Abstract
Background
Low birth weight and gestational maternal smoking have been linked with reduced lung function in children in many cross sectional studies. However, these associations have not yet been assessed with repeated measurements of lung function. Our aim was to investigate the effects of birth weight, gestational age, and gestational maternal smoking on lung function in children at age 10 and 18 years.
Methods
In the Isle of Wight birth cohort spirometry was performed at age 10 and 18 years. Information on birth weight and gestational age were obtained from hospital records. Mothers were asked about smoking during pregnancy. We employed linear mixed models to estimate the effect of these risk factors on repeated measurements of lung function. We considered maternal asthma, sex, neonatal intensive care unit admission, height, socio-economic status, personal smoking in participants at age 18, body mass index and environmental tobacco smoke exposure as potential confounders. Finally, we used path analysis to determine links between birth weight, gestational age and gestational maternal smoking on lung function at age 10 and 18 years.
Results
Linear mixed models showed that with every 1 kg increase in birth weight, Forced expiratory volume in one second (FEV1) increased by 42.62 ± 17.15 mL and Forced expiratory flow between 25% and 75% of forced vital capacity (FEF25-75) increased by 95.51 ± 41.19 mL at age 18 years after adjusting for potential confounders. Path analysis suggested that birth weight had positive direct effects on FEV1 and FEF25-75 and positive indirect effect on Forced vital capacity (FVC) at 10 years which were carried forward to 18 years. Additionally, results also suggested a positive association between gestational age and FEV1, FVC and FEF25-75 at ages 10 and 18 years and an inverse association between gestational smoke exposure and FEV1/FVC ratio and FEF25-75 at age 18 years.
Conclusions
Higher birth weight and gestational age were associated with higher FEV1, FVC and FEF25-75 and gestational smoking was associated with reduced FEV1/FVC ratio and FEF25-75. The use of path analysis can improve our understanding of underlying “causal’ pathways among different prenatal and childhood factors that affect lung function in both pre-adolescent and adolescent periods.
Keywords: Lung function, birth weight, maternal smoking during pregnancy, path analyses
INTRODUCTION
The ‘Barker hypothesis’ also known as ‘fetal origins of adult disease’ hypothesis, states that adverse exposures encountered during intrauterine life can result in permanent changes in physiology which may result in increased risk of chronic diseases in adulthood [1]. Barker et al. showed that, fetal and infant growths are associated with lung function in adults and low birth weight (LBW) may increase the risk of death from chronic obstructive lung disease [2]. Other studies have also shown that LBW and very low birth weight (VLBW) are associated with reduced lung function in children [3–7]. Two more studies have found a positive relationship between continuous birth weight measures and lung function in children [8, 9]. In adults, the findings are mixed, some studies reported a significant positive linear trend between birth weight and lung function [10–13], while other studies found no association [14].
The process of lung development begins in the intrauterine period and continues well into late adolescence/early adulthood. Therefore, intrauterine exposures affecting lung development during fetal life, for example maternal smoking, may have a long term negative impact on lung function. Additionally, maternal smoking during pregnancy is known to result in pre-term births and LBW in full term babies [15, 16]. Thus, maternal smoking during pregnancy, gestational age and birth weight are correlated and birth weight may be in the pathway between in-utero exposure to maternal smoking and lung function. However, there is disagreement on whether maternal smoking during pregnancy has independent effect on reduction of lung function in childhood [17–19]. Previous studies while investigating association between birth weight and lung function have adjusted for the effect of maternal smoking during pregnancy without addressing the fact that birth weight may be an intervening variable. Similarly, height which is a significant determinant of lung function may also act as an intervening variable in the path between birth weight and lung function as many pediatric studies have shown a positive association between birth weight and growth of height during childhood [20, 21].
Lung function during childhood and adolescent periods is determined by complex relationships between several factors that need to be taken into account simultaneously. However, adjusting for intervening variables as confounders not only distorts the causal pathway but also leads to an over-adjustment bias [22]. The inconsistent results in association between birth weight and lung function in the above mentioned studies may be attributed to the use of traditional regression analyses, which do not take into consideration the directional or non-directional relationships between various observed factors. To elucidate these complex relationships, use of path analysis provides a novel approach. A variable representing the response in one equation can act as a risk factor in another equation, thus allowing the inclusion of intervening or mediating variables in the model. Finally, simultaneously solving multiple linear regression equations generates direct, indirect and total effects of each variable on the outcome, which can be used to develop a causal path diagram.
To gain better understanding of the relationship between birth weight, maternal smoking, and lung function in children at age 10 and 18 years, we analyzed data from the Isle of Wight (IOW) birth cohort. We explored these associations first by using linear regression, followed by linear mixed models and path analysis in which we assessed complex relationships between different prenatal and childhood factors that may affect the association between birth weight and lung function.
MATERIALS AND METHODS
Study population
Between January 1989 and February 1990, 1,536 mothers/child pairs were contacted to be enrolled in the IOW birth cohort. After obtaining informed written consent 1,456 were enrolled and available for follow-up at 1, 2, 4, 10 and 18 years of age. Among them, 1,121 children were tested for spirometry either at 10 (n = 981) or 18 years of age (n = 838) or both (n = 698) The IOW cohort is described in detail elsewhere [23–25].
Birth weight and other measurements
Information on birth weight, gestational age, and admission to neonatal intensive care unit (NICU) were obtained from the hospital records. Information on maternal smoking during gestation, sex of the child, and maternal history of asthma was ascertained after delivery. We considered maternal smoking during gestation, maternal history of asthma, sex, admission to NICU, height, socio-economic status (SES), personal smoking in children at age 18, body mass index (BMI) and environmental tobacco smoke (ETS) exposure at age 10 and 18 as potential confounders or intervening variables. Information on the SES was based on the following three variables: (a) the British socioeconomic classes (1–6) derived from parental occupation reported at birth; (b) the number of children in the index child’s bedroom (collected at age 4 years); and (c) family income at age 10 years [25]. Height and weight were measured before spirometric tests at age 10 and 18 years; BMI was calculated. To address the differential growth pattern in height in boys and girls we considered an interaction term between height and sex. Exposure to ETS at age 10 and 18 was inquired from questions of “any smoking in the household”. Active smoking at age 18 years was ascertained from the study participants at age 18.
Lung function
Lung function tests were conducted at 10 and 18 years of age. Forced vital capacity (FVC), Forced expiratory volume in one second (FEV1), Forced expiratory flow between 25% and 75% of forced vital (FEF25-75) and Peak expiratory flow rate (PEFR) were measured using a Koko Spirometer and software with a portable desktop device (both PDS Instrumentation, Louisville, KY, USA). Spirometry was performed and evaluated according to the American Thoracic Society (ATS) criteria. Children were required to be free of respiratory infection for two weeks and not to be taking any oral corticosteroids and were advised to abstain from any β-agonist medication for six hours and from caffeine intake for at least 4 hours [23].
Statistical analysis
Firstly, to determine effects of birth weight and maternal smoking during pregnancy on lung function at cross-sectional level we used standard linear regression technique separately at ages 10 and 18 years. Next, we used linear mixed models for repeated measurements on cohort of children who were tested for lung function either at age 10 or18 years or both. Unstructured covariance structure matrix was selected based on lowest Akaike information criteria and the Bayesian Schwarz information criterion after considering unstructured, compound symmetry and autoregressive covariance structure matrices. All models were adjusted for above mentioned confounders. The models assessing the relationship between maternal smoking in-utero and lung function and gestational age and lung function were not adjusted for birth weight. We selected the confounders that changed the estimates of main exposures (birth weight, exposure to in-utero maternal smoking and gestational age) by 10%. We also included an interaction term between sex and height since the relationship between height and lung function varies by sex [26]. To control for type-I error due to multiple comparisons the significance level was set at alpha=0.025 whenever interaction term between height and sex was included in the model. Otherwise significance level of alpha=0.05 was maintained for rest of the models.
To address the issue of missing data on one or more confounders we used multiple imputations to generate ten new datasets. All datasets were analyzed separately for both linear regressions and linear mixed models. Finally all results were combined and valid statistical inferences were generated using MIANALYZE.
Path analysis
As mentioned earlier birth weight and height act as intervening variables on two separate pathways from maternal smoking during pregnancy and lung function and linear mixed models do not address the issue of intervening variables. Adjusting on these variables may lead to biased estimates. Therefore, we explored the relationships between birth weight, maternal smoking during pregnancy and lung function at age 10 and 18 years by linear path analysis using Covariance Analysis of Linear Structural Equations. Since data on covariates was missing completely at random we used Full Information Maximum Likelihood (FIML) method to determine parameter estimates. The adequacy of model fit was determined by several statistics: a Chi-square p-value > 0.05 for the difference between the theoretical and the empirical model, comparative fit index (CFI) > 90, adjusted goodness of fit index (GFI) > 90 and root mean square error of approximation (RMSEA) < 0.06. The data were analyzed using the SAS statistical package (version 9.3; SAS Institute, Cary, NC, USA).
RESULTS
There were no significant differences between full IOW cohort and the sample of participants who were tested for lung function at either age [Table 1]. In total 1,121 children had spirometry tests done either at age 10 years (n = 981) or 18 years (n = 838) or both (n = 698). The average birth weight was 3.4 ± 0.5 kg and 22.7 % children were exposed to maternal smoking in-utero.
Table 1.
Comparison of baseline characteristics for children with spirometry either at age 10 or 18 with total IOW cohort
| Participants | Total IOW cohort (N=1,536) n (%) / n (mean ± s.d.) |
Sample with spirometry either at age 10 or 18 (N=1,121) n (%) / n (mean ± s.d.) |
p-value | |
|---|---|---|---|---|
| Sex | Male | 786 (51.2) | 557 (49.7) | 0.4499 |
| Female | 750 (48.8) | 564 (50.3) | ||
|
| ||||
| Maternal smoking | Yes | 384 (25.3) | 253 (22.7) | 0.1236 |
| No | 1137 (74.8) | 864 (77.3) | ||
| Missing | 15 | 4 | ||
|
| ||||
| Low birth weight | Yes | 61 (4.1) | 36 (3.3) | 0.3049 |
| No | 1433 (95.9) | 1053 (96.7) | ||
| Missing | 42 | 32 | ||
|
| ||||
| ETS at age 10 yrs | Yes | 561 (42.1) | 440 (41.2) | 0.6364 |
| No | 771 (57.9) | 629 (58.8) | ||
| Missing | 204 | 52 | ||
|
| ||||
| Admission to NICU | Yes | 142 (11.5) | 92 (10.2) | 0.3481 |
| No | 1092 (88.5) | 808 (89.8) | ||
| Missing | 302 | 221 | ||
|
| ||||
| Smoking at age 18 years | Yes | 368 (28.8) | 276 (26.8) | 0.3003 |
| No | 910 (71.2) | 752 (73.2) | ||
| Missing | 258 | 93 | ||
|
| ||||
| Socio-economic status | Lowest | 209 (15.4) | 160 (14.6) | 0.7796 |
| Middle | 1037 (76.4) | 850 (77.6) | ||
| Highest | 111 (8.2) | 85 (7.8) | ||
| Missing | 179 | 26 | ||
|
| ||||
| Maternal asthma | Yes | 163 (10.7) | 76 (10.9) | 0.8345 |
| No | 1355 (89.3) | 618 (89.1) | ||
| Missing | 18 | 4 | ||
|
| ||||
| Birth weight kg | Birth weight kg | 1511 (3.39 ± 0.52) | 1103(3.41 ± 0.51) | 0.3906 |
| Missing | 25 | 18 | ||
|
| ||||
| Height cm | At age 10 yrs | 1043 (138.9 ± 6.2) | 1026 (138.9± 6.2) | 0.3646 |
| Missing | 493 | 95 | ||
|
| ||||
| At age 18 yrs | 994 (171.2 ± 9.5) | 918 (171.0± 9.3) | 0.5918 | |
| Missing | 542 | 203 | ||
|
| ||||
| Weight kg | At age 10 yrs | 1043 (35.2 ± 7.5) | 1026 (35.2± 7.5) | 0.9616 |
| Missing | 493 | 95 | ||
|
| ||||
| At age 18 yrs | 970 (67.8 ± 13.7) | 897 (67.8 ± 13.6) | 0.9909 | |
| Missing | 566 | 224 | ||
|
| ||||
| BMI kg/m2 | At age 10 yrs | 1043 (18.1 ± 3.0) | 1026 (18.1 ± 2.9) | 0.9307 |
| Missing | 493 | 95 | ||
|
| ||||
| At age 18 yrs | 964 (23.2 ± 4.3) | 896 (23.2 ± 4.3) | 0.9291 | |
| Missing | 572 | 225 | ||
IOW: Isle of Wight
ETS: Environmental tobacco smoke
NICU: Neonatal Intensive Care Unit
BMI: Body Mass Index
Linear regression at age 10 and 18 years of age
At 10 years of age, with every 1 kg increase in birth weight there was a significant increase in FEV1, FVC and FEF25-75 [Table 2]. There was also a significant increase in FEV1/FVC ratio with every one week increase in gestational age but no significant effect of maternal smoking during pregnancy on lung function at age 10 years. At 18 years of age, birth weight showed a positive association with FEV1 and FVC and those who were exposed to maternal smoking in-utero had an increase in FVC and hence significant decrease in FEV1/FVC ratio.
Table 2.
Association of birth weight, gestational age and gestational maternal smoking with lung function: cross-sectional analysis
| Predictor | FEV1 (mL) | FVC (mL) | FEV1/FVC (%) | FEF25-75 (mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Est. ± SE | P-value | Est. ± SE | P-value | Est. ± SE | P-value | Est. ± SE | P-value | |
| At 10 years of age (n=981) | ||||||||
|
| ||||||||
| Birth weight (kg)† | 44.4 ± 17.3 | 0.0107 | 39.4 ± 18.1 | 0.0302 | 0.3 ± 0.4 | 0.4977 | 89.4 ± 41.3 | 0.0306 |
| Gestational age (weeks)§ | 2.9 ± 5.1 | 0.5672 | −4.0 ± 5.4 | 0.4617 | 0.3 ± 0.1 | 0.0477 | 19.1 ± 12.2 | 0.1178 |
| Maternal smoking during pregnancy: Yes‡ | −7.4 ± 17.0 | 0.6664 | −6.3 ± 18.0 | 0.7270 | −0.1 ± 0.4 | 0.9118 | −63.1 ± 40.8 | 0.1222 |
| At 18 years of age (n=838) | ||||||||
|
| ||||||||
| Birth weight (kg)† | 80.2 ± 38.5 | 0.0375 | 100.1 ± 42.7 | 0.0193 | −0.4 ± 0.6 | 0.5131 | 90.4 ± 84.3 | 0.2839 |
| Gestational age (weeks)§ | 11.2 ± 10.8 | 0.2995 | −3.8 ± 12.0 | 0.7507 | 0.3 ± 0.2 | 0.1380 | 34.1 ± 23.6 | 0.1483 |
| Maternal smoking during pregnancy: Yes‡ | 42.4 ± 40.1 | 0.2907 | 109.4 ± 44.8 | 0.0149 | −1.3 ± 0.6 | 0.0421 | −88.8 ± 87.4 | 0.3099 |
Models adjusted for maternal smoking during pregnancy, sex, height, age, gestational age, maternal history of asthma, admission to Neonatal Intensive Care Unit, smoking at 18 years of age, height*sex
Models adjusted for sex, height, age, gestational age, maternal history of asthma, admission to Neonatal Intensive Care Unit, smoking at 18 years of age, height*sex
Models adjusted for sex, height, age, maternal smoking during pregnancy, maternal history of asthma, admission to Neonatal Intensive Care Unit, smoking at 18 years of age, height*sex.
FEV1: Forced expiratory volume in one second
FVC: Forced vital capacity
FEF25-75: Forced expiratory flow between 25% and 75% of forced vital capacity
Linear mixed models
Results from linear mixed models (repeated measurements) showed that with every 1 kg increase in birth weight, FEV1 and FEF25-75 increased by 42.62 ± 17.15 mL and 95.51 ± 41.19 mL, respectively at age 18 years [Table 3]. The models were adjusted for maternal smoking during pregnancy, sex, height, age, gestational age, maternal history of asthma, admission to NICU, smoking at 18 years of age and the interaction between height and sex. We found no effect of maternal smoking during pregnancy or gestational age on any lung function parameters.
Table 3.
Association of birth weight, gestational age and gestational maternal smoking with lung function: repeated-measurement analysis
| Predictor | FEV1 (mL) | FVC (mL) | FEV1/FVC (%) | FEF25-75 (mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Est. ± SE | P-value | Est. ± SE | P-value | Est. ± SE | P-value | Est. ± SE | P-value | |
| (n=1121) | ||||||||
| Birth weight (kg)† | 42.6 ± 17.4 | 0.0145 | 35.0 ± 18.1 | 0.0536 | 0.1 ± 0.4 | 0.8747 | 95.5 ± 41.2 | 0.0207 |
| Gestational age (weeks)§ | 3.1 ± 5.2 | 0.5515 | −3.3 ± 5.5 | 0.5484 | 0.2 ± 0.1 | 0.0885 | 18.7 ± 12.1 | 0.1242 |
| Maternal smoking during pregnancy: Yes‡ | 0.5 ± 17.0 | 0.9781 | −0.1 ± 18.0 | 0.9964 | −0.3 ± 0.4 | 0.4694 | −48.0 ± 39.9 | 0.2284 |
Models adjusted for maternal smoking during pregnancy, sex, height, age, gestational age, maternal history of asthma, admission to Neonatal Intensive Care Unit, smoking at 18 years of age, height*sex
Models adjusted for sex, height, age, gestational age, maternal history of asthma, admission to Neonatal Intensive Care Unit, smoking at 18 years of age, height*sex
Models adjusted for sex, height, age, maternal smoking during pregnancy, maternal history of asthma, admission to Neonatal Intensive Care Unit, smoking at 18 years of age, height*sex.
Path analysis
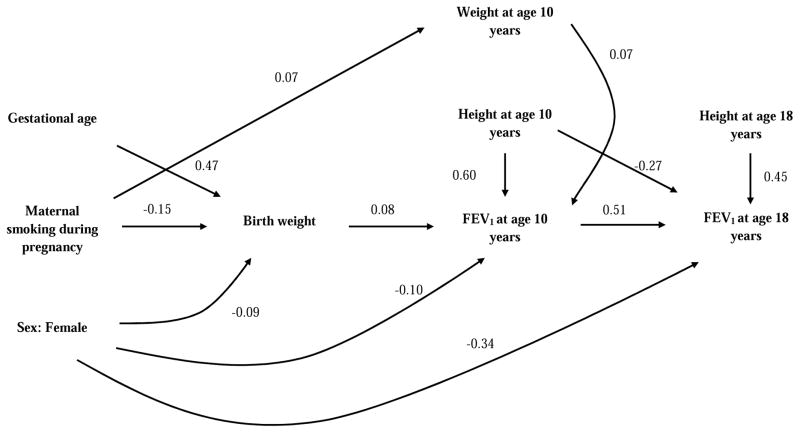
Figure 1 and 2 illustrate statistically significant direct effects (path coefficients) of each factor on FEV1 and FEF25-75, respectively. Detailed information on direct and indirect effect of each factor on lung function parameter is provided in the online supplement. In figure 1, path coefficients suggested a positive direct effect of birth weight on FEV1 at age 10, but no direct effect at age 18 years. However, since FEV1 at age 10 years has a positive direct effect on FEV1 at age 18 years presumably the effect of birth weight on FEV1 at age 10 was carried forward (indirect effect) to age 18 years as indicated by a positive total effect (direct + indirect) of birth weight on FEV1 at 18 years (Table 2 in online supplement). On the other hand, maternal smoking during pregnancy had no significant direct or indirect effects on FEV1 either at age 10 or 18 years (Table 1 and 2 in online supplement); however, it may have an inverse indirect effect on FEV1 through reduction in birth weight. Additionally, gestational age also had positive indirect and total effects on FEV1 both at ages 10 and 18 years (Table 1 and 2 in online supplement).
Figure 1.
Path diagram - association of birth weight, gestational age and gestational maternal smoking with FEV1.
This analytical path diagram shows statistically significant standardized direct effects (path coefficient) of birth weight, gestational age and maternal smoking status during pregnancy on FEV1 at 10 and 18 years.
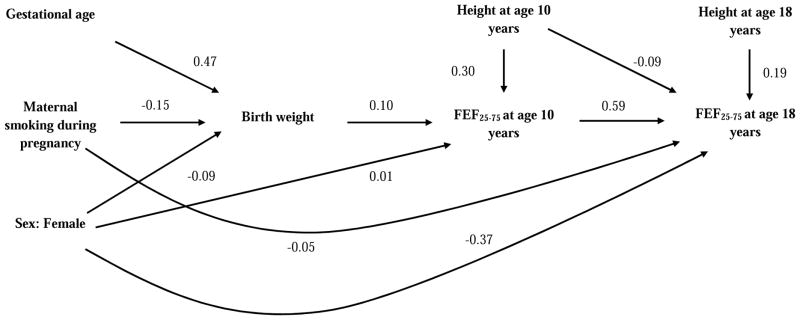
Figure 2.
Path diagram - association of birth weight, gestational age and gestational maternal smoking with FEF25-75.
This analytical path diagram shows statistically significant standardized direct effects (path coefficient) of birth weight, gestational age and maternal smoking status during pregnancy on FEF25-75 at 10 and 18 years.
Figure 2 shows that birth weight had a positive direct effect on FEF25-75 at age 10, which was carried forward to age 18 years. Another significant finding was that of a direct negative effect of exposure to maternal smoking in-utero on FEF25-75 at age 18 years but not at age 10 years which was not seen in FEV1. Gestational age also had a positive indirect effect on FEF25-75 at age 10 years but not at age 18 years (Table 1 and 2 in online supplement). We also found that birth weight had a positive indirect effect on FVC at age 18 years and exposure to maternal smoking in-utero had a positive direct effect on FVC and therefore negative direct effect on the FEV1/FVC ratio (Table 2 in online supplement).
DISCUSSION
We studied the IOW birth cohort to assess the association between birth weight and gestational smoking on lung function at 10 and 18 years. Using linear mixed models for repeated measurements we found that there was significant increase in FEV1 and FEF25-75 with every 1 kg increase in birth weight at age 18 years after adjusting for potential confounders. We did not find any significant association of maternal smoking during pregnancy with lung function after adjusting for other potential confounders. The results of the linear path analysis were different from the linear mixed models. Path analysis showed that birth weight had positive effects not only on FEV1and FEF25-75 but also on FVC either directly or indirectly through various pathways. Additionally, path analysis also showed that maternal smoking during pregnancy had direct negative associations with the FEV1/FVC ratio (due to increase in FVC) and FEF25-75 and that gestational age was positively linked with FEV1 and FVC through birth weight.
Relationship between birth weight and lung function in children
Most previous studies assessing relationship between birth weight and lung function conducted in children focused on investigating effects of LBW and VLBW on respiratory health, since these are well-known risk factors for increased morbidity and mortality in infants. Findings from these studies showed that children born with LBW and VLBW children had significantly lower lung function [3, 7] and volumes [6, 27] along with increased bronchial hyper-responsiveness [7] when compared to normal birth weight. However, approaches using dichotomized birth weight do not provide information on whether there is linear relationship between birth weight and lung function. Rona et al. investigated the association between continuous birth weight and lung function and demonstrated a positive linear association between birth weight adjusted for gestational age and FEV1 and FVC at age 10 years in children of 5–11 years of age [8]. Our findings showing a positive association between birth weight and FEV1 and FVC are consistent with those of Rona et al. However, in contrast to our results, Rona et al. did not find any significant association between birth weight and FEF25-75. Sonnenschein et al. examined the association of children’s growth pattern with asthma and lung function [9] and found that at 8 years of age higher birth weight was strongly associated with higher FVC, FEV1 and FEF25-75 z-scores and at age 15 years with higher FVC and reduced FEV1/FVC and FEF25-75 /FVC ratios [9]. Our findings from linear regression models at age 10 years are comparable to those of Sonnenschein et al. at 8 years. However, our results using appropriate models for repeated measurements, such as linear mixed models and path analysis, showed that birth weight also had positive association with FEV1 and FEF25-75 in addition to FVC, at age 18 years.
Relationship between maternal smoking during pregnancy, gestational age, birth weight and lung function: Path analysis
The results from previous studies exploring the relationship between maternal smoking during pregnancy and lung function are mixed. Some studies have suggested reduction in lung function in children exposed to maternal smoking in-utero [17, 28] while other studies reported no such association [19, 29]. While most studies have not adjusted for birth weight in their statistical models, Hayatbakhsh et al. found a reduction in lung function in boys of age 21 years even after adjusting for birth weight [30]. One can argue that birth weight and maternal smoking are in the same ‘causal pathway’ related to intra-uterine growth retardation and both should not be used in the same model. In these situations, use of traditional analysis does not allow to discern the independent effects of risk factors (maternal smoking and birth weight) on the outcome (lung function). In standard regression analysis each variable is identified as either risk or effect prior to analysis and statistical relationship between these variables are based on a conditional expected value. These models do not take into consideration the temporal sequence and thus are ill-suited for modeling relationships which are composed of effects mediated through intervening variables. Linear path analyses on the other hand accommodate intervening variables in the analysis [31]. Using path analysis, we found that exposure to maternal smoking in-utero was associated directly with reduction in FEF25-75 and increase in FVC. These findings were not evident in linear mixed models. Additionally, path analysis also showed that even though birth weight did not have any significant direct effect on FVC at age 18 years it did have significant indirect effect [Table 2 online supplement].
One of the main findings of our study was the positive association between birth weight and FEF25-75 at age 18 years which measures airflow in small airways; this association was not shown by Sonnenschein et al. in adolescents of age 15 years [9]. Birth weight is a surrogate marker for intrauterine growth and gestational age and maternal smoking during pregnancy have significant adverse effect on birth weight. Hence the association between birth weight and lung function may reflect the underlying association between gestational age and maternal smoking during pregnancy with lung function. Previous studies have shown that higher gestational age was associated with higher FEF25-75 [9] and maternal smoking during pregnancy was associated with reduced FEF25-75 [17, 28, 30]. Our path analysis results are consistent with these findings [Figure 2]. The underlying patho-physiological mechanisms are not fully understood. Although histopathological studies have shown that broncho-pulmonary dysplasia, a hallmark of respiratory distress syndrome in premature babies, is characterized by formation of hyaline membrane in small airways, enlargement and oversimplification of alveoli and increase in interstitial thickening leading to a reduction in elastic recoil [32, 33]. Additionally, an animal model presented by Rehan et al. to study the effects of maternal smoking on fetal lung development showed that in-utero exposure to tobacco smoke alters the normal homeostatic epithelial-mesenchymal interaction in the developing alveolus, resulting in production of myofibroblasts in larger as well as smaller airways, which is a common finding in asthma and chronic lung disease [34].
Our results from cross-sectional analysis at age 18 years (Table 2) and path analysis (Table 2 in online supplement) showed a positive association between maternal smoking and higher FVC, comparable to the previous studies, which have also shown an association between maternal smoking during pregnancy and higher FVC in children [26, 35, 36]. Studies have suggested that maternal smoking during pregnancy or exposure to parental smoking during early childhood may cause disproportional growth of lung parenchyma and airways known as dysynaptic growth of lungs in children [35, 37–39].
One of the major limitations of this study is that the information on smoking during pregnancy was self-reported and was not verified by objective measurements like urine cotinine levels. Studies have shown that self-reported smoking status during pregnancy grossly underestimates the true prevalence of smoking during pregnancy [40, 41]. Therefore, it is likely that the prevalence of smoking during pregnancy in our study is underestimated which may in turn bias our findings related to maternal smoking and lung function towards null. The second important limitation of this study was that there was a lack of information on number of cigarettes smoked per day and duration of smoking by mothers during pregnancy. Therefore, we were not able to assess the dose-response relationship between in-utero exposure to smoking and lung function. The strength of this study is that we analyzed lung function measurements in pre-adolescents and adolescents, thus covering an important period of lung development. In addition, cohort members who participated in lung function testing were not different from the participant of the overall birth cohort. To our knowledge no other study has investigated the risk of gestational and neonatal conditions on lung function in the adolescence with repeated measurements and path-analytical models. As information on birth weight, gestational age and maternal smoking during pregnancy were recorded soon after birth; a recall bias is unlikely. To address a few missing information, multiple imputations were used. This is a robust method to overcome the problem of missing data and to generate unbiased estimates. We also used linear mixed models which were more appropriate than simple linear regression models as they also consider individual change in lung function across time. Use of path analysis allowed us to include intervening variables in models whose inclusion in linear mixed models would normally produce biased estimates.
CONCLUSIONS
In conclusion, we found that higher birth weight was significantly associated, either directly or indirectly, with higher FEV1, FVC and FEF25-75 in adolescents at age 18 years. Though we did not find significant associations between maternal smoking and lung function through standard linear mixed models, the more appropriate path analysis showed that that gestational smoke exposure does have negative effects on FEV1/FVC ratio due to increase in FVC and on FEF25-75 at age 18 years. With path analysis, we gained insight and better understanding about the underlying links between various prenatal and early childhood factors that affect lung function. Our results suggest that the beneficial effects of favorable fetal growth, which is reflected by birth weight, goes beyond lung function changes in early childhood years. Future research investigating effects of gestational smoking, birth weight and growth on lung function should consider employing path analyses models to disentangle the complex relationships between these determinants of lung function.
Supplementary Material
Highlights.
Use of path analysis to detect underlying “causal’ pathways between prenatal and early childhood factors that affect lung function is proposed.
Birth weight and gestational age act as intervening variables in association between in-utero exposure to maternal smoking and lung function.
Higher birth weight and gestational age have favorable effects while in-utero exposure to maternal smoking have adverse effects on lung function even in late adolescence.
Acknowledgments
FUNDING
This study is funded by the National Institute of Health (NIH) R01 AI091905 (principle investigator W. Karmaus). The 10-yr follow-up of this study was funded by National Asthma Campaign, UK (grant no. 364) and the 18-yr follow-up by NIH R01 HL082925 (principle investigator S.H. Arshad).
The authors gratefully acknowledge the cooperation of the children and parents who participated in this study, and appreciate the hard work of the Isle of Wight research team in collecting data.
LIST OF ABBREVIATIONS
- LBW
Low birth weight
- VLBW
Very low birth weight
- IOW
Isle of Wight
- NICU
Neonatal intensive care unit
- SES
Socio-economic status
- BMI
Body mass index
- ETS
Environmental tobacco smoke
- FVC
Forced vital capacity
- FEV1
Forced expiratory volume in one second
- FEF25-75
Forced expiratory flow between 25% and 75% of forced vital capacity
- PEFR
Peak expiratory flow rate
- ATS
American Thoracic Society
- FIML
Full information maximum likelihood
- CFI
Comparative fit index
- GFI
Goodness of fit index
- RMSEA
Root mean square error of approximation
APPENDIX A
Footnotes
COMPETING INTERESTS
None declared.
AUTHOR’S CONTRIBUTIONS
WK and SHA was involved in the design of the study, development, and preparation of data. PB under the guidance of WK analyzed data and wrote the first draft of the manuscript. WK, SHA, GR, RK, FM and SB discussed data analyses and interpretation and contributed to subsequent versions of the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pallavi Balte, Email: balte.p@gmail.com.
Wilfried Karmaus, Email: karmaus1@memphis.edu.
Graham Roberts, Email: g.c.roberts@soton.ac.uk.
Ramesh Kurukulaaratchy, Email: r.j.kurukulaaratchy@soton.ac.uk.
Frances Mitchell, Email: frances.mitchell@iow.nhs.uk.
S Hasan Arshad, Email: s.h.arshad@soton.ac.uk.
References
- 1.Barker DJP, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. The Lancet. 1986;327(8489):1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand D, Stevenson CJ, West CR, Pharoah POD. Lung function and respiratory health in adolescents of very low birth weight. Archives of disease in childhood. 2003;88(2):135–138. doi: 10.1136/adc.88.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan MH, Levison H, Swyer PR. Pulmonary function in infants and children following the acute neonatal respiratory distress syndrome. Bulletin de physio-pathologie respiratoire. 1973;9(6):1587. [PubMed] [Google Scholar]
- 5.Hoo A-F, Stocks J, Lum S, Wade AM, Castle RA, Costeloe KL, Dezateux C. Development of lung function in early life: influence of birth weight in infants of nonsmokers. American journal of respiratory and critical care medicine. 2004;170(5):527–533. doi: 10.1164/rccm.200311-1552OC. [DOI] [PubMed] [Google Scholar]
- 6.Kitchen WH, Olinsky A, Doyle LW, Ford GW, Murton LJ, Callanan C, Slonim L. Respiratory health and lung function in 8-year-old children of very low birth weight: a cohort study. Pediatrics. 1992;89(6):1151–1158. [PubMed] [Google Scholar]
- 7.Wjst M, Popescu M, Trepka MJ, Heinrich J, Wichmann H. Pulmonary function in children with initial low birth weight. Pediatric allergy and immunology. 1998;9(2):80–90. doi: 10.1111/j.1399-3038.1998.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 8.Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306(6881):817–820. doi: 10.1136/bmj.306.6881.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenschein-van der Voort AMM, Howe LD, Granell R, Duijts L, Sterne JAC, Tilling K, Henderson AJ. Influence of childhood growth on asthma and lung function in adolescence. Journal of Allergy and Clinical Immunology. 2014 doi: 10.1016/j.jaci.2014.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards CA, Osman LM, Godden DJ, Campbell DM, Douglas JG. Relationship between birth weight and adult lung function: controlling for maternal factors. Thorax. 2003;58(12):1061–1065. doi: 10.1136/thorax.58.12.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancox RJ, Poulton R, Greene JM, McLachlan CR, Pearce MS, Sears MR. Associations between birth weight, early childhood weight gain and adult lung function. Thorax. 2009;64(3):228–232. doi: 10.1136/thx.2008.103978. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor DA, Ebrahim S, Smith GD. Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;60(10):851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein CE, Kumaran K, Fall CH, Shaheen SO, Osmond C, Barker DJ. Relation of fetal growth to adult lung function in south India. Thorax. 1997;52(10):895–899. doi: 10.1136/thx.52.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaheen SO, Sterne JAC, Tucker JS, du Florey VC. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998;53(7):549–553. doi: 10.1136/thx.53.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal A, Scherrer JF, Grant JD, Sartor CE, Pergadia ML, Duncan AE, Madden PAF, Haber JR, Jacob T, Bucholz KK. The effects of maternal smoking during pregnancy on offspring outcomes. Preventive medicine. 2010;50(1):13–18. doi: 10.1016/j.ypmed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the US. American journal of preventive medicine. 2010;39(1):45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, Avol E, Peters JM. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55(4):271–276. doi: 10.1136/thorax.55.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaakkola JJK, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. American journal of public health. 2004;94(1):136–140. doi: 10.2105/ajph.94.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherrill DL, Martinez FD, Lebowitz MD, Holdaway MD, Flannery EM, Herbison GP, Stanton WR, Silva PA, Sears MR. Longitudinal effects of passive smoking on pulmonary function in New Zealand children. American journal of respiratory and critical care medicine. 1992;145(5):1136–1141. doi: 10.1164/ajrccm/145.5.1136. [DOI] [PubMed] [Google Scholar]
- 20.Eide MG, Øyen N, Skjaerven RR, Nilsen ST, Bjerkedal T, Tell GS. Size at birth and gestational age as predictors of adult height and weight. Epidemiology. 2005;16(2):175–181. doi: 10.1097/01.ede.0000152524.89074.bf. [DOI] [PubMed] [Google Scholar]
- 21.Sørensen HT, Sabroe S, Rothman KJ, Gillman M, Steffensen FH, Fischer P, Serensen TIA. Birth weight and length as predictors for adult height. American Journal of Epidemiology. 1999;149(8):726–729. doi: 10.1093/oxfordjournals.aje.a009881. [DOI] [PubMed] [Google Scholar]
- 22.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology (Cambridge, Mass) 2009;20(4):488. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto-Ramírez N, Alexander M, Karmaus W, Yousefi M, Zhang H, Kurukulaaratchy RJ, Raza A, Mitchell F, Ewart S, Arshad SH. Breastfeeding is associated with increased lung function at 18 years of age: a cohort study. European Respiratory Journal. 2012;39(4):985–991. doi: 10.1183/09031936.00037011. [DOI] [PubMed] [Google Scholar]
- 24.Yousefi M, Karmaus W, Zhang H, Roberts G, Matthews S, Clayton B, Arshad SH. Relationships between age of puberty onset and height at age 18 years in girls and boys. World Journal of Pediatrics. 2013;9(3):230–238. doi: 10.1007/s12519-013-0399-z. [DOI] [PubMed] [Google Scholar]
- 25.Ogbuanu IU, Karmaus W, Arshad SH, Kurukulaaratchy RJ, Ewart S. Effect of breastfeeding duration on lung function at age 10 years: a prospective birth cohort study. Thorax. 2009;64(1):62–66. doi: 10.1136/thx.2008.101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatric pulmonology. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy JD, Edward LJ, Bates DJ, Martin AJ, Dip SN, Haslam RR, McPhee AJ, Staugas RE, Baghurst P. Effects of birthweight and oxygen supplementation on lung function in late childhood in children of very low birth weight. Pediatric pulmonology. 2000;30(1):32–40. doi: 10.1002/1099-0496(200007)30:1<32::aid-ppul6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham J, Dockery DW, Speizer FE. Maternal smoking during pregnancy as a predictor of lung function in children. American Journal of Epidemiology. 1994;139(12):1139–1152. doi: 10.1093/oxfordjournals.aje.a116961. [DOI] [PubMed] [Google Scholar]
- 29.Dijkstra L, Houthuijs D, Brunekreef B, Akkerman I, Boleij J. Respiratory Health Effects of the Indoor Environment in a Population of Dutch Children1–3. Am Rev Respir Dis. 1990;142:1172–1178. doi: 10.1164/ajrccm/142.5.1172. [DOI] [PubMed] [Google Scholar]
- 30.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64(9):810–814. doi: 10.1136/thx.2009.116301. [DOI] [PubMed] [Google Scholar]
- 31.Gunzler D, Chen T, Wu P, Zhang H. Introduction to mediation analysis with structural equation modeling. Shanghai archives of psychiatry. 2013;25(6):390. doi: 10.3969/j.issn.1002-0829.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agrons GA, Courtney SE, Stocker JT, Markowitz RI. Lung Disease in Premature Neonates: Radiologic-Pathologic Correlation 1. Radiographics. 2005;25(4):1047–1073. doi: 10.1148/rg.254055019. [DOI] [PubMed] [Google Scholar]
- 33.Coalson JJ. Seminars in perinatology: 2006. Elsevier; 2006. Pathology of bronchopulmonary dysplasia; pp. 179–184. [DOI] [PubMed] [Google Scholar]
- 34.Rehan VK, Asotra K, Torday JS. The effects of smoking on the developing lung: insights from a biologic model for lung development, homeostasis, and repair. Lung. 2009;187(5):281–289. doi: 10.1007/s00408-009-9158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Wypij D, Gold DR, Speizer FE, Ware JH, Ferris BG, Dockery DW. A longitudinal study of the effects of parental smoking on pulmonary function in children 6–18 years. American Journal of Respiratory and Critical Care Medicine. 1994;149(6):1420–1425. doi: 10.1164/ajrccm.149.6.8004293. [DOI] [PubMed] [Google Scholar]
- 36.Tager IB, Weiss ST, Rosner B, Speizer FE. Effect of parental cigarette smoking on the pulmonary function of children. American Journal of Epidemiology. 1979;110(1):15–26. doi: 10.1093/oxfordjournals.aje.a112783. [DOI] [PubMed] [Google Scholar]
- 37.Chan KN, Noble-Jamieson CM, Elliman A, Bryan EM, Silverman M. Lung function in children of low birth weight. Archives of disease in childhood. 1989;64(9):1284–1293. doi: 10.1136/adc.64.9.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green M, Mead J, Turner JM. Variability of maximum expiratory flow-volume curves. Journal of Applied Physiology. 1974;37(1):67–74. doi: 10.1152/jappl.1974.37.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Mead J. Dysanapsis in Normal Lungs Assessed by the Relationship between Maximal Flow, Static Recoil, and Vital Capacity. American Review of Respiratory Disease. 1980;121(2):339–342. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- 40.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009:339. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ford RP, Tappin DM, Schluter PJ, Wild CJ. Smoking during pregnancy: how reliable are maternal self reports in New Zealand? Journal of epidemiology and community health. 1997;51(3):246–251. doi: 10.1136/jech.51.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.