Abstract
Purpose
The goal of this study was to compare the diffusion tensor imaging (DTI) metrics from an end-stage canine Krabbe brain evaluated by MR imaging ex vivo to those of a normal dog brain. We hypothesized that the white matter of the canine Krabbe brain would show decreased fractional anisotropy (FA) values and increased apparent diffusion coefficient (ADC) and radial diffusivity (RD) values.
Methods
An 11-week-old Krabbe dog was euthanized after disease progression. The brain was removed and was placed in a solution of 10% formalin. MR imaging was performed and compared to the brain images of a normal dog that was similarly fixed post-mortem. Both brains were scanned using similar protocols on a 7 T small-animal MRI system. For each brain, maps of ADC, FA, and RD were calculated for 11 white-matter regions and five control gray-matter regions.
Results
Large decreases in FA values, increases in ADC values, and increases in RD (consistent with demyelination) values, were seen in white matter of the Krabbe brain but not gray matter. ADC values in gray matter of the Krabbe brain were decreased by approximately 29% but increased by approximately 3.6% in white matter of the Krabbe brain. FA values in gray matter were decreased by approximately 3.3% but decreased by approximately 29% in white matter. RD values were decreased by approximately 27.2% in gray matter but increased by approximately 20% in white matter.
Conclusion
We found substantial abnormalities of FA, ADC, and RD values in an ex vivo canine Krabbe brain.
Keywords: Krabbe, DTI, MRI, FA, RD, ADC, canine
Introduction
Nearly 400 diseases have been described in the domestic dog, Canis familiaris, that are analogous to human diseases.1 Advances in the understanding of the dog genome have allowed for the identification of the molecular bases for many of these spontaneous, naturally occurring diseases. Discovery of the molecular bases for and characterization of the phenotype of rare monogenic disorders, such as lysosomal storage disorders in dogs, has led to better understanding of pathogeneses of these diseases, and to the development of experimental therapies for diseases that have no current cure.2 The mutations responsible for and the phenotype associated with the following lysosomal storage disorders in dogs have been identified: mucopolysaccharidosis (I, II, IIIA, IIIB, VI, and VII), neuronal ceroid-lipofuscinoses (CLN1, CLN2, CLN5, CLN6, CLN8, and CLN10), fucosidosis, globoid cell leukodystrophy (GLD), GM1 gangliosidosis, and GM2 gangliosidosis.3
Each lysosomal storage disorder may result from many different mutations in human patients, and may present with various ages of onset and rates of progression. In contrast, canine disease models allow for the study of disease pathogenesis in cohorts of animals with a single disease-causing mutation and with a predictable disease onset and progression. Specifically, the vast majority of lysosomal storage disorders have a primary neurological component, making the evaluation of central nervous system (CNS) disease paramount. The regular onset of CNS disease progression in dogs makes them a useful model in which to study neurodegenerative diseases. In contrast to murine models, the large size and sulcation pattern of the canine brain (far more similar to a human child’s than is a rodent brain) affords evaluation of neurodegeneration using clinically relevant tools that are routinely used to assess human patients, including neurological examinations, magnetic resonance imaging (MRI), and electrodiagnostic testing.
The canine model of GLD (Krabbe disease) has proven particularly valuable in understanding the disease in infants.4,5 Krabbe disease in West Highland white terriers and Cairn terriers is due to a spontaneous mutation in the galactosylceramidase (GALC) gene (c.473A > C) that results in the substitution of serine for tyrosine (p.S158Y) in galactosylceramindase (E.C. 3.2.1.46). A breeding colony of dogs heterozygous for the canine GALC mutation was established at the School of Veterinary Medicine of the University of Pennsylvania in 1997. Genotype can be determined at birth from whole blood using polymerase chain reaction (PCR) amplification of genomic DNA.5,6 Central and peripheral nervous system disease progression in the canine model of Krabbe closely recapitulates human disease; symptoms and signs include: head tremor, pelvic limb spinal ataxia, thoracic limb dysmetria, and hyporeflexia, progressing to pelvic limb paralysis and urinary incontinence.4,5 Clinical deterioration warrants euthanasia at about 16 weeks of age. MRI and quantitative magnetization transfer imaging of the brains of affected Krabbe dogs have shown changes consistent with CNS demyelination.4,5,7 Furthermore, magnetic resonance spectroscopy (MRS) has demonstrated decreases in N-acetylaspartate (NAA) and increases in choline (Cho), which are indicative of neuronal loss and abnormal myelin turnover.8 Pathological abnormalities include demyelination, reduced oligodendrocyte numbers, destruction of white matter, accumulation of globoid cells and gliosis within the white matter, inflammation, and enlargement of the cerebral ventricles.5,9 Enzyme analysis of nervous system tissue from affected dogs has shown deficient GALC activity.10 Lipid analysis shows a reduction in total lipid and sulfatide concentrations and increases in cerebroside and psychosine concentrations.5,10,11
Diffusion tensor imaging (DTI) is an MRI technique that provides a quantitative measurement of the microscopic motion of water molecules, thus allowing for the examination of the microstructural environment. The diffusion of water is hindered by structures such as cell membranes and, especially, myelinated axons. Hence, reasonable inferences (although not definitive conclusions) about the microstructure of tissue can be made. Compact, parallel arrays of myelinated axons, such as in the corpus callosum, provide the greatest impediment to diffusion perpendicular to an array of fibers but relatively freely allow water diffusion in the direction of the long axis of such structures. In this example, the microscopic water motion can be best assessed by three DTI measurements, i.e. the apparent diffusion coefficient (ADC), fractional anisotropy (FA), and radial diffusivity (RD). The ADC value measures the rate of microscopic water motion. Typically, in chronically abnormal tissue, the ADC value is elevated. FA measures the tendency for microscopic water motion to proceed in a non-random manner, i.e. have some degree of directionality. FA values are expected to be high in intact white-matter structures, especially those that have compact fibers oriented in a parallel array. Tissues in which disruption of compact white-matter pathways has occurred would be expected to show decreased FA values. RD is a measure of the rate of water motion perpendicular to the long axis of microscopic water motion. Such motion should be relatively low in highly myelinated structures and relatively high in unmyelinated structures. White-matter tissue with abnormal myelination would be expected to show increased RD values. Because gray matter is generally unmyelinated, one would not expect gray matter to exhibit such changes in a leukodystrophy brain.
In the described research we compared the DTI metrics from an end-stage canine Krabbe brain evaluated ex vivo and compared those metrics to the DTI metrics of a normal dog brain. We hypothesized that the white matter of the canine Krabbe brain would show decreased FA values and increased ADC and RD values and that these changes would not be seen in gray matter, which served as a control tissue.
Materials and methods
Specimen acquisition and preparation
The affected dog was raised in the National Referral Center for Animal Models of Human Genetic Disease of the School of Veterinary Medicine of the University of Pennsylvania (NIH OD P40-10939) under National Institutes of Health and United States Department of Agriculture (USDA) guidelines for the care and use of animals in research. The experimental protocol was approved by the University’s Institutional Animal Care and Use Committee. The 11-week-old male Krabbe dog was euthanized after disease progression had resulted in pelvic limb paralysis. The Krabbe canine was administered intravenous (IV) heparin 500 U and then euthanized using an overdose of IV barbiturates. Then, 1 L of 0.9% sodium chloride solution was given through the left ventricle while blood was drained form the right atrium. The brain was then removed from the skull and was placed in a solution of 10% formalin and shipped to the site where MR imaging would be performed. Finally, the brain was transferred to a solution of 1% ProHance® (Bracco Diagnostics Inc, Monroe Township, NJ) in 10% formalin and allowed to continue to fix for four weeks.
The normal male canine was collected from Iowa State University, Department of Animal Science, and was produced as part of an NIH funded project (R01 NS085381) under National Institutes of Health and USDA guidelines for the care and use of animals in research. The experimental protocol was approved by the University's Institutional Animal Care and Use Committee. At 6 weeks of age was administered IV heparin (0.5 ml, 500 U/dog) 10 minutes before euthanasia. It was then anesthetized with Euthasol® (200 mg/kg), until respiration and cardiac activity had stopped and corneal and pedal reflexes were absent. Perfusion catheters were placed in each of the carotid arteries, the arteries clamped below the catheter placement and the abdominal aorta cut. The dog was perfused first with a 0.9% sodium chloride solution and then with a solution of 10% Gadavist® (Bayer Healthcare Pharmaceuticals, Whippany, NJ) in 10% formalin. Following perfusion, most of the skin, subcutaneous tissue, and muscle were removed from the head. The head was then placed in a 10% formalin solution and allowed to post-fix for 24 hours at room temperature. After 24 hours, the head was transferred to a solution of 1% Gadavist in 10% formalin and shipped to the imaging facility at room temperature. Upon receipt of the head, we carefully removed the skull using an atraumatic technique and placed it in a solution of 1% ProHance (Bracco Diagnostics Inc) in 10% formalin.
Two weeks before image acquisition, both brains were immersed in a solution of 1% ProHance in phosphate-buffered saline (pH 7.4) in order allow the tissue to rehydrate and to reduce the T1 relaxation time and allow for a shorter imaging acquisition.
Imaging
Both brains were scanned using similar protocols on a 7T small-animal MRI system (Magnex Scientific, Yarnton, Oxford, UK) equipped with 670 mT/m Resonance Research gradient coils (Resonance Research Inc, Billerica, MA) using a 65 mm internal diameter quadrature radiofrequency (RF) coil (M2M Imaging, Cleveland, OH). The system was controlled with a General Electric Signa console (GE Medical Systems, Milwaukee, WI). The images were acquired using a custom-designed, six-direction, diffusion-weighted, spin-echo pulse sequence (repetition time (TR) = 100 ms, echo time (TE) = 18.1 ms, number of excitations (NEX) = 1, b = 1506 s/mm2). The acquisition matrix was optimized to fit the dimensions of each canine brain and adjusted for a field of view producing a Nyquist-limited isotropic voxel size of 100 µm for the normal brain and 200 µm for the Krabbe brain. Diffusion preparation was accomplished using a modified Tanner-Stejskal diffusion-encoding scheme with a pair of unipolar, half-sine diffusion gradient waveforms. Total acquisition time was approximately 40 hours for the normal brain and 12 hours for the Krabbe brain.
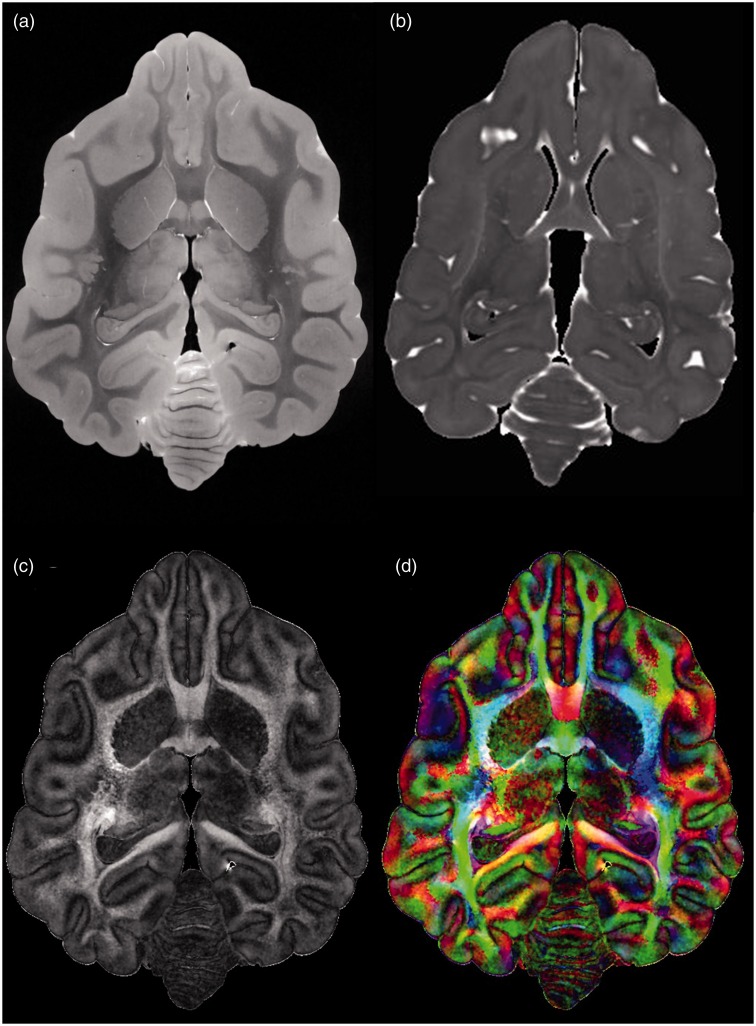
Following image acquisition, the data for each brain were then smoothed using the SUSAN de-noising algorithm implemented in FSL with a 3 × 3 × 3 voxel kernel. For each brain, maps of ADC, FA, color direction, and the three eigenvalues were constructed using Diffusion Toolkit (www.trackvis.org) (Figure 1).
Figure 1.
Representative diffusion tensor imaging axial images acquired from the normal brain. (a) B0 image, i.e. a diffusion image acquired before diffusion gradients are employed. (b) An apparent diffusion coefficient (ADC) image. (c) A fractional anisotropy image. (d) Directional color map image in which color indicates the direction of the principal axis of diffusion, with the principal axis in the left-right direction designated by the color red, that in the anterior-posterior direction designated by green, and that in the dorsal-ventral direction designated by blue.
DTI metric acquisition and comparison
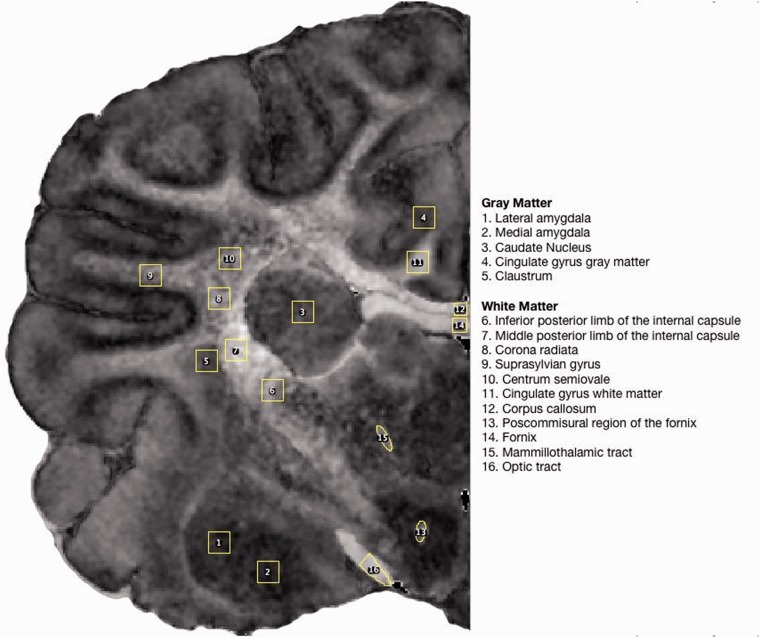
The FA, ADC, and all three eigenvalue maps for both brains were opened in ImageJ (http://imagej.nih.gov/ij/). We designated a total of 16 regions of interest (ROIs) that were intended to sample diverse neural tissues, including five gray-matter ROIs and 11 subcortical and deep white-matter ROIs (Figure 2). These ROIs were adapted from a previous study comparing DTI and histology in the canine brain.12 One set of ROIs was drawn on the FA map from the normal brain, and then applied to the normal ADC and all three normal eigenvalue maps and then used to measure mean values for each DTI metric within each ROI. Some of the smaller ROIs were translated, but not stretched or sheered, to account for small differences between the FA, ADC, and eigenvalue maps. A second set of ROIs was drawn on the FA map of the Krabbe brain, and the same process as was used for the normal brain was repeated to measure mean DTI values in all 16 ROIs. Comparisons were then made between the Krabbe and normal brains.
Figure 2.
Examples of the region of interest designations for all brain regions evaluated, drawn on a coronal fractional anisotropy (FA) image from the normal brain.
Values were obtained in a number of gray-matter regions to serve as controls based on the generally accepted fact that DTI is sensitive to abnormalities of white matter but not gray matter.
Results
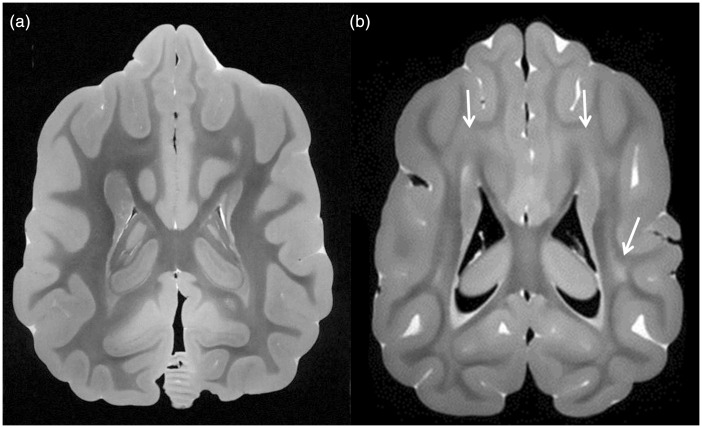
An example of images from the normal brain and the Krabbe brain is shown in Figure 3. Note that the Krabbe brain shows extensive areas of hyperintense signal within white matter, consistent with dysmyelination, in a pattern similar to that seen in humans.
Figure 3.
Comparison of white matter in a normal canine brain and a canine affected by Krabbe disease. (a) Axial B0 image from diffusion imaging sequence (essentially a T2-weighted image) from the normal canine shows dark signal throughout white matter, extending up to the subcortical regions, consistent with normal myelination. (b) Axial B0 image from the Krabbe brain shows large regions of hyperintense signal within deep white matter and subcortical white matter (arrows), consistent with impaired myelination, in a pattern that is similar to that of humans with markedly progressed Krabbe disease.
The DTI findings both for the normal brain and the Krabbe brain are outlined in Table 1. The differences between the two brains are outlined in Table 2, which shows marked decreases in FA values. On average, ADC values in gray matter of the Krabbe brain were decreased by approximately 29% but increased by approximately 3.6% in white matter of the Krabbe brain (as would be expected in abnormal white matter). On average, FA values in gray matter of the Krabbe brain were decreased by approximately 3.3% but decreased by approximately 29% in white matter of the Krabbe brain (as would be expected in abnormal white matter). On average, RD values were decreased by approximately 27.2% in Krabbe gray matter but increased by approximately 20% in white matter of the Krabbe brain (as would be expected in abnormal white matter).
Table 1.
DTI metrics obtained from all 16 ROIs in both brains. First five are gray-matter regions and last 11 are white-matter regions.
| FA | ADC | RD | |
|---|---|---|---|
| Normal | |||
| Lateral amygdala | 1.35E-01 | 4.79E-04 | 4.46E-04 |
| Medial amygdala | 1.21E-01 | 4.83E-04 | 4.59E-04 |
| Caudate nucleus | 2.31E-01 | 4.35E-04 | 3.92E-04 |
| Cingulate gyrus gray matter | 2.05E-01 | 5.05E-04 | 4.58E-04 |
| Claustrum | 2.37E-01 | 5.00E-04 | 4.39E-04 |
| Inferior posterior internal capsule | 4.55E-01 | 3.05E-04 | 2.22E-04 |
| Middle posterior internal capsule | 6.82E-01 | 2.27E-04 | 1.20E-04 |
| Corona radiata | 4.78E-01 | 2.46E-04 | 1.73E-04 |
| Suprasylvian lobe | 3.65E-01 | 3.94E-04 | 3.25E-04 |
| Centrum semiovale | 4.11E-01 | 2.61E-04 | 2.03E-04 |
| Cingulate gyrus white matter | 5.42E-01 | 3.88E-04 | 2.56E-04 |
| Corpus callosum | 5.45E-01 | 3.44E-04 | 2.25E-04 |
| Post-commissural fornix | 3.32E-01 | 4.47E-04 | 3.59E-04 |
| Fornix | 5.24E-01 | 3.81E-04 | 2.57E-04 |
| Mammillothalamic tract | 3.49E-01 | 3.59E-04 | 2.97E-04 |
| Optic tract | 6.22E-01 | 4.37E-04 | 2.57E-04 |
| Krabbe | |||
| Lateral amygdala | 1.13E-01 | 3.29E-04 | 3.16E-04 |
| Medial amygdala | 1.30E-01 | 3.03E-04 | 2.85E-04 |
| Caudate nucleus | 2.16E-01 | 3.49E-04 | 3.08E-04 |
| Cingulate gyrus gray matter | 1.77E-01 | 3.84E-04 | 3.46E-04 |
| Claustrum | 2.66E-01 | 3.78E-04 | 3.37E-04 |
| Inferior posterior internal capsule | 3.37E-01 | 3.00E-04 | 2.32E-04 |
| Middle posterior internal capsule | 3.16E-01 | 3.20E-04 | 2.66E-04 |
| Corona radiata | 2.47E-01 | 3.07E-04 | 2.63E-04 |
| Suprasylvian lobe | 2.89E-01 | 3.43E-04 | 2.93E-04 |
| Centrum semiovale | 1.44E-01 | 3.55E-04 | 3.35E-04 |
| Cingulate gyrus white matter | 3.97E-01 | 3.72E-04 | 2.77E-04 |
| Corpus callosum | 3.27E-01 | 3.67E-04 | 3.15E-04 |
| Post-commissural fornix | 2.84E-01 | 3.12E-04 | 2.49E-04 |
| Fornix | 4.73E-01 | 4.19E-04 | 2.96E-04 |
| Mammillothalamic tract | 2.70E-01 | 2.77E-04 | 2.41E-04 |
| Optic tract | 6.66E-01 | 4.06E-04 | 1.90E-04 |
DTI: diffusion tensor imaging; ROIs: regions of interest; FA: fractional anisotropy; ADC: apparent diffusion coefficient; RD: radial diffusivity.
Table 2.
Differences between Krabbe DTI metrics and normal brain DTI metrics. The first five regions listed are gray-matter regions and the remaining 11 regions are white matter regions. Increases greater than 25% and decreases greater than −25% are highlighted in bold type.
| Difference between Krabbe and normal | FA | ADC | RD |
|---|---|---|---|
| Lateral amygdala | −16.30% | −31.35% | −29.23% |
| Medial amygdala | 7.44% | −37.34% | −37.83% |
| Caudate nucleus | −6.49% | −19.87% | −21.37% |
| Cingulate gyrus gray matter | −13.66% | −23.97% | −24.38% |
| Claustrum | 12.24% | −24.32% | −23.13% |
| Inferior posterior internal capsule | −25.93% | −1.57% | 4.38% |
| Middle posterior internal capsule | −53.67% | 41.04% | 121.19% |
| Corona radiata | −48.33% | 24.77% | 51.40% |
| Suprasylvian lobe | −20.82% | −12.78% | −10.02% |
| Centrum semiovale | −64.96% | 35.96% | 64.93% |
| Cingulate gyrus white matter | −26.75% | −3.97% | 8.20% |
| Corpus callosum | −40.00% | 6.63% | 39.69% |
| Post-commissural fornix | −14.46% | −30.17% | −30.53% |
| Fornix | −9.73% | 9.81% | 15.23% |
| Mammillothalamic tract | −22.64% | −22.89% | −18.87% |
| Optic tract | 7.07% | −6.99% | −26.17% |
DTI: diffusion tensor imaging; FA: fractional anisotropy; ADC: apparent diffusion coefficient; RD: radial diffusivity.
We selected an arbitrary threshold of a 25% increase in ADC values, a 25% decrease in FA values and a 25% increase in RD values to indicate substantial abnormalities. Differences of greater than 25% are shown in bold type in Table 2. As the table indicates, no 25% increases in ADC values were seen in gray matter but such increases were found in two white-matter regions, i.e. the middle posterior internal capsule and the centrum semiovale. No 25% decreases in FA values were seen in gray matter but such decreases were found in six white-matter regions, with the greatest changes seen in the centrum semiovale, internal capsule, and corpus callosum. These findings indicate substantial changes in microstructural organization of white matter in the Krabbe brain. No 25% increases in RD values were seen in gray matter but such increases were found in four white-matter regions, i.e. the middle posterior internal capsule, centrum semiovale, corona radiate, and the corpus callosum. Such increases in RD are taken by many investigators to represent decreased myelination. Substantial changes in all three metrics (i.e. 25% increases both in the ADC values and RD values and 25% decreases in FA values) were seen in the middle posterior internal capsule and the centrum semiovale.
Discussion
In this study, we hypothesized that DTI metrics would substantially differ between the white matter of the canine Krabbe brain studied and that of a normal canine brain. Specifically, we hypothesized that FA values would be decreased and ADC values and RD values would be increased in white matter, but not gray matter, of the Krabbe brain. Our hypothesis was validated in all instances. In white-matter regions in the Krabbe brain, large decreases in FA values were found. In general, large increases in RD, consistent with demyelination, were seen in the Krabbe brain and, again, solely in white matter. Notably, all changes in gray matter RD were negative, i.e. the opposite result that would be expected in demyelination. Large increases in ADC values were, indeed, seen in the white matter of the Krabbe brain (and not in gray matter), but the results were more variable than for the FA and RD changes. Unexpectedly, a large decrease in RD values was seen in the optic tract, which would suggest increased, rather than decreased, myelination. Both optic nerve atrophy and optic nerve enlargement have been described in human Krabbe disease patients.13,14 The optic nerve enlargement is thought to be due to accumulation of the globoid cells that are diagnostic of Krabbe disease.13 The etiology of decreased RD values in the optic tract is uncertain but may represent a reparative response to a previous demyelinating process.
Although psychomotor regression due to cerebral hemisphere disease is a significant component of Krabbe disease and many lysosomal storage diseases, cerebral disease has not been accurately modeled in any system. Behavioral testing as a means to determine psychomotor regression is difficult to perform in affected Krabbe animals because of the walking difficulty produced by the disease. Therefore, a noninvasive, quantitative imaging tool, such as DTI, which can be used independently of the clinical status of the subject, has distinct advantages.
Based on our previous observations, the brains of Krabbe dogs show atrophy as evidenced by widening of cerebral sulci and dilation of the ventricular system (unpublished data). Histologically, massive numbers of globoid cells within the brain’s white matter are present, most prominently in the centrum semiovale and corpus callosum, along with extensive astrocytosis and lymphocytic perivascular cuffing. Severe loss of myelin is typically seen throughout the cerebral white matter; the centrum semiovale, internal capsule, and corpus callosum are most severely affected, although a thin region of normal-appearing myelin often remains laterally in the corona radiata. However, gray matter typically appears normal.
In previous studies, we have demonstrated our ability to measure myelination abnormalities, neuronal loss, and gliosis in canine Krabbe as well as in other canine and feline models of lysosomal storage diseases using MR imaging, magnetization transfer imaging, and single-voxel spectroscopy7,8,15–20 (preliminary data) using methodologies that can easily be translated into assessment of pediatric leukodystrophies. Here, we show data on quantitative evaluation of the canine brain using DTI.12,21–23 Notably, using the canine model, it is also possible to directly compare MR data with histopathological data.12,21
In summary, using high-resolution DTI, we found substantial abnormalities of FA, ADC, and RD values in an ex vivo canine Krabbe brain that have similarities to findings in human Krabbe brains.24–26 Based on our findings, MR imaging of the ex vivo canine Krabbe brain appears to be a suitable model for evaluation of imaging changes in this disease which may be useful in monitoring disease progression and treatment response.
Acknowledgments
The authors would like to acknowledge JK Jens and EM Snella for animal care and sample preparation, and students at Iowa State University and University of Pennsylvania School of Veterinary Medicine for animal care.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by the following grants: NIH/NCRR P40 RR02512-17 (PI Charles Vite, DVM, PhD), National Referral Center for Animal Models of Human Genetic Disease and NIH R01 NS085381 (PI Patricia Dickson, MD), Neuroimaging and Neuropathology of Mucopolysaccharidosis I.
References
- 1.Shearin AL, Ostrander EA. Leading the way: Canine models of genomics and disease. Dis Model Mech 2010; 3: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Switonski M. Dog as a model in studies on human hereditary diseases and their gene therapy. Reprod Biol 2014; 14: 44–50. [DOI] [PubMed] [Google Scholar]
- 3.Skelly BJ, Franklin RJ. Recognition and diagnosis of lysosomal storage diseases in the cat and dog. J Vet Intern Med 2002; 16: 133–141. [DOI] [PubMed] [Google Scholar]
- 4.Cozzi F, Vite CH, Wenger DA, et al. MRI and electrophysiological abnormalities in a case of canine globoid cell leucodystrophy. J Small Anim Pract 1998; 39: 401–405. [DOI] [PubMed] [Google Scholar]
- 5.Wenger DA, Victoria T, Rafi MA, et al. Globoid cell leukodystrophy in Cairn and West Highland white terriers. J Hered 1999; 90: 138–142. [DOI] [PubMed] [Google Scholar]
- 6.Victoria T, Rafi MA, Wenger DA. Cloning of the canine GALC cDNA and identification of the mutation causing globoid cell leukodystrophy in West Highland White and Cairn terriers. Genomics 1996; 33: 457–462. [DOI] [PubMed] [Google Scholar]
- 7.McGowan JC, Haskins M, Wenger DA, et al. Investigating demyelination in the brain in a canine model of globoid cell leukodystrophy (Krabbe disease) using magnetization transfer contrast: Preliminary results. J Comput Assist Tomogr 2000; 24: 316–321. [DOI] [PubMed] [Google Scholar]
- 8.Vite CH, Cross JR. Correlating magnetic resonance findings with neuropathology and clinical signs in dogs and cats. Vet Radiol Ultrasound 2011; 52(1 Suppl 1): S23–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jortner BS, Jonas AM. The neuropathology of globoid-cell leucodystrophy in the dog. A report of two cases. Acta Neuropathol 1968; 10: 171–182. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher TF, Suzuki K, Martin FB. Galactocerebrosidase activity in canine globoid leukodystrophy. Neurology 1977; 27: 758–766. [DOI] [PubMed] [Google Scholar]
- 11.Cantuti Castelvetri L, Maravilla E, Marshall M, et al. Mechanism of neuromuscular dysfunction in Krabbe disease. J Neurosci 2015; 35: 1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei PT, Leong D, Calabrese E, et al. Diffusion tensor imaging of neural tissue organization: Correlations between radiologic and histologic parameters. Neuroradiol J 2013; 26: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain SA, Zimmerman HH, Abdul-Rahman OA, et al. Optic nerve enlargement in Krabbe disease: A pathophysiologic and clinical perspective. J Child Neurol 2011; 26: 642–644. [DOI] [PubMed] [Google Scholar]
- 14.Beslow LA, Schwartz ES, Bonnemann CG. Thickening and enhancement of multiple cranial nerves in conjunction with cystic white matter lesions in early infantile Krabbe disease. Pediatr Radiol 2008; 38: 694–696. [DOI] [PubMed] [Google Scholar]
- 15.Vite CH, McGowan JC, Braund KG, et al. Histopathology, electrodiagnostic testing, and magnetic resonance imaging show significant peripheral and central nervous system myelin abnormalities in the cat model of alpha-mannosidosis. J Neuropathol Exp Neurol 2001; 60: 817–828. [DOI] [PubMed] [Google Scholar]
- 16.Vite CH, McGowan JC, Niogi SN, et al. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol 2005; 57: 355–364. [DOI] [PubMed] [Google Scholar]
- 17.Vite CH, Magnitsky S, Aleman D, et al. Apparent diffusion coefficient reveals gray and white matter disease, and T2 mapping detects white matter disease in the brain in feline alpha-mannosidosis. AJNR Am J Neuroradiol 2008; 29: 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnitsky S, Vite CH, Delikatny EJ, et al. Magnetic resonance spectroscopy of the occipital cortex and the cerebellar vermis distinguishes individual cats affected with alpha-mannosidosis from normal cats. NMR Biomed 2010; 23: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradbury AM, Gray-Edwards HL, Shirley JL, et al. Biomarkers for disease progression and AAV therapeutic efficacy in feline Sandhoff disease. Exp Neurol 2015; 263: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vite CH, Nestrasil I, Mlikotic A, et al. Features of brain MRI in dogs with treated and untreated mucopolysaccharidosis type I. Comp Med 2013; 63: 163–173. [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J, Dickson P, Calabrese E, et al. Predicting degree of myelination based on diffusion tensor imagining of canines with mucopolysaccharidosis type I. Neuroradiol J 2015; 28: 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leong D, Calabrese E, White LE, et al. Correlation of diffusion tensor imaging parameters in the canine brain. Neuroradiol J 2015; 28: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce TT, Provenzale JM. Evaluation of apparent diffusion coefficient thresholds for diagnosis of medulloblastoma using diffusion-weighted imaging. Neuroradiol J 2014; 27: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo AC, Petrella JR, Kurtzberg J, et al. Evaluation of white matter anisotropy in Krabbe disease with diffusion tensor MR imaging: Initial experience. Radiology 2001; 218: 809–815. [DOI] [PubMed] [Google Scholar]
- 25.McGraw P, Liang L, Escolar M, et al. Krabbe disease treated with hematopoietic stem cell transplantation: Serial assessment of anisotropy measurements—initial experience. Radiology 2005; 236: 221–230. [DOI] [PubMed] [Google Scholar]
- 26.Provenzale JM, Escolar M, Kurtzberg J. Quantitative analysis of diffusion tensor imaging data in serial assessment of Krabbe disease. Ann N Y Acad Sci 2005; 1064: 220–229. [DOI] [PubMed] [Google Scholar]