Abstract
The presentation of carbon monoxide poisoning is non-specific and highly variable. Hyperbaric oxygen therapy is used for the treatment of this condition. Various reports show the occurrence of self-limiting seizures after carbon monoxide poisoning and as a consequence of hyperbaric oxygen therapy. Contrary to the seizures, status epilepticus has been rarely observed in these conditions. The exact pathophysiology underlying seizures and status epilepticus associated with carbon monoxide poisoning and hyperbaric oxygen therapy is not really clear, and some elements appear to be common to both conditions. We describe a case of non-convulsive status epilepticus in a patient with carbon monoxide poisoning treated with hyperbaric oxygen therapy. The mechanism, MRI findings and implications are discussed.
Keywords: Carbon monoxide poisoning, seizures, status epilepticus, hyperbaric oxygen therapy, MRI
Introduction
Carbon monoxide (CO) poisoning is one of the most difficult medical emergencies to diagnose. Symptoms are non-specific and in low levels of exposure generally consist of headache, myalgia, dizziness or neuropsychological impairment. Severe exposures result in confusion, loss of consciousness, coma, seizures or death.1 Numerous cases of CO-induced, self-limiting, isolated seizures have been described, but there is no information concerning the incidence of this phenomenon in adults.2,3 To our knowledge, there are only two reported cases of acute CO toxicity-induced status epilepticus in adults.4,5 On the basis of the available data, including biochemical studies and one rigorous clinical trial,6 hyperbaric oxygen therapy (HBO2) is recommend by several authors1 for patients with CO poisoning. HBO2 therapy is associated with an increased risk of generalized tonic-clonic convulsive (GTC) seizures in approximately 0.002–0.035% of patients undergoing treatment.7 To our knowledge, status epilepticus following treatment with HBO2 has been reported in only one case.8 We describe a case of a patient presenting non-convulsive status epilepticus after acute CO poisoning studied with MRI and treated with HBO2.
Case report
A 52-year-old woman was admitted as an emergency at 22:30 h after being found unresponsive 30 min earlier at home. She had been healthy in the morning. Her husband had been repairing the boiler in the afternoon. She had called a friend around 16:00 h and told her she was feeling the worst headache of her life. The patient could not be reached by telephone during the next hours, so rescue was called. It was estimated that about 6 h had elapsed from the beginning of the symptoms to the arrival of the fire brigade. The latter noted that the patient was lethargic and confused. There was no history of psychiatric illness, drug misuse, previous suicidal attempts or epilepsy.
On arrival at our Emergency Room, the patient was unconscious, with a Glasgow Coma Scale (GCS) score of 3/15. A vital signs examination revealed an oral temperature of 37℃, a regular pulse of 125 beats/min, blood pressure of 90/60 mmHg, pulse oximetry 99% in room air.
Her haemoglobin level was 14.2 g/dl and leukocyte count was 22 × 1000 cells/l with neutrophilia (87.9%). Creatinine kinase level was 256 UI/l (normal range 10–167 UI/l), creatinine kinase-MB isoenzyme level 10 ng/ml (normal range 0.5–3.6), and troponin I was 0.580 ng/ml (normal range <0.045 ng/ml). Urine toxicology screening tests gave negative results. Other results of her general routine examination were unremarkable. Her arterial blood gases in room air showed pH 7.42, PaCO2 33 mmHg, PaO2 48 mmHg, base excess was −3.1 mmol/l, SaO2 95.8% and carboxyhaemoglobin (COHb) 32%. An electrocardiogram showed sinus tachycardia with no ST segment changes. The results of a brain computed tomography scan showed loss of grey/white matter distinction and sulcal effacement, suggesting diffuse cerebral oedema.
CO poisoning was suspected because of the increased level of COHb (32%) and 100% oxygen administration was initiated. Meanwhile, inspection of the patient’s house showed an important leakage of fumes.
Twenty-five minutes following the start of O2 therapy, the arterial blood gases improved: pH 7.41, PaCO2 36 mmHg, PaO2 405 mmHg, base excess was −1.8 mmol/l, SaO2 100% and COHb 22.9%. At that point in time, on neurological examination, the patient did not respond to voice but she did respond to pain induced in her extremities (GCS 6), both pupils were miotic, and Babinski’s reflex was positive on the right side.
One hour later, since the patient had started to have GTC seizure, endotracheal intubation was instituted. Her convulsions were abated after sedation with thiopental. Following a consultation with a specialist, the patient was transferred to the nearest Department of Hyperbaric Medicine. The patient underwent three hyperbaric oxygen sessions (an initial session of 150 min with 100% oxygen at 2 atm absolute, followed by two sessions, respectively of 120 min and 60 min at 1 atm absolute, separated by an interval of 6 and 12 h). Following HBO2 the patient had spontaneous respiration and the tube was removed.
Despite normalization of the arterial blood gases parameters and the absence of sedatives or paralytic medications, the patient’s mentation continued to oscillate. On neurological examination she was alert, mutacic and responsive to painful stimuli. There was a loss of contact with no movement abnormalities, lip smacking, posturing or facial grimacing. An electroencephalogram (EEG) was performed and showed diffuse high amplitude paroxysmal sharp waves at 2.5–3 Hz. Non-convulsive status epilepticus was suspected. Within 5 min, 500 mg intravenous (i.v.) Levetiracetam (LEV) was therefore administered. After 20 min, an additional 500 mg LEV was infused over 15 min. Anti-oedema therapy was set with 8 mg/day of betamethasone and 200 cc/day of mannitol. LEV 500 mg twice a day has been instituted as maintenance therapy. After the administration of the first 500 mg i.v. LEV, the patient reported a substantial relief, and after the second infusion of 500 mg LEV she felt that she was back to her normal self.
On day 3, as the patient regained consciousness, a new EEG showed an increase of 8–12 Hz alpha activity, whereas the amount of 1–4 Hz activity persisted (4–6 s sequences).
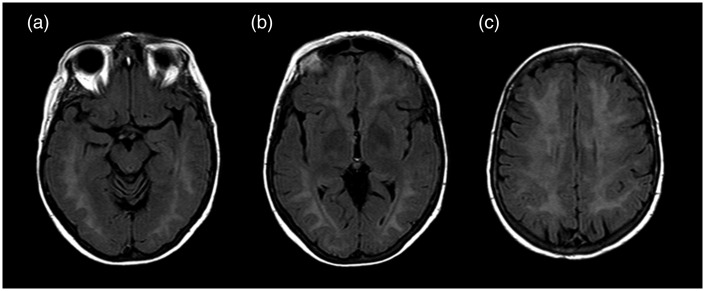
Magnetic resonance (MR) examination was performed by a 1.5 T scan; it showed a diffuse, confluent hyperintensity on T2-weighted sequences involving predominantly periventricular white matter, with a partial involvement of corpus callosum, without interest of internal and external capsules (Figure 1).
Figure 1.
T2 FLAIR (FLuid Attenuated Inversion Recovery) on axial plan at three different levels: (a) hyppocampal cortex; (b) globus pallidus; (c) semioval center at 3 days from CO poisoning. The figure shows a widespread increase in T2 signal intensity at bi-hemisferic periventricular white matter without involvement of the basal ganglia structures. However, this alteration of white matter intensity is not specific to CO poisoning.
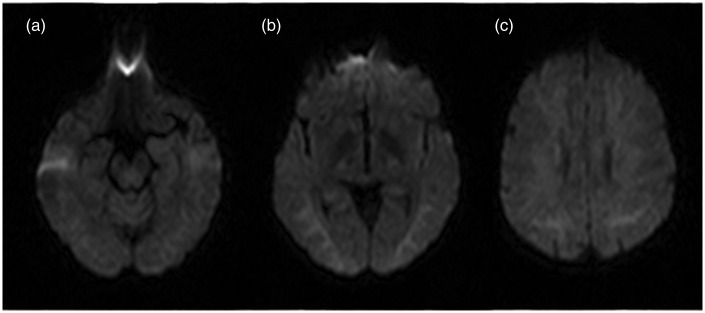
Diffusion weighted sequence showed a moderate increase of signal intensity at bilateral frontal white matter and at semioval centres (Figure 2), due to a T2-shine-through rather than a true ischaemia, sparing the brain stem and the cerebellum as well as the grey matter structures.
Figure 2.
DWI (Diffusion Weighted Imaging) on axial plan at three different levels: (a) hyppocampal cortex; (b) globus pallidus; (c) semioval center at 3 days from CO poisoning. This sequence shows a mild signal intensity at white matter of these three different levels referred to as T2-shine trough effect and no to ischemic lesion. Also, the ADC (Apparent Diffusion Coefficient) maps did not show alteration consistent with an ischemic event.
On day 4, the last performed EEG showed normal alpha activity and the disappearance of 1–4 Hz activity. A week later, when the patient was discharged, the neurological examination was normal.
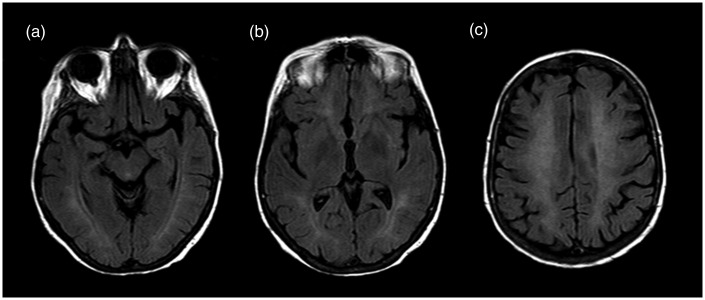
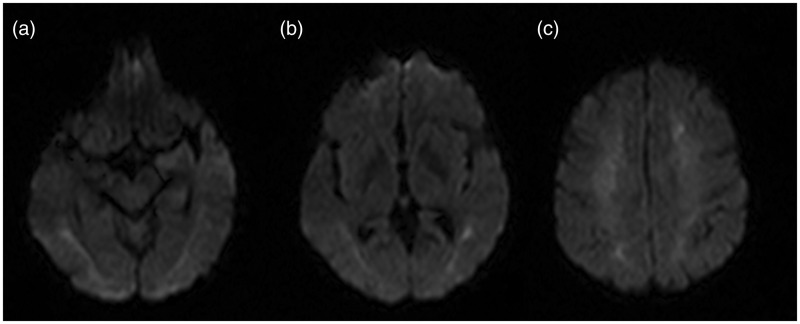
About a month after the CO poisoning we performed a MR examination which confirmed a diffuse hyperintensity of T2-FLAIR (FLuid Attenuated Inversion Recovery) of white matter referred to a diffuse leukoencephalopathy (Figure 3). Also, we reported diffusion weighted images a month after CO poisoning (Figure 4).
Figure 3.
T2 FLAIR (FLuid Attenuated Inversion Recovery) on axial plan at three different levels: (a) hyppocampal cortex; (b) globus pallidus, (c) semioval center at 30 days from CO poisoning. The MR examination did not show significant changes in the T2 signal intensity compared to the previous MR exam.
Figure 4.
DWI (Diffusion Weighted Imaging) on axial plan at three different levels: (a) hyppocampal cortex; (b) globus pallidus, (c) semioval center at 30 days from CO poisoning.
Discussion
CO diffuses quickly into the blood by way of the lungs, causes injury and an adaptive response that continue after COHb levels have returned to normal. CO causes hypoxaemia through the formation of carboxyhaemoglobin and a leftward shift of the oxyhaemoglobin dissociation curve. CO binds to haemoproteins such as cytochrome C oxidase, impairing mitochondrial function and contributing to hypoxia. Brain hypoxia elevates levels of excitatory amino acids (N-methyl-d-aspartate) and increases brain nitrite levels causing oxidative stress, necrosis and apoptosis.1
Seizures are generally thought to occur when COHb level reaches more than 40%,9 but the level does not necessarily correlate with the severity of symptoms or the prognosis.4 The incidence of isolated seizures after CO poisoning is well supported by the literature. Seizures may result from cellular hypoxia and cerebral vasodilatation, which can also lead to cerebral oedema. Unlike the self-limited focal seizure activity, only two cases of status epilepticus secondary to acute CO poisoning have been reported to date.4,5
HBO2 is recommended for patients with serious CO poisoning. Repeated exposures to HBO2 may be responsible for central nervous system (CNS) toxicity and for the decrease in the seizure threshold leading to epileptic clinical manifestation. The mechanism of GTC seizures after HBO2 depends on various mechanisms. Under HBO2 conditions, production of nitric oxide (NO) by NO synthase would increase approximately four- to five-fold in hippocampal and striatal regions. Increased NO production inhibits glutamic acid decarboxylase and results in a decrease in gamma-aminobutyric acid levels. NO can also produce toxicity through the induction of adenosine triphosphate depletion and the increase of intracellular free calcium, leading to membrane potential disturbances and depolarization.8 Furthermore, cerebral blood flow during HBO2 increases immediately before signs of CNS toxicity and seizure. This probably depends on hyperoxia and hypocarbia, which act as potent vasoconstrictors. The same mechanism could be responsible for the decreased threshold for generalized seizures during hyperventilation.7
Our patient presented GTC seizures after CO poisoning and developed a non-convulsive status epilepticus after HBO2. Her electro-clinical epileptic activity was abated until recovery with the instauration of anti-epileptic and anti-oedemigene therapy. It is difficult to establish whether the electro-clinical state of our patient has been determined by CO poisoning or by HBO2 or if the relief was determined by anti-epileptic or anti-oedemigene therapy. Furthermore the description of EEG activity after CO exposure is not clearly outlined in the literature, but, in general, resembles the evolution observed after an anoxic insult.10
MR imaging shows abnormal signal intensity of white matter in 12%–37% of patients, but the alteration is not specific on the severity of intoxication.11–13
The hippocampal and cortex involvement are correlated with a poor prognosis in the delayed phase of CO poisoning due to irreversible damage of grey matter.14
Our patient had the same involvement and abnormal signal intensity of white matter in the acute phase (between 24 h and seven days) and the delayed phase (up 22 days) with no alteration of grey matter’s signal intensity; these neuroradiological findings agree with the absence of neurological symptoms to discharge.
Conclusion
In conclusion, non-convulsive status epilepticus is uncommon both after CO poisoning and after HBO2. The supposed mechanisms underlying the epileptogenic role of both events are similar. The current report supports the idea that EEG should be performed when mental status fails to improve after HBO2 because status epilepticus can have atypical and misleading presentation, and MRI is useful to support these procedures.
Acknowledgment
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. A consent form has been signed by the parent/guardian of the case allowing publication of the report. Author contributions: 1. Guarantor of integrity of the entire study: RF, FL; 2. Study concepts and design: SN, SM, FL; 3. Literature research: EP, FG, CL; 4. Clinical studies: FDG, SM, CL; 5. Manuscript preparation: SN, FG, EP; 6. Manuscript editing: FDG, SM.
Conflict of interest
We declare that we have no conflict of interest. We certify that we have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. We declare that we have no financial support from commercial sources or pecuniary interest in such enterprises that could pose a conflict of interest.
References
- 1.Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med 2009; 360: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 2.Durnin C. Carbon monoxide poisoning presenting with focal epileptiform seizures. Lancet 1987; 1: 1319. [DOI] [PubMed] [Google Scholar]
- 3.Herman LY. Carbon monoxide poisoning presenting as an isolated seizure. J Emerg Med 1998; 16: 429–432. [DOI] [PubMed] [Google Scholar]
- 4.Brown KL, Wilson RF, White MT. Carbon monoxide-induced status epilepticus in an adult. J Burn Care Res 2007; 28: 533–536. [DOI] [PubMed] [Google Scholar]
- 5.Abdulaziz S, Dabbagh O, Arabi Y, et al. Status epilepticus and cardiopulmonary arrest in a patient with carbon monoxide poisoning with full recovery after using a neuroprotective strategy: A case report. J Med Case Rep 2012; 6: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med 2002; 347: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 7.Doherty MJ, Hampson NB. Partial seizure provoked by hyperbaric oxygen therapy: Possible mechanisms and implications. Epilepsia 2005; 46: 974–976. [DOI] [PubMed] [Google Scholar]
- 8.Seckin M, Gurgor N, Beckmann YY, et al. Focal status epilepticus induced by hyperbaric oxygen therapy. Neurologist 2011; 17: 31–33. [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Nagai K. Carbon-monoxide poisoning presenting as an afebrile seizure. Pediatr Neurol 2000; 22: 330–331. [DOI] [PubMed] [Google Scholar]
- 10.Neufeld MY, Swanson JW, Klass DW. Localized EEG abnormalities in acute carbon monoxide poisoning. Arch Neurol 1981; 38: 524–527. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson RB, Hopkins RO, Cleavinger HB, et al. White matter hyperintensities and neuropsychological outcome following carbon monoxide poisoning. Neurology 2002; 58: 1525–1532. [DOI] [PubMed] [Google Scholar]
- 12.Pavese N, Napolitano A, De Iaco G, et al. Clinical outcome and magnetic resonance imaging of carbon monoxide intoxication. A long-term follow-up study. Ital J Neurol Sci 1999; 20: 171–178. [DOI] [PubMed] [Google Scholar]
- 13.Lin WC, Lu CH, Lee YC, et al. White matter damage in carbon monoxide intoxication assessed in vivo using diffusion tensor MR imaging. AJNR Am J Neuroradiol 2009; 30: 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beppu T. The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: A review of the literature. AJNR Am J Neuroradiol 2014; 35: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]