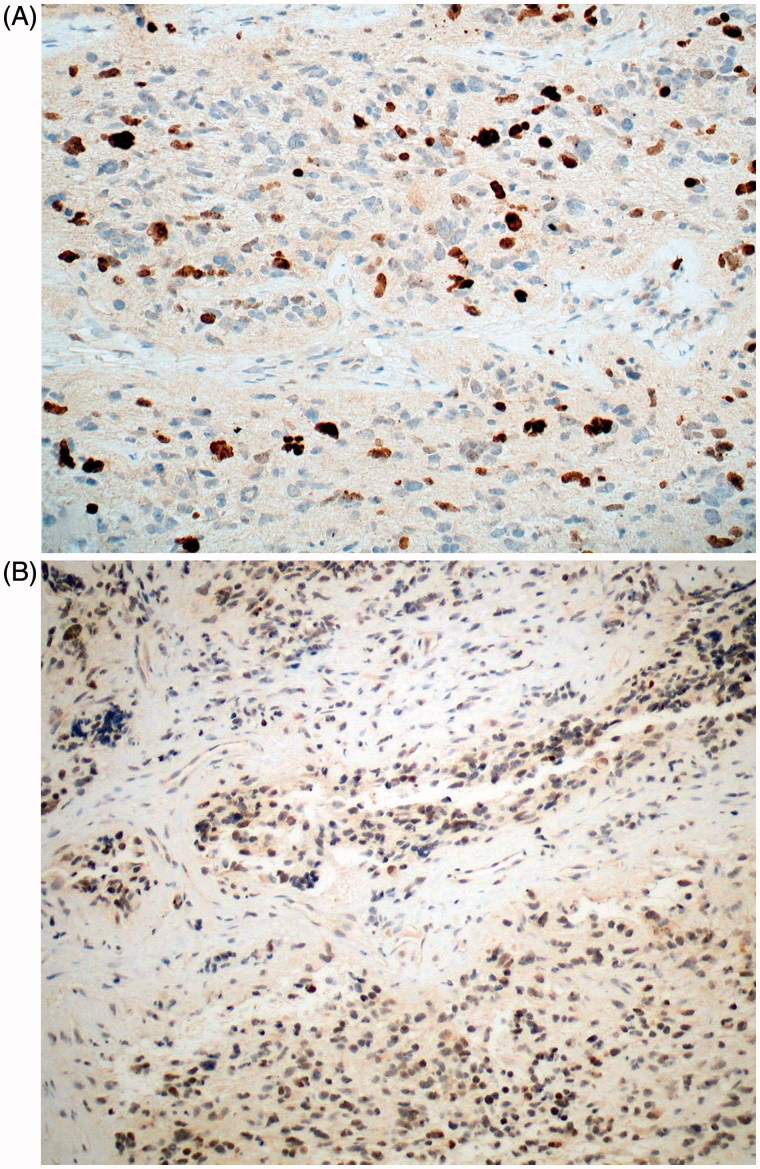
FIGURE 6.
Case 1. (A) Tumour shows prominent Ki-67 nuclear labelling (anti-Ki-67; ×200). (B) Many nuclei are p53 immunoreactive (anti-p53; ×200).
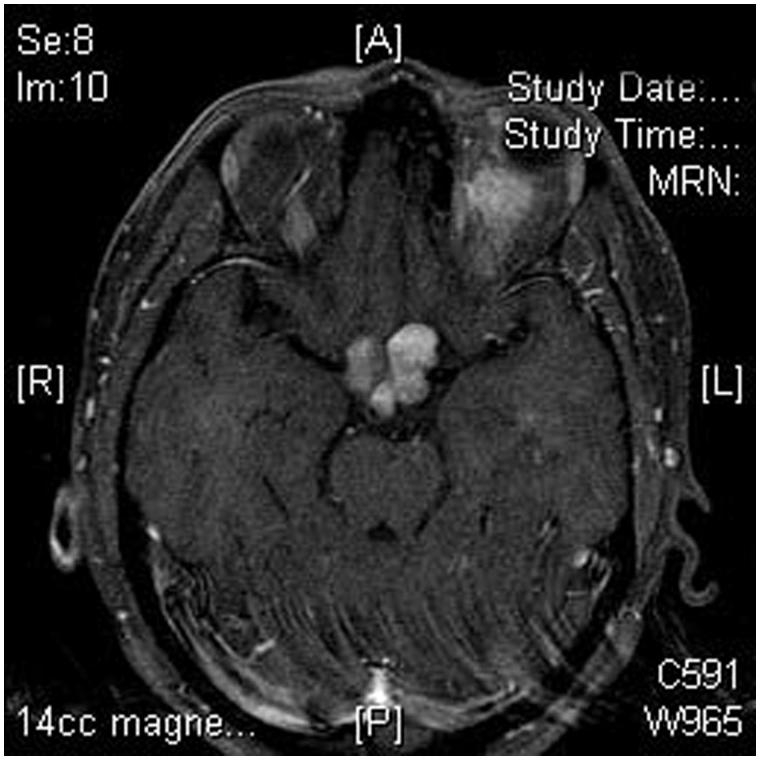
The patient began radiotherapy and temodar chemotherapy. Two months later, at 10 months after presentation, visual acuity was 20/50 right eye and no light perception left eye. Fundus examination revealed visible infiltration of the optic disc with retinal vein occlusion (Figure 7). Perimetry showed a temporal defect in the right eye (Figure 8). MRI showed extension of the tumour to involve the chiasm and optic tracts (Figure 9). Malignant transformation was suspected. She died from tumour-related causes 1 year later.
FIGURE 7.
Case 1. Colour fundus photograph of the left optic disc, showing oedema and probable infiltration, with retinal venous congestion and diffuse retinal haemorrhages consistent with central retinal vein occlusion.
FIGURE 8.
Case 1. Automated perimetry, right eye, showing temporal hemianopic defect with midline margination.
FIGURE 9.
Case 1. Axial MRI, brain and orbits, T1-weighted with fat suppression and contrast. There is expansion and enhancement of both optic nerves and chiasm by tumour.
Case 2
A 45-year-old woman reported a 1-month history of photopsia and transient visual obscurations right eye. Visual acuity was 20/20 in each eye, with equivocal right RAPD, mild optic disc oedema (Figure 10), and minimal blind spot enlargement on perimetry (Figure 11). MRI demonstrated slightly widened and diffusely enhancing right intraorbital optic nerve (Figure 12).
FIGURE 10.
Case 2. Colour fundus photograph of the right optic disc, showing diffuse oedema.
FIGURE 11.
Case 2. Automated perimetry, showing mild blind spot enlargement, right eye.
FIGURE 12.
Case 2. Axial MRI, brain, T1-weighted with contrast. The right intraorbital optic nerve shows mild enhancement.
Over the next 8 months, the transient visual obscurations increased, but visual acuity remained 20/20, with only minor visual field loss (Figure 13), and no change in RAPD or optic disc oedema. CBC, ESR, VDRL, fluorescent treponemal antibody (FTA), ACE, and Lyme titres were normal, and cerebrospinal fluid (CSF) evaluation revealed only slightly elevated protein of 59 with 1 oligoclonal band. At 9 months after presentation, she noted a rapid decrease in vision right eye to 20/200, with worsened visual field (Figure 14). A short course of systemic steroid treatment produced no benefit, and within another month, visual acuity was no light perception right eye, with worsened optic disc oedema. MRI showed increased diameter of the intraorbital optic nerve (Figure 15). An optic nerve biopsy was performed.
FIGURE 13.
Case 2. Automated perimetry, showing mild blind spot enlargement and minimal generalised depression, right eye.
FIGURE 14.
Case 2. Automated perimetry, showing severe generalised depression, right eye.
FIGURE 15.
Case 2. Axial MRI, orbits, T1-weighted with contrast. The right intraorbital optic nerve shows increased diameter and enhancement on axial (left) and coronal fat suppressed (right) images.
Sections showed relative preservation of some portions of the optic nerve, with infiltration in other areas (Figure 16A, arrows, and B) by a highly cellular tumour with moderate to pronounced celluar atypia (Figure 16C). Pilocytic features consistent with glioma were noted, but additionally, a population of ganglion cells was present (Figure 16D), with synaptophysin positivity consistent with ganglioglioma (Figure 17A and B). Immunohistochemistry showed strongly positive GFAP. Ki-67 reactivity was present in approximately 2–3% of the cells (Figure 17C, arrows). The tumour was p53 negative. Features were consistent with a WHO Grade 1 tumour.
FIGURE 16.
Case 2. (A) Relative preservation of normal architecture of the optic nerve; however, arrows at upper right show disruption of this architecture by an infiltrative tumour (haematoxylin-eosin; ×20). (B) Preserved septation of the optic nerve, with slightly increased cellularity at right (haematoxylin-eosin; ×100). (C) xxx (same magnification as B) shows markedly increased cellularity, with moderate to pronounced cytologic atypia (haematoxylin-eosin; ×100). (D) Atypical ganglion cells are noted in some regions of the tumour (haematoxylin-eosin; ×280).
FIGURE 17.
Case 2. (A, B) Synaptophysin immunoreactivity is prominent on the membranes of atypical ganglion cells (anti-synaptophysin; ×50 and ×180, respectively). (C) Ki-67 labelling index is low (arrows denote immunoreactive nuclei) (anti-Ki-67; ×50).
Follow-up at 2 years showed no recurrence.
Case 3
A 31-year-old man reported a 1-month history of decreased vision right eye. Visual acuity was 20/20 in each eye, with a right RAPD, mild optic atrophy, and minimal visual field depression on perimetry (Figure 18). MRI demonstrated enlargement and enhancement of the optic nerve and sheath from globe to orbital apex (Figure 19). Over the next 10 months, visual acuity deteriorated to 20/50 right eye, with no change in fundus appearance, but mild worsening of visual field defect (Figure 20). Repeat MRI showed increase in diameter and extent of the optic nerve lesion to involve the intracranial segment to 5 mm from the chiasm (Figure 21). Four months later, he underwent fractionated stereotactic conformal radiation, 5040 cGy over 6 weeks.
FIGURE 18.
Case 3. Automated perimetry, showing mild generalised depression right eye.
FIGURE 19.
Case 3. Axial MRI, brain, T1-weighted with contrast. The right intraorbital optic nerve shows increased diameter, tortuosity, and mild enhancement.
FIGURE 20.
Case 3. Automated perimetry, showing increased superotemporal and mild generalised depression right eye.
FIGURE 21.
Case 3. Axial MRI, orbits, T1-weighted with fat suppression and contrast. The right optic nerve is increased in diameter, with moderate enhancement extending to the optic canal.
Two months after completing therapy, visual acuity was no light perception. The optic disc was oedematous (Figure 22), and MRI showed further extension of intracranial tumour nearly to the chiasm (Figure 23). An excisional biopsy of the right optic nerve was performed.
FIGURE 22.
Case 3. Colour fundus photograph of the right optic disc, showing diffuse oedema, with retinal venous congestion and scattered retinal haemorrhages.
FIGURE 23.
Case 3. Axial MRI, orbits, T1-weighted with fat suppression and contrast. The right optic nerve has further increased in diameter, with central hypointense region consistent with necrosis. The tumour now extends intracranially, with focal expansion of the optic nerve near the chiasm.
Sections showed infiltration of the optic nerve in some segments (Figure 24A) by a moderately cellular tumour with mild cytologic atypia (Figure 24B and C) and hyalinised vessels (Figure 24C, arrows). Regions with a loose reticular pattern and others with a more compact pattern were seen (Figure 24D). Regions of oligodendrocytic cells (Figure 25A) and others with pilocytic pattern were noted (Figure 25B). A low Ki-67 reactivity index (4–5%) was seen (Figure 25C, arrows), as well as p53 negativity. The tumour was strongly positive for GFAP (Figure 25D). Features were consistent with a WHO Grade 1 pilocytic astrocytoma.
FIGURE 24.
Case 3. (A) Whole-mount preparation showing low power views of the tumour; two of the fragments show retained configuration/architecture of the ON, whereas fragment at lower left shows a “dumbbell configuration” (haematoxylin-eosin; ×6). (B) Tumour is moderately cellular with mild cytologic atypia (haematoxylin-eosin; ×50). (C) Prominently hyalinised blood vessels are noted (arrows) within the tumour (haematoxylin-eosin; ×50). (D) Tumour has a biphasic appearance, with a loose reticular pattern (left) and more compact appearance (right) (haematoxylin-eosin; ×100).
FIGURE 25.
Case 3. (A) Some areas of the tumour show an oligodenroglioma-like pattern (haematoxylin-eosin; ×100). (B) Others show cells with elongated, piloid cytoplasm (haematoxylin-eosin; ×140). (C) Immunohistochemistry shows a low Ki-67 labelling index (arrows mark immunoreactive nuclei) (anti-Ki-67; ×50) and (D) strong GFAP immunoreactivity (GFAP; ×100).
Follow-up at 19 months showed no recurrence.
Discussion
The differentiation of benign from malignant central nervous system (CNS) glial tumours may be difficult. While the typical pilocytic astrocytoma of childhood has generally non-invasive features and Ki-67 and p53 reactivity of 1–2%, WHO Grade II tumours, with some invasiveness and higher immunoreactivity, may be difficult to characterise (Table 1). Case 1 was classified as benign (Grade II) by morphologic criteria, but showed high reactivity to both Ki-67 and p53, suggesting an aggressive tumour, which was eventually borne out after an indolent initial clinical course. Cases 2 and 3 were classified as Grade I, but followed aggressive clinical courses, with tumour growth and complete loss of vision over 1–1.5 years.
TABLE 1.
Selected CNS gliomas: Comparison of histopathology and immunostaining characteristics.
| Selected CNS tumour | WHO grade | Histopathology | MIB-1/Ki-67 LI | p53 |
|---|---|---|---|---|
| Pilocytic astrocytoma | I | Biphasic: piloid and spongy glial cell regions | <10% | <5% |
| Diffuse astrocytoma | II | +Diffuse infiltration, cytoplasmic atypia | <10% | <5% |
| Anaplastic astrocytoma | III | +Anaplasia, mitotic activity | >10% | >5% |
| Glioblastoma | IV | +Microvascular proliferation and/or necrosis | >10% | >5% |
| Ganglioglioma | I | Uniform, monomorphic glial cell and ganglion cell components, diffuse infiltration, rare mitoses | <5% | <5% |
| Anaplastic ganglioglioma | III | +Anaplasia. Increased mitotic activity, vascular proliferation, necrosis | >5% | >5% |
Immunostaining characteristics are general guidelines and must be utilised in conjunction with histopathologic features.
While MIB-1 (Ki-67) is routinely utilised in the evaluation of intracranial tumours, it has not been incorporated into their routine grading, for several reasons9,10:
MIB-1/Ki-67 labelling indices are usually estimated rather than rigorously assessed; therefore, substantial inter-observer variability comes into play.
The indices are assessed in different laboratories using a variety of antibodies and staining protocols, leading to additional variability in the results.
A given tumour may have variable Ki-67 labelling indices throughout the neoplasm, leading to over- or underestimation of its overall growth potential.
Tumours of the same WHO grade will often show tremendous variations in labelling indices for proliferating markers, rendering them of limited value in realistically predicting tumour prognosis.
Thus, in cases such as ours, low or moderate indices of proliferation may not accurately predict a tumour’s clinical aggressiveness.
While cases of malignant (WHO Grade III or IV) glioma are well documented in the literature and are most common in adulthood, cases of benign (WHO Grade I or II) are rarely seen or reported in this age group. They are often initially misdiagnosed as optic neuritis or non-arteritic anterior ischaemic optic neuropathy (NAION); MRI may demonstrate enhancement of the intraorbital optic nerve, ruling out the diagnosis of NAION, but the appearance may precisely mimic optic neuritis early in the course, as in our Cases 1 and 2. In certain cases, the length of optic nerve involvement on imaging and the lack of response to steroids may mimic neuromyelitis optica. The persistent optic disc oedema and progressive downhill course in spite of therapy, with progressive enlargement of the optic nerve lesion on MRI, later distinguish them.
Wulc and associates4 described seven cases seen over a 38-year period at Moorfields Eye Hospital and National Hospitals for Nervous Diseases, London. Ages ranged from 18 to 61 years, and histologic findings were consistent with pilocytic astrocytoma in all cases. Visual acuity was initially no light perception in three cases, light perception in one, and 6/9 in one, progressing to no light perception over 8 months. Tumialan and associates6 reported a 17-year-old woman who presented with acute pain and visual loss, a cecocentral scotoma, and enhancement of the optic nerve on MRI, all consistent with optic neuritis. She subsequently demonstrated deterioration of vision and increased size of the optic nerve abnormality on MRI over 1 year, prompting biopsy, which revealed pilocytic astrocytoma. Shapey and associates7 described a 20-year-old woman with a 6-month history of visual deterioration culminating in further loss to no light perception over several weeks. Biopsy revealed pilocytic astrocytoma. Pasol and associates8 reported a 75-year-old man with multiple vasculopathic risk factors who experienced sudden visual loss to the level of count fingers, associated with a RAPD and optic disc oedema consistent with NAION. Over the next 4 months, vision deteriorated to no light perception, with persistent optic disc oedema. MRI revealed mild-to-moderate enhancement and enlargement of the intraorbital and intracranial optic nerves suggestive of optic neuritis, but the lesion continued to enlarge despite corticosteroid therapy, and biopsy of the optic nerve 1 year after visual loss was consistent with pilocytic astrocytoma.
Biopsy is frequently performed to rule out malignancy, particularly in cases with rapid enlargement of tumour, but establishing the diagnosis of even a Grade I glioma in these cases is essential to support proper management and prevent contralateral involvement. Rarely, malignant transformation occurs later in the course,11,12 as was presumed in our Case 1; early diagnosis may therefore be lifesaving.
Acknowledgements
This work was supported in part by an unrestricted grant from the Research to Prevent Blindness, Inc.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Note: Figures 5–7, 10–12, and 15–25 appear in colour online at http://informahealthcare.com/oph.
References
- 1.Dutton JJ. Gliomas of the anterior visual pathway. Surv Ophthalmol 1994;38:427–452 [DOI] [PubMed] [Google Scholar]
- 2.Hoyt WF, Meshel LG, Lessell S, Schatz NJ, Suckling RD. Malignant optic glioma of adulthood. Brain 1973;96:121–132 [DOI] [PubMed] [Google Scholar]
- 3.Wabbels B, Demmler A, Seitz J, Woenckhaus M, Bloss HG, Lorenz B. Unilateral adult malignant glioma. Graefes Arch Clin Exp Ophthalmol 2004;242:741–748 [DOI] [PubMed] [Google Scholar]
- 4.Wulc AE, Bergin DJ, Barnes D, Scaravilli F, Wright JE, McDonald WI. Orbital optic nerve glioma in adult life. Arch Ophthalmol 1989;107:1013–1016 [DOI] [PubMed] [Google Scholar]
- 5.Hoyama E, Cruz AA, Colli BO, Matos JR, Chahud F. Isolated low grade pilocytic astrocytoma of the optic nerve in the elderly: case report. Arq Bras Oftalmol 2008;71:97–100 [DOI] [PubMed] [Google Scholar]
- 6.Tumialan LM, Dhall SS, Biousse V, Newman NJ. Optic nerve glioma and optic neuritis mimicking one another: case report. Neurosurgery 2005;57:E190. [DOI] [PubMed] [Google Scholar]
- 7.Shapey J, Danesh-Meyer HV, Kaye AH. Diagnosis and management of optic nerve glioma. J Clin Neurosci 2011;18:1585–1591 [DOI] [PubMed] [Google Scholar]
- 8.Pasol J, Sternau L, Luetner P, Giannini C. Rapid progressive unilateral visual loss in an elderly man. J Neuro-Ophthalmol 2010;30:188–192 [DOI] [PubMed] [Google Scholar]
- 9.Prayson RA. The utility of MIB-1/Ki-67 immunostaining in the evaluation of central nervous system neoplasms. Adv Anat Pathol 2005;12:144–148 [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz SI, Jin T, Prayson RA. Role of MIB 1 in predicting survival in patients with glioblastoma. J Neuro-Oncol 2006;76:193–200 [DOI] [PubMed] [Google Scholar]
- 11.Zoeller GK, Braithwaite CD, Sandberg DI. Malignant transformation of an optic pathway glioma without prior radiation therapy. J Neurosurg Pediatr 2010;5:507–510 [DOI] [PubMed] [Google Scholar]
- 12.Mullanaey J, Walsh J, Lee WR, Adams JH. Recurrence of astrocytoma of optic nerve after 48 years. Br J Ophthalmol 1976;60:539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]