Abstract
OBJECTIVES
To determine the cost-effectiveness of two nutrition interventions on food, beverage, and supplement intake and body weight.
DESIGN
Randomized, controlled trial.
SETTING
Five skilled nursing home facilities.
PARTICIPANTS
Long-stay residents with orders for nutrition supplementation (N = 154).
INTERVENTION
Participants were randomized into a usual care control group, an oral liquid nutrition supplement (ONS) intervention group, or a snack intervention group. Research staff provided ONS, according to orders or a variety of snack foods and beverages twice per day between meals, 5 days per week for 24 weeks and assistance to promote consumption.
MEASUREMENTS
Research staff independently weighed residents at baseline and monthly during the 24-week intervention. Resident food, beverage and supplement intake and the amount of staff time spent providing assistance were assessed for 2 days at baseline and 2 days per month during the intervention using standardized observation and weighed intake procedures.
RESULTS
The ONS intervention group took in an average of 265 calories more per day and the snack intervention group an average of 303 calories more per day than the control group. Staff time required to provide each intervention averaged 11 and 14 minutes per person per offer for ONS and snacks, respectively, and 3 minutes for usual care. Both interventions were cost-effective in increasing caloric intake, but neither intervention had a significant effect on body weight, despite positive trends.
CONCLUSION
Oral liquid nutrition supplements and snack offers were efficacious in promoting caloric intake when coupled with assistance to promote consumption and a variety of options, but neither intervention resulted in significant weight gain.
Keywords: long-term care, nutrition intervention, oral liquid nutrition supplements, unintentional weight loss
Inadequate food and fluid intake is a common problem for long-term care (LTC) residents and can lead to weight loss, hospitalization, and death.1–4 The most common intervention is the use of oral liquid nutrition supplements (ONSs), although there is limited controlled evidence of the efficacy of supplements in increasing caloric intake and weight in LTC residents.5,6 Results from ONS intervention trials may be mixed because of variation in implementation. Studies using research staff for supplement provision show significant gains in daily intake or weight in nutritionally at-risk LTC residents.7–9 In contrast, studies relying on LTC staff show that supplements are not provided consistent with orders, resident adherence is poor, and effects on weight are none to modest.10–13 It has been suggested that inadequate staffing contributes to inconsistent supplement delivery and insufficient staff assistance to promote consumption in daily LTC practice.11,13 Few studies report the staff time necessary to promote ONS consumption and weight gain.
Two recent studies suggest that offering residents a variety of ONSs and other food and beverage options (snacks) between meals results in greater caloric intake and body weight.14,15 One randomized trial with 76 LTC residents showed that a combination of mealtime assistance and snack offers between meals resulted in greater intake and weight when trained research staff provided both interventions twice per day for 24 weeks, but each intervention also required significantly more staff time than usual care.14 A separate randomized trial with 63 LTC residents directly compared the cost-effectiveness of ONS with that of a variety of snack options between meals twice per day for 6 weeks.15 Results showed that both interventions increased between-meal caloric intake and, again, required more staff time than usual care. The snack intervention was marginally more cost-effective than the supplement intervention in increasing caloric intake, but neither intervention had a significant effect on body weight.15 The lack of an effect on body weight could be due to the small sample size or the limited duration of the intervention, although it also could be due to the medical instability and comorbidity of this frail population, most of whom decline over time. Other studies have suggested that multiple chronic conditions and routine medications with anorexigenic side effects are common in the LTC population and significantly contribute to unintentional weight loss.2,16–18
It is unclear which nutrition intervention is more cost-effective in increasing caloric intake and weight in LTC residents.19 The cost-effectiveness component is critical to further evaluation studies because supplements represent a significant financial cost to LTC facilities because many residents have an ONS order, yet LTC staffing resources may limit quality care provision to all residents in need.13–15,20–22 It is also unclear to what extent weight loss can be prevented in a LTC population with multiple comorbidities. The purpose of this study was to examine the cost-effectiveness of two between-meal interventions on resident daily caloric intake and weight. The following research questions were addressed:
What is the effect of each intervention on between-meal and total daily caloric intake?
What is the effect of each intervention on body weight?
How much staff time is required to implement each intervention?
Which of the two interventions is more cost-effective in increasing caloric intake?
METHODS
Setting and Recruitment
Participants were recruited from four for-profit and one nonprofit facility housing a total of 784 LTC residents (average occupancy rate 88.5%). Nurses’ aide-level staff to resident ratios, as the directors of nursing reported, ranged from six to 11 residents per nurses’ aide during the day and eight to 15 residents per nurses’ aide in the evening. Total staffing levels (licensed nurses plus nurse aides) ranged from 2.9 to 5.0 hours per resident per day, with registered nurse staffing ranging from 0.5 to 1.0 hour per resident per day.
Figure 1 shows the flow of participants through the study. Two hundred seventy-six residents met inclusion criteria, which required residents to be long stay (non-Medicare), capable of oral intake, and not receiving end-of-life (hospice) care. Eligible residents were also required to have an order for nutrition supplementation because of nutrition risk.
Figure 1.
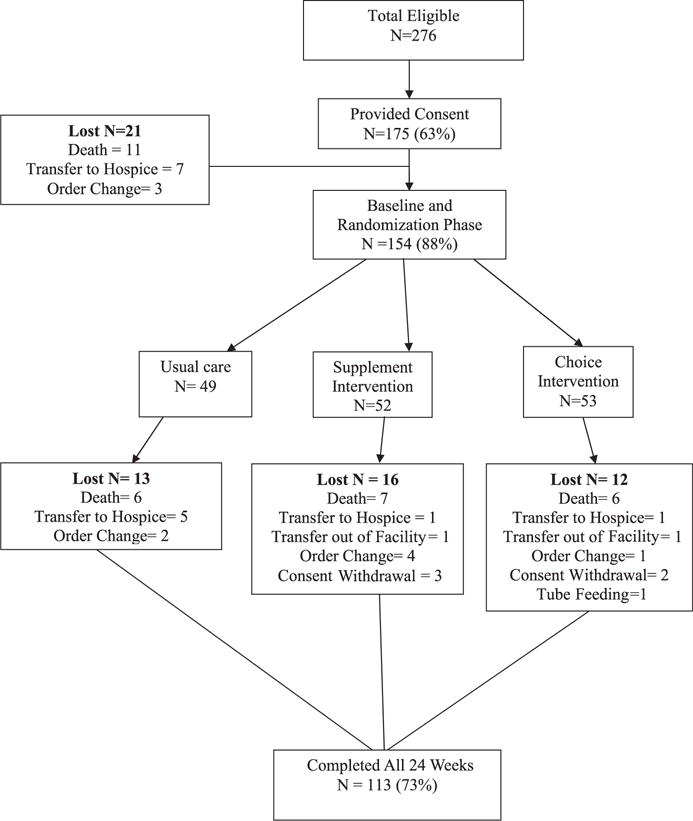
Study participant flowchart.
Written consent was obtained from the resident or the resident’s responsible party for 175 (63%) eligible residents. The Vanderbilt University institutional review board approved study procedures. Twenty-one participants were lost from the study after consent (Figure 1). The remaining 154 participants were randomized into one of the following three groups using a computer-generated random numbers table: usual care control (n = 49), supplement intervention (n = 52), or snack intervention (n = 53). After randomization, an additional 41 participants were lost from the study (Figure 1), with comparable attrition between the three groups. The remaining 113 participants completed all 24 intervention weeks.
Measures
Descriptive information was retrieved from each participant’s medical record, and information on participants’ need for assistance with eating was retrieved from their most-recent Minimum Data Set (MDS) assessment (Section G, Item 1 h) and scored from 0 (completely independent) to 4 (completely dependent). Recent weight loss history also was retrieved from the MDS (Section K, Item 3a) and defined as 5% or greater in the last 30 days or 10% or greater in the last 180 days.23 Research staff assessed participants’ cognitive status using the Mini-Mental State Examination (MMSE), which is a standardized, performance-based assessment with a score range from 0 (severely impaired or comatose) to 30 (cognitively intact).24
Body Weight and Height
Research staff assessed body weight at baseline and monthly throughout the 24-week intervention using a standardized protocol14,15,25 that required research staff to weigh participants in the morning, before breakfast but after incontinence care, wearing clean bed clothes using the facility scale calibrated to 0. Assessments of participants’ body weights were used to calculate body mass index (BMI) and estimate daily caloric requirements. (Refer to Table 1 footnotes for formulas.) A BMI less than 21.0 kg/m2 was considered indicative of being underweight and at nutrition risk.26 Monthly weight was also used to determine weight changes from baseline to after the intervention. The last monthly weight was used as the resident’s final weight for those lost from the study before completion.
Table 1.
Participant Characteristics According to Group and Overall (N = 154)
| Measure | Usual Care Control, n = 49 | Supplement Intervention, n = 52 | Snack Intervention, n = 53 | Overall, N = 154 |
|---|---|---|---|---|
| Demographic | ||||
| Female, n (%) | 41 (83.7) | 41 (78.9) | 42 (79.2) | 124 (80.5) |
| White, n (%) | 38 (77.6) | 80.8(42) | 77.4(41) | 121 (78.6) |
| Age, mean ± SD | 81.9 ± 13.5 | 85.2 ± 10.7 | 82.2 ± 11.3 | 83.1 ± 11.9 |
| Length of stay, years, mean ± sd | 4.6 ± 4.7 | 3.0 ± 3.1 | 3.4 ± 4.2 | 3.6 ± 4.0 |
| Medical | ||||
| Diagnosis of depression, n (%) | 34 (69.4) | 29 (55.8) | 28 (52.8) | 91 (59.1) |
| Diagnosis of dementia, n (%) | 39 (79.6) | 43 (82.7) | 38 (71.7) | 120 (77.9) |
| Diagnosis of dysphagia | 55.1(27) | 63.5(33) | 45.3(24) | 84 (54.5) |
| ≥1 anorexigenic medications, n (%) | 32 (65.3) | 32 (61.5) | 32 (60.4) | 96 (62.4) |
| Mini-Mental State Examination total score, mean ± SD (range 0–30) | 14.4 ± 7.9 | 9.4 ± 7.7 | 11.2 ± 8.4 | 11.3 ± 8.4 |
| Nutritional | ||||
| Special diet order, n (%)a | 34 (69.4) | 82.7(43) | 40 (75.5) | 76 (117) |
| Number of days order in place, mean ± SD | 522.5 ± 429.6 | 355.9 ± 347.0 | 368.1 ± 346.8 | 407.2 ± 376.4 |
| Minimum Data Set | ||||
| Eating dependency rating ≥1, n (%)b | 40 (81.6) | 46 (88.5) | 73.6(39) | 125 (81.2) |
| Recent weight loss ≥5% in 30 days or ≥10% in 180 days, n (%) | 4 (8.2) | 8 (15.4) | 13.2(7) | 19 (12.3) |
| Body mass index < 21.0 kg/m2c | 33.3 (16/48) | 30.0 (15/50) | 26.9(14/52) | 30 (45/150) |
| Estimated daily caloric requirements, kcal, mean ± SDd | 1,300.0 ± 272.7 | 1,263.3 ± 313.2 | 1,339.1 ± 344.8 | 1,306.2 ± 321.0 |
Included any restrictions (no added salt, no concentrated sugars) or altered texture (ground, mechanical soft, puree, thickened liquids).
Rating of need for staff supervision or minimal, extensive, or total assistance to eat.
Could not be calculated for four participants who had missing data for height.
Men = kg × 23, women = kg × 21.
SD = standard deviation.
Research staff also independently estimated each participant’s height using the Chumlea formula based on the measurement of knee height27 because most LTC residents are unable to stand in an upright position independently. Knee height was measured to the nearest 0.1 cm using a tape measure. Measurements were taken from the bottom of the right heel, just below the lateral malleolus of the fibula, to the top of the knee, with the knee and ankle at a 90° angle. Three consecutive measurements were taken to ensure reliability, and the average of the three measures was used to estimate height.
Oral Food and Beverage Intake
Food, beverage, and supplement intake were measured for each participant based on standardized observations and weighed intake methods that had been found to be reliable and valid in prior studies.13–15,28–30 Trained research staff observed all three scheduled meals and between-meal periods (morning, afternoon, evening) on 2 weekdays during the same week for a total of six meal and six between-meal observations per participant at baseline (12 total observations per participant) and monthly after the intervention (12 total observations per participant per month for 6 months). Each observation period lasted from the time of meal, snack, or supplement delivery to the time staff removed items, which averaged 1.5 hours per period. Research staff recorded the amount of staff time spent providing any type of assistance to promote consumption (e.g., setup, verbal cuing, physical assistance) using a stop watch (to record minutes and seconds for each interval of assistance).13–15
On the same 2 days on which observations were conducted, research staff also weighed all foods, beverages, and supplements provided during and between meals using a calibrated digital scale. Specifically, individual items were weighed before being served and then again after consumption (±0.1 g) outside the direct view of the resident. Intake data from the before and after weights (±0.1 g) were entered into the Nutrition Data System for Research software, along with facility menu items and corresponding recipes (NDS-R version 2008, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) for a precise measure of caloric intake.
Intervention Protocol: 24 Weeks
Research staff provided supplements or snacks consistent with each participant’s diet orders twice per day (morning and afternoon), 5 weekdays per week for 24 consecutive weeks (240 total possible intervention episodes per participant). Participants in the supplement intervention group were offered a variety of available flavors, including liquid and solid supplement options, during each intervention episode. Participants in the snack intervention group were offered a variety of foods (e.g., yogurts, pudding) and beverages (e.g., assorted juices, liquid supplements) during each intervention episode.
Research staff provided both intervention groups with assistance according to a standardized protocol to enhance independence and intake.14,15,22 The following cost-relevant information was documented at baseline and daily throughout the 24-week intervention: staff time to provide supplements or snacks between meals (minutes and seconds) and provide assistance to promote consumption, resident refusal rates, and cost of snacks and supplements (per facility costs). Research staff also documented reasons for missed intervention episodes (e.g., resident in hospital) throughout the 24 weeks (possible 240 total episodes per person).
Data Analyses
All baseline characteristics (Table 1) of participants who completed (n = 113) and were lost from (n = 62) the study were compared using Kruskal-Wallis tests for continuous variables and Pearson chi-square tests for categorical variables. These comparisons were also conducted between groups (usual care, supplement intervention, snack intervention) at baseline. Multivariate, repeated-measures regression analyses were used to examine intervention effects on caloric intake and body weight in the three groups while controlling for the effects of selected covariates (baseline value, facility site, depression diagnosis). In preliminary analyses, there was no evidence of a time (month) effect on caloric intake or body weight. Thus, the effect of time was ignored in the final analyses. A compound symmetry variance-covariance structure was used to account for within-subject correlations between the monthly caloric intake and weight measurements. The effects of each intervention relative to the control were tested using multivariate Wald (F) tests and the empirical variance estimator. Wald 95% confidence intervals (CIs) were computed for the corresponding effect estimates. The primary analysis was an intention-to-treat analysis for all randomized participants who had at least one complete postintervention measure for each outcome (e.g., minimally completed the initial month after intervention to include six meal and six snack observations). All analyses were performed in SAS for Windows version 9.4 (SAS Institute, Inc., Cary, NC).
Cost-Effectiveness Analysis
The cost-effectiveness analysis used the monthly weighed intake data for between-meal and total daily calorie intake (kcal) as the measures of effect. Increasing calorie intake is a therapeutic goal, but the specific dollar value of health improvements associated with increasing caloric intake is not known. Thus, the cost-effectiveness analysis addressed the economically most efficient method of increasing calorie intake. The daily incremental costs in dollars and cents and gains in calorie intake for each intervention group in comparison to the control group were calculated using weighted least squares (WLS) regression. The outcome variables were the average daily between-meal and total calorie intake and between-meal costs per person during the 24-week intervention period. Daily between-meal costs were calculated as the sum of additional daily food, beverage, and supplement costs between meals and associated labor costs for delivery and assistance. Labor cost was the product of staff time (minutes) per episode of care (supplement or snack delivery twice per day/person) and the average earning rate of certified nursing assistants (CNAs), who typically provide supplement and snack delivery.13,15 In 2012, the Bureau of Labor Statistics estimate for the hourly rate of CNAs at nursing homes was $11.66.31 Assuming a 10% fringe benefit rate, the hourly rate was adjusted to $12.83 per hour, or $0.214 per minute.
The treatment variables in the WLS regression analyses were the two dichotomous variables for the supplement and snack group membership (with the usual care control group as their shared reference group). The regression analyses adjusted for the baseline values of the outcome measures (daily between-meal and total calorie intake and cost per episode of care) as well as other baseline characteristics, including sex, age, ethnicity (non-Hispanic white vs minority), depression diagnosis, dementia diagnosis, and facility site.
The weight in the WLS analysis was the number of postintervention months with nonmissing values of the outcome measures (range 1–6). For instance, if a participant’s calorie intake was recorded for the first 5 months after the intervention but missing for the last month, a weight of 5 was assigned. Thus, a larger weight (smaller variance) was assigned to participants who remained in the study longer. The only reasons for a missing calorie intake value within a given study month were due to dropping out status (e.g., transferred out of facility or to hospice, death, feeding tube insertion, discontinuation of nutrition supplementation order) or a prolonged hospitalization (>3 weeks) during that study month.
The uncertainty in the cost-effectiveness was determined using cost-effectiveness acceptability curves (CEAcc), which is a method that builds on the net benefit (NB) approach.32,33 Given a monetary value (λ) of a 1-unit gain in calorie intake, the net benefit of the intervention was defined as: NB = λ × E−C, where E is the effectiveness (gain in calorie intake), and C is the total intervention cost. A distribution of costs and benefits was obtained by bootstrapping the trial data to generate the CEAcc.34 Participants were randomly selected with replacement, keeping their own individual costs and calorie gains. A total of 1,000 pairs of mean calorie gains and costs were generated using bootstrapping for both intervention groups, and the NB estimated for each pair as λ ranged from $0.00 to $0.10 in increments of $0.005. The proportion of bootstrapped pairs with NB greater than 0 is the probability each intervention was cost-effective conditional on the assumed monetary value of calorie gains. Those probabilities were subsequently plotted for every value of λ, producing the CEAcc.33,34
RESULTS
Participants and Setting
Table 1 shows the characteristics of study participants overall (N = 154) and according to group. Participants were 81% female and 79% white, with an average age of 83 ± 11.9 and an average length of stay of 3.7 ± 4.1 years. Fifty-nine percent had a diagnosis of depression, and 55% had a diagnosis of dysphagia. Participants had moderate to severe cognitive impairment, as evidenced by an average MMSE score of 11 ± 8.4, and 78% had a dementia diagnosis. Sixty-two percent had at least one routine medication with anorexigenic side effects.16,18
Seventy-six percent had an order for a special diet, and LTC staff rated 81% as requiring assistance to eat. Orders for nutrition supplementation had already been in place for longer than a year, on average, at the time of the study. Thirty-percent had a BMI indicative of being underweight at baseline, and 12% had MDS documentation of recent weight loss. Participants had an estimated calorie requirement of approximately 1,300 ± 321 kcal/d. (See Table 1 footnotes for formula.)35 There were no significant differences between participants who completed (n = 113) and those lost from (n = 62) the study for any of the characteristics shown in Table 1, although there were significant differences between groups at baseline, despite randomization, for length of stay (P = .03) and MMSE score (P = .005).
Intervention Effects on Between-Meal and Total Caloric Intake
On average, participants in both intervention groups received approximately 84% (202/240) of the total possible intervention episodes. The most common reason for a “missed” episode was the resident being out of the facility because of hospitalization or a medical appointment. Overall, participants averaged 1.5 ± 4.9 days in the hospital during 6 intervention months, with no differences between groups in frequency or duration of hospitalizations.
Both interventions had a significant effect on between-meal caloric intake (F = 56.71, P < .001) from baseline to each subsequent month of intervention relative to usual care (Figure 2). The between-meal intake of the snack and supplement intervention groups was, on average, 302.6 (95% CI = 242.1–363.2) and 265.2 (95% CI = 204.0–326.4) calories per person per day higher than that of the usual care control group, respectively. The observed increase in between-meal calorie intake occurred within the first month for both groups and was maintained over the 6 months of intervention (Figure 2). Each intervention also had a significant effect on total calorie intake (meal + between-meal calories, F = 9.19, P < .001). The average total calorie intake of the snack intervention group was 288.3 (95% CI = 144.6–432.0) higher than that of the usual care group, and that of the supplement intervention group was 253.2 (95% CI = 109.3–397.1) higher than that of the usual care group. Meal intake remained comparable from baseline to after the intervention for all three groups. Thus, the gains in calorie intake in both intervention groups were due to the increase in calories between meals from supplements and snacks as part of each intervention.
Figure 2.
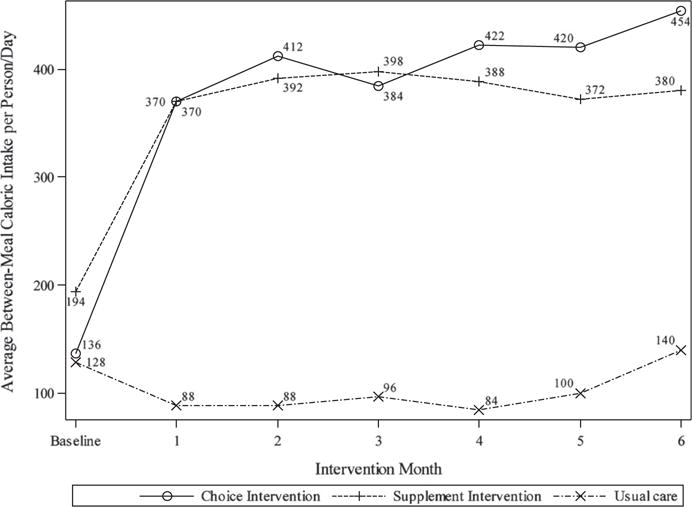
Average between-meal caloric intake by group over time.
Intervention Effects on Body Weight
There was not a significant effect of either intervention on body weight from baseline to after the intervention. The average weight change was a gain of 1.8 (95% CI =−0.5–4.1) pounds in the supplement intervention group and 1.1 (95% CI = 1.1–3.4) pounds in the snack intervention group and a loss of 0.5 (95% CI = −5.3–4.3) pounds in the control group. Thus, there was a trend for weight maintenance or gain in both intervention groups and weight loss in the usual care control group but comparable variability in all three groups.
Staff Time to Provide Snacks and Supplements Between Meals
Each intervention resulted in a significantly greater frequency of staff offers of snacks or supplements between meals and the associated staff time spent to promote consumption than usual care. On average, participants received 1.3 ± 1.0 offers per person per day between meals at baseline (N = 154), and LTC staff spent an average of 1.5 ± 1.5 minutes per person per offer to promote consumption of served items, which consisted primarily of beverages (e.g., juice, water) and supplements. This infrequent delivery was in the context of physician or dietitian orders to receive nutrition supplementation two to three times per day for all participants. In comparison, each intervention group consistently received two offers per person per day from research staff, and assistance time was a significantly greater 7.9 (95% CI = 6.12–9.6) minutes per person per offer than usual care for the snack intervention group (mean 8.7 ± 8.4) and 5.2 (95% CI = 3.5–6.8) minutes per person per offer more than usual care (mean 6.0 ± 4.7) for the supplement intervention group (F = 42.72, P < .001). The staff time per person per offer did not change significantly over the 6 intervention months for either group. Refusal rates averaged 19% for both intervention groups and also did not change significantly over time. The amount of assistance that LTC staff provided during meals remained comparable for all three groups from baseline to intervention, with an average of less than 10 minutes per person per meal at all measurement points and all meals.
Cost-Effectiveness Analysis
Table 2 shows the between-meal costs for each intervention and the control group. Baseline costs were low across all participants because of infrequent delivery (≤1 per person/day) and minimal to no staff assistance to promote consumption (<2 minutes per person per offer). The intervention costs were $2.54 per person per day higher than usual care for the supplement group and $3.85 higher for the snack group (both P < .001). The incremental cost-effectiveness ratios were 103 kcal per dollar for the supplement intervention and 79 kcal per dollar for the snack intervention. On average, the supplement intervention cost approximately 0.1 cent per calorie gained, and the snack intervention cost approximately 0.8 cents per calorie gained. Thus, the snack intervention resulted in a larger caloric gain but also had a higher total cost. This increase in cost was due to the greater number of snack items given per person per day and the associated staff time to provide assistance for multiple items, in particular food items. Specifically, the snack group received an average of 4.12 ± 2.0 snack items per person per day (two items per offer, 1 food + 1 beverage), whereas the supplement group received an average of 2.5 ± 1.1 items (one supplement per offer) (t = −12.22, P < .001).
Table 2.
Average Between-Meal Costs per Person per Day and Differences According to Group (N = 154)
| Group (n) | Baseline | Intervention | Adjusted Difference, Mean (Standard Error) |
|---|---|---|---|
| Mean ± Standard Deviation | |||
| Usual care (49) | 1.29 ± 1.10 | 1.23 ± 0.81 | |
| Supplement (52) | 1.45 ± 0.78 | 3.34 ± 1.38 | 2.54 (0.39)a |
| Snacks (53) | 1.18 ± 0.77 | 4.44 ± 2.36 | 3.85 (0.38)a |
Costs include cost of supplement and snack items and labor costs for delivery and assistance.
P < .001.
The results of the cost-effectiveness analysis showed the same pattern for both effectiveness measures (average between-meal and total caloric gain). The CEAcc for the two interventions, which show the probability that each intervention is worthwhile (has a net benefit) as a function of the dollar value assigned to caloric gain, are shown in Figure 3 for total caloric intake gains. The y axis begins at 0% probability, indicating that neither intervention is worthwhile if each calorie gained is assigned a low value, and increases to 100%, indicating that both interventions are worthwhile if each calorie gained is assigned a high value. As the dollar value of caloric gain increases, the number of bootstrapped samples where the value of the gain is greater than the cost (a net benefit) also increases. Figure 3 shows that the supplement intervention has a larger probability of a net benefit than the snack intervention if the value of caloric gain is low. For example, given a 1 cent value per unit of caloric gain, the probability of a net benefit is approximately 57% for the supplement intervention and 18% for the snack intervention, but as the dollar value of an additional unit of caloric gain increases, the difference in the probability of a net benefit between the two interventions quickly diminishes. For example, given a value of 2 cents per unit of caloric gain, the probability of a net benefit is approximately 98% for the supplement intervention and 94% for the snack intervention, which makes the two interventions comparable.
Figure 3.
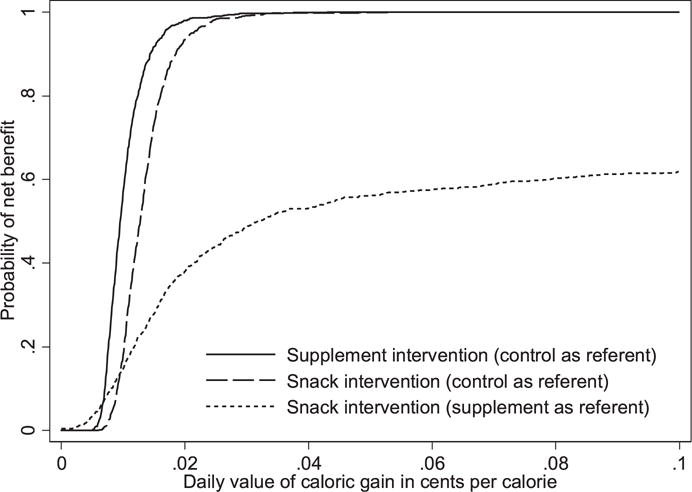
Cost-effectiveness acceptability curves for each intervention.
DISCUSSION
This controlled trial, which included precise measures of caloric intake and body weight, showed that two between- meal interventions were both efficacious in improving daily caloric intake when provided consistently and coupled with assistance to promote consumption and a variety of options. Although the cost of each intervention was significantly higher than usual care, baseline data indicated that usual care consisted of infrequent delivery of between-meal supplements or snacks and suboptimal assistance, which was inconsistent with participants’ dietary orders and regulatory guidelines. Furthermore, Although an additional $2 to $4 per resident per day may exceed the amount many nursing homes currently spend on supplement or snack delivery, each intervention reflects a modest cost when compared with other more-invasive nutrition interventions (e.g., feeding tubes, appetite stimulant medications). Moreover, family members of LTC residents have rated the provision of snacks and improvements in staff assistance as strongly preferable for improving oral intake.36
The results of a prior pilot study suggested that the snack intervention was marginally more cost-effective than supplements and that residents preferred it, as lower refusal rates evidenced. In this prior study, supplement options were limited to the specific type that the facility prescribed (all one type of liquid supplement) and traditional available flavors within the facility (e.g., strawberry, chocolate, vanilla).15 In the current study, options were expanded according to type (e.g., assorted liquid and solid options) and flavors, including nontraditional flavors (e.g., orange cream, cappuccino, butter pecan). It is likely that the availability of options in the current study contributed to comparable low refusal rates and high cost-effectiveness for both interventions. In LTC settings where ONS is the most common intervention for poor oral intake and weight loss and an intervention that remains in place for a prolonged period of time (> 1 year, on average), the importance of variety for quality of life should be paramount.36,37
The lack of a significant effect on body weight was surprising in this study in light of the gains in caloric intake for both intervention groups. A prior controlled trial of improved feeding assistance provided during and between meals twice per day, 5 days per week for 24 weeks resulted in significant improvements in weight and BMI.14 Mealtime assistance averaged less than 10 minutes per person per meal throughout this study for all three groups. Other studies have shown that residents in need of assistance require an average of 20 to 30 minutes per meal to ensure adequate intake.14,15,22,38 Thus, it is likely that feeding assistance improvements also need to occur during regularly scheduled meals in addition to supplement and snack delivery between meals to affect the body weight of LTC residents with multiple chronic conditions and medications that may contribute to unintentional weight loss, in addition to frequent hospitalizations for acute illnesses.16–18
An important limitation of this study is that it was not designed to detect the cost-effectiveness of nutrition intervention on total LTC costs or other outcomes associated with poor caloric intake, such as pressure ulcers, hospitalizations, and mortality.1,19 In addition, overall attrition was high (35%) but comparable between groups and similar to that found in prior prospective LTC studies.14,15
Even in the absence of significant effects on weight, offering residents a variety of options between meals should be considered an essential part of daily care practice to ensure optimal nutritional health and quality of life.37 Although ONS orders remain ubiquitous in LTC, there is a growing trend toward providing residents with “real food first.”39 Staff provision of options also is more consistent with the recent emphasis on culture change and new federal regulations related to resident-centered care.40,41 Moreover, federal regulations allow LTC facilities to cross-train nonnursing personnel to provide feeding assistance to residents during and between meals. This approach provides facilities with a feasible way to augment their existing, often limited, nurse aide staffing to improve nutritional care practices within the constraints of available resources.42,43
Acknowledgments
This research was supported by Agency for Healthcare Research and Quality (AHRQ) Grant 1R01HS018580–01 awarded to Dr. Sandra F. Simmons (Principal Investigator) and National Center for Research Resources Grant UL 1 RR024975–01 and is now at the National Center for Advancing Translational Science Grant 2 UL 1 TR000445–06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The authors thank the nursing home facilities, residents, and their families for allowing us to work closely with them as part of this project.
Dr. Sandra Simmons has served as a professional consultant for Nestle, which makes nutritional supplement products, but no portion of this study was funded or otherwise supported by Nestle. Dr. Heidi Silver has grant funding support from Nestle and other nutrition-based companies, but none of these other grant funds supported her work on this study. Dr. John Schnelle has provided expert testimony on staffing levels in long-term care but none related to this project or any of the facilities that participated in this project.
Sponsor’s Role: There was no role of the sponsor in any aspect of this study beyond funding.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Dr. Sandra F. Simmons served as the Principal Investigator on the AHRQ-funded study, so she led all aspects of this study from concept and design, oversight of data collection and analyses and preparation of this manuscript. Drs. John F. Schnelle and Heidi Silver were co-investigators on the AHRQ study, so each had a significant role on this project. Dr. Silver’s nutrition laboratory analyzed all of the nutrient intake data. Drs. Matt Shotwell and Xulie Lio were biostatisticians responsible for several of the analyses and corresponding write-up of analysis section and results tables. Drs. Emmett Keeler and Ruopeng An conducted the cost-effectiveness analysis and prepared this section of the manuscript and related figures. Brittany Kuertz served as the project coordinator of the study, organized all data collection tasks, provided oversight on data entry and data cleaning procedures, prepared study databases for the biostatistics and cost-effectiveness analyses, and created results tables.
References
- 1.Agarwal E, Ferguson M, Banks M, et al. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: Results from the Nutrition Care Day Survey 2010. Clin Nutr. 2013;32:737–745. doi: 10.1016/j.clnu.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Blaum CS, Fries BE, Fiatarone MA. Factors associated with low body mass index and weight loss in nursing home residents. J Gerontol A Biol Sci Med Sci. 1995;50A:M162–M168. doi: 10.1093/gerona/50a.3.m162. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson RP, O’Conner P, Crabtree B, et al. Serum albumin and prealbumin as predictors of clinical outcomes of hospitalized elderly nursing home residents. J Am Geriatr Soc. 1993;41:545–549. doi: 10.1111/j.1532-5415.1993.tb01893.x. [DOI] [PubMed] [Google Scholar]
- 4.Thomas JM, Cooney LM, Fried TR. Systematic review: Health-related characteristics of elderly hospitalized adults and nursing home residents associated with short-term mortality. J Am Geriatr Soc. 2013;61:902–911. doi: 10.1111/jgs.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milne AC, Potter J, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009;(2):CD003288. doi: 10.1002/14651858.CD003288.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanson LC, Ersek M, Gilliam R, et al. Oral feeding options for people with dementia: A systematic review. J Am Geriatr Soc. 2011;59:463–472. doi: 10.1111/j.1532-5415.2011.03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiaterone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 8.Lauque S, Battandier FA, Gillette S, et al. Improvement of weight and fat-free mass with oral nutritional supplementation in patients with Alzheimer’s disease at risk for malnutrition: A prospective randomized study. J Am Geriatr Soc. 2004;52:1702–1707. doi: 10.1111/j.1532-5415.2004.52464.x. [DOI] [PubMed] [Google Scholar]
- 9.Young KWH, Greenwood CE, Reekum RV, et al. Providing nutrition supplements to institutionalized seniors with probable Alzheimer’s disease is least beneficial to those with low body weight status. J Am Geriatr Soc. 2004;52:1305–1312. doi: 10.1111/j.1532-5415.2004.52360.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson LE, Dooley PA, Gleick JB. Oral nutritional supplement use in elderly nursing home patients. J Am Geriatr Soc. 1993;41:947–952. doi: 10.1111/j.1532-5415.1993.tb06760.x. [DOI] [PubMed] [Google Scholar]
- 11.Kayser-Jones J, Schell ES, Porter C, et al. A prospective study of the use of liquid oral dietary supplements in nursing homes. J Am Geriatr Soc. 1998;46:1378–1386. doi: 10.1111/j.1532-5415.1998.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 12.Lad H, Gott M, Gariballa S. Elderly patients compliance and health professionals’ views and attitudes towards prescribing sip-feed supplements. J Nutr Health Aging. 2005;9:310–314. [PubMed] [Google Scholar]
- 13.Simmons SF, Patel AV. Nursing home staff delivery of oral liquid nutritional supplements to residents at risk for unintentional weight loss. J Am Geriatr Soc. 2006;54:1372–1376. doi: 10.1111/j.1532-5415.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 14.Simmons SF, Keeler E, Xiaohui ZM, et al. Prevention of unintentional weight loss in nursing home residents: A controlled trial of feeding assistance. J Am Geriatr Soc. 2008;56:1466–1473. doi: 10.1111/j.1532-5415.2008.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons SF, Zhuo X, Keeler E. Cost-effectiveness of two nutrition interventions in nursing home residents: A pilot intervention. J Nutr Health Aging. 2010;14:367–372. doi: 10.1007/s12603-010-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morley JE, Kruenzle D. Causes of weight loss in a community nursing home. J Am Geriatr Soc. 1994;42:583–585. doi: 10.1111/j.1532-5415.1994.tb06853.x. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan DH, Johnson LE, Bopp MM, et al. Prognostic significance of monthly weight fluctuations among older nursing home residents. J Gerontol A Biol Sci Med Sci. 2004;59A:633–639. doi: 10.1093/gerona/59.6.m633. [DOI] [PubMed] [Google Scholar]
- 18.Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician. 2014;89:718–722. [PubMed] [Google Scholar]
- 19.Milte RK, Miller MD, Crotty M. Economic evaluation for protein and energy supplementation in adults: Opportunities to strengthen the evidence. Eur J Clin Nutr. 2013;67:1243–1250. doi: 10.1038/ejcn.2013.206. [DOI] [PubMed] [Google Scholar]
- 20.Schnelle JF, Cretin S, Saliba D, et al. Appropriateness of Minimum Nurse Staffing Ratios in Nursing Homes. Vol. 2. Cambridge, MA: Abt Associates, Inc; 2000. Minimum nurse aide staffing required to implement best practice care in nursing homes. Health Care Financing Administration; pp. 141–1468. [Google Scholar]
- 21.Schnelle JF, Simmons SF, Harrington C, et al. Relationship of nursing home staffing to quality of care. Health Serv Res. 2004;39:225–250. doi: 10.1111/j.1475-6773.2004.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons SF, Schnelle JF. Individualized feeding assistance care for nursing home residents: Staffing requirements to implement two interventions. J Gerontol A Biol Sci Med Sci. 2004;59A:966–973. doi: 10.1093/gerona/59.9.m966. [DOI] [PubMed] [Google Scholar]
- 23.Research Triangle Institute International. MDS 3.0 Quality Measures: User’s Manual. Research Triangle Park, NC: Research Triangle Institute International; 2012. [Google Scholar]
- 24.Molloy DW, Alemayehu E, Roberts R. A standardized Mini-Mental State Examination (SMMSE): Its reliability compared to the traditional Mini-Mental State Examination (MMSE) Am J Psychiatry. 1991;148:102–105. doi: 10.1176/ajp.148.1.102. [DOI] [PubMed] [Google Scholar]
- 25.Simmons SF, Peterson E, You C. The accuracy of monthly weight assessments in nursing homes: Implications for the identification of weight loss. J Nutr Health Aging. 2009;13:284–288. doi: 10.1007/s12603-009-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiaterone Singh MA, Rosenberg IH. Nutrition and aging. In: Hazzard WR, Blass JP, Ettinger WH Jr, et al., editors. Principles of Geriatric Medicine and Gerontology. 4th. New York: McGraw-Hill Health Professions Division; 1998. [Google Scholar]
- 27.Chumlea WC. Methods of nutritional anthropometric assessment for special groups. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manuals. Champaign, IL: Human Kinetic Books; 1988. [Google Scholar]
- 28.Bingham BSA, Cassidy A, Cole TJ, et al. Validation of weighed records and other methods of dietary assessment using the 24 h urine nitrogen technique and other biological markers. Br J Nutr. 1995;73:531–550. doi: 10.1079/bjn19950057. [DOI] [PubMed] [Google Scholar]
- 29.Silver HJ, Dietrich MS, Castellanos VH. Increased energy density of the home-delivered lunch meal improves 24-hour nutrient intakes in older adults. J Am Diet Assoc. 2008;108:2084–2089. doi: 10.1016/j.jada.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Simmons SF, Babinou S, Garcia E, et al. Quality assessment in nursing homes by systematic direct observations: Feeding assistance. J Gerontol A Biol Sci Med Sci. 2002;57A:M665–M671. doi: 10.1093/gerona/57.10.m665. [DOI] [PubMed] [Google Scholar]
- 31.National Occupational Employment and Wage Estimates. U.S. Department of Labor, Bureau of Labor Statistics; May, 2006. [on-line], Available at http://www.bls.gov/oes/current/oes311012.htm Accessed April 25, 2014. [Google Scholar]
- 32.Stinnett A, Mullahy J. Net health benefits: A new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18:S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 33.Lothgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ. 2000;9:623–630. doi: 10.1002/1099-1050(200010)9:7<623::aid-hec539>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 35.Silver H, Wall R, Hollingsworth E, et al. Simple kcal/kg formula is comparable to prediction equations for estimating resting energy expenditure in older cognitively impaired long term care residents. J Nutr Health Aging. 2013;17:39–44. doi: 10.1007/s12603-012-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons SF, Lam H, Rao G, et al. Family members’ preferences for nutrition interventions to improve nursing home residents’ oral food and fluid intake. J Am Geriatr Soc. 2003;51:69–74. doi: 10.1034/j.1601-5215.2002.51012.x. [DOI] [PubMed] [Google Scholar]
- 37.Crogan NL, Pasvogel A. The influence of protein-calorie malnutrition on quality of life in nursing homes. J Gerontol A Biol Sci Med Sci. 2003;58A:159–164. doi: 10.1093/gerona/58.2.m159. [DOI] [PubMed] [Google Scholar]
- 38.Simmons SF, Schnelle JF. Feeding assistance needs of long-stay nursing home residents and the staff time to provide care. J Am Geriatr Soc. 2006;54:919–924. doi: 10.1111/j.1532-5415.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 39.Pioneer Network, New Dining Practice Standards, Food and Dining Clinical Standards Task Force. A Rothschild Regulatory Task Force. 2011 Aug; [on-line]. Available at https://www.pioneernetwork.net/Providers/DiningPracticeStandards/ Accessed September 18, 2013.
- 40.Centers for Medicare and Medicaid Services. Nursing Homes—Issuance of Revisions to Interpretive Guidance at Several Tags, as Part of Appendix PP, State Operations Manual (SOM), and Training Materials. Letter to State Survey Agency Directors [on-line] Available at http://www.cms.gov/SurveyCertificationGenInfo/downloads/SCletter09_31.pdf Accessed April 26, 2011.
- 41.Doty MM, Koren MJ, Sturla EL. Findings from the Commonwealth Fund 2007 National Survey of Nursing Homes. New York, NY: Commonwealth Fund; 2008. Culture Change in Nursing Homes: How Far Have We Come? [on-line]. Available at http://www.commonwealthfund.org/Content/Publications/Fund-Reports/2008/May/Culture-Change-in-Nursing-Homes—How-Far-Have-We-Come—Findings-From-The-Commonwealth-Fund-2007-Nati.aspx Accessed may 23, 2011. [Google Scholar]
- 42.Simmons SF, Bertrand R, Shier V, et al. A preliminary evaluation of the paid feeding assistant regulation: Impact on feeding assistance care process quality in nursing homes. Gerontologist. 2007;47:184–192. doi: 10.1093/geront/47.2.184. [DOI] [PubMed] [Google Scholar]
- 43.Bertrand B, Porchak T, Moore T, et al. The nursing home Dining Assistant program: A demonstration project. J Gerontol Nurs. 2010;23:1–10. doi: 10.3928/00989134-20100730-04. [DOI] [PubMed] [Google Scholar]


