Abstract
Renal angiomyolipomata associated with tuberous sclerosis complex are often bilateral, multiple and progressive. They cause significant morbidity and mortality in older children and adults. Surveillance and pre-emptive treatment reduce this risk. Recent research suggests treatment with mammalian target of rapamycin inhibitors is better at preventing bleeding, recurrence, and preserving renal function than percutaneous embolization.
Keywords: angiomyolipoma, therapy, tuberous sclerosis complex
Introduction
Tuberous sclerosis complex (TSC) is a multisystem disorder that affects about 1 in 10,000 individuals worldwide [EMA, 2010]. It is caused by mutations in one of two genes, TSC1 and TSC2, causing a very variable spectrum of problems including: epilepsy; intellectual disability; autistic spectrum disorder and other neuropsychiatric problems; skin, heart, lung and kidney lesion [Curatolo et al. 2008].
Renal involvement is a major cause of morbidity and mortality in TSC [Shepherd et al. 1991] (Table 1). The severity of the conditions caused by this genetic disease means that TSC not only affects 1:10,000 individuals, but also has a profound effect on 1:10,000 families. The renal disease prevalence is such that 80% of patients have renal angiomyolipomata, leading to life-threatening bleeding in 25%, and can be associated with renal failure. Most patients’ angiomyolipomata are multiple, bilateral and progressive [Bissler and Kingswood, 2004]. In contrast, sporadic renal angiomyolipomata are twice as common but usually occur in an older age group, and are single, small and rarely progress to cause significant morbidity [Bissler and Kingswood, 2004].
Table 1.
TSC renal disease features.
| • Angiomyolipomata approximately 80% and most common cause of death in adults with TSC1,2 |
| • Risk of bleeding 25–50%3,4 |
| • LAM ~30–80% of women1,2 |
| • Kidney cancer ~1–3% |
| • Polycystic kidney 5% |
| • Needing dialysis ~1% |
| • Reduced kidney function 40% (Adults)5 |
| • High blood pressure 27% (Adults)5 |
| – 1. Franz et al. (2010) Neuropediatrics 41: 199–208. |
| – 2. Dixon et al. (2011) Nephron Exp Nephrol 118: e15–e20. |
| – 3. Kessle et al. (1998) Eur Urol 33: 572–575. |
| – 4. Mouded et al. (1978) J Urol 119: 684–688. |
| – 5. Kingswood et al. CPRD Eur Assoc Urol 201. |
LAM, lymphangioleiomyomatosis.
Medical care of a TSC patient is often splintered between multiple subspecialists; not uncommonly the practitioners also have very limited experience with the disease. The purpose of this review is to critically assess the published and presented data on treatment pertaining to TSC-related angiomyolipomata. This aspect of patient care is particularly important following the discovery that dysregulation of the mammalian target of rapamycin (mTOR) is fundamental to the pathogenesis of TSC [Tee et al. 2003], and because mTOR inhibitors have been proposed as an alternative to surgery or merely supportive treatment.
Methodology
Literature search strategy
The literature search included three topics: everolimus, sirolimus and surgery/embolization. Three separate searches from 2000 to 2014 were performed, using Ovid, with the following search criteria.
AML or Angiomyolipoma plus TSC and treatment plus Surgery or Embolization.
AML or Angiomyolipoma plus TSC and treatment plus Sirolimus.
AML or Angiomyolipoma plus TSC and treatment plus Everolimus.
All titles and abstracts were inspected. Duplicates within each search were removed, as were duplicates between searches. Publications on topics that did not include renal disease (e.g. topics such as epilepsy, subependymal giant cell astrocytoma (SEGA), pulmonary lymphangioleiomyomatosis (LAM), neuropsychiatric problems, molecular mechanisms in animal models and studies in carcinoma) were removed. Papers that were reviews without any original data were excluded, but their references were inspected to find any possible missing references. Original papers, conference abstracts and case reports have been included in this review. Abstracts that have been superseded by more detailed later publications were removed (Figures 1–3).
Figure 1.

Search 1: surgery/embolization and relevant natural history.
Of these 26 relevant publications; 10 were papers, 9 abstracts and seven single case reports.
Figure 2.

Search 2: sirolimus treatment.
Of these 7 relevant publications; 2 were papers, 1 abstract and 4 single case reports.
Figure 3.

Search 3: everolimus treatment.
Of these 11 relevant publications; 2 were papers, 9 abstracts, there were no single case reports.
The initial search found 204 publications. There were 38 duplicates and 11 reviews. Of the rest, only 58 were about treatment or natural history of TSC renal disease or angiomyolipomata. Of these 14 were papers, 19 abstracts and 25 case series.
These results were combined with relevant results from the previous systematic literature search carried out in 2012 in preparation for writing the new international guidelines for the surveillance and management of TSC [Krueger et al. 2013a]. That review used the PUBMED and SCOPUS databases to find all papers published between 1997 and March 2012 which included the terms ‘tuberous sclerosis’ and ‘diagnosis or therapy’ +/− ‘humans’. The search returned 2692 references; of which 604 included information on renal disease in TSC. These 604 references were reviewed again to find any papers relevant to this search that had not already been included. There were 14 of these.
Natural history of TSC associated renal angiomyolipomata
Data from TOSCA (a worldwide database study, now including 2226 subjects) is consistent with the adverse role of renal disease in the health of a patient with TSC. Analysis of the first 508 patients revealed 53% had been diagnosed with renal angiomyolipomata [Kingswood et al. 2014c], despite the young median age of 16 years. The relatively low prevalence of haemorrhage (4.8%), pain (3.7%), impaired renal function (3%) and hypertension (4.4%), is probably explained by the high incidence of pre-emptive treatment of angiomyolipomata (28%), in addition to the young age of the subjects [Kingswood et al. 2014c]. Significant and life-threatening renal complications are common in TSC patients with angiomyolipomata [Cappell et al. 2014].
In addition to the risks from haemorrhage, premature impairment of glomerular filtration rate (GFR) has been reported in up to 40% of subjects [Kingswood et al. 2014a]. Figure 4 shows that these 40% (with GFR <60 ml/min) effectively have a level of renal function that would be expected if they were 30 years older [Kingswood et al. 2014a].
Figure 4.
Prevalence of CKD in the overall TSC population by age compared with the general UK population.
Data from December 1998 to November 2003 as reported by Stevens et al. [2007] and from Kingswood et al. [2014b].
CKD, chronic kidney disease; TSC, tuberous sclerosis complex.
The study by Nikolskaya and colleagues [Nikolskaya et al. 2014] showed that renal impairment occurs in the absence of overt bleeding from angiomyolipomata or intervention (Table 2). This underscores the need to use methods that preserve kidney function when treating angiomyolipomata pre-emptively, to prevent bleeding.
Table 2.
Distribution of potential predictors of bleeding in patients with AML who had or not had bleeding. From Nikolskaya et al. [2014].
| Parameters | Bleeding | No bleeding | p value |
|---|---|---|---|
| Number of patients | 56 | 112 | NA |
| Age (years) | 34 (5.9–66.4) | 23.2 (1.7–56.6) | 0.0001 |
| GFR initial level | 77.5 (31–124) | 84.9 (45–109) | 0.45 |
| GFR final level | 51.6 (10–89) | 55.8 (13–89) | 0.65 |
Cross-sectional analysis of renal function and possible role of haemorrhage. Follow-up duration of for both groups varied widely but was similar. Haemorrhage alone did not impact loss of renal function.
AML, angiomyolipoma; GFR, glomerular filtration rate; NA, not applicable.
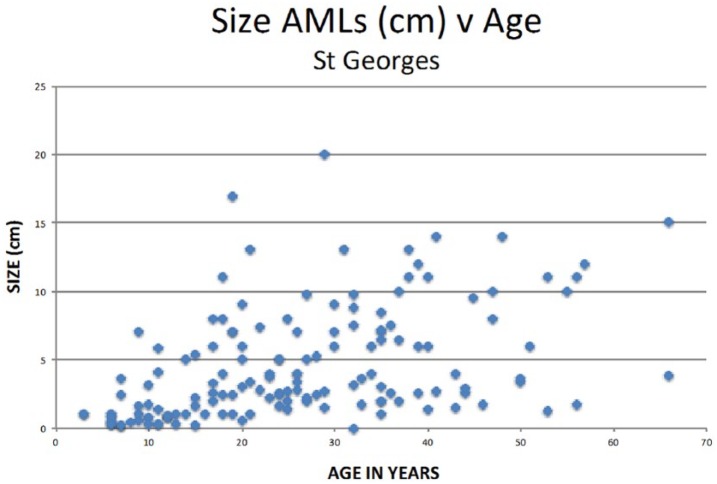
Angiomyolipomata progressively enlarge from early childhood onwards (Figure 5) with an apparent growth spurt in teenage/early adulthood (Figure 6). In older adults about 33% stop growing [Cox et al. 2012]. Data from Cox and colleagues [Cox et al. 2012] suggest that it is angiomyolipomata that are still enlarging (and >30 mm) that are most at risk of haemorrhage; and the number needed to treat (NNT) to prevent one bleed is low (see Table 3).
Figure 5.
The largest angiomyolipoma in individual patients versus age from Cox et al. [2012].
Figure 6.
Growth velocity of angiomyolipomata versus age from Cox et al. [2012].
Table 3.
Risk of AML bleeding stratified by growth/no growth. From Cox et al. [2012].
| 9.5 Year Follow Up (n = 54) |
| • Growth 34 (63%) |
| • bleed 11 (20%)/3 (6%) commenced mTOR inhibitor |
| • no bleed 20 (37%) |
| • No growth 20 (37%) |
| • bleed 2 (4%) |
| • no bleed 18 (33%) |
| χ2 = 6.7, p < 0.01 |
| • NNT: for patients with growing AMLs, the NNT to prevent one bleed is 3.2 |
AML, angiomyolipoma; mTOR, mammalian target of rapamycin; NNT, number needed to treat.
Embolization
Prior to the availability of mTOR inhibitors, surgery or percutaneous embolization were the only options available for pre-emptive treatment of angiomyolipomata [Nelson and Sanda, 2002]. Surgery was considered second choice in TSC associated angiomyolipomata because of the technical difficulties caused by their multifocal bilateral nature [Sooriakumaran et al. 2010].
The main problem with embolization is the trade-off between completely occluding the angiomyolipoma blood supply, with the risk of infarcting normal surrounding renal tissue, versus a more conservative approach which minimises collateral damage but increases the risk of recurrence. In addition, embolization of a single lesion does not prevent other lesions from progressing. There are also the risks of short-term complications (post-embolization syndrome, acute renal failure and infection) [Sooriakumaran et al. 2010].
There have not been any controlled trials of embolization, nor any trials to compare treatment modalities (surgery, embolization & mTOR inhibitor treatment). There have been a number of small case series of embolization that contain enough detail to draw some tentative conclusions. These are listed in Table 4. Of the 125–132 embolizations reported in TSC patients, 32 (24–26%) had a recurrence. However, these were short-term findings and, as the paper by Kothary and colleagues [Kothary et al. 2005] pointed out, the mean time to recurrence of critical problems from angiomyolipomata post embolization was 78 months, which was longer than most of the follow up periods reported in the case series. One recent paper reviewed the long-term outcome (median 15.8 years) in 351 TSC patients from a single clinic, 244 of who had angiomyolipomata that were systematically pre-emptively treated with embolization [Eijkemans et al. 2015]. This confirmed that the outcome was suboptimal and patients in that clinic are now treated with mTOR inhibitor as a first line instead.
Table 4.
Case series of treatment of renal angiomyolipomata by embolization.
Given that, in at least 40% of patients, TSC associated angiomyolipomata are: multiple, bilateral, show an aggressive growth pattern in a majority of patients; that the recurrence rate post-embolization is at least 24% but probably higher over the longer-term; and that premature loss of kidney function is a long-term risk in TSC renal disease and can be exacerbated by embolization, the 96 expert TSC physicians who drew up the new international guidelines felt that an mTOR inhibitor should be first choice for pre-emptive treatment of angiomyolipomata, and embolization should be reserved for angiomyolipomata that are acutely bleeding [Krueger et al. 2013a].
The wisdom of this pharmacological strategy is supported by the findings reported by Zonnenberg and colleagues [Zonnenberg et al. 2014]. This investigation supervised care of a group of 351 adults in a TSC specialist clinic in Utrecht, over an average follow-up period of 15.8 years, undertaking a policy of active pre-emptive embolization. A total of 144 patients had one or more embolizations for angiomyolipomata greater than 3.5 cm. The median age at end of the follow-up period was 39.8 years. Of the 29 deaths, nine (31%) were due to renal complications, making it the most common cause of death in this group. The overall mortality was significantly higher than the Dutch general population. Although embolization managed individual large angiomyolipomata as a strategy to improve mortality, it did not prevent the progression renal disease [Zonnenberg et al. 2014]. In addition, Zonnenberg and colleagues have previously reported that a significant number of these patients with a high angiomyolipoma burden needed dialysis or transplantation [van der Wal et al. 2012].
Sirolimus
The initial studies of the efficacy of mTOR inhibitors in animal models and in humans with TSC were carried out using sirolimus (see the references cited below). In TSC renal disease, there were only three protocol specified phase II studies in humans: using the patients as their own controls [Bissler et al. 2008; Davies et al. 2011; Dabora et al. 2011]. There has also been a single phase III (placebo-controlled) study in pulmonary LAM, which is arguably a renal disease because the angiomyolipoma or LAM cells may metastasize from renal lesion to the lung [Henske, 2003]. However, the MILES study did not consider renal outcomes, although sirolimus was highly efficacious in halting deterioration in lung disease in both an open-label trial [Bissler et al. 2008] and a placebo-controlled trial [McCormack et al. 2011].
The renal studies all showed that sirolimus was very effective at not only preventing angiomyolipomata from enlarging, but also significantly reducing their size, while the medication was taken continuously. When discontinued after 12 months [Bissler et al. 2008; Dabora et al. 2011] the angiomyolipomata started to enlarge again, demonstrating that the mTORC1 inhibitors need to be continued. No studies of stopping treatment after a few years’ therapy have yet been undertaken.
Everolimus
The phase II studies of sirolimus for TSC renal disease led to a landmark phase III study: Exist-2. Publication of the initial results [Bissler et al. 2013] showed good efficacy and acceptable side effects (minor side effects being frequent but tolerable, and serious side effects rare); which led to the licensing of everolimus for the treatment of angiomyolipomata in adults in the USA and Europe [EMA, 2012]. Previously, everolimus had gained a provisional license for the treatment of SEGA in TSC: which was confirmed following the publication of the Exist-1 study [Franz et al. 2012]. Subsequently, everolimus has been adopted into widespread use, worldwide, in clinical practice for these two indications [Nicholson and Wood, 2014; Narayanan et al. 2013]: as recommended in the international clinical guidelines [Krueger et al. 2013a].
Analysis of the continuing Exist-2 study has shown that efficacy increases over time (see Figure 7) [Bissler et al. 2014a]. In contrast, adverse events have diminished dramatically over time (see Table 5) [Bissler et al. 2014a, 2014b; Kingswood et al. 2013]. This may be due in part to increasing tolerance of normal cells to everolimus (as opposed to the TSC null cells which remain exquisitely sensitive), and partly due to the dosing strategy of lowering the dose in those who had recurrent or persistent side effects [Bissler et al. 2013].
Figure 7.
Longer-term efficacy of everolimus in Exist-2, from Bissler et al. [2014a].
Table 5.
Incidence of side effects over time in Exist-2 from Bissler et al. [2014a].
| EXIST-2 adverse events by preferred term and year of emergence occurring in >10% of patients: 2.45-year update | ||||
|---|---|---|---|---|
|
Everolimus
| ||||
| Adverse event, n (%) |
⩽12 months
|
13–24 months
|
25–36 months
|
37–48 months
|
| (n = 112) | (n = 101) | (n = 77) | (n = 18) | |
| Stomatitis | 46 (41.1) | 9 (8.9) | 2 (2.6) | 0 (0.0) |
| Nasopharyngitis | 36 (32.1) | 19 (18.8) | 14 (18.2) | 5 (27.8) |
| Acne | 28 (25.0) | 8 (7.9) | 3 (3.9) | 0 (0.0) |
| Headache | 26 (23.2) | 11 (10.9) | 3 (3.9) | 0 (0.0) |
| Hypercholesterolaemia | 25 (22.3) | 9 (8.9) | 6 (7.8) | 3 (16.7) |
| Aphthous stomatitis | 21 (18.8) | 14 (13.9) | 6 (7.8) | 1 (5.6) |
| Fatigue | 19 (17.0) | 2 (2.0) | 2 (2.6) | 0 (0.0) |
| Cough | 18 (16.1) | 4 (4.0) | 4(5.2) | 0 (0.0) |
| Diarrhoea | 17 (15.2) | 6 (5.9) | 3 (3.9) | 0 (0.0) |
| Nausea | 17 (15.2) | 5 (5.0) | 0 (0.0) | 2 (11.1) |
| Mouth ulceration | 17 (15.2) | 3 (3.0) | 2 (2.6) | 0 (0.0) |
| Urinary tract infection | 16 (14.3) | 14 (13.9) | 6 (7.8) | 1 (5.6) |
| Vomiting | 15 (13.4) | 7 (6.9) | 1 (1.3) | 1 (5.6) |
| Hypertension | 14 (12.5) | 3 (3.0) | 3 (3.9) | 1 (5.6) |
| Oedema peripheral | 12 (10.7) | 8 (7.9) | 4 (5.2) | 0 (0.0) |
| Amenorrhoea | 12 (10.7) | 7 (6.9) | 3 (3.9) | 0 (0.0) |
| Leukopenia | 12 (10.7) | 6 (5.9) | 0 (0.0) | 0 (0.0) |
| Back pain | 12 (10.7) | 5 (5.0) | 2 (2.6) | 1 (5.6) |
| Blood lactate dehydrogenase increased | 12 (10.7) | 2 (2.0) | 1 (1.3) | 0 (0.0) |
| Hypophosphataemia | 11 (9.8) | 5 (5.0) | 4 (5.2) | 2 (11.1) |
The dose or plasma level of everolimus did not correlate with the incidence of side effects [Kingswood et al. 2014c]. Nor did they correlate with efficacy, except for one significant relationship between the concentration at 2 h post-dose (Cmin) and the percentage decrease in total volume of renal angiomyolipomata in the early phase of the study [Zonnenberg et al. 2012], such that for every doubling of Cmin there was a 10% extra decrease in angiomyolipoma total volume. This implies that for TSC renal disease the doses used (and plasma levels achieved) in most patients were higher than needed, and the main effect of the higher doses was to shrink angiomyolipomata slightly more rapidly.
The MILES and Malinowska studies found a relationship between vascular endothelial growth factor D (VEGF-D), a vascular promoting cytokine, and treatment with sirolimus [McCormack et al. 2011; Malinowska et al. 2013]. Exist-2 tested the relationship between a number of potential biomarkers, angiomyolipoma mass and treatment. This confirmed a highly significant correlation between percentage change in VEGF-D and decrease in angiomyolipoma mass on mTORC1 inhibitor treatment, with a weaker correlation between VEGF-D change and increase in angiomyolipoma mass on placebo (see Figure 8) [Bissler et al. 2013]. There was also a significant correlation between angiomyolipoma size at baseline, and both VEGF-D and collagen IV plasma levels [Bissler et al. 2013; Budde et al. 2013]. The changes in these biomarkers may be useful in future clinical practice in order to measure response, and also may help to explain the protection from haemorrhage that treatment with mTOR inhibitors confers. No patient in Exist-2 has had a renal haemorrhage after commencing on everolimus; the study is now completing its fifth year.
Figure 8.
The relationship between the vascular growth promoting cytokine VEGF-D and change in angiomyolipoma mass in the Exist-2 study from Bissler et al. [2013].
VEGF-D, vascular endothelial growth factor D.
Another finding from the longer-term data in Exist-2 was that the mean GFR did not significantly change (see Figure 9) [Bissler et al. 2014c]. Some individuals (most of whom started the trial with a GFR <30 ml/min) did have deterioration in renal function; but in most GFR remained stable or improved [Bissler et al. 2014c]. This is a very encouraging finding in a group of individuals who would otherwise have been at high risk of developing chronic kidney disease.
Figure 9.
The effect of everolimus on renal function in Exist-2 from Bissler et al. [2014c].
Although Exist-1 was primarily designed to test the efficacy and safety of treating SEGA in children with TSC [Franz et al. 2012], the exploratory endpoint of the effect of everolimus on angiomyolipomata greater than 1 cm was also analysed. In the 44 subjects (median age 12.5 years) with at least one angiomyolipoma greater than one centimetre, 80% on everolimus and 0% of those on placebo had a decrease in total volume of target angiomyolipomata of >50%. In addition, a decrease of more than 30% was found in all of the patients taking everolimus, and in only 17% of those on placebo [Kingswood et al. 2014b]; also see Figure 10. This clearly shows that everolimus is highly effective in preventing angiomyolipoma progression in children with TSC. The side-effect profile of the patients in Exist-1 has also been relatively mild and tolerable [Franz et al. 2014].
Figure 10.
The effect of everolimus on angiomyolipomata in a paediatric population from Kingswood et al. [2014c].
One initial concern when designing studies with mTORC1 inhibitors was that the mTOR pathway is of such fundamental significance in cellular metabolism that an mTORC1 inhibitor might cause a deterioration in other aspects of TSC. In fact, the opposite has been demonstrated. The MILES study showed Sirolimus was highly efficacious for pulmonary LAM, as did one of the phase II angiomyolipoma studies [McCormack et al. 2011; Bissler et al. 2008]. In addition, an ongoing study has shown similar findings for treatment with everolimus [Goldberg et al. 2014]. Exist-2 also examined the effect of everolimus on skin rash as a secondary endpoint; the rash showed a significant improvement, varying from moderate to marked on everolimus, but not on placebo [Bissler et al. 2013].
Seizures are the major cause of morbidity and mortality in TSC. Work in animal models and case studies suggested mTORC1 inhibitors might have a beneficial effect. This was proven in a phase II study of the effect of everolimus in resistant seizures, for which it proved highly efficacious [Krueger et al. 2013b]. The utility of this and the risk/benefit ratio is currently being explored in an Exist-3 study [ClinicalTrials.gov identifier: NCT01713946].
Finally, case reports of improvements in neurocognition and autistic spectrum disorder, as well as work in animal models, has led to ongoing studies to assess the magnitude of the effect of everolimus on these two complications of TSC in the TRON study in the UK [ClinicalTrials.gov identifier: NCT01954693], the RAPIT study in Holland [ClinicalTrials.gov identifier: NCT01730209], and a further study in the USA [ClinicalTrials.gov identifier: NCT01289912].
Conclusion
The aggressive nature of renal angiomyolipomata in patients with TSC and their natural history justifies the recommendation of the international guidelines [Krueger et al. 2013a] proposing pre-emptive treatment with an mTOR inhibitor as first choice of therapy. All the evidence suggests that this will not only prevent the high morbidity and mortality due to renal complications, but may also confer other significant benefits in TSC.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
John J. Bissler, FedEx Chair of Excellence, Director, Tuberous Sclerosis Center of Excellence, Director, Division of Nephrology at St. Jude Children’s Research Hospital and LeBonheur Children’s Hospital, University of Tennessee Health Science Center, Professor of Pediatrics, 51 North Dunlap Street, Memphis, TN 38103, USA.
John C. Kingswood, Royal Sussex County Hospital, Brighton, UK
References
- Bishay V., Crino P., Wein A., Malkowicz S., Trerotola S., Soulen M., et al. (2010) Embolization of giant renal angiomyolipomas: technique and results. J Vasc Int Radiol 21: 67–72. [DOI] [PubMed] [Google Scholar]
- Bissler J., Kingswood J. (2004) Renal angiomyolipomata. Kidney Int 66: 924–934. [DOI] [PubMed] [Google Scholar]
- Bissler J., Kingswood J., Radzikowska E., Zonnenberg B., Frost M., Belousova E., et al. (2013) Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381: 817–824. [DOI] [PubMed] [Google Scholar]
- Bissler J., Kingswood J., Radzikowska E., Zonnenberg B., Frost M., Belousova E., et al. (2014a) Everolimus for renal angiomyolipoma associated with tuberous sclerosis complex (TSC): EXIST-2 3-year follow-up. Eur Urol Suppl 13: e1139. [Google Scholar]
- Bissler J., Kingswood J., Radzikowska E., Zonnenberg B., Frost M., Sauter M., et al. (2014b) Everolimus for renal angiomyolipoma associated with tuberous sclerosis complex: Exist-2 long-term efficacy and safety. Nephrol Dial Transplant 0: 1–9. [Google Scholar]
- Bissler J., Kingswood J., Zonnenberg B., Frost M., Belousova E., Sauter M., et al. (2014c). Effect of everolimus on renal function in patients with tuberous sclerosis complex (TSC): results from exist-1 and exist-2. Nephrol Dial Transpl 29: iii43–iii44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissler J., McCormack F., Young L., Elwing J., Chuck G., Leonard J., et al. (2008) Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358: 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde K., Kingswood J., Brechenmacher T., Stein K., Chen D., Bissler J. (2013) Predictive value of angiogenic biomarkers in tuberous sclerosis complex (TSC) patients with renal angiomyolipoma (AML). Eur Urol Suppl 12: e981–e982. [Google Scholar]
- Cappell K., Song X., Liu Z., Eynullayeva E., Gregory C., Prestifilippo J., et al. (2014) Natural history of patients with tuberous sclerosis complex related renal angiomyolipoma. Value Health 17: A56. [DOI] [PubMed] [Google Scholar]
- Chick C., Tan B., Cheng C., Taneja M., Lo R., Tan Y., et al. (2010) Long-term follow-up of the treatment of renal angiomyolipomas after selective arterial embolization with alcohol. BJU Int 105: 390–394. [DOI] [PubMed] [Google Scholar]
- Cox J., Kingswood J., Mbundi J., Attard G., Patel U., Saggar A., et al. (2012) The natural history of renal angiomyolipomata (AMLs) in tuberous sclerosis complex (TSC). Nephrol Dial Transpl 27: ii325. [Google Scholar]
- Curatolo P., Bombardieri R., Jozwiak S. (2008) Tuberous sclerosis. Lancet 372: 657–668. [DOI] [PubMed] [Google Scholar]
- Dabora S., Franz D., Ashwal S., Sagalowsky A., Dimario F., Jr., Miles D., et al. (2011) Multicenter phase 2 trial of sirolimus for tuberous sclerosis: Kidney angiomyolipomas and other tumors regress and VEGF- D levels decrease. PLoS One 6: e23379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D., de Vries P., Johnson S., McCartney D., Cox J., Serra A., et al. (2011) Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res 17: 4071–4081. [DOI] [PubMed] [Google Scholar]
- Eijkemans M., van der Wal W., Reijnders L., Roes K., van Waalwijk van Doorn-Khosrovani S., Pelletier C., et al. (2015) Long-term follow-up assessing renal angiomyolipoma treatment patterns, morbidity, and mortality: an observational study in tuberous sclerosis complex patients in the Netherlands. Am J Kidney Dis 66: 638–645. [DOI] [PubMed] [Google Scholar]
- El-Assmy A., Abou-El-Ghar M., Mosbah A., El-Refaie H., El-Diasty T. (2011) Efficacy, complications and long-term outcomes of selective arterial embolization of symptomatic giant renal angiomyolipoma. Curr Urol 5: 179–184. [Google Scholar]
- Ewalt D., Diamond N., Rees C., Sparagana S., Delgado M., Batchelor L., et al. (2005) Long-term outcome of transcatheter embolization of renal angiomyolipomas due to tuberous sclerosis complex. J Urol 174: 1764–1766. [DOI] [PubMed] [Google Scholar]
- Franz D., Belousova E., Sparagana S., Bebin E., Frost M., Kuperman R., et al. (2012) Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381: 125–132. [DOI] [PubMed] [Google Scholar]
- Franz D., Belousova E., Sparagana S., Bebin E., Frost M., Kuperman R., et al. (2014) Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol 15: 1513–1520. [DOI] [PubMed] [Google Scholar]
- Goldberg H., Harari S., McCormack F. (2014) Efficacy and safety of everolimus for the treatment of lymphangioleiomyomatosis: a phase II study. In The LAM Foundation 17th Annual International Lymphangioleiomyomatosis Research Conference and Patient and Family Educational LAMposium, 28–30 March 2014, Chicago, IL, USA. [Google Scholar]
- Haber G., Lemaitre L., Hancart C., Delomez J., Faucon H., Biserte J., et al. (2005) Selective arterial embolization of renal angiomyolipoma for the prophylaxis and the treatment of hemorrhage: Retrospective study of 24 cases. Eur Urol Suppl 4: 51. [Google Scholar]
- Hashimoto M., Kanou T., Ohuchi Y., Nakamura K., Kotani K., Sugihara S., et al. (2003) Evaluation of arterial embolization for renal angiomyolipoma. Jpn J Clin Radiol 48: 1201–1205. [Google Scholar]
- Henske E. (2003) Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosome Can 38: 376–381. [DOI] [PubMed] [Google Scholar]
- Kessler O., Gillion G., Neuman M., Engelstein D., Winkler H., Baniel J. (1998) Management of renal angiomyolipoma: analysis of 15 cases. Eur Urol 33: 572–575. [DOI] [PubMed] [Google Scholar]
- Kingswood J., Demuth D., Nasuti P., Lucchese L., Gray E., Magestro M. (2014a) Real-world assessment of renal involvement in tuberous sclerosis complex (TSC) patients in the United [Google Scholar]
- Kingdom (UK). Eur Urol Suppl 13: e318–e318a. [Google Scholar]
- Kingswood J., Jozwiak S., Belousova E., Frost M., Kuperman R., Bebin E., et al. (2014b) The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo-controlled, phase 3 trial EXIST-1. Nephrol Dial Transpl 29: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingswood J., Zonnenberg B., Frost M., Cheung W., Wang J., Brechenmacher T., et al. (2013) Pharmacokinetics and exposure-safety relationship of everolimus in patients with renal angiomyolipoma (AML) associated with tuberous sclerosis complex (TSC) or sporadic lymphangioleiomyomatosis. Nephrol Dial Transpl 28: i316. [Google Scholar]
- Kingswood J., Zonnenberg B., Sauter M. (2014c) TOSCA-tuberous sclerosis registry to increase disease awareness. Nephrol Dial Transpl 29: iii384. [Google Scholar]
- Koo K., Kim W., Ham W., Lee J., Ju H., Choi Y. (2010) Trends of presentation and clinical outcome of treated renal angiomyolipoma. Yonsei Med J 51: 728734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary N., Soulen M., Clark T., Wein A., Shlansky-Goldberg R., Crino P., et al. (2005) Renal angiomyolipoma: long-term results after arterial embolization. J Vasc Int Radiol 16: 45–50. [DOI] [PubMed] [Google Scholar]
- Krueger D., Northrup H. and International Tuberous Sclerosis Complex Consensus Group. (2013a) Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D., Wilfong A., Holland-Bouley K., Anderson A., Agricola K., Tudor C., et al. (2013b) Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol 74: 679–687. [DOI] [PubMed] [Google Scholar]
- Lazarov R., De Kort G., Van Moorselaar R. (2002) Persistent renal bleeding treated with selective vascular embolisation with preservation of renal function. Ned Tijdschr Geneeskd 146: 994–999. [PubMed] [Google Scholar]
- Lee S., Hsu H., Chen Y., Huang C., Wong Y., Wang L., et al. (2009) Embolization of renal angiomyolipomas: Short-term and long-term outcomes, complications, and tumor shrinkage. Cardiovasc Intervent Radiol 32: 1171–1178. [DOI] [PubMed] [Google Scholar]
- Lee W., Kim T., Chung J., Han J., Kim S., Park J. (1998) Renal angiomyolipoma: embolotherapy with a mixture of alcohol and iodized oil. J Vascu Int Radiol 9: 255–261. [DOI] [PubMed] [Google Scholar]
- Leong S., Keeling A., McGrath F., Lee M. (2010) Transcatheter embolisation of renal angiomyolipoma. Ir J Med Sci 179: 211–216. [DOI] [PubMed] [Google Scholar]
- Malinowska I., Lee N., Kumar V., Thiele E., Franz D., Ashwal S., et al. (2013) Similar trends in serum VEGF-D levels and kidney angiomyolipoma responses with longer duration sirolimus treatment in adults with tuberous sclerosis. PLoS One 8: e56199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack F., Inoue Y., Moss J., Singer L., Strange C., Nakata K., et al. (2011) Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 364: 1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourikis D., Chatziioannou A., Antoniou A., Kehagias D., Gikas D., Vlahos L. (1999) Selective arterial embolization in the management of symptomatic renal angiomyolipomas. Eur J Radiol 32: 153–159. [DOI] [PubMed] [Google Scholar]
- Narayanan S., Gee M., Paul E., Thiele E. (2013) Identifying renal imaging biomarkers of mTOR inhibitor therapy for tuberous sclerosis complex. Pediatr Radiol 43: S430. [Google Scholar]
- Nelson C., Sanda M. (2002) Contemporary diagnosis and management of renal angiomyolipoma. J Urol 168: 1315–1325. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Wood S. (2014) Everolimus for tuberous sclerosis complex-associated angiomyolipomas: a case series. BJU Int. 113: 88–89. [Google Scholar]
- Nikolskaya N., Cox J., Kingswood J. (2014) CKD in TSC patients with different renal phenotypes. Nephrol Dial Transpl. 29: iii350. [Google Scholar]
- Ramon J., Rimon U., Garniek A., Golan G., Bensaid P., Kitrey N., et al. (2009) Renal angiomyolipoma: long-term results following selective arterial embolization. Eur Urol 55: 1155–1162. [DOI] [PubMed] [Google Scholar]
- Seyam R., Bissada N., Kattan S., Mokhtar A., Aslam M., Fahmy W., et al. (2008) Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology 72: 1077–1082. [DOI] [PubMed] [Google Scholar]
- Shepherd C., Gomez M., Lie J., Crowson C. (1991) Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 66: 792–796. [DOI] [PubMed] [Google Scholar]
- Sooriakumaran P., Gibbs P., Coughlin G., Attard V., Elmslie F., Kingswood C., et al. (2010) Angiomyolipomata: challenges, solutions, and future prospects based on over 100 cases treated. BJU Int 105: 101–106. [DOI] [PubMed] [Google Scholar]
- Tee A., Anjum R., Blenis J. (2003) Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/AKT-dependent and -independent phosphorylation of tuberin. J Biol Chem 278: 37288–37296. [DOI] [PubMed] [Google Scholar]
- Vallejo B., Herrera T., Domenech C., Lafuente P., de Ramírez T., Robles M. (2008) Renal angiomyolipoma: presentation, treatment and results of 20 cases. Actas Urol Esp 32: 307–315. [DOI] [PubMed] [Google Scholar]
- Van der Wal W., Over E., de Wit G., Roes K., Schotsman J., Khosrovani S., et al. (2012) Side-effects and impact of embolization on morbidity and mortality in TSC patients with renal angiomyolipomas. Presented at International Research Conference in TSC, Naples 2012. [Google Scholar]
- Williams J., Racadio J., Johnson N., Donnelly L., Bissler J. (2006) Embolization of renal angiomyolipomata in patients with tuberous sclerosis complex. Am J Kidney Dis 47: 95–102. [DOI] [PubMed] [Google Scholar]
- Zonnenberg B., Cheung W., Urva S., Wang J., Frost M., Kingswood C., et al. (2012) Pharmacokinetics/pharmacodynamics of everolimus in patients with renal angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis. Nephrol Dial Transpl 27: ii325–ii326. [Google Scholar]
- Zonnenberg B, Eijkemans M., Reijnders L., Khosrovani S., Magestro M. (2014) Presentation of renal angiomyolipoma and mortality in tuberous sclerosis in the Netherlands. Nephrol Dial Transpl 29: iii43. [Google Scholar]