Summary
CD4+ T cells are the master regulators of adaptive immune responses, and many autoimmune diseases arise due to a breakdown of self-tolerance in CD4+ T cells. Activation of CD4+ T cells is regulated by not only the binding of peptide-major histocompatibility complexes to T-cell receptor but also costimulatory signals from antigen-presenting cells. Recently, there has been progress in understanding the extracellular and intracellular mechanisms that are required for implementation and maintenance of T-cell tolerance. Understanding of the molecular mechanisms underlying T-cell tolerance will lead to development of pharmacological approaches either to promote the tolerance state in terms of autoimmunity or to break tolerance in cancer.
Keywords: tolerance, costimulation, E3 ubiquitin ligases, transcription, epigenetics
Introduction
T-cell activation and tolerance are tightly regulated to ensure effective elimination of foreign antigen while maintaining immune tolerance to self-antigens. Immunological tolerance in T cells is maintained by various mechanisms to prevent autoimmune diseases. This is initially mediated in thymus, where self-reactive T cells are deleted by negative selection (1). Although most autoreactive T cells are eliminated by this mechanism, it is incomplete. Additional peripheral tolerance mechanisms thus exist to prevent autoreactive reactions. Peripheral tolerance is regulated by both T-cell-extrinsic (regulatory T cells and tolerogenic dendritic cells) and -intrinsic (signaling defects, apoptosis, phenotype skewing, transcriptional, and epigenetic) mechanisms (1).
T cells are regulated by extracellular signals delivered by antigen-presenting cells (APCs) and delicate intracellular signal transducers and regulators. Costimulatory molecules on APCs are important in regulation of T-cell clonal activation and function. CD28 and inducible costimulator (ICOS) are important costimulatory receptors required for T-cell activation and function (2–5). Many negative costimulatory signals such as cytotoxic T-lymphocyte antigen-4 (CTLA4), programmed death-1 (PD-1), B7S1, B7-H3, and so on have been discovered and regulate the induction of T-cell tolerance (4, 6).
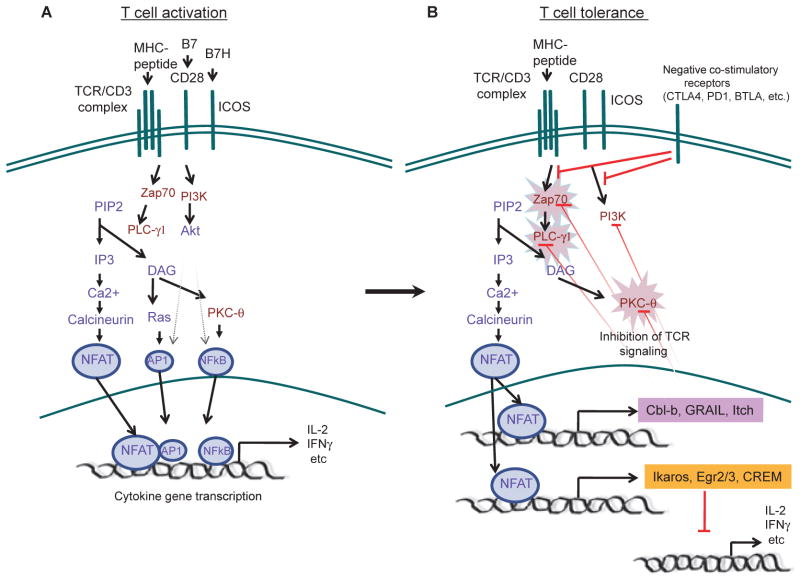
In T cells, several E3 ubiquitin ligases, including Cbl-b, Itch, and GRAIL, have been shown to negatively regulate T-cell receptor (TCR) signaling (7–10). These proteins can specifically target TCR signaling components, such as phospholipase C-γ (PLC-γ), protein kinase C-θ (PKC-θ), ζ-chain (TCR) associated protein kinase 70 kDa (Zap70), CD3ζ, or p85, leading to unresponsiveness, or anergy. The hallmark of tolerant T cells is their inability to proliferate or to produce cytokines in response to TCR engagement. Although this defect can be explained by negative role of E3 ubiquitin ligases in TCR signaling, growing evidence suggests that transcriptional or even epigenetic mechanisms are involved to actively repress cytokine gene transcription. Transcriptional repressors such as Ikaros, cAMP responsive element modulator (CREM), early growth response 2 (Egr2), and Egr3 are recruited to the interleukin-2 (IL-2) gene and repress its expression through a mechanism that involves histone deacetylation and DNA and histone methylation (11–13) (Fig. 1). Thus, peripheral T-cell tolerance may represent another state or fate of naive T cells when they encounter self-antigens and molecules highly up-regulated in tolerant T cells may regulate the induction and maintenance of tolerance.
Fig. 1. Molecular mechanisms of T-cell tolerance.
(A). Activation of T cells by both TCR and positive costimulatory receptor signaling via several major pathways, leading to activation of multiple transcriptional factors, such as NFAT, AP1, and NF-κB, that are important for activation of cytokine gene transcription. (B) TCR engagement without CD28- or ICOS-mediated costimulation or in the presence of strong negative costimulation signals results in attenuated T-cell signaling. For example, in the absence of AP-1, NFAT provoked the expression of tolerance-inducing genes: E3 ubiquitin ligases (Cbl-b, GARIL and Itch) and transcriptional repressors (Egr2, Egr3, Ikaros and CREM). E3 ubiquitin ligases inhibit TCR signaling. In addition, transcriptional repressors are recruited to cytokine genes and repress their transcription.
In this review, we summarize the findings on the roles of costimulation in T-cell tolerance. We will also discuss the mechanisms of induction of tolerance-related genes and the function of proteins encoded by those genes. In addition, we review the transcriptional and epigenetic control of the tolerant T cells.
Costimulation and regulation of tolerance
In contrast to the robust responses to pathogen-associated antigens, presentation of self-antigens to T cells can result in T-cell tolerance, such as clonal deletion or anergy (14, 15). Anergy was first discovered in T-helper 1 (Th1) clones when the TCR/CD3 complex was engaged in the absence of costimulation, which resulted in hyporesponsiveness to secondary TCR stimulation (1, 16). Based on this phenomenon, a two signal model for T-cell activation was proposed in which, in addition to the MHC–peptide complexes, a costimulatory ‘second’ signal is required for T-cell activation (1, 16). In addition to positive costimulatory molecules, more and more negative costimulatory molecules have been recently identified, and the decision of T-cell tolerance or function may be determined by these factors together.
CD28/CTLA4
The CD28 receptor on naive T cells has long been regarded to deliver a crucial costimulatory signal for T-cell activation (2). It binds to B7.1 (CD80) and B7.2 (CD86) on activated APCs. Mice deficient in CD28 or both of its ligands B7.1 and B7.2 (hereafter as B7-deficient mice) were severely impaired in CD4+ T-cell proliferation (17, 18). In these mice, however, reduced levels of effector cytokines were still produced (19–21). A second receptor for B7-CTLA4 is induced on activated T cells and serves as a negative regulator of T-cell activation and proliferation (22). Mice deficient in CTLA4 died at neonatal stage owing to massive T-cell activation and infiltration into tissues.
B7H-ICOS
ICOS is the third member of the CD28 family expressed on activated T cells (23, 24). A ligand for ICOS, B7h (also named B7RP-1), has been described to be expressed on B cells, macrophages, endothelial cells, and in non-lymphoid tissues (24, 25). Analysis of mice deficient in ICOS or its ligand, B7h, revealed that this pathway, although not globally required for CD4+ T-cell activation and effector differentiation, regulates their selective effector function (3, 26–28). Nurieva et al. (27) demonstrated that ICOS, via enhancing the nuclear factor of activated T cells c1 (NFATc1) expression at an early stage of T-cell activation, regulates c-Maf expression and hence IL-4 expression at the effector stage. In addition, the analysis of the signaling mechanisms whereby costimulation regulates NFATc1 expression revealed that a phosphatidylinositol 3 kinase (PI3K)-Itk-PLCγ1-Ca2+pathway initiated by CD28 and ICOS leads to the induction of Nfatc1 P1 promoter activity (29).
Recently, the ICOS pathway was also found to be important in the generation of CXCR5+ follicular helper T cells (Tfh), a unique T-cell subset regulating germinal center reactions and humoral immunity (30). Consistently, Nurieva et al. (31) found that absence of ICOS ligand in B cells led to a greatly reduced frequency of CXCR5+ Tfh cells and germinal center B cells. In addition to CXCR5 expression, the expression of IL-21 cytokine by T cells in the absence of ICOS-B7h interaction was also greatly reduced in these mice.
It has been shown that IL-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis (32, 33). Using collagen-induced arthritis (CIA) model, Nurieva et al. (Nurieva R and Dong C, unpublished data) found that CII-specific IL-21 production was greatly reduced in ICOS-deficient mice. In addition, in CIA model blockade of IL-21 or IL-21R ameliorated disease and also lowered levels of IL-6 and IL-17 (34). Considering that in the CIA model ICOS is important for both IL-17 and IL-21 expression, ICOS may potentially regulate IL-17 production through IL-21 expression. In support of this idea, several groups reported recently that IL-21 produced by Th17 cells plays a critical role in generation of IL-17-producing cells in vitro and in vivo (35–37).
Immune tolerance is regulated by anti-inflammatory regulatory T (Treg) cells. A key phenotype of these suppressors cell is the secretion of IL-10, and ICOS has an important role in costimulating production of IL-10 (23). ICOS–ICOS-ligand interaction is important in the control of IL-10-producing Treg cells and peripheral T-cell tolerance (38–41). In addition, recent studies suggest that Treg cells may suppress auto-immune development in an ICOS-dependent manner (33, 42).
Overlapping function of CD28 and ICOS pathways in T cells
Absence of B7 or ICOS costimulation resulted only in defective immune functions, but absolute T-cell tolerance was not observed. To better understand the molecular mechanisms that determine T-cell function or tolerance, the roles of positive and negative costimulatory molecules was collectively examined (4). Nurieva et al. (4) found that CD4+ or CD8+ T cells activated without CD28 and ICOS costimulation were completely impaired in their effector function. Biochemical characterization revealed that these cells were anergic and deficient in TCR signal transduction leading to gene transcription. Moreover, these cells impaired in expression of transcriptional factors that regulate effector differentiation and cytokine expression. On the other hand, expression of two E3 ubiquitin ligases such as Cbl-b and Itch was significantly induced in these cells (4). Interestingly, both CD4+ and CD8+ T cells after activation without CD28 and ICOS signals expressed GRAIL, an E3 ubiquitin ligase whose expression was previously found to be associated with CD4+ T-cell anergy in vitro and in vivo (43, 44). Therefore, these CD4+ and CD8+ cells shared some characteristics with anergic Th1 cells previously characterized (1, 7, 44, 45). Thus, when antigen-specific naive T cells were activated in the absence of CD28 and ICOS costimulation, instead of differentiating into effector cells, they likely developed into stably tolerized T cells with both TCR signaling and gene transcription defects.
Nurieva et al. (4) demonstrated that T-cell tolerance resulting from blockade of CD28 and ICOS signaling requires the action of negative costimulatory molecules PD-1, B7-H3, and B7S1. PD-1, B7-H3, and B7S1 have been shown to regulate T-cell activation thresholds, and loss of their action often resulted in greater susceptibility to autoimmune diseases (46–49) (see below). T-cell proliferation and effector function was partially restored by inhibiting PD-1, B7H3, and B7S1. Thus, the immune tolerance is not only mediated by lack of positive costimulatory molecule but also by the presence of negative ones.
PD-1
PD-1 is an inhibitory receptor expressed on activated T cells, which binds to PD-L1 (B7-H1) and PD-L2 (B7-DC) (50). PD-L1 is expressed in hemopoietic cells and can be upregulated upon activation (51). PD-L1 is also found in several tissues including pancreatic islets, heart, endothelium, small intestine, and placenta (52). In contrast, PD-L2 expression is restricted to macrophages and DCs and can be upregulated upon activation with IFN-γ, GM-CSF, and IL-4 (51). The expression pattern of PD1 ligands suggests overlapping and differential roles in immune regulation. Interestingly, Martin-Orozco et al. (53) found that CD11c+CD8α+ DCs, which were previously shown to cross-present pancreatic antigens to CD8+ T cells in pancreatic lymph nodes, express PD-L1 but not PD-L2, whereas CD11c+CD11b+CD8α− cells express PD-L1 and PD-L2.
The spontaneous autoimmunity observed in PD-1-deficient mice indicates its critical function in immune tolerance (46). PD-1 blockade or deficiency resulted in accelerated type-I diabetes (T1D) in NOD/LtJ mice (54, 55), although it is not clear whether PD-1 functions in resistant animals to mediate protection against autoimmune diabetes. Investigations on the role of PD-1 ligands in tolerance, however, have led to contradictory results, with evidence supporting their roles as negative regulators in some scenarios and positive regulators in others (50). Recently, it was reported that the PD-L1 protein expressed on islet cells mediates peripheral tolerance and prevents autoimmune attack in NOD/LtJ mice (56). Previously, an opposite conclusion was drawn on the transgenic expression of PD-L1 in the islet cells of C57BL/6J mice, where PD-L1 in the islet cells appears to promote spontaneous autoimmunity (57). Martin-Orozco et al. (53) further analyzed the function of PD-1 and its ligands in the regulation of CD8+ T-cell tolerance to tissue antigens. Blockade of PD-1 in the RIP-mOVA mice resulted in autoimmune diabetes mediated by adoptively transferred OT-I T cells. PD-L1 but not PD-L2 was required for CD8+ T-cell tolerance induction. Blockade of PD-1/PD-L1 interaction during the priming phase did not significantly affect divisions of autoreactive T cells but greatly enhanced their effector differentiation.
To analyze the role of PD-L2 in T-cell activation and potential role in tolerance, Zhang et al. (58) generated and analyzed a PD-L2-deficient mouse. Antigen-presenting cells from PD-L2 knockout (KO) mice were more potent in activation of T cells in vitro, which depended on interaction with PD-1. PD-L2 KO mice also exhibited increased activation of both CD4+ and CD8+ T cells in vivo after immunization. In addition, T-cell tolerance to an oral antigen was abrogated in PD-L2 KO mice. These results thus demonstrate that PD-L2 negatively regulates T cells in immune responses and plays an essential role in immune tolerance (58).
B7-H3
B7-H3 was first reported to be expressed by human dendritic cells and to stimulate human T-cell proliferation and IFN-γ production (59). Mouse B7-H3 appears to be broadly expressed lymphoid and non-lymphoid tissues, and its expression on dendritic cells was found further upregulated following LPS treatment (48, 60). Human and mouse B7-H3 binds to an unidentified receptor expressed on activated but not naive T cells (59, 60). It was initially reported that human B7-H3 provided a positive costimulation to enhance T-cell activation (59). However, later studies found that both VC and VCVC forms of human B7-H3 inhibited CD4+ T-cell proliferation and downregulated cytokine production upon TCR activation (61). Two groups found that mouse B7-H3 protein inhibited T-cell activation and effector cytokine production in both CD4+ and CD8+ T cells (48, 49). In vivo, mice deficient in B7-H3 or treated with antagonistic antibodies exhibited exacerbated EAE disease characterized by excessive inflammatory infiltrates in the central nervous system (CNS) (48, 49). Interestingly, B7-H3-deficient mice developed severe airway inflammation in conditions in which T-helper cells differentiated toward Th1 type rather than Th2. B7-H3 expression was consistently enhanced by IFN-γ but suppressed by IL-4 in dendritic cells. Thus, B7-H3 might provide a negative feedback mechanism for Th1-mediated responses (49).
B7S1
B7S1/B7x/B7-H4 belongs to the B7 superfamily and is also widely expressed in lymphoid and non-lymphoid tissues (47, 62, 63). B7S1 engaged to an unidentified receptor on activated but not naive T cells, which is distinct from CD28, CTLA4, PD-1, and BTLA (47, 62, 63). In vitro study indicates that B7S1 serves as a negative regulator of T cells by inhibiting their proliferation and IL-2 production (1, 47, 63). In vivo blockade of B7S1 led to enhanced T-dependent immune responses and exacerbation of experimental autoimmune encephalitis (EAE) (47). Therefore, B7S1 is a novel negative costimulator and regulates the threshold of T-cell activation.
Signaling basis of T-cell tolerance
Multiple molecular pathways required for the induction of tolerance (64). In recent years, the E3 ubiquitin ligases (Cbl-b, Itch, and GRAIL) have been acknowledged as key molecules implicated in T-cell activation and tolerance (7, 8, 45). NFAT plays a central role in T-cell tolerance induction (65). NFAT cooperates in the nucleus with AP-1 (Fos-Jun) to stimulate the expression of many genes, encoding cytokines, chemokines, and other products essential in the productive immune response. However, NFAT in the absence of its transcriptional partner AP-1 imposes a genetic program of T-cell tolerance. This regulation involves the calcineurin-dependent upregulation of three E3 ubiquitin ligases: Itch, Cbl-b, and GRAIL (8). These ligases regulate the proteolitic degradation of central components in the TCR signaling cascade like protein kinase C-θ (PKC-θ), phospholipase C-γ1 (PLC-γ1), and Zap-70. (8) Because of degradation of key of TCR signaling components, mature immunological synapse between T cells and APCs cannot be maintained, and the inability to sustain the stable APC contact further reduced the antigen responses of tolerant T cells. Recent evidence has suggested that E3 ubiquitin ligases are crucial regulators of T-cell tolerance.
Cbl-b
Cbl-b is a member of mammalian Cbl family proteins that consist of c-Cbl, Cbl-b, and Cbl-3 (66, 67). Proteins of this family contain a RING finger domain that recruits E2 enzyme to help transfer ubiquitin (Ub) to the target protein. Cbl-b mRNA and protein are upregulated in T cells during tolerance induction (8). Interestingly, in recent publication, microarray analyses revealed Egr-2 and Egr-3 as key factors responsible for the induction of Cbl-b (11). Consistent with idea that Cbl-b is important in the development of T-cell anergy, loss of Cbl-b results in impaired induction of T-cell tolerance both in vitro and in vivo (10). Cbl-b-deficient T cells display hyperresponsiveness to TCR stimulation in the absence of CD28 costimulatory signal, suggesting that mutation of Cbl-b uncouples T-cell proliferation and IL-2 production from the requirement of costimulation (68–70). In molecular terms, Cbl-b-induced ubiquitination of p85, the regulatory subunit of PI3K, prevents its interaction to CD28 and TCRζ (71). This results in downregulation of Akt-dependent and PKCθ-dependent NF-κB activation (72) and additionally abrogates Vav1-mediated TCR-clustering necessary for T-cell activation (70). In addition, Cbl-b plays a negative role in Crk-L-C3G signaling pathway in response to TCR stimulation (73). Cbl-b dependent ubiquitination of Crk-L inhibits its association to C3G, which leads to inhibition of activity of LFA-1 and to destabilization of immune synapse. Downregulation of PLC-γ and PKC-θ expression found to occur during restimulation of anergic cells from wildtype mice but not from Cbl-b-deficient mice, suggesting that these two molecules might be targets for Cbl-b anergic functions (8).
Cbl-b-deficient T cells show reduced sensitivity to Treg cellmediated suppression (74). Natural Treg cells from Cbl-b-deficient mice do not show any impairment in development of function. However, Cbl-b-deficient T cells are partially resistant to Treg and TGF-β-mediated immunosuppression. TGF-β-induced SMAD activation appears to be impaired in Cbl-b-deficient T cells, which might explain why Cbl-b deficient CD4+ T cells are partially resistant to TGF-β-mediated suppression of proliferation.
The strong evidence that Cbl-b is a key factor in peripheral T-cell tolerance was confirmed by genetic studies (10, 68, 69). Cbl-b-deficient mice display high susceptibility to spontaneous and peptide-induced autoimmune diseases. Cbl-b-deficient mice starting from 3 to 6 months of age develop spontaneous autoimmunity characterized by auto-antibody production, infiltration T and B lymphocytes into multiple organs (69). Importantly, these mice are highly susceptible to peptide-induce autoimmunity, such as EAE (68), autoimmune arthritis (10), and diabetes (75). Interestingly, in vivo, Cbl-b-deficient mice are resistant to antigen-specific anergy (10). Repetitive exposure of P14 TCR transgenic mice to the high dose of soluble cognate peptide p33 results in the induction of T-cell anergy. In contrast, repeated challenge of P14 TCR transgenic Cbl-b-deficient mice is lethal due to massive activation of CD8+ T cells (10). These results have highlighted the essential role of Cbl-b in T-cell tolerance.
Itch
Itch is a monomeric protein that belongs to the homologous E6AP carboxy terminus (HECT) family of E3 ubiquitin ligases (76, 77). Mice deficient in Itch developed a spontaneous severe dermatitis-type inflammation with constant itching of skin (78). This phenotype is characterized by production of Th2 cytokines IL-4 and IL-5 by Itch-deficient T cells and enhanced serum concentration of IgG1 and IgE. A recent report suggests that Itch disruption in αβ and γδ T cells causes expansion of B1b lymphocytes leading to IgM elevation, and initiates IgE production, respectively (79). Molecularly, Itch controls Th2 differentiation by targeting JunB, a Th2-specific transcriptional factor, for ubiquitin-dependent degradation (80). Itch-deficient Th2 cells are resistant to ionomyc-ininduced anergy in vitro and in vivo (81). These results suggest that Itch plays an important role in Th2 tolerance. Additional study suggests that MEKK1-JNK signaling pathway regulates Itch-mediated tolerogenic process in Th2 cells (81).
Itch regulates T-cell anergy by targeting PLCγ and PKCθ, two key TCR signaling molecules induced by Ca2+/calceneurin signaling (8). Itch mRNA level is induced during T-cell anergy in NFAT-dependent manner. Anergizing stimuli redistribute newly expressed Itch from the cytosol to endosomal compartment, where Itch associates with PLCγ and PKCθ and mediates their ubiquitin-mediated degradation. Downregulation of these signaling proteins is associated with the inability to sustain a stable immunological synapse and with T-cell unresponsiveness after TCR engagement.
Itch plays a critical role in the regulation of TGFβ signaling and Foxp3 expression in CD4+ T cells (82). Itch-deficient T cells are resistant to Treg-dependent immunosuppression: Itch-deficient Tregs differentiated in the presence TGFβ have less Foxp3 and are unable to suppress airway inflammation. Itch is involved in the generation of Treg cells by targeting ubiquitination of the transcription factor TIEG1 (TGF-β-induced early gene 1 product) and promoting its transcriptional activation, which in turn appears to bind and transactivate the Foxp3 promoter. This non-proteolytic pathway is important in the regulation of Th2-mediated allergic responses.
GRAIL
GRAIL (encoded by Rnf128) is a type I transmembrane protein localized to the endosomal compartment with homology to RING finger proteins whose expression was previously associated with T-cell anergy induction (8, 43, 44). While largely undetectable in naive T cells, GRAIL mRNA and protein are rapidly upregulated in T cells exposed to antigen in the absence of costimulation or ionomycin in vitro and after peptide administration in tolerogenic fashion in vivo (44). Consistent with the notion that GRAIL regulates T-cell anergy, overexpression of GRAIL in T-cell hybridomas or in primary cells sufficient to abrogate T-cell proliferation and IL-2 expression (44). Moreover, expression of an enzymatically inactive form of GRAIL in primary T cells prevented T-cell anergy in vivo (43). Rho guanine dissociation inhibitor (RhoGDI) has been identified as a potential substrate of GRAIL. Stable T-cell lines expressing dominant-negative RhoA mimicked the GRAIL-mediated IL-2 inhibition phenotype, and T cells expressing constitutively active RhoA were able to overcome GRAIL-mediated inhibition of IL-2 expression (83). Yeast two-hybrid screening revealed that GRAIL is associated with and regulated by two isoforms of the ubiquitin-specific protease otubain-1 (84). Otubain-1 and its splice variant otubain-1ARF-1 play apposing roles in regulation of GRAIL and its ability to inhibit IL-2 expression in T cells. Overexpression of otubain-1 in CD4+ T cells results in small GRAIL expression and large production of IL-2, whereas otubain-1 ARF-1 overexpression in T cells stabilized GRAIL expression and reduced IL-2 production. Thus, balance between varied isoforms of otubain-1 in T cells determines GRAIL level and therefore the outcome of antigenic challenge. Furthermore, recent studies have proposed that GRAIL targets and ubiquitinates transmembrane proteins such as CD40, CD151, and CD81 (85, 86).
In addition to tolerant CD4+ T cells, GRAIL is highly expressed in Treg cells, and retroviral overexpression of GRAIL in an Ova-specific CD4+ T-cell line allows the transduced T cells to acquire the capacity to suppress T-cell responses (87). Thus, GRAIL appears to be essential for T-cell immuno-tolerance.
The physiological function of GRAIL in immune regulation was addressed recently by genetic studies. Nurieva et al. (88) generated and analyzed GRAIL-deficient mice and found they were resistant to immune tolerance induction in vitro and in vivo and exhibited greater susceptibility to autoimmune diseases that wildtype mice. GRAIL-deficient mice at 18–20 months of age developed autoimmune symptoms characterized by splenomegaly and increased sizes of mesenteric lymph nodes, infiltration lymphocytes in the lung, kidney, and liver, high titers of dsDNA antibodies in the sera, and enhanced expression of pro-inflammatory cytokines by splenic CD4+ T cells. In addition, Nurieva et al. (88) determined an intrinsic role of GRAIL in CD4+ T cells in controlling EAE.
Two independent groups demonstrated that GRAIL is required for immune tolerance induction in vivo (88, 89). Oral tolerance (form of peripheral tolerance) was abolished in GRAIL-deficient mice fed with ovalbumin (88, 89). Moreover, injection of GRAIL-deficient OTII TCR transgenic mice with a high dose of Ova peptide could not result in T-cell tolerance (88). Thus, these data indicate the GRAIL controls antigen-specific CD4+ T-cell tolerance in vivo.
Nurieva et al. (88) found that GRAIL mRNA is upregulated during normal T cell activation even under non-tolerant conditions. These observations suggest that GRAIL function might not be restricted to T-cell anergy, which is supported by another study (89). Analysis of GRAIL-deficient mice showed that they are hyper-responsive to immunization with foreign as well as self-antigens. Thus, GRAIL critically controls the thresholds of T-cell activation, and as a consequence of GRAIL deficiency, immune responses to exogenous as well as endogenous stimuli are increased. On the one hand, this may have pathological implications in predisposing autoimmune responses. On the other hand, modulation of GRAIL expression and function may help boost immune responses to infection and cancer.
E3 ubiquitin ligases including Cbl-b and Itch negatively regulate T-cell responses by targeting multiple components of TCR signaling pathway for degradation. Nurieva et al. (88) found that hyper-activation of GRAIL-deficient T cells is selectively associated with their inefficiency in TCR down-modulation (88). In contrast, expression of other TCR proximal signaling components, such as PKCθ and PLCγ1, were not increased in the absence of GRAIL. In addition, TCR downregulation by GRAIL was dependent on the E3 ligase activity and mediated by proteosome-mediated degradation (88). These results suggest that GRAIL, located in the endosomal compartment, may target endocytosed TCR-CD3 complex via ubiquitination and proteasome-mediated degradation.
A previous study indicates the elevated expression of GRAIL in Treg cells (87). Nurieva et al. (88) found that GRAIL was not necessary for development of Treg cells but was required for their suppressive function. Unlike Cbl-b-deficient T cells (74), naive GRAIL-deficient CD4+ T cells could be suppressed by WT Treg cells, suggesting that GRAIL deficiency might not affect TGF-β signaling (88). In addition, reduced suppressive function was associated with elevated expression of Th17 cell-specific genes such as those that encode IL-17, IL-21, and RORγ (88). IL-21 was sufficient in upregulating Th17 cell-specific genes in Treg cells. These studies indicate a critical role of GRAIL in control of IL-21 expression in Treg cells.
NFATc1 is strongly induced upon T-cell activation and controls numerous genes that are involved in T-cell effector function (90). Nurieva et al. (88) found that GRAIL-deficient naive and Treg cells after TCR activation expressed substantially higher amounts of mRNA and protein of NFATc1 compared to WT cells, whereas the activation of other factors in AP-1 and NFκB pathways were normal. In contrast to naive T cells, increased expression of NFATc1 in GRAIL-deficient Treg cells was associated with just enhanced expression of Th17 cell genes. These results thus indicate GRAIL as an essential regulator of T-cell tolerance by regulating naive T-cell tolerance and Treg cell function.
Transcriptional regulation of T-cell tolerance
In addition to signaling defect or anergy in tolerant T cells, growing evidence has revealed that tolerant T cells also exhibit unique transcriptional features, compared with effector T cells. In the absence of costimulation, overactivated NFAT without appropriate AP-1 activation has been shown to upregulate many tolerance-associated transcriptional factors (like Egr-2, Egr-3, Ikaros, CREM, p50, ZEB1, Blimp-1, Tob, and Smads) that may play important roles in controlling T-cell tolerance (8–13).
Egr-2 and Egr-3
One of the immediate targets of NFAT-mediated transcription following calcium flux in T cells is the Egr family gene. Of the four members, Egr-2 and Egr-3 activated by NFAT sequentially drives Fas ligand (FasL) expression in T cells, which suggested they may act as a negative regulator to maintain T-cell homeostasis (91). In a recent study of in vitro stimulation Th1 clone A.E7 by specific antigen pigeon cytochrome c epitope (PCC), Powell and colleagues found that Egr-2 and Eg-r3 were rapidly upregulated and persisted for 2–5 days in tolerant T cells (11, 92). Overexpression of these two factors was able to block IL-2 and T-cell functions. Tolerance-related E3 ligase Cbl-b was also upregulated by Egr-2 and Egr-3. Moreover, Egr3-deficient T cells are resistant to tolerance induction (11). Conditional deletion of Egr-2 in T cells will lead mice to develop a lupus-like autoimmune disease with anti-nuclear antibodies, glomerulonephritis, and infiltration of IFN-γ and IL-17-producing T cells into multiple organs (93). Therefore, transcriptional complexes of Egr proteins and NFAT are responsible for T cells tolerance program.
Ikaros and ZEB1
The transcriptional regulation of IL-2 gene has always been the key issue of T-cell tolerance. T-cell activation in the absence of CD28 signals blocks the full ERK and JNK MAPK cascades, results in defective assembly of transcriptional factors in IL-2 promoter, and leads to poor production of IL-2 (94, 95). In addition, tolerant T cells suffered from active, dominant repression of IL-2 transcription. In a T-cell tolerance induction assay, it was demonstrated that Ikaros, a zinc finger binding transcriptional factor, was highly expressed in anergic T cells or naive T cells (96). In this quiescent state, Ikaros exhibits its suppressive function through marking the nucleosomes across the IL-2 promoter with hypoacetylation. There are at least two functional Ikaros-binding sites in the IL-2 promoter, one contains a GGGA core consensus element at TCEd/NFIL-2C region, and another one is located within the TATA box of proximal promoter of IL-2, where it recruits the HDAC complex such as NURD, Sin3A, Sin3B, and CtBP, to deacetylate both histone H3 and H4 at IL-2 promoter rapidly and stably. However, in Ikaros-deficient naive T cells, the histones at theIL-2 promoter are constitutively acetylated, and IL-2 transcription is fully ready for TCR-only signal. Conversely, overexpression of Ikaros in effector CD4+ T cells enhances histone deacetylation and silences IL-2 expression in response to TCR/CD28 signals, therefore promoting T-cell anergy (96). Similarly to Ikaros, another transcriptional repressor ZEB1 (zinc finger E-box-binding protein) binds to NRE-A (negative regulatory element, located at −100) at the IL-2 promoter, where it associates with CtBP-2 (co-repressor C-terminal binding protein 2) and HDAC1 to repress IL-2 transcription (97).
CREB/CREM and p50
Another inhibitory transcriptional factor CREM (cAMP response element modulator) is induced during tolerance induction (98). Unlike its isoform CREB (cAMP response element binding protein) that is induced during T-cell activation, CREM lacks a transactivation domain and can specially bind to AP-1 site (−180) at the IL-2 promoter in complex with CREB, subsequently leads to repression of IL-2 transcription (98). Thus, the binding of NFAT with AP-1 versus CREM could switch IL-2 transcription positively or negatively. Similarly, p50–p50 homodimer, an NF-κB family member, generally compete with p65–p50 heterodimers to inhibit gene transcription (99). In resting T cells, the default homodimers p50–p50s are present in the nucleus, bound to IL-2 promoter at TCEd/NFIL-2C element, and suppress IL-2 transcription (100). These homodimers can also interact with HDACs to render hypoacetylation at the IL-2 promoter and sequester IL-2 at epigenetic level (101). In an in vivo superantigen-induced T-cell tolerance model, p50–p50 homodimers were found complexed with Bcl-3 to inhibit IL-2 (101).
Blimp-1
B lymphocyte-induced maturation protein-1 (Blimp-1), a transcriptional repressor containing a SET domain and a zinc finger domain, had been previously known to plays critical roles in regulating B-cell terminal differentiation, plasma cell formation, and maintenance (102). Recent studies showed that Blimp-1 was involved in T-cell homeostasis and differentiation (103, 104). Upon T-cell activation, Blimp-1 is upregulated by TCR signaling plus the autoregulatory loop from IL-2 stimulation, and then it attenuates T-cell proliferation by direct repression of IL-2 and Fos protein (105). Mice with a T-cell-specific deletion of Blimp-1 display hyperresponsiveness and fatal colitis (102). In addition, Blimp-1 was shown to attenuate IFN-γ and T-bet transcription in Th1 sublineage through direct binding with target promoters (102). However, whether Blimp-1 regulates chromatin structure remains unsolved and needs further investigation.
Tob and Smad
Another transcriptional factor that appears to regulate T-cell tolerance is Tob, an anti-proliferative protein BTG (B-cell translocation gene) family member (106). Tob is constitutively expressed in resting T cells and is downregulated during activation. In vitro enforced expression of Tob suppressed effector cytokines expression such as IFN-γ, IL-4, and IL-10. Meanwhile, T-cell proliferation was inhibited due to cell cycle blocking. In Tob-transfected Jurkat T cells, positive regulators of cell cycle including cyclin E, cyclin A, and Cdk2 was significantly reduced, whereas negative regulator p27kip1 was tuned up. In concert with Smads (mainly Smad2 and Smad4), Tob can bind to negative regulatory element NRE-A (−105) at the IL-2 promoter and suppress IL-2 transcription. Conversely, when Tob was deleted in CD4+ T cells, Smads was barely bound to the IL-2 promoter, and IL-2 production can be increased even in the absence of CD28 costimuation, which resulted in resistance to anergy induction (106). Therefore, Tob synergized with Smads to inhibit CD3 and CD28-mediated T-cell activation.
Epigenetic control of T-cell tolerance
T-cell tolerance is defined by active, dominant repression of effector cytokines and cell proliferation (1). These characteristics can be inherited from precursors to their daughter cells, which suggests an epigenetic mechanism regulates the program of T-cell tolerance. The epigenetic process includes chromatin modification and chromatin remodeling, which defines the status of the position and compact of nucleosomes, post-translational histone modifications, and the DNA methylation (107).
Histone deacetylation in anergic CD4+ T cells
Histone acetylation is associated generally with transcriptional activation, whereas histone deacetylation represents repression. The dynamic status of chromatin acetylation depends on activities of both histone acetyltransferases (HATs) and histone deacetyltransferases (HDACs) (107).
Unlike productive T-cell activation, where the promoter and enhancer regions of the IL-2 locus exhibit rapid acetylation, CD4+ T cells stimulated with TCR signal alone fail to acetylate and remodel nucleosomes of the IL-2 promoter, which results in poor IL-2 transcription (108). Histone acetylation at IL-2 locus in CD4+ T cells requires signals from both TCR and CD28 costimulation, which is demonstrated by the evidence that CD4+ T cells anergized either in vivo by superantigen or in vitro by stimuli (CTLA4-Ig, anti-TCR antibodies or Ionomycin) exhibit hypoacetylation at the IL-2 promoter (109, 110). However, the lack of histone acetylation in anergic cells is not simply a passive readout of signaling defects. Several groups proved an active involvement of the deacetylation process. In comparison with naive T cells, when activated T cells were restimulated under anergic conditions, increased histone deacetylation was observed at the IL-2 promoter, which is associated with enhanced recruitments of HDAC1 and HDAC2 (12, 109). In the meantime, HAT activities in anergic CD4+ T cells were intact, because constitutive gene CD3ε in anergic cells exhibits the same level of histone acetylation as in activated cells (109–111). Therefore, hypoacetylation at the IL-2 locus in anergic CD4+ T cells is caused by increased deacetylation and intact acetylation.
The IFN-γ locus in anergic T cells is also imprinted with a repressive marker of hypoacetylation, which resulted in the significant reduction of IFN-γ (110, 112, 113). Unlike naive T cells, the hypoacetylation status in anergic T cells is caused by deacetylation. When the HDAC inhibitor trichostatin A (TSA) was incubated together with T cells during anergy induction, both acetylation at the IFN-γ locus and IFN-γ production were restored, which suggested that the deacetylation process played an important role to program anergy (12, 109). CD8+ T cells activated in the absence of CD4+ T cells were unresponsive to secondary antigenic challenges and exhibited hypoacetylation at the IFN-γ locus (114). Histone hypoacetylation at the IFN-γ and IL-2 gene loci contributes to maintain the closed chromatin structures and thus presents formidable barriers for transcriptional activities.
Histone methylation
Compared with histone acetylation, histone methylation is more specific and complex. Histone acetylation is almost invariably associated with gene activation. However, histone methylations may render the chromatin with permissive, repressive, or even poised states (107). For example, histone H3 trimethylation at lysine 4 (H3K4me3) is generally associated with permissive chromatins, while H3K27me3 is associated with repressive chromatins. In Th1 cells, H3K4me3 specifically marks IFN-γ and T-bet gene loci, whereas IL-4 and Gata-3 loci are imprinted with H3K27me3 (115). Genome-wide histone modification patterns have been profiled in programming T-cell differentiation (115–117). However, little is known about the histone modification during tolerance program. An unpublished study by Andrew’s group (12) demonstrated that the IL-2 promoter from anergic T cells was marked with another repressive marker, H3K9me3, which suggests that histone modifications is involved in programming T-cell tolerance.
DNA methylation at IL-2 and IFN-γ locus
DNA methylation is another layer of epigenetic regulation. CpG methylation is usually associated with gene repression, and removal of CpG methylation is associated with gene activation. In naive CD4+ and CD8+ T cells, CpG was found hypermethylated in the promoter of IL-2 and IFN-γ (111, 112, 114, 118). Similarly, it appears that CpG residues at the IL-2 promoter and enhancer are methylated in tolerant T cells (110, 118). Tolerant CD4+ and CD8+ T cells threefold more methylation within IL-2 promoter compared with activated T cells (111, 114). Loss of DNA methyltransferase Dnmt1 leads to excessive production of IL-2, Th1 and Th2 cytokines (119). On the other hand, T-cell development and activation depends on DNA demethylation, a process that occurs kinetically and is highly correlated with effector cytokine production (119, 120).
Conclusions
T-cell tolerance is associated with a unique set of gene expression, which is correlated with expression of negative regulatory factors and epigenetic modulations. Based on DNA microarray techniques, many ‘repressive’ factors are unveiled, and they act as integral transducer of the extracellular signals to actively program tolerance. Through changing chromatin with histone modification, DNA methylation and nucleosome positioning, epigenetic modifications facilitate heritable and stable T-cell programming and eventually determine cell fate. Recently developed techniques to study chromatin at the genome-wide scale will speed up the studies of epigenomes in tolerant T cells. Ultimately, the understanding and manipulation of T-cell tolerance should lead to development of pharmacological approaches to prevent autoimmunity, chronic infection, and cancer.
Acknowledgments
We thank our colleagues in my group and our many collaborators for their scientific contributions to the knowledge described in this review. The work is supported by research grants from NIH (to CD and RIN). RIN was a recipient of a Scientist Development Grant from the American Heart Association; XL is a recipient of an Odyssey Postdoctoral Fellowship from the MD Anderson Cancer Center. CD is Leukemia and Lymphoma Society Scholar and a Trust Fellow of the MD Anderson Cancer Center. The authors declare no conflicts of interest.
References
- 1.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Dong C, Nurieva RI. Regulation of immune and autoimmune responses by ICOS. J Autoimmun. 2003;21:255–260. doi: 10.1016/s0896-8411(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 4.Nurieva R, et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharpe A. Costimulation and regulation of autoimmunity and tolerance. J Ped Gastroenterol Nutr. 2005;40(Suppl):S20–S21. doi: 10.1097/00005176-200504001-00011. [DOI] [PubMed] [Google Scholar]
- 7.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 8.Heissmeyer V, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Zha Y, Gajewski T. Molecular regulation of T-cell anergy. EMBO Rep. 2008;9:50–55. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon MS, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Safford M, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 12.Wells A. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol. 2009;182:7331–7341. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 13.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 14.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev. 2001;1:126–134. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 15.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 16.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 17.Shahinian A, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 18.Borriello F, et al. B7-1 and B7-2 have over-lapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 19.Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998;161:2762–2771. [PubMed] [Google Scholar]
- 20.Tada Y, et al. CD28-deficient mice are highly resistant to collagen-induced arthritis. J Immunol. 1999;162:203–208. [PubMed] [Google Scholar]
- 21.Girvin AM, et al. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164:136–143. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- 22.Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 23.Hutloff A, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 24.Yoshinaga SK, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 25.Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7. 2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- 26.Dong C, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–102. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 27.Nurieva RI, et al. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 28.Nurieva RI, Mai XM, Forbush K, Bevan MJ, Dong C. B7h is required for T cell activation, differentiation, and effector function. Proc Natl Acad Sci USA. 2003;100:14163–14168. doi: 10.1073/pnas.2335041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurieva RI, et al. A costimulation-initiated signaling pathway regulates NFATc1 transcription in T lymphocytes. J Immunol. 2007;179:1096–1103. doi: 10.4049/jimmunol.179.2.1096. [DOI] [PubMed] [Google Scholar]
- 30.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 31.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Shen W, Kong K, Liu Z. Interleukin-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis. Scand J Immunol. 2006;64:515–522. doi: 10.1111/j.1365-3083.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 33.Gotsman I, et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 34.Young DA, et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 2007;56:1152–1163. doi: 10.1002/art.22452. [DOI] [PubMed] [Google Scholar]
- 35.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 38.Akbari O, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 39.Massey EJ, Sundstedt A, Day MJ, Corfield G, Anderton S, Wraith DC. Intranasal peptideinduced peripheral tolerance: the role of IL-10 in regulatory T cell function within the context of experimental autoimmune encephalomyelitis. Vet Immunol Immunopathol. 2002;87:357–372. doi: 10.1016/s0165-2427(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 40.Faria AM, Maron R, Ficker SM, Slavin AJ, Spahn T, Weiner HL. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor-beta/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20:135–145. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto K, et al. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J Immunol. 2005;175:7341–7347. doi: 10.4049/jimmunol.175.11.7341. [DOI] [PubMed] [Google Scholar]
- 42.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seroogy CM, et al. The gene related to anergy in lymphocytes, an E3 ubiquitin ligase, is necessary for anergy induction in CD4 T cells. J Immunol. 2004;173:79–85. doi: 10.4049/jimmunol.173.1.79. [DOI] [PubMed] [Google Scholar]
- 44.Anandasabapathy N, et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 45.Heissmeyer V, Rao A. E3 ligases in T cell anergy–turning immune responses into tolerance. Sci STKE. 2004;2004:pe29. doi: 10.1126/stke.2412004pe29. [DOI] [PubMed] [Google Scholar]
- 46.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 47.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 48.Prasad DV, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 49.Suh W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 50.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 52.Liang SC, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 53.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8 + T cell tolerance to tissue antigens. J Immunol. 2006;177:8291–8295. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 54.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subudhi SK, et al. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest. 2004;113:694–700. doi: 10.1172/JCI19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, et al. Regulation of T cell activation and tolerance by PD-L2. Proc Natl Acad Sci USA. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chapoval AI, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 60.Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 61.Ling V, et al. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82:365–377. doi: 10.1016/s0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 62.Sica GL, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 63.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rangachari M, Penninger JM. Negative regulation of T cell receptor signals. Curr Opin Pharmacol. 2004;4:415–422. doi: 10.1016/j.coph.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 66.Keane MM, Rivero-Lezcano OM, Mitchell JA, Robbins KC, Lipkowitz S. Cloning and characterization of cbl-b: a SH3 binding protein with homology to the c-cbl protooncogene. Oncogene. 1995;10:2367–2377. [PubMed] [Google Scholar]
- 67.Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 68.Chiang YJ, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 69.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 70.Krawczyk C, et al. Cbl-b is a negative regulator of receptor clustering and raft aggregation in T cells. Immunity. 2000;13:463–473. doi: 10.1016/s1074-7613(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 71.Fang D, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 72.Qiao G, et al. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol. 2008;28:2470–2480. doi: 10.1128/MCB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang W, Shao Y, Fang D, Huang J, Jeon MS, Liu YC. Negative regulation of T cell antigen receptor-mediated Crk-L-C3G signaling and cell adhesion by Cbl-b. J Biol Chem. 2003;278:23978–23983. doi: 10.1074/jbc.M212671200. [DOI] [PubMed] [Google Scholar]
- 74.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 75.Yokoi N, et al. Cbl-b is a major susceptibility gene for rat type 1 diabetes mellitus. Nat Genet. 2002;31:391–394. doi: 10.1038/ng927. [DOI] [PubMed] [Google Scholar]
- 76.Perry WL, Hustad CM, Swing DA, O’Sullivan TN, Jenkins NA, Copeland NG. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat Genet. 1998;18:143–146. doi: 10.1038/ng0298-143. [DOI] [PubMed] [Google Scholar]
- 77.Bernassola F, Karin M, Ciechanover A, Melino G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell. 2008;14:10–21. doi: 10.1016/j.ccr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Fang D, et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol. 2002;3:281–287. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- 79.Parravicini V, Field AC, Tomlinson PD, Basson MA, Zamoyska R. Itch−/− alphabeta and gammadelta T cells independently contribute to autoimmunity in Itchy mice. Blood. 2008;111:4273–7282. doi: 10.1182/blood-2007-10-115667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartenstein B, Teurich S, Hess J, Schenkel J, Schorpp-Kistner M, Angel P. Th2 cell-specific cytokine expression and allergeninduced airway inflammation depend on JunB. EMBO J. 2002;21:6321–6329. doi: 10.1093/emboj/cdf648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Venuprasad K, et al. Convergence of Itch-induced ubiquitination with MEKK1-JNK signaling in Th2 tolerance and airway inflammation. J Clin Invest. 2006;116:1117–1126. doi: 10.1172/JCI26858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venuprasad K, et al. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol. 2008;9:245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su L, Lineberry N, Huh Y, Soares L, Fathman CG. A novel E3 ubiquitin ligase substrate screen identifies Rho guanine dissociation inhibitor as a substrate of gene related to anergy in lymphocytes. J Immunol. 2006;177:7559–7566. doi: 10.4049/jimmunol.177.11.7559. [DOI] [PubMed] [Google Scholar]
- 84.Soares L, et al. Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat Immunol. 2004;5:45–54. doi: 10.1038/ni1017. [DOI] [PubMed] [Google Scholar]
- 85.Lineberry N, Su L, Soares L, Fathman CG. The single subunit transmembrane E3 ligase gene related to anergy in lymphocytes (GRAIL) captures and then ubiquitinates transmembrane proteins across the cell membrane. J Biol Chem. 2008;283:28497–28505. doi: 10.1074/jbc.M805092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schartner JM, Singh AM, Dahlberg PE, Nettenstrom L, Seroogy CM. Recurrent superantigen exposure in vivo leads to highly suppressive CD4+CD25+ and CD4+CD25−T cells with anergic and suppressive genetic signatures. Clin Exp Immunol. 2009;155:348–356. doi: 10.1111/j.1365-2249.2008.03827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacKenzie DA, et al. GRAIL is up-regulated in CD4+ CD25+ T regulatory cells and is sufficient for conversion of T cells to a regulatory phenotype. J Biol Chem. 2007;282:9696–9702. doi: 10.1074/jbc.M604192200. [DOI] [PubMed] [Google Scholar]
- 88.Nurieva RI, et al. The E3 ubiquitin ligase GRAIL regulates T cell tolerance and regulatory T cell function by mediating T cell receptor-CD3 degradation. Immunity. 2010;32:670–680. doi: 10.1016/j.immuni.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kriegel MA, Rathinam C, Flavell RA. E3 ubiquitin ligase GRAIL controls primary T cell activation and oral tolerance. Proc Natl Acad Sci USA. 2009;106:16770–1775. doi: 10.1073/pnas.0908957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chuvpilo S, et al. Autoregulation of NFATc1/A expression facilitates effector T cells to escape from rapid apoptosis. Immunity. 2002;16:881–895. doi: 10.1016/s1074-7613(02)00329-1. [DOI] [PubMed] [Google Scholar]
- 91.Rengarajan J, et al. Sequential Involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- 92.Harris JE, et al. Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells. J Immunol. 2004;173:7331–7338. doi: 10.4049/jimmunol.173.12.7331. [DOI] [PubMed] [Google Scholar]
- 93.Zhu B, et al. Early growth response gene 2 (Egr-2) controls the self-tolerance of T cells and prevents the development of lupuslike autoimmune disease. J Exp Med. 2008;205:2295–2307. doi: 10.1084/jem.20080187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li W, Whaley CD, Mondino A, Mueller DL. Blocked Signal Transduction to the ERK and JNK Protein Kinases in Anergic CD4+ T Cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 95.Boussiotis VA, Barber DL, Lee BJ, Gribben JG, Freeman GJ, Nadler LM. Differential association of protein tyrosine kinases with the T cell receptor is linked to the induction of anergy and its prevention by B7 family-mediated costimulation. J Exp Med. 1996;184:365–376. doi: 10.1084/jem.184.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros Enforces the Costimulatory Requirement for IL2 Gene Expression and Is Required for Anergy Induction in CD4+ T Lymphocytes. J Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Lee S, Teh CE-Y, Bunting K, Ma L, Shannon MF. The transcription repressor, ZEB1, cooperates with CtBP2 and HDAC1 to suppress IL-2 gene activation in T cells. Int Immunol. 2009;21:227–235. doi: 10.1093/intimm/dxn143. [DOI] [PubMed] [Google Scholar]
- 98.Powell JD, Lerner CG, Ewoldt GR, Schwartz RH. The -180 site of the IL-2 promoter is the target of CREB/CREM binding in T cell anergy. J Immunol. 1999;163:6631–6639. [PubMed] [Google Scholar]
- 99.Liu X, Ye L, Christianson GJ, Yang J-Q, Roopenian DC, Zhu X. NF-κB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J Immunol. 2007;179:2999–3011. doi: 10.4049/jimmunol.179.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang S, Tran A, Grilli M, Lenardo M. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992;256:1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- 101.Grundstrom S, Anderson P, Scheipers P, Sundstedt A. Bcl-3 and NFκB p50-p50 homodimers act as transcriptional repressors in tolerant CD4+ T cells. J Biol Chem. 2004;279:8460–8468. doi: 10.1074/jbc.M312398200. [DOI] [PubMed] [Google Scholar]
- 102.Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–169. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- 103.Martins GA, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 104.Kallies A, et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 105.Martins GiA, Cimmino L, Liao J, Magnusdottir E, Calame K. Blimp-1 directly represses Il2 and the Il2 activator Fos, attenuating T cell proliferation and survival. J Exp Med. 2008;205:1959–1965. doi: 10.1084/jem.20080526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tzachanis D. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat Immunol. 2001;2:1174–1182. doi: 10.1038/ni730. [DOI] [PubMed] [Google Scholar]
- 107.Kouzarides T. Chromatin Modifications and Their Function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Bunting K, Wang J, Shannon MF, Gerald L. Vitamins & Hormones. Burlington, MA: Academic Press; 2006. Control of Interleukin-2 Gene Transcription: A Paradigm for Inducible, Tissue-Specific Gene Expression; pp. 105–145. [DOI] [PubMed] [Google Scholar]
- 109.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas RM, Saouaf SJ, Wells AD. Superantigen-induced CD4+ T cell tolerance is associated with DNA methylation and histone hypo-acetylation at cytokine gene loci. Genes Immun. 2007;8:613–618. doi: 10.1038/sj.gene.6364415. [DOI] [PubMed] [Google Scholar]
- 111.Thomas RM, Gao L, Wells AD. Signals from CD28 Induce Stable Epigenetic Modification of the IL-2 Promoter. J Immunol. 2005;174:4639–4646. doi: 10.4049/jimmunol.174.8.4639. [DOI] [PubMed] [Google Scholar]
- 112.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 113.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. TH cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 114.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-γ loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 115.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Schones DE, et al. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132:887–898. doi: 10.1016/j.cell.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol. 2003;4:235–240. doi: 10.1038/ni887. [DOI] [PubMed] [Google Scholar]
- 119.Makar KW, Wilson CB. DNA methylation is a nonredundant repressor of the Th2 effector program. J Immunol. 2004;173:4402–4406. doi: 10.4049/jimmunol.173.7.4402. [DOI] [PubMed] [Google Scholar]
- 120.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]