Abstract
We have previously shown that pycnogenol (PYC) increases antioxidants, decreases oxidative stress, suppresses neuroinflammation and enhances synaptic plasticity following traumatic brain injury (TBI). Here, we investigate the effects of PYC on cognitive function following a controlled cortical impact (CCI). Adult Sprague-Dawley rats received a CCI injury followed by an intraperitoneal injection of PYC (50 or 100 mg/kg). Seven days post trauma, subjects were evaluated in a Morris water maze (MWM) and evaluated for changes in lesion volume. Some animals were evaluated at 48h for hippocampal Fluoro-jade B (FJB) staining. The highest dose of PYC therapy significantly reduced lesion volume, with no improvement in MWM compared to vehicle controls. PYC failed to reduce the total number of FJB positive neurons in the hippocampus. These results suggest that the reduction of oxidative stress and neuroinflammation are not the key components of the secondary injury that contribute to cognitive deficits following TBI.
Keywords: bioflavonoids, head injury, natural compounds, cortical contusion, water maze, recovery of function
1. INTRODUCTION
Traumatic brain injury (TBI) is a global health problem that is financially crippling. In the United States alone, it is estimated that approximately 1.7 million individuals will suffer from some form of TBI [14]. Following the initial trauma, a secondary injury cascade begins leading to the loss of brain connectivity, neuronal death, and reduced cognitive function. Because of the complexity surrounding the many components of the secondary injury cascade, it is now recognized that a multifaceted or combinational therapeutic approach is necessary [26]. A new field using complementary and alternative medical therapeutic approaches appears to be very promising following TBI [18]. An increasing number of natural compounds, with multifaceted pharmacological effects, may provide essential neuroprotection and increased cellular health following TBI to promote greater recovery [6, 10, 23, 36, 50, 52–54].
Pycnogenol® (PYC) is a patented combinational bioflavonoid extracted from the French maritime pine, Pinus maritima, which has well documented antioxidant and anti-inflammatory properties [25, 33, 38]. One of the mechanisms behind PYC’s ability to suppress inflammation is its ability to inhibit the NF-κB and AP-1 pathway, which suppresses the activation of microglia [13, 15]. In addition, PYC modulates nitric oxide (NO) production through the suppression of inducible nitric oxide synthase (iNOS), a key enzyme of NO [13]. Several studies have documented PYC’s ability to inhibit apoptosis [22, 34, 48, 49].
We have previously shown that PYC is effective in significantly reducing components of the secondary injury cascade including, oxidative stress, neuroinflammation, loss of synaptic proteins and synaptic dysfunction in both the cortex and hippocampus [4, 31, 42]. It is unclear whether PYC-related changes in the secondary injury cascade translate into true neuroprotection and improvement in cognitive ability. The purpose of the present study was to investigate if post trauma therapy with PYC can offset injury-related cognitive dysfunction and protect neurons in the cortex and hippocampus.
2. Materials and methods
2.1 Animal model
Adult male Sprague-Dawley rats (n = 38, 275–300 g; Harlan Labs, Indianapolis, IN) were housed in group cages (2 per cage) on a 12-h light/dark cycle with free access to food and water. All experimental protocols involving animals were approved by the University of Kentucky Animal Use and Care Committee. Cortical contusions were carried out under isoflurane anesthesia (2%) as previously described [5]. Briefly, following a midline incision, a 6 mm diameter craniotomy was made lateral to midline and midway between bregma and lambda. The skull disk was removed without disturbing the dura. The exposed brain was then contused. All injuries were produced using a pneumatic controlled cortical impact device (TBI 0310; Precision Systems and Instrumentation, Fairfax Station, VA) with a hard stop Bimba cylinder (Bimba Manufacturing, Monee, IL) and a 5 mm beveled impactor tip. The depth of the impact was set at 2.0 mm with a velocity of 3.5 m/sec and a dwell time of 500 msec. After the impact, the craniotomy site was sealed with an 8 mm disc formed from clear polyester and MASCOT adhesive. Following injury, animals were treated with PYC (generously provided by Horphag Research, Hoboken, NJ) (50 mg or 100 mg/kg) or vehicle (6% dimethyl sulfoxide in physiological saline). Animals were treated with PYC or vehicle with three i.p. injections (15 min, 3 h, 6 h) after the injury as previously described using the same batch of PYC [42]. Sham operated animals were subjected to a craniotomy and three i.p. injections.
2.2 Morris Water Maze (MWM)
A total of 28 animals were used in these experiments: Sham + vehicle (n = 7); TBI+ vehicle (n = 7); TBI+low PYC (n = 7); TBI+high PYC (n = 7). Seven days following the injury, animals were acquisition trained in a Morris Water Maze (MWM) as previously described [43]. Briefly, animals were trained to locate a 13.5 cm in diameter circular black plastic platform in a featureless black pool 127 cm (diameter) × 56 cm (height). Nontoxic black powdered tempera paint was added to the water (23–25°C) to obscure the goal platform located 1 cm below the water surface. A video camera recorded swimming during each trial. Each recording was processed by a Videomex V system (Columbus Instruments, Columbus, OH). The maze was divided conceptually into four quadrants, and the hidden platform was always located in the SE quadrant, approximately 30 cm from the pool wall.
Animals were given five consecutive days of testing with four trials each day and a five minute intertrial interval. For each trial, rats were placed in the pool facing the perimeter of the tank and allowed to search for the platform. If unable to find the platform within the allotted time (120 sec), they were guided to it and remained on it for 10 sec before returned to a holding cage. Rats were started from one of the four different quadrants on each trial with the starting location randomized across trials. Latency and path length to find the platform were recorded with the Videomex system and used to measure performance on each trial. After the final trial on day 5, the submerged platform was removed and each animal was given a 30 sec probe test. The percent time the animal swam in the maze quadrant that previously contained the platform was computed.
2.3 Cortical Tissue Sparing
Cortical damage was assessed blindly with respect to treatment group using an unbiased estimate of tissue sparing as a measure of change in injury volume [51]. Briefly, after MWM testing (day 12), animals were overdosed with Fatal-Plus (Med-Vet International, Mettawa, IL) and transcardially perfused with 4% paraformaldehyde. Brains were cryoprotected and coronal sections (50 µm) cut with a freezing microtome. Twelve equidistant sections throughout the anterior-posterior extent of the damaged hemisphere were stained with cresyl violet and subjected to morphological analysis (Scion Image 4.0.2, Frederick, MD). Quantitative determination of the volume of cortical tissue sparing used the Cavalieri method [28]. On each section, the total cortical area was determined for the entire hemisphere independently. Both the ipsilateral and contralateral hemispheres were evaluated. The amount (percent) of damage (spared tissue) is calculated by dividing the volume of the cortex ipsilateral to the injury site by the cortical volume of the same region in the contralateral (uninjured) hemisphere. In this regard, each animal serves as its own control and histological artifacts such as shrinkage or swelling of tissue that might occur during tissue processing are negated. All quantitative results are reported as mean percent tissue sparing.
2.4 Fluoro-Jade B (FJB)
Assessment of degenerating neurons in the hippocampus was determined using FJB as previously described [3]. Only animals treated with the high dose of PYC (100 mg/kg) were compared to the TBI+ vehicle cohort. Briefly, 48h post trauma, rats (TBI+vehicle n = 5; TBI+high PYC n = 5) were overdosed with Fatal-Plus and the brains processed as above. Twelve equidistant sections throughout the hippocampus, with a variable starting location, were stained for FJB (HistoChem Inc., Jefferson, AR) according to the method of Schmued [45, 46]. The total number of FJB-positive neurons was determined blindly with respect to treatment group using unbiased stereology with well described anatomical boundaries [2]. The sections were examined with an Olympus BX50 microscope using blue (450–490 nm) excitation light. Counts were limited to the dorsal and ventral leaf of the dentate gyrus granule cell layer and the CA3 region.
2.5 Statistics
Both the escape latency and path length MWM data on acquisition days 1 and 5 separately and also tissue sparing were evaluated for possible differences using a one-way analysis of variance (ANOVA) and the Fisher-Hayter [17] post hoc test. Group means were graphed ± SD. Possible differences in group FJB staining used a Mann-Whitney U-test. Significance for all statistical comparison was set at p < 0.05.
3. Results
3.1 Morris water maze
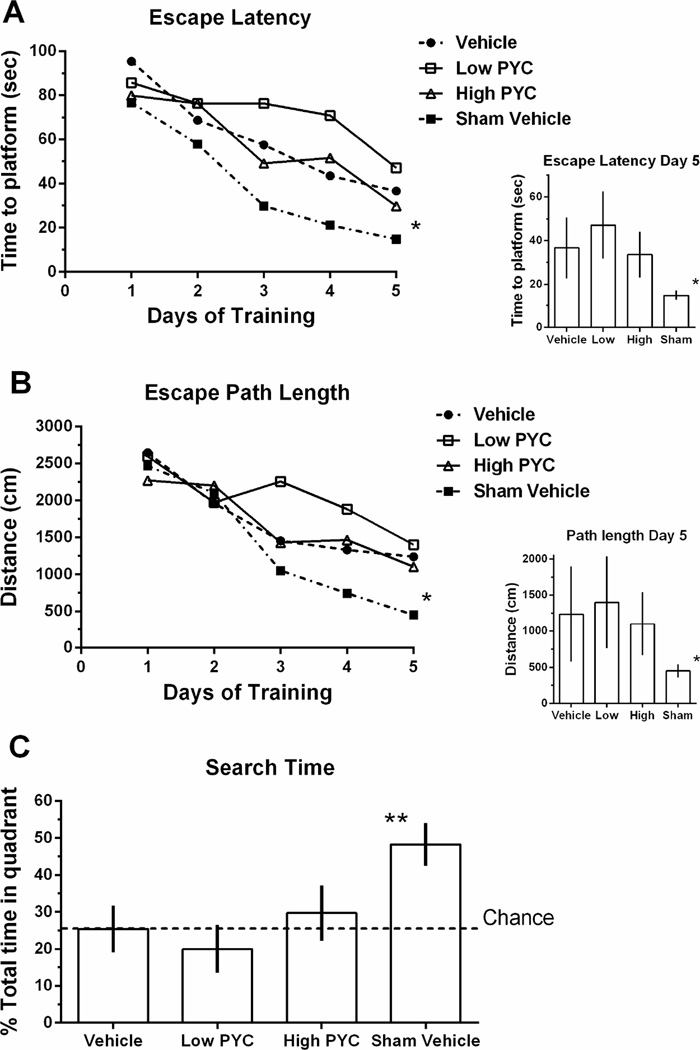
All four groups showed improvement in ability to locate the submerged platform over the five days of acquisition training (Fig. 1A–B). A one-way ANOVA showed that there were no group differences on the first day of acquisition training for both the escape latency [F(3,24) = 1.096, p > 0.05] and for path length traveled [F(3,24) = 0.600, p > 0.05]. On day 5 of acquisition training, the analysis revealed a significant difference between groups for both escape latency [F(3,24) = 5.469, p < 0.005] and path length traveled [F(3,24) = 4.665, p < 0.01]. Post hoc analysis showed that the Sham+vehicle treatment group performed significantly better than all other groups (p < 0.05) for both escape latency and path length traveled, and that TBI+ high PYC group was not significantly different from the TBI+vehicle group (p > 0.05) (See insets in Figures 1A–B). The vehicle treated TBI group performed significantly better than the TBI+low PYC group in escape latency (p < 0.05) but not total path length. Mean percent search time during the probe test was significantly different [F(3,24) = 24.679; p < 0.0001] (Fig. 1C). Post hoc analysis showed that the Sham treated group spent significantly more time in the correct quadrant (p < 0.001). The injured groups were not significantly different from each other (p > 0.05).
Figure 1.
Beginning seven days post injury, animals were trained to locate a submerged platform in a standard Morris water maze. (A) The time to locate the submerged platform (Escape Latency) was averaged over the four trials on each day of training. Inset shows the different groups’ performance on day 5. (B) Total distance required to find the submerged platform (Escape Path Length) was averaged over the four trials on each day of training. The inset shows the different groups’ performance on day 5. (C) Following the final acquisition trial on day five of training in the Morris water maze, animals were tested for spatial memory during a probe trial. Horizontal dashed line represents chance performance. Points represent group means (n=7/group) * p < 0.05 ** 0.01 Sham vehicle compared to all other groups. Bars represent group mean ± SD.
3.2 Tissue sparing following TBI
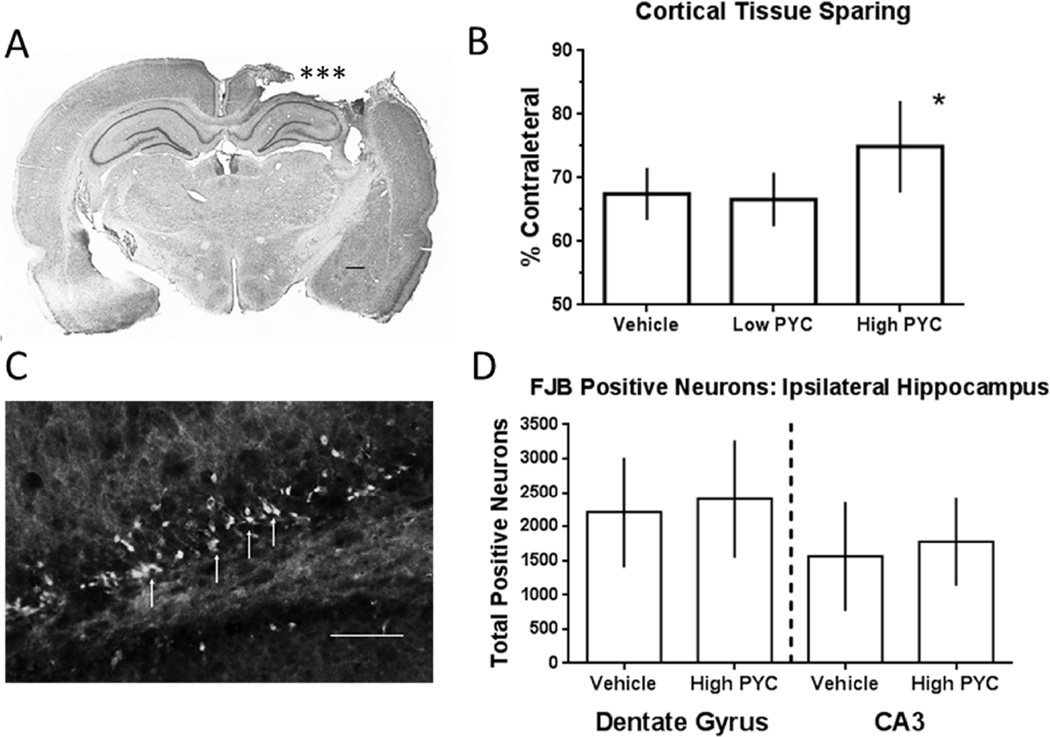
All injured animals showed an obvious loss of cortical tissue ipsilateral to the cortical impact. An ANOVA revealed a significant group difference in cortical tissue sparing [F(2,18) = 4.245, p < 0.05] (Fig. 2A–B). Post hoc analysis showed that the TBI+high PYC group had significantly more spared cortical tissue than the low PYC or vehicle treated animals (p < 0.05). The TBI−low PYC group was not significantly different from the vehicle treated animals (p > 0.05).
Figure 2.
(A) Photomicrograph of a coronal cresyl violate stained section showing a representative lesion resulting from a unilateral cortical impact at 12 days post injury. Tissue loss (***) on the right side resulted from the cortical impact. T (B) Changes in injury volume assessed by measuring the total amount of spared tissue using unbiased stereology. The high dose PYC group showed greater tissue sparing. The sham group is not included because there was no injury. (C) Photomicrograph of a FJB stained coronal section in the dentate gyrus. The arrows indicate a few of the FJB-positive stained granule cells ipsilateral to the injury. (D) Estimates of the total number of FJB positive neurons in two different regions of the hippocampus. There was no significant difference between the vehicle and high-PYC treatment group. * p < 0.05 compared to other groups. Calibration bar = 100 µm. Bars represent group mean ± SD.
3.3 FJB staining following TBI
FJB staining was evaluated in the hippocampal formation 2 days after a cortical contusion. All injured animals had FJB-positive neurons in the ipsilateral hippocampus in both the dentate gyrus (Fig. 2C) and CA3 regions. Only animals treated with the high dose of PYC were evaluated based upon the cortical tissue sparing results. A Mann-Whitney U test failed to demonstrate a significant difference for either the granule cell layer (p > 0.05) or the CA3 region (p > 0.05) (Fig. 2D).
4. Discussion
PYC has previously been shown to reduce oxidative stress, spare hippocampal synaptic proteins, and protect hippocampal synaptic function [4, 31, 42]. In the present study, two different doses of PYC were used to assess cognitive function. Neither dose of PYC significantly improved MWM performance compared to the TBI+vehicle treated controls. These same animals were subsequently analyzed for possible cortical tissue sparing as a measure of neuroprotection. The high PYC treated group showed increased tissue sparing compared to the vehicle treated animals. Animals treated with the low dose of PYC showed no improvement. Because the MWM is considered to be a hippocampal dependent task and the high dose indicated cortical tissue sparing, neuroprotection in the hippocampus was explored with FJB staining. The analysis failed to demonstrate a PYC-related neuroprotection in these hippocampal neuronal layers.
The present results do not support our previous post-trauma PYC studies demonstrating significant alterations of some aspects of the secondary injury cascade following a moderate TBI. We previously reported that an i.p. injection sequence, identical to that used in this study, significantly decreased oxidative stress, neuroinflammation, and spared synaptic proteins in both the cortex and hippocampus within 96h of the injury [42]. The same type of cortical contusion injury paradigm was used with the same parameters, the same batch of PYC, and the same strain of adult rat. Although there was some cortical neuroprotection, it did not appear to be comparable to the levels observed for spared synaptic proteins [42]. In the previous investigation, the assessment of synaptic proteins utilized the penumbra as opposed to the cortex directly below the injury location. There are several instances where a natural compound has been shown to significantly reduce secondary injury cascades but fail to improve cognitive performance in the MWM [1, 11, 21]. Somewhat surprising is the fact that caffeic acid, which is one of the main components of PYC, has been shown to significantly reduce lesion volume and improve cognition when administered post-trauma [56]. Previous studies using an extended treatment with PYC have reported significant enhancement in cognition in both animals [20, 24] and humans [8, 9, 39].
Following TBI, there is a significant reduction in the total number of neurons in the hippocampal CA3 region [3, 7]. This neuronal loss results in a transient loss of synaptic contacts and synaptic strength eventually recovers [30]. We have previously shown that PYC, when administered following TBI, significantly enhances synaptic efficacy in the injured hippocampal CA3-CA1 pathway [31] suggesting a positive role for PYC. The hippocampus is well known for its plasticity [41] and previously shown to support synaptogenesis in the denervated CA1 region following TBI [44]. Our previous studies indicated that PYC treatment might accelerate synaptogenesis [31] while failing to totally protect the vulnerable CA3 neurons as evidenced by the FJB staining. It is puzzling why PYC failed to facilitate the MWM acquisition task. Unlike many motor skills that show a transient deficit following TBI and spontaneously recover, deficits in the MWM have been shown to be long lasting [32]. It may also be the case that increased hippocampal plasticity in the PYC treated animals, as evidenced by enhanced levels of synaptic proteins [4, 42], may not have had time to properly reorganize. The benefits of PYC therapy may require an extended recovery time such as 30 days. Alternatively, it is well known that injury-induced axon sprouting can lead to detrimental consequences such as spasticity, epilepsy, pain, or no discernible behavioral change [12, 16, 19, 27, 29, 35, 37, 40, 47, 55].
The failure to observe a beneficial effect of PYC in the MWM maze task may have a much greater implication. These results may indicate that the simple suppression of neuroinflammation and oxidative stress may not be enough to improve cognitive ability in this model of TBI. Because PYC has been shown to suppress NO through the inhibition of iNOS and also the NF-kB/AP-1 pathway, these pathways may be only partial players in the secondary injury cascade following TBI. Future experiments would have to probe whether or not PYC may be effective with a less severe injury.
Highlights.
A combinational bioflavonoid, pycnogenol, was used as a therapy enhance cognition following brain injury
Pycnogenol significantly reduced TBI-related cortical injury volume
Fluoro-jade B staining failed to demonstrate hippocampal neuroprotection
Pycnogenol administered post-injury did not improve Morris water maze acquisition
Acknowledgments
Work supported by the Kentucky Spinal Cord and Head Injury Research Trust Fund (12-16A) and NIH Grant R21NS66117-2.
Abbreviations
- ANOVA
analysis of variance
- CCI
controlled cortical impact
- FJB
fluoro-jade B
- iNOS
inducible nitric oxide synthase
- MWM
Morris water maze
- NO
nitric oxide
- PYC
pycnogenol
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Alder J, Fujioka W, Giarratana A, Wissocki J, Thakkar K, Vuong P, Patel B, Chakraborty T, Elsabeh R, Parikh A, Girn HS, Crockett D, Thakker-Varia S. Genetic and pharmacological intervention of the p75NTR pathway alters morphological and behavioural recovery following traumatic brain injury in mice. Brain Inj. 2016;30:48–65. doi: 10.3109/02699052.2015.1088963. [DOI] [PubMed] [Google Scholar]
- 2.Amaral D, Witter M. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. New York: Academic Press; 1995. pp. 443–493. [Google Scholar]
- 3.Anderson KJ, Miller KM, Fugaccia I, Scheff SW. Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp. Neurol. 2005;193:125–130. doi: 10.1016/j.expneurol.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Ansari MA, Roberts KN, Scheff SW. Dose- and time-dependent neuroprotective effects of Pycnogenol following traumatic brain injury. J. Neurotrauma. 2013;30:1542–1549. doi: 10.1089/neu.2013.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aruoma OI, Bahorun T, Jen LS. Neuroprotection by bioactive components in medicinal and food plant extracts. Mutat. Res. 2003;544:203–215. doi: 10.1016/j.mrrev.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin SA, Gibson T, Callihan CT, Sullivan PG, Palmer E, Scheff SW. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical disector method for cell counting. J. Neurotrauma. 1997;14:385–398. doi: 10.1089/neu.1997.14.385. [DOI] [PubMed] [Google Scholar]
- 8.Belcaro G, Dugall M, Ippolito E, Hu S, Saggino A, Feragalli B. The COFU3 Study. Improvement in cognitive function, attention, mental performance with Pycnogenol(R) in healthy subjects (55–70) with high oxidative stress. J. Neurosurg. Sci. 2015;59:437–446. [PubMed] [Google Scholar]
- 9.Belcaro G, Luzzi R, Dugall M, Ippolito E, Saggino A. Pycnogenol(R) improves cognitive function, attention, mental performance and specific professional skills in healthy professionals aged 35–55. J. Neurosurg. Sci. 2014;58:239–248. [PubMed] [Google Scholar]
- 10.Bigford GE, Del Rossi G. Supplemental substances derived from foods as adjunctive therapeutic agents for treatment of neurodegenerative diseases and disorders. Adv. Nutr. 2014;5:394–403. doi: 10.3945/an.113.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dash PK, Moore AN, Moody MR, Treadwell R, Felix JL, Clifton GL. Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J. Neurotrauma. 2004;21:1573–1583. doi: 10.1089/neu.2004.21.1573. [DOI] [PubMed] [Google Scholar]
- 12.Emery DL, Royo NC, Fischer I, Saatman KE, McIntosh TK. Plasticity following injury to the adult central nervous system: is recapitulation of a developmental state worth promoting? J. Neurotrauma. 2003;20:1271–1292. doi: 10.1089/089771503322686085. [DOI] [PubMed] [Google Scholar]
- 13.Fan B, Dun SH, Gu JQ, Guo Y, Ikuyama S. Pycnogenol Attenuates the Release of Proinflammatory Cytokines and Expression of Perilipin 2 in Lipopolysaccharide-Stimulated Microglia in Part via Inhibition of NF-kappaB and AP-1 Activation. PLoS One. 2015;10:e0137837. doi: 10.1371/journal.pone.0137837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faul M, Xu L, Wald M, Coronodo V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitilizations and Death 2002–2006. Centr. Disease Contr. Prevent. 2010 [Google Scholar]
- 15.Gu JQ, Ikuyama S, Wei P, Fan B, Oyama J, Inoguchi T, Nishimura J. Pycnogenol, an extract from French maritime pine, suppresses Toll-like receptor 4-mediated expression of adipose differentiation-related protein in macrophages. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1390–E1400. doi: 10.1152/ajpendo.90543.2008. [DOI] [PubMed] [Google Scholar]
- 16.Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayter AJ. The maximum familywise error rate of teh Fisher's least significant difference test. J. Mer. Stat. Assoc. 1986;81:1000–1004. [Google Scholar]
- 18.Hernandez TD, Brenner LA, Walter KH, Bormann JE, Johansson B. Complementary and alternative medicine (CAM) following traumatic brain injury (TBI): Opportunities and challenges. Brain Res. 2016;1640:139–151. doi: 10.1016/j.brainres.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Hunt RF, Scheff SW, Smith BN. Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp. Neurol. 2009;215:243–252. doi: 10.1016/j.expneurol.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishrat T, Parveen K, Hoda MN, Khan MB, Yousuf S, Ansari MA, Saleem S, Islam F. Effects of Pycnogenol and vitamin E on cognitive deficits and oxidative damage induced by intracerebroventricular streptozotocin in rats. Behav. Pharmacol. 2009;20:567–575. doi: 10.1097/FBP.0b013e32832c7125. [DOI] [PubMed] [Google Scholar]
- 21.Itoh T, Tabuchi M, Mizuguchi N, Imano M, Tsubaki M, Nishida S, Hashimoto S, Matsuo K, Nakayama T, Ito A, Munakata H, Satou T. Neuroprotective effect of (−)-epigallocatechin-3-gallate in rats when administered pre- or post-traumatic brain injury. J. Neural Transm. (Vienna) 2013;120:767–783. doi: 10.1007/s00702-012-0918-4. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi MS, Han D, Packer L. Antioxidants and herbal extracts protect HT-4 neuronal cells against glutamate-induced cytotoxicity. Free Radic. Res. 2000;32:115–124. doi: 10.1080/10715760000300121. [DOI] [PubMed] [Google Scholar]
- 23.Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012;6:81–90. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Zhang Y, Lau BH. Pycnogenol improves learning impairment and memory deficit in senescence-accelerated mice. J. Anti-Aging Med. 1999;2:349–355. [Google Scholar]
- 25.Maimoona A, Naeem I, Saddiqe Z, Jameel K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J. Ethnopharmacol. 2011;133:261–277. doi: 10.1016/j.jep.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 26.Margulies S, Hicks R. L. Combination Therapies for Traumatic Brain Injury Workshop, Combination therapies for traumatic brain injury: prospective considerations. J. Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCouch GP, Austin GM, Liu CN, Liu CY. Sprouting as a cause of spasticity. J. Neurophysiol. 1958;21:205–216. doi: 10.1152/jn.1958.21.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Mouton P. Principles and practicies of unbiased stereology: an introduction for bioscientists. Baltimore: Johns Hopkins University Press; 2002. [Google Scholar]
- 29.Nishibe M, Barbay S, Guggenmos D, Nudo RJ. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J. Neurotrauma. 2010;27:2221–2232. doi: 10.1089/neu.2010.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris CM, Scheff SW. Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. J. Neurotrauma. 2009;26:2269–2278. doi: 10.1089/neu.2009.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris CM, Sompol P, Roberts KN, Ansari M, Scheff SW. Pycnogenol protects CA3-CA1 synaptic function in a rat model of traumatic brain injury. Exp. Neurol. 2016;276:5–12. doi: 10.1016/j.expneurol.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osier ND, Carlson SW, DeSana A, Dixon CE. Chronic Histopathological and Behavioral Outcomes of Experimental Traumatic Brain Injury in Adult Male Animals. J. Neurotrauma. 2015;32:1861–1882. doi: 10.1089/neu.2014.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 34.Peng QL, Buz'Zard AR, Lau BH. Pycnogenol protects neurons from amyloid-beta peptide-induced apoptosis. Brain Res. Mol. Brain Res. 2002;104:55–65. doi: 10.1016/s0169-328x(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez JJ. The role of axonal sprouting in functional reorganization after CNS injury: lessons from the hippocampal formation. Restor. Neurol. Neurosci. 2001;19:237–262. [PubMed] [Google Scholar]
- 36.Rather MA, Bhat BA, Qurishi MA. Multicomponent phytotherapeutic approach gaining momentum: Is the "one drug to fit all" model breaking down? Phytomedicine. 2013;21:1–14. doi: 10.1016/j.phymed.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Reeves TM, Smith DC. Reinnervation of the dentate gyrus and recovery of alternation behavior following entorhinal cortex lesions. Behav. Neurosci. 1987;101:179–186. doi: 10.1037//0735-7044.101.2.179. [DOI] [PubMed] [Google Scholar]
- 38.Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002;40:158–168. doi: 10.5414/cpp40158. [DOI] [PubMed] [Google Scholar]
- 39.Ryan J, Croft K, Mori T, Wesnes K, Spong J, Downey L, Kure C, Lloyd J, Stough C. An examination of the effects of the antioxidant Pycnogenol on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J. Psychopharmacol. 2008;22:553–562. doi: 10.1177/0269881108091584. [DOI] [PubMed] [Google Scholar]
- 40.Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann. Neurol. 2001;50:708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- 41.Scheff SW. Synaptic reorganization afer injury: the hippocampus as a model system. In: Seil FJ, editor. Neural Regen. Transplan. New York: Alan R. Liss; 1989. pp. 137–156. [Google Scholar]
- 42.Scheff SW, Ansari MA, Roberts KN. Neuroprotective effect of Pycnogenol(R) following traumatic brain injury. Exp. Neurol. 2013;239:183–191. doi: 10.1016/j.expneurol.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- 44.Scheff SW, Price DA, Hicks RR, Baldwin SA, Robinson S, Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J. Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- 45.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 46.Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol. Pathol. 2000;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- 47.Shetty AK. Entorhinal axons exhibit sprouting in CA1 subfield of the adult hippocampus in a rat model of temporal lobe epilepsy. Hippocampus. 2002;12:534–542. doi: 10.1002/hipo.10031. [DOI] [PubMed] [Google Scholar]
- 48.Siler-Marsiglio KI, Paiva M, Madorsky I, Serrano Y, Neeley A, Heaton MB. Protective mechanisms of pycnogenol in ethanol-insulted cerebellar granule cells. J. Neurobiol. 2004;61:267–276. doi: 10.1002/neu.20057. [DOI] [PubMed] [Google Scholar]
- 49.Siler-Marsiglio KI, Shaw G, Heaton MB. Pycnogenol and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. J. Neurobiol. 2004;59:261–271. doi: 10.1002/neu.10311. [DOI] [PubMed] [Google Scholar]
- 50.Spencer JP. Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc. Nutr. Soc. 2008;67:238–252. doi: 10.1017/S0029665108007088. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan PG, Thompson M, Scheff SW. Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 2000;161:631–637. doi: 10.1006/exnr.1999.7282. [DOI] [PubMed] [Google Scholar]
- 52.Vafeiadou K, Vauzour D, Spencer JP. Neuroinflammation and its modulation by flavonoids. Endocr. Metab. Immune Disord Drug Targets. 2007;7:211–224. doi: 10.2174/187153007781662521. [DOI] [PubMed] [Google Scholar]
- 53.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3:115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vonder Haar C, Peterson TC, Martens KM, Hoane MR. Vitamins and nutrients as primary treatments in experimental brain injury: Clinical implications for nutraceutical therapies. Brain Res. 2016;1640:114–129. doi: 10.1016/j.brainres.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: a systems, network and cellular evaluation. Neuroscience. 2005;133:1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 56.Zhao J, Pati S, Redell JB, Zhang M, Moore AN, Dash PK. Caffeic Acid phenethyl ester protects blood-brain barrier integrity and reduces contusion volume in rodent models of traumatic brain injury. J. Neurotrauma. 2012;29:1209–1218. doi: 10.1089/neu.2011.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]