Abstract
Impairment of autophagy-lysosomal pathways (ALPs) is increasingly regarded as a major pathogenic event in neurodegenerative diseases, including Parkinson’s disease (PD). ALP alterations are observed in sporadic PD brains and in toxic and genetic rodent models of PD-related neurodegeneration. In addition, PD-linked mutations and post-translational modifications of α-synuclein impair its own lysosomal-mediated degradation, thereby contributing to its accumulation and aggregation. Furthermore, other PD-related genes, such as leucine-rich repeat kinase-2 (LRRK2), parkin, and phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1), have been mechanistically linked to alterations in ALPs. Conversely, mutations in lysosomal-related genes, such as glucocerebrosidase (GBA) and lysosomal type 5 P-type ATPase (ATP13A2), have been linked to PD. New data offer mechanistic molecular evidence for such a connection, unraveling a causal link between lysosomal impairment, α-synuclein accumulation, and neurotoxicity. First, PD-related GBA deficiency/mutations initiate a positive feedback loop in which reduced lysosomal function leads to α-synuclein accumulation, which, in turn, further decreases lysosomal GBA activity by impairing the trafficking of GBA from the endoplasmic reticulum-Golgi to lysosomes, leading to neurodegeneration. Second, PD-related mutations/deficiency in the ATP13A2 gene lead to a general lysosomal impairment characterized by lysosomal membrane instability, impaired lysosomal acidification, decreased processing of lysosomal enzymes, reduced degradation of lysosomal substrates, and diminished clearance of autophagosomes, collectively contributing to α-synuclein accumulation and cell death. According to these new findings, primary lysosomal defects could potentially account for Lewy body formation and neurodegeneration in PD, laying the groundwork for the prospective development of new neuroprotective/disease-modifying therapeutic strategies aimed at restoring lysosomal levels and function.
Keywords: Parkinson’s disease, ATP13A2, glucocerebrosidase, lysosome, neurodegeneration, Lewy body
Lysosomes are dynamic acidic organelles that contain hydrolytic enzymes capable of degrading intracellular components through several degradation pathways, including endocytosis, phagocytosis, and autophagy1,2 (Fig. 1). Lysosomes are responsible for the clearance of long-lived proteins, such as aggregate-prone α-synuclein among others, and for the removal of old or damaged organelles, such as mitochondria. Both α-synuclein aggregation and mitochondrial dysfunction are considered major pathogenic events in Parkinson’s disease (PD).3–5 Increasing evidence indicates that impairment of lysosomal function may contribute to the pathogenesis of several neurodegenerative diseases, including PD.6 Here, we review recent data, mostly derived from genetic alterations in lysosomal-related genes, supporting a potential pathogenic role for lysosomal dysfunction in PD.
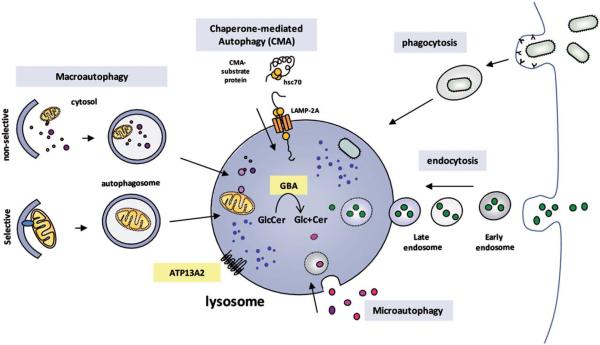
FIG. 1.
Lysosomal degradation pathways are illustrated. Several degradation pathways, including endocytosis, phagocytosis, and autophagy, converge at the lysosome as a final destination. Autophagy is a tightly regulated process by which certain intracellular components are recycled inside lysosomes. Various types of autophagy, including microautophagy and macroautophagy and chaperone–mediated autophagy (CMA), differ in their mechanisms and functions. Microautophagy involves the sequestration and degradation of complete regions of the cytosol (including proteins and organelles) through invaginations and tubulations of the lysosomal membrane. In macroautophagy, intracellular components are enclosed in a double membrane vesicle called the autophagosome. This vesicle is then fused with lysosomes, wherein hydrolytic enzymes complete the degradation of the sequestered material. Macroautophagy can exert a nonselective degradative effect by engulfing in–bulk portions of the cytosol and can constitute a more selective mechanism whereby specific substrates are recognized by distinct autophagic adapters and selectively degraded. In CMA, specific cytosolic proteins that contain a KFERQ–like consensus sequence directly cross the lysosomal membrane via a specific membrane receptor, LAMP-2A, assisted by chaperones, including Hsc70. Glucocerebrosidase (GBA) is an intralysosomal enzyme that catalyzes the conversion of the glycolipid glucosylceramide into glucose and ceramide inside lysosomes. ATP13A2 is a transmembrane type 5 P–type adenosine triphosphatase (ATPase) protein present in lysosomal membrane.
Dysregulation of the Autophagy-Lysosome System in PD
Neurons are particularly sensitive to alterations in protein degradation pathways. Constitutive autophagy is essential for neuronal survival, because its genetic inactivation selectively in neurons leads to the formation of ubiquitinated intracellular inclusions and cell loss in mutant mice.7–9 Implicating an impairment of lysosomal activity in PD, a reduced number of intraneuronal lysosomes, decreased levels of lysosomal-associated proteins (cathepsin D, lysosomal-associated membrane protein 1 [LAMP-1], LAMP-2a, and heat shock cognate 71 kDa protein [Hsc70]) and accumulation of undegraded auto-phagosomes (APs) have been observed in postmortem brain samples from patients with idiopathic PD and toxin and genetic rodent models of PD.10–14
In addition, impaired lysosomal-mediated clearance of APs has been reported in cultured dopaminergic neurons generated from reprogrammed induced pluripotent stem cells (iPSCs) derived from skin fibroblasts of sporadic and genetic PD patients.15 Mechanistic studies in 1-methyl-4-phenyl-11.2.3.6-tetrahydropyridine (MPTP)-treated mice revealed that PD-linked lysosomal deficiency preceded cell death and was instrumental in the impairment of autophagy and in overall dopaminergic neurodegeneration.16 In these animals, pharmacologic reactivation of autophagy-lysosomal pathways (ALPs) with rapamycin resulted in an increased number of functional lysosomes, reversed AP accumulation, and attenuated dopaminergic cell death.13,17 Further demonstrating a deleterious role of impaired lysosomal/autophagic degradation in relation to PD, directed genetic deletion of an essential autophagy gene, autophagy related 7 (Atg7), within catecholaminergic neurons in mice resulted in decreased striatal dopamine; abnormal presynaptic neurotransmission; and age-dependent axonal morphologic alterations, motor deficits, and neurodegeneration.18–21 Remarkably, these animals also developed presynaptic α-synuclein accumulations, suggesting that macroautophagy may play a critical role in axons, whereas other degradative pathways (such as chaper-one-mediated autophagy [CMA] or the ubiquitin-proteasome system [UPS]) may have a more prominent role in cell bodies. α-Synuclein is a major constituent of Lewy bodies (LBs) and Lewy neurites and is believed to play a significant pathogenic role in both familial and idiopathic forms of PD. Although it was originally thought that α-synuclein was exclusively degraded by the UPS, we now know that this protein can also be degraded inside lysosomes, through CMA, or through endocytosis22–27 (Fig. 2). The signals responsible for sending α-synuclein to either 1 or another degradation pathway are not yet fully understood, particularly in neurons, but depend on several factors intrinsic to the status of the protein, such as: (1) its folding state (unfolded, properly folded, or misfolded), (2) its localization (cytosolic, associated to membranes, or even extracellular), (3) the presence of post-translational modifications (unmodified, ubiquitinated, phosphorylated, nitrated, oxidized, or dopamine-modified), and (4) its oligomeric state (monomeric, oligomeric, protofibrillar, fibrillar, or aggregated).25,28 All these factors, together with possible interactions with different chaperones and co-chaperones, determine the degradation destiny of α-synuclein. Although macroautophagy is able to degrade different forms of α-synuclein, it has been recently reported that α-synuclein, in turn, can directly impair macroautophagy both in vitro and in vivo.29–31 Furthermore, PD-linked pathologic α-synuclein (ie, mutated, post-translationally modified, or oligomeric/ aggregated) can directly impair UPS and lysosomal functions, resulting in defective clearance and subsequent accumulation of abnormal α-synuclein species and other UPS/lysosomal substrates.23,32,33 Hence, α-synuclein accumulation in PD may represent both a cause and a consequence of impaired proteolytic activity in this disease. It is noteworthy that the lysosomal enzyme cathepsin-D, the most active protease in degradation of α-synuclein, is neuroprotective against α-synuclein–induced dopaminergic neurodegeneration in a Caenorhabditis elegans model, and genetic ablation of this enzyme in mutant mice leads to α-synuclein accumulation.34,35 In addition to α-synuclein, other PD-related genes recently have been linked to ALP alterations (Fig. 2). For instance, PD-linked mutations in leucine-rich repeat kinase-2 (LRRK2) have been associated with impaired autophagy by an as yet unknown mechanism. In addition, PD-linked mutations in the phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) and parkin genes have been shown to disrupt the coordinated normal regulatory role of these molecules at promoting autophagic degradation of dysfunctional mitochondria, thereby leading to the deleterious consequences of defective mitophagy. Taken together, these observations strongly support the concept that the ALP may be impaired in PD.
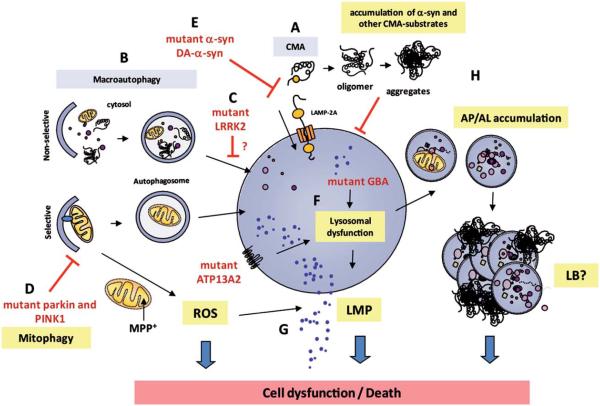
FIG. 2.
Lysosomal deficiency is illustrated in Parkinson’s disease (PD). a–Synuclein can be degraded by various proteolytic pathways within the cell, including autophagy and the ubiquitin proteasome system. Lysosomes can degrade different types of a–synuclein species by means of different pathways, including macroautophagy, chaperone–mediated autophagy (CMA), and endocytosis. (A) Soluble or wild–type a–synuclein are preferentially degraded in the lysosome by CMA, whereas (B) macroautophagy can degrade both soluble and large protein complexes that contain modified or oligomeric forms of a–synuclein. (C) PD-linked mutations in leucine-rich repeat kinase-2 (LRRK2) have been associated with impaired autophagy by an as yet unknown mechanism. (D) In addition, PD-linked mutations in phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) and Parkin have been shown to disrupt the coordinated normal regulatory role of these molecules at promoting autophagic degradation of dysfunctional mitochondria, thereby leading to defective mitophagy. (E) PD-linked A30P or A53T α-synuclein mutants and dopamine-modified wildtype (WT) α-synuclein (DA-a-syn) block CMA activity, resulting in insufficient lysosomal clearance of α-synuclein and other CMA-substrates. (F) Mutations in lysosomal-associated genes (glucocerebrosidase [GBA], ATP13A2) directly cause lysosomal impairment and can be associated with PD-like neurodegeneration. (G) Enhanced reactive-oxygen species (ROS) production caused by mitochondrial neurotoxin 1-methyl-4-phenylpyridine (MPP)-positive or α-synuclein oligomers induces abnormal lysosomal membrane permeabilization (LMP) and disruption of lysosomal membrane integrity. (H) Overall, lysosomal dysfunction leads to the accumulation of toxic/aggregated α-synuclein, dysfunctional organelles, and undegraded/ partly degraded autophagosomes (AP) and autophagolysosomes (AL), all of which may result in Lewy body (LB) formation and/or neurodegeneration.
Lysosomal-Related Genetic Alterations and PD
Although the above-reported data associate lysosomal insufficiency with PD, genetic analyses also indicate that lysosomal impairment may play a primary pathogenic role in this disease. In particular, mutations in 2 genes that encode lysosomal proteins, including the enzyme glucocerebrosidase (GBA) and lysosomal type 5 P-type ATPase (ATP13A2), have been linked to PD—the former as an important risk factor for PD through a multicenter genetic analysis,36 and the latter through linkage in rare families with prominent parkinsonism.37–39 Recent data offer mechanistic molecular evidence for such a connection (Fig. 2).
1. GBA
Loss-of-function mutations in the gene encoding GBA cause Gaucher disease (GD), the most common lysosomal storage disorder. GBA catalyzes the conversion of the glycolipid glucosylceramide into glucose and ceramide inside lysosomes. Conditional knock-out mice of GBA in the central nervous system develop neuronal loss associated with microgliosis, indicating a critical role of GBA in neuronal survival.40 Glucosyl-ceramide levels are increased in the brains of these animals. Carrier status of a single mutant GBA allele is a significant risk factor for PD36,41 and for dementia with LBs.42 Conversely, patients with GD, although clinically different from PD, not infrequently exhibit parkinsonism, α-synuclein–immunoreactive LBs, and loss of melanized dopaminergic neurons.41,43 Intralysosomal accumulation of glucosylceramide has been proposed as the most likely pathogenic mechanism linked to GBA loss-of-function homozygous mutations.44 However, GBA mutations linked to an increased risk of PD are usually present only in the heterozygous state (ie, patients who carry 1 wild-type GBA allele and, thus, have at least 50% of normal enzyme function).45 In addition, it has been reported that GBA is a component of LBs.46
Recent mechanistic studies indicate that GBA can influence α-synuclein processing through both gain-of-function and loss-of-function mechanisms. Loss of GBA activity in mouse primary cortical neurons and in human neurons derived from iPSCs from a patient with GD resulted in glucosylceramide accumulation, decreased lysosomal degradation, and subsequent accumulation of α-synuclein, promoting α-synuclein oligomer formation and neurotoxicity.47 α-Synuclein accumulations, in turn, impair the trafficking of GBA from the endoplasmic reticulum-Golgi to lysosomes, thereby resulting in further decreased lysosomal GBA activity.47 Thus, loss of GBA creates a positive feedback loop of reduced lysosomal function and α-synuclein accumulation that ultimately leads to neurodegeneration.48 In another study, overexpression of several GBA mutants in cultured cell lines did not alter GBA activity but also resulted in α-synuclein accumulations, which were reversed by inducing autophagy with rapamycin or by promoting GBA translocation into lysosomes with the GBA chaperone isofagomine.49 Taken together, these results indicate that both GBA gain of function and loss of function can promote pathologic α-synuclein accumulations and that restoring/enhancing normal GBA activity may hold promise as a potential therapeutic strategy for PD and other synucleinopathies. In this regard, it has been demonstrated that adeno-associated, virus-mediated expression of exogenous GBA attenuates α-synuclein pathology and cognitive deficits in a genetic mouse model of GD.50 However, it is worth noting that, although individuals with GD and parkinsonism exhibit synucleinopathy, the majority of patients with GD (either homozygous or heterozygous) do not develop either synucleinopathies or parkinsonism.43,46
2. ATP13A2
Mutations in the ATP13A2/PARK9 gene have been linked to autosomal recessive, levodopa-responsive parkinsonism with nigrostriatal-pallidal pyramidal neurodegeneration (Kufor–Rakeb syndrome [KRS]).37,51 However, there is wide phenotypic heterogeneity in patients with KRS, depending on the type of ATP13A2 mutation, thus indicating a high level of complexity of this disorder. To date, no brain histopathology data from ATP13A2-mutant patients have been reported, thereby precluding the assessment of α-synuclein pathology in these patients.
The ATP13A2 gene encodes a lysosomal ATPase involved in selective active transport of cations across diverse biologic membranes.52,53 Genetic studies in yeast suggest that ATP13A2 yeast ortholog is involved in protecting cells against manganese toxicity and, more broadly, heavy metals.54 Conversely, ATP13A2 also confers protection against α-synuclein misfolding in mammalian cells and attenuates α-synuclein toxicity in Caenorhabditis elegans and in primary dopaminergic cell cultures.55 Thus, these results suggest a potential link between these 2 PD-associated pathogenic pathways.
Likewise, a general protective role for ATP13A2 against a wide variety of cellular stresses, such as mitochondrial complex I impairment, oxidative stress, and proteasomal stress, has been demonstrated.56 It is hypothesized that missense or truncation mutations in the ATP13A2 gene exert their pathogenic effect by causing loss of ATP13A2 function due to impaired targeting of ATP13A2 to lysosomes.39,57,58 Studies in KRS patient-derived fibroblasts and ATP13A2-deficient cell lines revealed a general lysosomal impairment characterized by instability of the lysosomal membrane, impaired lysosomal acidification, decreased proteolytic processing of lysosomal enzymes, reduced degradation of lysosomal substrates, and diminished lysosomal-mediated clearance of AP, all of which were associated with cell death. All these effects were rescued by restoring the expression of wild-type ATP13A2 in ATP13A2-depleted cells.59–61 In both ATP13A2-mutant or ATP13A2-defective cells, impaired lysosomal proteolysis resulted in a marked accumulation of α-synuclein.59,60 Silencing of endogenous α-synuclein attenuated toxicity in ATP13A2-depleted neurons.60 Conversely, cell death induced by ATP13A2 knockdown was greatly enhanced by α-synuclein overexpression.59 Relevant to PD, lentiviral vector-mediated ATP13A2 knockdown in primary mesencephalic dopaminergic neurons resulted in selective dopaminergic, but not GABAergic, neurodegeneration.59 In addition, ATP13A2 levels were decreased in postmortem PD nigral samples in which 90% of LBs exhibited a positive signal for ATP13A2 in their core and were surrounded by more peripherally located α-synuclein.59
Overall, these results indicate a pathogenic role of ATP13A2 deficiency in lysosomal function and cell viability. In addition, other studies have indicated that loss of ATP13A2 function may also induce mitochondrial defects, likely because of decreased mitochondrial turnover secondary to impaired mitophagy.62,63 ATP13A2 and some of its interacting partners have been identified as modifiers of α-synuclein toxicity in yeast 2-hybrid systems and RNA interference screens in worms64,65 and, thus, may represent potential therapeutic targets for the development of new strategies aimed at modulating ATP13A2-related pathways in PD.
Concluding Remarks and Future Directions
Increasing evidence indicates that impaired lysosomal function, which is essential to maintain proper protein and organelle quantity and quality within cells, may play an important role in the pathogenesis of PD. Lysosomal defects could potentially account not only for dopaminergic cell dysfunction/death but also for the presence of α-synuclein–containing LBs. The identification of AP/lysosomal markers as components of LBs in patients with sporadic PD, including LC3,13,14 LAMP-1,12 LAMP-2a,14 cathepsin-D,12 VPS35,66 GBA,46 and ATP13A2,59 raises the possibility that LBs, the origin and significance of which remain unknown, may seed around impaired lysosomes and/or undegraded APs and grow in size by the continuous deposition of lysosomal/AP-derived, undegraded material as the disease progresses. Consistent with this, (1) LBs contain abnormal mitochondria, autophagy-related molecules, lysosomes, and vacuolar structures12,13,67; (2) patients with GD can exhibit α-synuclein–immunoreactive LBs similar to those found in PD46,68; (3) specific environments inside membranous and vesicular structures, such as a molecularly crowded milieu, are more prone to α-synuclein aggregation67,69,70; and (4) ubiquitin, which has been identified as 1 of the main components of LBs, was originally associated with the proteasome degradation pathway, but we now know that ubiquitin is a tag that can also target intracellular components for its degradation by some forms of selective autophagy.71
Although lysosomal impairment represents only 1 aspect of the many potential facets of PD pathogenesis, the results reviewed here raise the possibility that enhancement/restoration of lysosomal-mediated degradation may prove beneficial for PD. It is important to note, however, that, based on the current results, strategies/drugs aimed at activating autophagy solely by increasing AP formation without concomitant increases in lysosomal function could result in further cellular damage, rather than benefit, in the context of PD. Instead, therapeutic modulation of autophagy in PD should be aimed at the late steps of the ALP (ie, improving the efficiency of AP maturation and substrate digestion) by boosting AP maturation, fusion with lysosome, and lysosomal biogenesis, trafficking, and function.72,73 In this regard, autophagy induction with the mammalian target of rapamycin (mTOR)-inhibiting drug rapamycin or with mTOR-independent autophagy enhancers, such as lithium and trehalose, provide neuroprotection in several in vitro and in vivo genetic and toxic models of PD17,74–77 and have been shown to exert part of their proautophagy actions by enhancing lysosomal activation and AP clearance, and not solely by increasing new AP formation.13,17,78 Similarly, viral-vector–mediated expression of autophagy regulators, such as beclin-1, has been shown to reduce α-synuclein accumulations and synaptic pathology in α-synuclein transgenic mice by enhancing autophagic activity.79 Overexpression of transcription factor EB (TFEB), a master activator of the ALP,80,81 also reportedly protects cultured cells against parkinsonian neurotoxins.13 Overall, those studies lay the groundwork for the potential development of novel therapeutic strategies aimed at restoring lysosomal-mediated degradation in PD.
Acknowledgments
Funding Agencies: This work was supported by a Marie Curie Reintegration Grant from the European Commission (FP7-PEOPLE-2009-ERG256303; to B.D.); by a Fondation pour la Recherche Médicale grant (to B.D.), by Agence Nationale de la Recherche grants (ANR-08-MNP-018; ANR-07-MNP-Trafinlid; to E.B.); by the Volkswagen Foundation, the Hermann and Lilly Schilling Foundation, and MEFOPA (FP7) (to C.K.); by Fondo de Investigación Sanitaria-Instituto de Salud Carlos III, Spain (to M.V. and M.M.-V.); by Ministerio de Investigación, Desarrollo e Inovación, Spain (to M.M.-V); and by US National Institutes of Health, National Institute of Neurological Disorders and Stroke grants (R01NS060123, NS060809, RNS055683) and the Michael J. Fox Foundation (to Z.Y.). This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (to M.R.C.) and by National Institutes of Health grant 1R15NS075684 (to G.A.C.)
EB has equity stake in Motac holding Ltd. (Manchester, UK), receives consultancy payments from Motac Neuroscience Ltd. (Manchester, UK), has received grants from the Agence Nationale de la Recherche (France), the Michael J. Fox Foundation for Parkinson Research (USA), the European Commission and is a member of the scientific advisory board of the Michael J. Fox Foundation for Parkinson Research (USA) and of the France Parkinson association. CK is a member of the editorial board of “Neurology” and has served as editor of the “Continuum Issue Neurogenetics 2008” and as faculty at the Annual Meetings of the American Academy of Neurology since 2004. CK is a consultant to Centogene and received honoraria for speaking from Boehringer Ingelheim and Orion Pharma. CK is the recipient of a career development award from the Hermann and Lilly Schilling Foundation. CK is funded by the Volkswagen Foundation, the Deutsche Forschungsgemeinschaft, the Possehl Foundation and received institutional support from the University of Luebeck for genetics research.
Footnotes
Relevant conflicts of interest/financial disclosures: All other authors have nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 2.Lubke T, Lobel P, Sleat DE. Proteomics of the lysosome. Biochim Biophys Acta. 2009;1793:625–635. doi: 10.1016/j.bbamcr.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rochet JC, Hay BA, Guo M. Molecular insights into Parkinson’s disease. Prog Mol Biol Transl Sci. 2012;107:125–188. doi: 10.1016/B978-0-12-385883-2.00011-4. [DOI] [PubMed] [Google Scholar]
- 4.Cookson MR, Bandmann O. Parkinson’s disease: insights from pathways. Hum Mol Genet. 2010;19(R1):R21–R27. doi: 10.1093/hmg/ddq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perier C, Vila M. Mitochondrial biology and Parkinson’s disease [serial online] Cold Spring Harb Perspect Med. 2012;2:a009332. doi: 10.1101/cshperspect.a009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tofaris GK. Lysosome-dependent pathways as a unifying theme in Parkinson’s disease. Mov Disord. 2012;27:1364–1369. doi: 10.1002/mds.25136. [DOI] [PubMed] [Google Scholar]
- 7.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 8.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 9.Komatsu M, Wang QJ, Holstein GR, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anglade P, Vyas S, Javoy-Agid F, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histology and histopathology. 1997;12:25–31. [PubMed] [Google Scholar]
- 11.Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13:473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Dehay B, Bove J, Rodriguez-Muela N, et al. Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, et al. Chaper-one-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I, et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vila M, Bove J, Dehay B, Rodriguez-Muela N, Boya P. Lysosomal membrane permeabilization in Parkinson disease. Autophagy. 2011;7:98–100. doi: 10.4161/auto.7.1.13933. [DOI] [PubMed] [Google Scholar]
- 17.Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez D, Torres CA, Setlik W, et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–284. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman LG, Lachenmayer ML, Wang J, et al. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci. 2012;32:7585–7593. doi: 10.1523/JNEUROSCI.5809-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed I, Liang Y, Schools S, Dawson VL, Dawson TM, Savitt JM. Development and characterization of a new Parkinson’s disease model resulting from impaired autophagy. J Neurosci. 2012;32:16503–16509. doi: 10.1523/JNEUROSCI.0209-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue K, Rispoli J, Kaphzan H, et al. Macroautophagy deficiency mediates age-dependent neurodegeneration through a phospho-tau pathway. Mol Neurodegener. 2012;7:48. doi: 10.1186/1750-1326-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 23.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 24.Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA. Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem. 2010;285:13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci. 2011;31:14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tofaris GK, Kim HT, Hourez R, Jung JW, Kim KP, Goldberg AL. Ubiquitin ligase Nedd4 promotes alpha-synuclein degradation by the endosomal-lysosomal pathway. Proc Natl Acad Sci U S A. 2011;108:17004–17009. doi: 10.1073/pnas.1109356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xilouri M, Brekk OR, Stefanis L. Alpha-synuclein and protein degradation systems: a reciprocal relationship. Mol Neurobiol. 2013;47:537–4551. doi: 10.1007/s12035-012-8341-2. [DOI] [PubMed] [Google Scholar]
- 28.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2012;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winslow AR, Chen CW, Corrochano S, et al. alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crews L, Spencer B, Desplats P, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy [serial online] PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Yu WH, Dorado B, Figueroa HY, et al. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am J Pathol. 2009;175:736–747. doi: 10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emmanouilidou E, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiol Aging. 2010;31:953–968. doi: 10.1016/j.neurobiolaging.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Vicente M, Talloczy Z, Kaushik S, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao L, Hamamichi S, Caldwell KA, et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity [serial online] Mol Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cullen V, Lindfors M, Ng J, et al. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo [serial online] Mol Brain. 2009;2:5. doi: 10.1186/1756-6606-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez A, Heimbach A, Grundemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 38.Di Fonzo A, Chien HF, Socal M, et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- 39.Park JS, Mehta P, Cooper AA, et al. Pathogenic effects of novel mutations in the P-type ATPase ATP13A2 (PARK9) causing Kufor-Rakeb syndrome, a form of early-onset parkinsonism. Hum Mutat. 2011;32:956–964. doi: 10.1002/humu.21527. [DOI] [PubMed] [Google Scholar]
- 40.Enquist IB, Lo Bianco C, Ooka A, et al. Murine models of acute neuronopathic Gaucher disease. Proc Natl Acad Sci U S A. 2007;104:17483–17488. doi: 10.1073/pnas.0708086104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 42.Goker-Alpan O, Giasson BI, Eblan MJ, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 43.Wong K, Sidransky E, Verma A, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Xu YH, Sun Y, Ran H, Quinn B, Witte D, Grabowski GA. Accumulation and distribution of alpha-synuclein and ubiquitin in the CNS of Gaucher disease mouse models. Mol Genet Metab. 2011;102:436–447. doi: 10.1016/j.ymgme.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson’s disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275:5767–5773. doi: 10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120:641–649. doi: 10.1007/s00401-010-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazzulli JR, Xu YH, Sun Y, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawson TM, Dawson VL. A lysosomal lair for a pathogenic protein pair[serial online] Sci Transl Med. 2011;3:91ps28. doi: 10.1126/scitranslmed.3002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cullen V, Sardi SP, Ng J, et al. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Ann Neurol. 2011;69:940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 50.Sardi SP, Clarke J, Kinnecom C, et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci U S A. 2011;108:12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lees AJ, Singleton AB. Clinical heterogeneity of ATP13A2 linked disease (Kufor-Rakeb) justifies a PARK designation. Neurology. 2007;68:1553–1554. doi: 10.1212/01.wnl.0000265228.66664.f4. [DOI] [PubMed] [Google Scholar]
- 52.Schultheis PJ, Hagen TT, O’Toole KK, et al. Characterization of the P5 subfamily of P-type transport ATPases in mice. Biochem Biophys Res Commun. 2004;323:731–738. doi: 10.1016/j.bbrc.2004.08.156. [DOI] [PubMed] [Google Scholar]
- 53.Ramonet D, Podhajska A, Stafa K, et al. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum Mol Genet. 2012;21:1725–1743. doi: 10.1093/hmg/ddr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt K, Wolfe DM, Stiller B, Pearce DA. Cd21, Mn21, Ni21 and Se21 toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochem Biophys Res Commun. 2009;383:198–202. doi: 10.1016/j.bbrc.2009.03.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gitler AD, Chesi A, Geddie ML, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Covy JP, Waxman EA, Giasson BI. Characterization of cellular protective effects of ATP13A2/PARK9 expression and alterations resulting from pathogenic mutants. J Neurosci Res. 2012;90:2306–2316. doi: 10.1002/jnr.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Podhajska A, Musso A, Trancikova A, et al. Common pathogenic effects of missense mutations in the P-Type ATPase ATP13A2 (PARK9) associated with early-onset Parkinsonism [serial online] PLoS One. 2012;7:e39942. doi: 10.1371/journal.pone.0039942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ugolino J, Fang S, Kubisch C, Monteiro MJ. Mutant Atp13a2 proteins involved in parkinsonism are degraded by ER-associated degradation and sensitize cells to ER-stress induced cell death. Hum Mol Genet. 2011;20:3565–3577. doi: 10.1093/hmg/ddr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dehay B, Ramirez A, Martinez-Vicente M, et al. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A. 2012;109:9611–9616. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, alpha-synuclein accumulation, and neurotoxicity. J Neurosci. 2012;32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dehay B, Martinez-Vicente M, Ramirez A, et al. Lysosomal dysfunction in Parkinson disease: ATP13A2 gets into the groove. Autophagy. 2012;8:1389–1391. doi: 10.4161/auto.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grunewald A, Arns B, Seibler P, et al. ATP13A2 mutations impair mitochondrial function in fibroblasts from patients with Kufor-Rakeb syndrome [serial online] Neurobiol Aging. 2012;33:1843.e1–e7. doi: 10.1016/j.neurobiolaging.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 63.Gusdon AM, Zhu J, Van Houten B, Chu CT. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis. 2012;45:962–972. doi: 10.1016/j.nbd.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamamichi S, Rivas RN, Knight AL, Cao S, Caldwell KA, Caldwell GA. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proc Natl Acad Sci U S A. 2008;105:728–733. doi: 10.1073/pnas.0711018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Usenovic M, Knight AL, Ray A, et al. Identification of novel ATP13A2 interactors and their role in α-synuclein misfolding and toxicity. Hum Mol Genet. 2012;21:3785–3794. doi: 10.1093/hmg/dds206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia Q, Liao L, Cheng D, et al. Proteomic identification of novel proteins associated with Lewy bodies. Front Biosci. 2008;13:3850–3856. doi: 10.2741/2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol Neurobiol. 2013;47:495–508. doi: 10.1007/s12035-012-8280-y. [DOI] [PubMed] [Google Scholar]
- 68.Yu WH, Cuervo AM, Kumar A, et al. Macroautophagy—a novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shtilerman MD, Ding TT, Lansbury PT., Jr Molecular crowding accelerates fibrillization of alpha-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry. 2002;41:3855–3860. doi: 10.1021/bi0120906. [DOI] [PubMed] [Google Scholar]
- 71.Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci U S A. 2008;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harrington AJ, Yacoubian TA, Slone SR, Caldwell KA, Caldwell GA. Functional analysis of VPS41-mediated neuroprotection in Caenorhabditis elegans and mammalian models of Parkinson’s disease. J Neurosci. 2012;32:2142–2153. doi: 10.1523/JNEUROSCI.2606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pivtoraiko VN, Harrington AJ, Mader BJ, et al. Low-dose bafilomycin attenuates neuronal cell death associated with autophagy-lysosome pathway dysfunction. J Neurochem. 2010;114:1193–1204. doi: 10.1111/j.1471-4159.2010.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim YH, Rane A, Lussier S, Andersen JK. Lithium protects against oxidative stress-mediated cell death in alpha-synuclein-overexpressing in vitro and in vivo models of Parkinson’s disease. J Neurosci Res. 2011;89:1666–1675. doi: 10.1002/jnr.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong N, Jia M, Chen C, et al. Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience. 2011;199:292–302. doi: 10.1016/j.neuroscience.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 76.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez-Navarro JA, Rodriguez L, Casarejos MJ, et al. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis. 2010;39:423–438. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Demarchi F, Bertoli C, Copetti T, et al. Calpain is required for macroautophagy in mammalian cells. J Cell Biol. 2006;175:595–605. doi: 10.1083/jcb.200601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spencer B, Potkar R, Trejo M, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sardiello M, Palmieri M, di Ronza A, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 81.Settembre C, Di Malta C, Polito VA, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]