Abstract
Background
Persistently active PKMζ has been implicated in maintaining spinal nociceptive sensitization that underlies pain hypersensitivity. However, evidence for PKMζ in the maintenance of pain hypersensitivity comes exclusively from short-term studies in males using pharmacological agents of questionable selectivity. The present study examines the contribution of PKMζ to long-lasting allodynia associated with neuropathic, inflammatory, or referred visceral and muscle pain in males and females using pharmacological inhibition or genetic ablation.
Results
Pharmacological inhibition or genetic ablation of PKMζ reduced mild formalin pain and slowly developing contralateral allodynia in nerve-injured rats, but not moderate formalin pain or ipsilateral allodynia in models of neuropathic and inflammatory pain. Pharmacological inhibition or genetic ablation of PKMζ also effectively reduced referred visceral and muscle pain in male, but not in female mice and rats.
Conclusion
We show pharmacological inhibition and genetic ablation of PKMζ consistently attenuate long-lasting pain hypersensitivity. However, differential effects in models of referred versus inflammatory and neuropathic pain, and in males versus females, highlight the roles of afferent input-dependent masking and sex differences in the maintenance of pain hypersensitivity.
Keywords: Sex differences, protein kinase M-zeta, PKC/Mζ knock-out, muscle pain, visceral pain, tactile allodynia, central sensitization, antinociception, analgesia
Introduction
Although many studies have revealed spinal signaling molecules (protein kinase C [PKC], calcium/calmodulin-dependent protein kinase II [CaMKII], and extracellular signal-regulated kinase [ERK]1/2) underlying the induction of hypersensitivity that accompanies chronic pain, few have identified molecules affecting its maintenance, which is critical for pain chronicity.1–3 Although a growing number of studies have implicated spinal PKMζ as key to the maintenance of pain hypersensitivity, this evidence comes mostly from experiments assessing short-lived nociception,4–9 while results from studies of longer lasting pain have been equivocal.6–8 Furthermore, all of these studies have utilized myristoylated-pseudosubstrate ζ-inhibitory peptide (ZIP), which has questionable specificity as mutant mice lacking PKMζ still exhibit hippocampal long-term potentiation (LTP) and perform memory tasks that are reversed by ZIP.10,11 Surprisingly, these mice have not been tested for a nociceptive phenotype. Notably, all studies examining the nociceptive role of PKMζ have used male rodents, despite a higher prevalence of chronic pain in females,12,13 and evidence for sex differences in hippocampal PKMζ’s role in spatial memory maintenance,14 as well as in other critical pain mechanisms.15,16 We examine here the contribution of spinal PKMζ to the maintenance of long-lasting pain, assessing the potential factor of ongoing afferent input to its differential manifestation in inflammatory and neuropathic pain models compared with referred pain after muscular and visceral injury. We also compare the effects of ZIP in rats to genetic disruption of PKMζ in mutant mice and for the first time examine PKMζ-dependent sex differences in the maintenance of pain hypersensitivity.
Materials and methods
Animals
Long Evans hooded rats (225–250 g, Charles River, St. Constant, QC) of both sexes were received and acclimatized at our animal housing facility at least seven days before the start of experiments. Methods were approved by the Animal Care Committee of McGill University and conformed to ethical guidelines of the Canadian Council on Animal Care and the International Association for the Study of Pain. Procedures for transgenic mice generation are as follows: While Prkcz−/− mice were devoid of exon 9 of the Prkcz gene, rendering the transcription of PKMζ and PKCζ mRNAs incomplete, heterozygous (Prkcz+/−) mice exhibited one wild type (WT) and one Prkcz allele. A vector targeting exon 9 of the mouse Prkcz gene was generated using a 129S6/SvEvTac mouse BAC clone from the RPC1-22 library and the plasmid pK-11 (Frt-PGKNeo-Frt-Loxp-pBSSK).17 Following chimera generation, exon 9 was deleted with the help of cre-loxp system and backcrossed for 11 generations into the C57BL/6 background. These breeder mice were obtained from the laboratory of Robert Messing, The University of Texas, Austin, for the development of our experimental mouse colony. Prkcz−/− and Prkcz+/− male and female mice were bred in-house to produce knockout, heterozygous, and WT off-spring, which were genotyped both at the start and end of experiments. Briefly, for genotyping, a 2 mm sample from the ear of restrained mice was obtained by using a commercially available ear punch that was previously cleaned with 70% ethanol. Ear punches were incubated overnight at 55℃ in 50 µL of lysis buffer. Proteinase K (20 mg/mL) was added immediately before incubation. After vortexing, the samples were then placed for 10 min at 100℃ in a PCR machine to denature the proteinase K. The resulting crude gDNA was subsequently genotyped by PCR amplification using KAPA2G Fast Hot Start Mastermix (Kapa Biosystems, Wilmington, MA) and a Bio-Rad T100 thermal cycler. WT forward primer was used at 300 nM: CCACACCCTCACCAACACT TTTTCC. Knockout forward primer was used at 275 nM: GGAACTTCGTCGACAT AACTTCGTATAGCATACAT. A common reverse primer was used at 1000 nM: CCTGCCCCAAGACACTTTCAGGGTTC. The reaction was supplemented with betaine HCl (Sigma-Aldrich, St. Louis, MO) at 1 M and bovine serum albumin (Ambion, Foster City, CA) at 1 µg/µL. Total reaction volume was 10 µL, and 1 µL of the crude gDNA prep was used. An initial denaturation at 95℃ for 3 min was followed by 40 cycles of amplification: 95℃ for 15 s, 64℃ for 30 s, and 68℃ for 1 min. The samples were run on a 1.5% agarose gel in TAE buffer with ethidium bromide and visualized in an Amersham Imager 600. The WT band migrated at 1.9 kb and the knockout band at 1.7 kb.
Pain models
Spared nerve injury
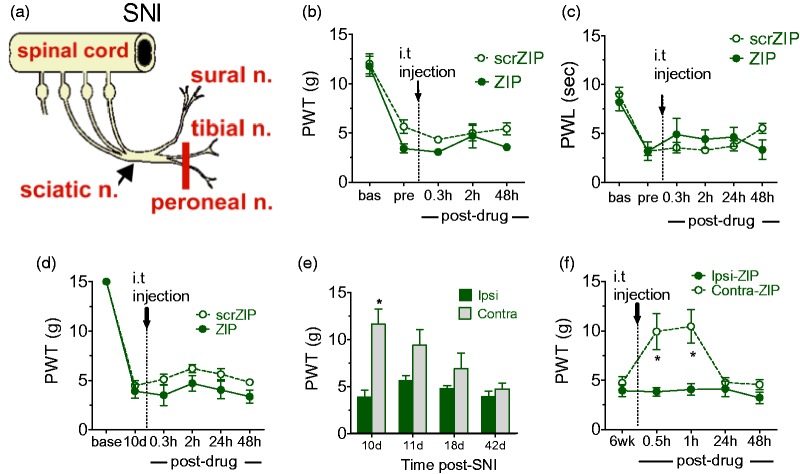
Neuropathic pain was induced in rats and transgenic mice using the SNI procedure of Decosterd and Woolf,18 by transecting the common peroneal and tibial nerve branches, sparing the adjacent sural and saphenous nerves (see Figure 1(a)). Rats received ZIP intrathecally (i.t.) 10 days or six weeks post-SNI. Paw withdrawal thresholds (PWTs) were measured using von Frey testing (described later) on both contralateral and ipsilateral paws. Transgenic mice were tested for PWTs until six weeks post-SNI.
Figure 1.
Effects of PKMζ inhibition in inflammatory and neuropathic pain models. (a) A diagram illustrating the SNI surgical procedure. (b and c) Absence of ZIP effects on CFA-induced thermal hyperalgesia (b) and mechanical allodynia (c). (d) PKMζ inhibition using i.t. ZIP at 10 days post-surgery does not reverse SNI-induced ipsilateral allodynia. (e) Time course of development of contralateral allodynia in SNI rats. (f) ZIP reduces contralateral allodynia for 60 min when injected at six weeks post-SNI. (b–f) Asterisks denote a significant difference between ipsilateral and contralateral paw withdrawal thresholds (*p < 0.05). All of the data were collected in male rats and are presented as mean ± S.E.M. Note. PKMζ = protein kinase M zeta; SNI = spared nerve injury; ZIP = ζ-inhibitory peptide; CFA = complete Freund’s adjuvant; scrZIP = scrambled ZIP.
Complete Freund’s adjuvant
Rats were injected i.pl. with 50 µL of 1 mg/mL complete Freund’s adjuvant (CFA) in the left hind paw. We examined the effects of i.t. ZIP on CFA-induced mechanical allodynia and thermal hyperalgesia using von Frey and Plantar tests (see later), respectively, until 48 h postinjection.
Intracolonic capsaicin
Induction of remote pain in rats using intracolonic (i.c.) capsaicin was as described previously.19 Briefly, a volume of 200 µL of 0.1% capsaicin (Sigma; dissolved in a solution consisting of 10% ethanol, 10% Tween 80, and 80% saline) was instilled into the colon via anus using a fine cannula (PE-20 polyethylene tubing) that was inserted up to a maximum length of 7 cm. ZIP or scrZIP effects were tested 20 min before or 1 h after a single i.c. capsaicin instillation. In the case of priming experiments, with two instillations spaced two weeks apart, drug effects were examined at 20 min prior to the first, two weeks after the first but 20 min before the second, and 24 h after the second challenging stimulus. For mice, 50 µL of 0.1% capsaicin (Sigma; in the same vehicle as above) was instilled into the colon using a thin cannula (PE-10 polyethylene tubing).20 The perianal area was covered with petroleum jelly prior to insertion of the cannula to avoid pre-injection irritation, and the cannula was inserted for 4 cm. ZIP effects in WT and heterozygous mice were examined until 24 h postinjection.
Intramuscular (i.m.) acidic saline
Two acidic saline (0.9% NaCl in sterile water for injection, pH adjusted to 4.0) injections, spaced five days apart, were administered to the gastrocnemius muscle in rats21 (100 µL) or mice (20 µL)22 to induce persistent hind paw mechanical allodynia. In rats, we examined the effects of i.t. ZIP, scrZIP, and NPC-15437 (Sigma), 24 h and one week after the second acidic saline challenge. The mice were tested for the time course of expression of mechanical allodynia over a period of four weeks.
Nociceptive testing
von Frey testing
Rats and mice were initially habituated for 20 and 60 min, respectively, to acrylic testing chambers (12 cm wide, 18 cm long, and 15 cm high) fitted with wire mesh floors (12 × 12 mm mesh size). Filaments (selected from a Semmes Weinstein Monofilament Kit, Stoelting, Wood Dale, IL) were applied in either ascending (after a negative response) or descending (after a positive response) force as necessary to determine the filament closest to the 50% threshold of response. Each filament was applied for 10 s (for rats) or 4 s (for mice) or until a flexion response occurred. The minimum stimulus intensity was 0.25 g and the maximum was 15.0 g. Based on the response pattern, and the force of the final filament (fifth stimulus after first direction change), the 50% threshold (gram) was calculated as: , where Xf = log(10 × filament buckling force in mg), k = value for the pattern of positive/negative responses, and δ = mean difference in log units between stimuli (here δ = 0.220, for more details, see Chaplan et al.23).
Plantar test
Briefly, rats were placed in clear plastic cubicles mounted on a glass plate and acclimatized for 20 min. A paw withdrawal response was induced by shining a radiant heat source directly under the plantar aspect of the hind paw using a modification of the method of Hargreaves et al.24 The intensity was preset so that a normal withdrawal response happens within a period of 10 s. To prevent tissue damage, a cutoff time of 20 s was set. The reported thermal paw withdrawal latency (PWL) was determined by calculating the mean of three consecutive PWL measures spaced 10 min apart.
Formalin test
A volume of 50 µL of 2%, 3.5%, or 5% formalin (Sigma, in 0.9% saline) was injected intraplantar (i.pl.) into the left hind paw of naïve or i.t. catheterized rats (see below). Naïve rats received ZIP or scrZIP by lumbar puncture 20 min prior to formalin; while for catheterized rats, drugs were administrated at the peak of the second phase of the formalin test via the i.t. catheter. Sustained nociceptive behaviors (SNBs) were assessed using the weighted means scoring methods of Dubuisson and Dennis25 every 2 min until 46 min. Transgenic mice received 25 µL formalin to the plantar hind paw at either 1% or 2% concentration. SNB scoring was performed blind to the genotype of the mice.
Drug administration
All drugs were administered in a volume of 20 µL into the intrathecal space by either lumbar puncture or an indwelling i.t. catheter. ZIP (Tocris Bioscience, Bristol, UK), and its inactive control peptide scrZIP (Tocris Bioscience, Bristol, UK), were dissolved in sterile water for injection and administered in a final dose of 10 nmol. For lumbar puncture, a 26½-gauge needle was inserted between the L5 and L6 vertebrae into the cauda equina in rats under isofluorane inhalation anesthesia (4% induction and 2% maintenance). Correct placement of the needle was verified by a brief flick of the tail at initial needle placement and on drug injection. For i.t. catheterization, a 5 cm polyethylene catheter (PE-32) was implanted sub-durally 4 cm to the lumbar enlargement with the help of a 23-gauge needle inserted between the L5/L6 vertebrae in rats under pentobarbital anesthesia. After removal of the needle, the outer end of the PE-32 catheter was glued to a 14 cm piece of PE-10 catheter. The tight connection between PE-10 and PE-32 catheters was verified by flushing saline through the connected catheters. The catheter was anchored in place by stitches over the skin until the time of testing. Rats were given a 24 h recovery period between catheter placement and formalin testing. The correct i.t. placement of the catheter was verified by the observation of a short-lived hind paw paralysis induced by lidocaine injection. Drugs were administered i.t. in a volume of 20 µL and flushed with the required volume of saline calculated according to length and diameter of the catheter.
Western immunoblotting
Spinal cord dorsal horns were quick frozen and homogenized twice for 5 s each in the presence of final sample buffer containing phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (Roche, Basel, Switzerland). Samples were incubated for 10 min at 95℃. After centrifugation (5 min at 13,000 rpm, room temperature), trichloroacetic acid precipitation was performed on the resulting supernatant. Samples were then incubated for 20 min on ice, centrifuged, and the resulting protein pellet was resuspended in 2× gel sample buffer (100–200 µL depending on size of the pellet). Samples were incubated for 10 min at 95℃ and separated on a 10% sodium dodecyl sulfate-polyacrylamide gel. Proteins were blotted onto a nitrocellulose membrane for 86 min. Membranes were incubated overnight with primary antibodies against total PKCζ, PKCλ, and PKMζ (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), the phosphorylated forms (1:100, Cell Signaling Technology, Danvers, MA), or actin (1:100,000, Chemicon, Rolling Meadows, IL). Filters were washed and incubated with a horseradish peroxidase-conjugated secondary antibody (1:2,000) overnight. After washing, filters were incubated with enhanced chemiluminescent substrate as recommended by the supplier (Abcam). Films were exposed between 5 s and 60 s and scanned using a Canon N650U image scanner. Relative band intensities were then quantified using Image J (NIH) software.
Statistical analysis
All data are presented as mean ± S.E.M. Statistical analyses were performed using SPSS. All time course data were analyzed using repeated measures analysis of variance followed by multiple comparisons with Bonferroni’s post hoc test. Statistical differences between treatment groups in western blots were obtained by comparisons of ImageJ results using Student’s t-test. Percent change from baseline was calculated using the formula: ([post-drug PWT − baseline PWT]/[baseline PWT]) × 100 for each animal. The resulting percent changes were analyzed for statistical differences using Student’s t-test. Graphs were constructed using GraphPad Prism (version 6.0).
Results
PKMζ inhibition in inflammatory and neuropathic pain models
We initially examined spinal PKMζ-dependent maintenance mechanisms in standard models of inflammatory (hind paw injection of complete Freund’s adjuvant [CFA]) and neuropathic (spared nerve injury [SNI], see Figure 1(a)) pain, in which pain hypersensitivity is evidenced by reduced PWLs or PWTs. Male Long Evans rats were treated with ZIP, or the scrambled control peptide scrZIP, i.t. 24 h after CFA and either 10 days or six weeks after SNI, in an attempt to reverse pain hypersensitivity or allodynia. Neither ZIP, nor scrZIP elevated ipsilateral mechanical PWTs (Figure 1(b)) or thermal PWLs (Figure 1(c)) of CFA rats or ipsilateral PWTs (Figure 1(d)) in SNI rats tested 10–12 days postsurgery. However, at six weeks, when allodynia has developed in the hind paw contralateral to the nerve injury (Figure 1(e)), ZIP produced transient, but highly significant increases in contralateral PWTs at 30 min (F1,10 = 10.627, p = 0.009) and 60 min (F1,10 = 12.438, p = 0.005), without effects on ipsilateral PWTs (Figure 1(f)).
PKMζ genetic ablation in a neuropathic pain models
Parallel assessment of post-SNI PWTs was performed in WT (Prkcz+/+) and mutant mice (Prkcz+/− and Prkcz−/−) with deletion of PKC/Mζ (Figure 2(a)). Analogous to ZIP-treated SNI rats, ipsilateral PWTs remained low (i.e., ipsilateral allodynia was present) in both WT and mutant mice for six weeks post-SNI (Figure 2(b)). However, similar to rats given ZIP, contralateral allodynia observed in Prkcz+/+ and Prkcz+/− mice at five and six weeks post-SNI was attenuated in Prkcz−/− mice (Genotype × Time: F10,65 = 2.809, p = 0.006) (Figure 2(c)).
Figure 2.
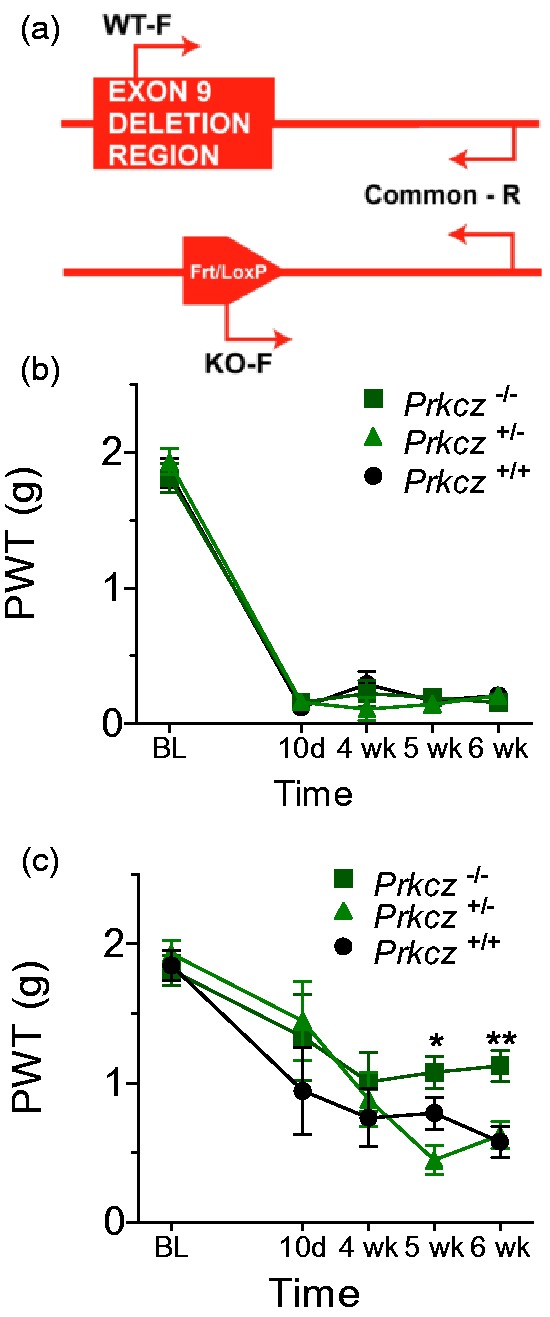
Effects of PKMζ genetic ablation in inflammatory and neuropathic pain models. (a) Schematic presentation of the Prkcz knockout methodology/genotyping strategy. (b) PKC/Mζ knockout (Prkcz−/− and Prkcz+/− mice) did not influence ipsilateral allodynia between 10 days and four weeks post-SNI. (c) Prkcz−/− mice display attenuated contralateral allodynia from five to six weeks post-SNI, whereas Prkcz+/+ and Prkcz+/− mice remain persistently allodynic until six weeks postsurgery. (b–c) Asterisks denote a difference between Prkcz−/− and Prkcz+/+ animals (*p < 0.05, **p < 0.01), daggers denote a difference between Prkcz−/− and Prkcz+/− animals (†p < 0.05). All of the data are presented as mean ± S.E.M. Note. PKMζ = protein kinase M zeta; PKC = protein kinase C; SNI = spared nerve injury.
PKMζ inhibition and genetic ablation in the formalin test
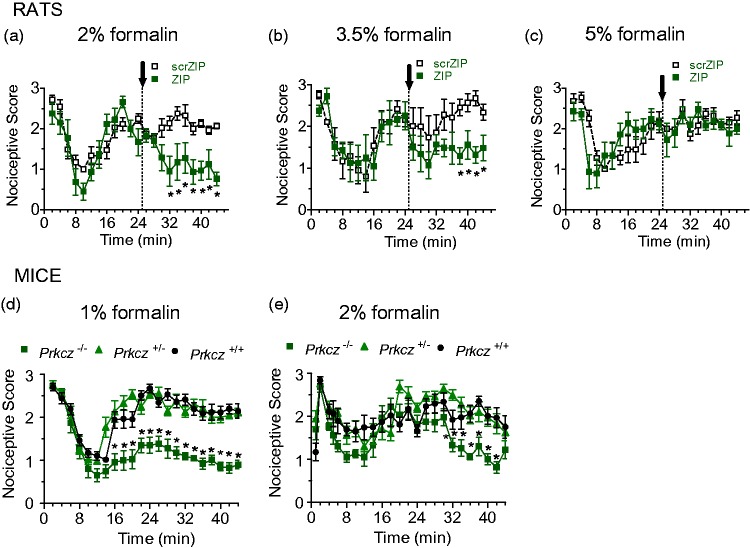
We hypothesized that the inability to demonstrate a role for PKMζ in ipsilateral neuropathic pain hypersensitivity was due to a masking of ZIP’s inhibitory effects by ongoing peripheral inputs from the nerve injury. To test this hypothesis, we examined the effects of posttreatment with i.t. ZIP or scrZIP in the formalin test in separate groups of rats that received increasing concentrations of i.pl. formalin to produce greater afferent inputs. We replicated our previous finding7 that i.t. ZIP, but not scrZIP, attenuated formalin-induced nociceptive behaviors when administered 25 min after a 2% formalin injection (Figure 3(a), Treatment × Time interaction: F20,210 = 3.089, p < 0.001). However, i.t. ZIP produced reduced analgesic effects in rats, given a higher, 3.5% concentration (treatment F1,20 = 13.62, p < 0.001; Figure 3(b)) and no effect after the highest, 5% i.pl. formalin injection (Figure 3(c)). Prkcz−/− mice, when compared with Prkcz+/− or Prkcz+/+ animals, showed dramatically reduced nociception after low concentration (1%) i.pl. formalin (Figure 3(d), genotype F2,28 = 94.617, p < 0.001), but only a minor suppression after administration of a higher concentration (2%) i.pl. formalin (Figure 3(e), Genotype × Time interaction: F88,1144 = 1.894, p < 0.001). Our formalin and SNI data thus suggest that the antinociceptive effect of PKMζ inhibition or silencing depends largely on the degree of ongoing peripheral inputs.
Figure 3.
Effects of PKMζ inhibition and genetic ablation in the formalin test. (a–c). Differential effects of formalin concentration on antinociceptive effects of PKMζ inhibition in the formalin test. ZIP treatment at 25 min post-2% formalin resulted in a significant reduction of nociceptive scores between 32 and 44 min post-formalin (a), while eliciting shorter effects (38–44 min) against 3.5% formalin (b) and no effects against 5% formalin (c). Asterisks indicate significant differences (p < 0.05) between ZIP- and scrambled ZIP-treated groups. (d) Prkcz−/− mice injected with 1% formalin showed a persistent decrease in nociceptive scores beginning 16 min postinjection until 44 min. Prkcz+/+ and Prkcz+/− mice exhibited the normally observed increase in formalin-induced nociceptive behaviors in the second phase. (e) Prkcz−/− mice injected with 2% formalin showed a weaker attenuation of formalin-induced nociception from 32 to 42 min. Prkcz+/+ and Prkcz+/− mice displayed higher second phase scores as in (d) (*p < 0.05 compared to Prkcz+/−; †p < 0.05 compared to Prkcz+/+ in c and d). (a–e) All of the data are presented as mean ± S.E.M. Note. PKMζ = protein kinase M zeta; ZIP = ζ-inhibitory peptide.
PKMζ inhibition in referred visceral pain
We have previously shown that i.t. ZIP both prevents and reverses allodynia that extends 24 h following i.pl. injection of capsaicin to the rat hind paw.7 We expanded on this cutaneous study to determine whether spinal PKMζ might also contribute to the maintenance of longer lasting referred pain associated with visceral injury. After rats were given an i.c. instillation of 200 µL of 0.1% capsaicin (Figure 4(a)), they developed a referred hind paw allodynia, evidenced by lowered PWTs for 24 h (Figure 4(b)). Intrathecal ZIP administered either 20 min before (Figure 4(c)) or 60 min after (Figure 4(d)) i.c. capsaicin prevented and reversed hypersensitivity, respectively, up to 24 h compared with ineffective scrZIP treatment (drug effect: F1,10 = 148.01, p < 0.001 and F1,10 = 102.303, p < 0.001, respectively).
Figure 4.
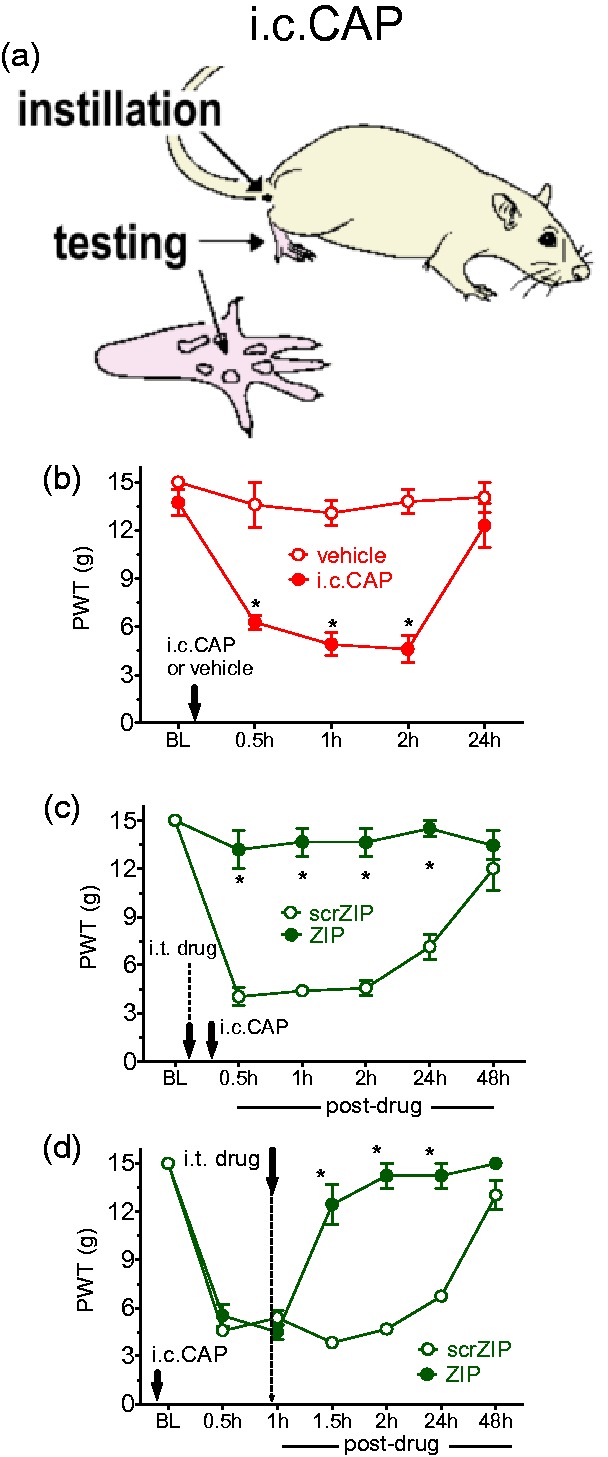
Effects of PKMζ inhibition in referred visceral pain. (a) Site of capsaicin instillation and testing of hind paw referred allodynia. (b) A single instillation of i.c. capsaicin (i.c. CAP) in male rats resulted in mechanical allodynia, with 50% withdrawal thresholds returning to baseline at 24 h postinstillation. (c and d) Pharmacological inhibition of PKMζ activity 20 min before (c) and 1 h after (d) i.c. capsaicin resulted in both prevention and reversal of mechanical allodynia, respectively, up to 24 h posttreatment. (b–d) Asterisks indicate a difference between ZIP- and scrambled ZIP-treated groups (*p < 0.05). All of the data were collected in male rats and are presented as mean ± S.E.M. Note. PKMζ = protein kinase M zeta; i.c. = intracolonic; ZIP = ζ-inhibitory peptide.
PKMζ inhibition in referred visceral pain with hyperalgesic priming
As the referred allodynia induced by i.c. capsaicin is short-lived, we employed a method known as hyperalgesic priming6,11 to induce longer lasting pain hypersensitivity. Thus, a second i.c. capsaicin instillation was performed two weeks after the first, resulting in referred allodynia persisting for six more days (Figure 5(a), Greenhouse–Geisser (GG) corrected injury × Time interaction: F2.215, 19.932 = 12.907, p < 0.001). Intrathecal ZIP and scrZIP effects were then assessed when administered as a 20 min pretreatment (i.e., induction), an intervening treatment (20 min before the second instillation (i.e., maintenance of the priming), or as a posttreatment 24 h following the second instillation (i.e., maintenance of the response to challenge). Significant antiallodynic ZIP effects were observed after pre- (Figure 5(b)), intervening- (Figure 5(c)), or posttreatment (Figure 5(d), GG corrected treatment × Time interaction: F7,80 = 3.032, p = 0.007; F3.869, 38.692 = 9.401, p < 0.001 and F3.673, 36.727 = 4.994, p = 0.003, respectively).
Figure 5.
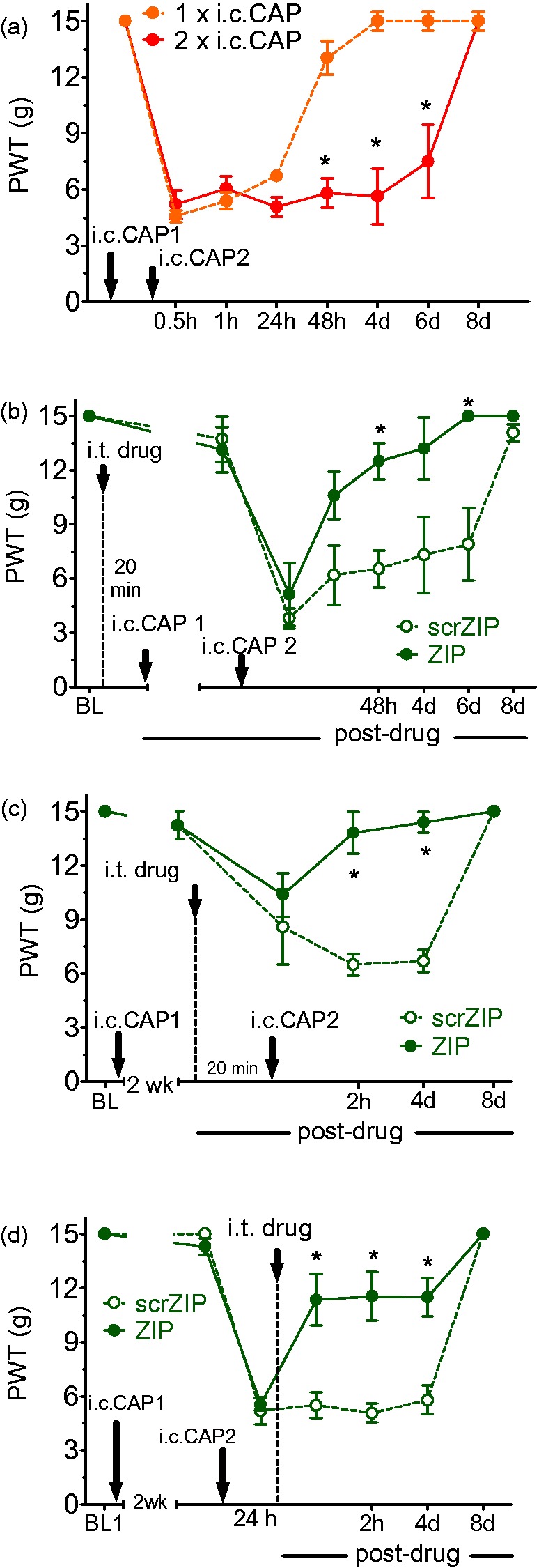
Effects of PKMζ inhibition in referred visceral pain with hyperalgesic priming. (a) A second i.c. capsaicin (i.c. CAP) instillation administered two weeks after the first resulted in the persistence of referred mechanical allodynia for up to six days post second instillation enabling us to study ZIP effects against long-lasting pain maintenance. (b–d) Long-lasting pain resulting from two repeated i.c. capsaicin instillations (spaced two weeks apart) was effectively attenuated by PKMζ inhibition, when ZIP was administered 20 min before the first priming stimulus (b), two weeks after the first and 20 min before the challenging stimulus (c), and 24 h after the challenging stimulus (d). In all three treatment scenarios, scrZIP treatment was observed to be ineffective. (a–d) Asterisks indicate a difference between treatment groups (*p < 0.05). All of the data were collected in male rats and are presented as mean ± S.E.M. Note. PKMζ = protein kinase M zeta; i.c. = intracolonic; ZIP = ζ-inhibitory peptide; scrZIP = scrambled ZIP.
PKMζ inhibition in referred muscle pain
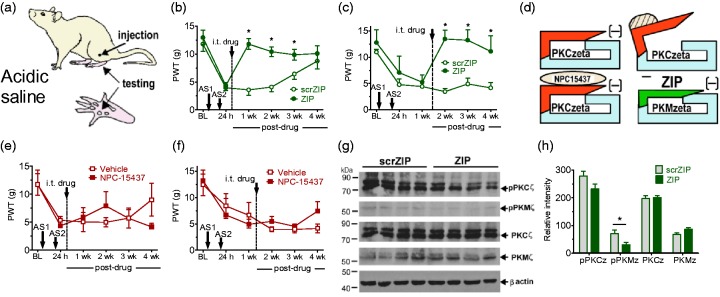
We next assessed the effects of i.t. ZIP or scrZIP in another referred pain model in which persistent allodynia develops in the rat hind paw following muscle injury produced by two injections of intramuscular (i.m.) acidic saline to the gastrocnemius muscle in the thigh (Figure 6(a)). We showed that i.t. ZIP, but not scrZIP, at either 24 h (Figure 6(b), F1,3 = 12.93, p < 0.001) or one week (Figure 6(c), F3,20 = 21.2, p < 0.001) postinjury resulted in a four-week reversal of allodynia. No antiallodynic effects were observed after administration of the full-length PKC inhibitor NPC-15437 (Figure 6(d)), given as either 24 h (Figure 6(e)) or one week (Figure 6(f)) posttreatment. Furthermore, i.t. ZIP, but not scrZIP, given one week postinjury, produced a significant decrease in the immunodensity of phosphorylated PKMζ in spinal cord dorsal horn, as determined by western blotting (two-tailed), t(6) = 2.521, p = 0.045 (Figures 6(g) and (h)). We conclude that a PKMζ-dependent mechanism is critical to the maintenance of long-lasting referred visceral and muscle pain, which is possible to demonstrate due to the remoteness between injury and test sites.
Figure 6.
Effects of PKMζ inhibition in referred muscle pain. (a) Sites of acidic saline (AS) injection and testing of hind paw referred allodynia. (b and c) ZIP treatment 24 h (b) or one week (c) after the second acidic saline injection resulted in a significant reversal of mechanical allodynia up to four weeks posttreatment compared with scrZIP. (d) Separate actions of ZIP versus NPC-15437 on PKMζ and full-length PKC, respectively. The cyan and red segments represent catalytic and regulatory domains, respectively. The dashed moiety denotes the second messenger. (e and f) NPC-15437 given either 24 h (e) or one week (f) after the second acidic saline injection was without any effect. (g and h) ZIP treatment at one week after the second acidic saline injection resulted in a significant reduction in phosphorylated PKMζ (pPKMζ), but had no effect on total PKMζ, total PKCζ, or pPKCζ, compared to the effects of scrZIP. Asterisks indicate a difference between ZIP and scrZIP (*p < 0.05). (b, c, e, f, and h) All of the data were collected in male rats and are presented as mean ± S.E.M. Note. PKMζ = protein kinase M zeta; i.c. = intracolonic; ZIP = ζ-inhibitory peptide; scrZIP = scrambled ZIP.
Sex differences after PKMζ genetic ablation in referred visceral pain
We next repeated the referred visceral pain experiments in PKC/Mζ mutant mice. In accordance with the pharmacological results obtained in rats, mutant male mice (Prkcz−/−) showed only a transient allodynia 30 min post i.c. capsaicin. In contrast, Prkcz+/+ and Prkcz+/− males remained persistently allodynic and recovered only at 24 h. ZIP treatment in both Prkcz+/+ and Prkcz+/− males resulted in PWTs similar to those observed in Prkcz−/− mice (Genotype × Time interaction: F16,210 = 14.12, p < 0.0001) (Figure 7(a)). Allodynia developed fully in i.c. capsaicin-treated female Prkcz−/− mice (Figure 7(b) and (c)), but weakly or not at all in males (Sex × Time interaction: F4,85 = 13.87, p = 0.001 and Genotype × Sex interaction: F4,85 = 10.87, p < 0.001, respectively—Figure 7(a)(c)). ZIP treatment reversed visceral referred pain in Prkcz+/+ and Prkcz+/− males at 1 and 2 h and additionally in Prkcz+/− males at 24 h (Figure 7(a)). To directly compare ZIP effects in male and female WT mice, one group of each was tested after i.c. capsaicn and given 10 nmol ZIP i.t. Treatment effects were found to differ by sex and time (F4,72 = 7.669, p < 0.0001, Figure 7(d)), males showing less allodynia than females after ZIP at 0.5 and 1 h after i.c. capsaicin (Figure 7(d)).
Figure 7.
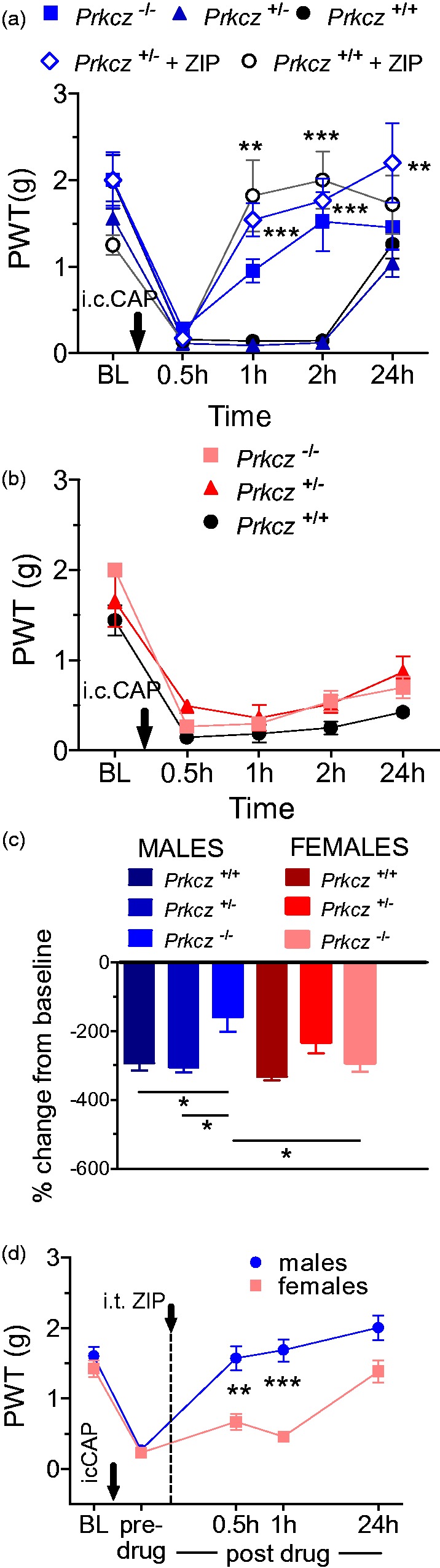
Sex differences after PKMζ genetic ablation in referred visceral pain. (a and b) Time course of development of mechanical allodynia in male transgenic mice after i.c. capsaicin (i.c. CAP) instillation. (a) A male-specific reversal of mechanical allodynia was observed after i.c. capsaicin instillation in Prkcz−/− mice. In addition, i.t. ZIP treatment in Prkcz+/+ and Prkcz+/− males resulted in the restoration of post-i.c. capsaicin PWTs to baseline values. Asterisks denote significant difference between ZIP-treated and untreated animals of the same genotype (**p < 0.01, ***p < 0.001). (b) Female knockouts displayed persistent allodynia after i.c. capsaicin throughout the 24 h period of testing. (c) Prkcz−/− male mice showed a smaller change in PWTs from baseline than Prkcz+/− or Prkcz+/+ males. Prkcz−/− female mice also show a larger change in PWTs from baseline than Prkcz−/− males (*p < 0.05). (d) ZIP treatment also produced a greater antiallodynic effect in Prkcz+/+ male, when compared with Prkcz+/+ female, mice (**p < 0.01, ***p < 0.001). (a–d) All of the data are presented as mean ± S.E.M. Males—blue shaded lines and bars; females—red/pink shaded lines and bars. Note. PKMζ = protein kinase M zeta; i.c. = intracolonic; i.t. = intrathecal; ZIP = ζ-inhibitory peptide; PWTs = paw withdrawal thresholds.
Sex differences after PKMζ genetic ablation in referred muscle pain
We next repeated the referred muscle pain experiments in our PKC/Mζ mutant mice. Once again, given the greater prevalence of muscle pain syndromes in females,13 and the reported sex differences for PKMζ’s role in memory maintenance,14 we again tested both male and female mutant mice. Consistent with referred allodynia after visceral injury, Prkcz−/− male mice displayed higher PWTs than their Prkcz+/+ and Prkcz+/− counterparts for four weeks after muscle injury (Genotype × Time interaction: F8,125 = 2.618, p = 0.011; Figure 8(a)). Persistent allodynia developed fully over four weeks in i.m. acidic saline-treated female Prkcz−/− mice (Figure 8(b) and (c)), but not in males (Figure 8(a) and (c); Sex × Time: F4,75 = 2.798, p = 0.032 and Sex × Time: F4,85 = 10.87, p < 0.001, respectively). Overall, our data indicate that PKC/Mζ ablation attenuated referred visceral pain more in male than in female mice.
Figure 8.
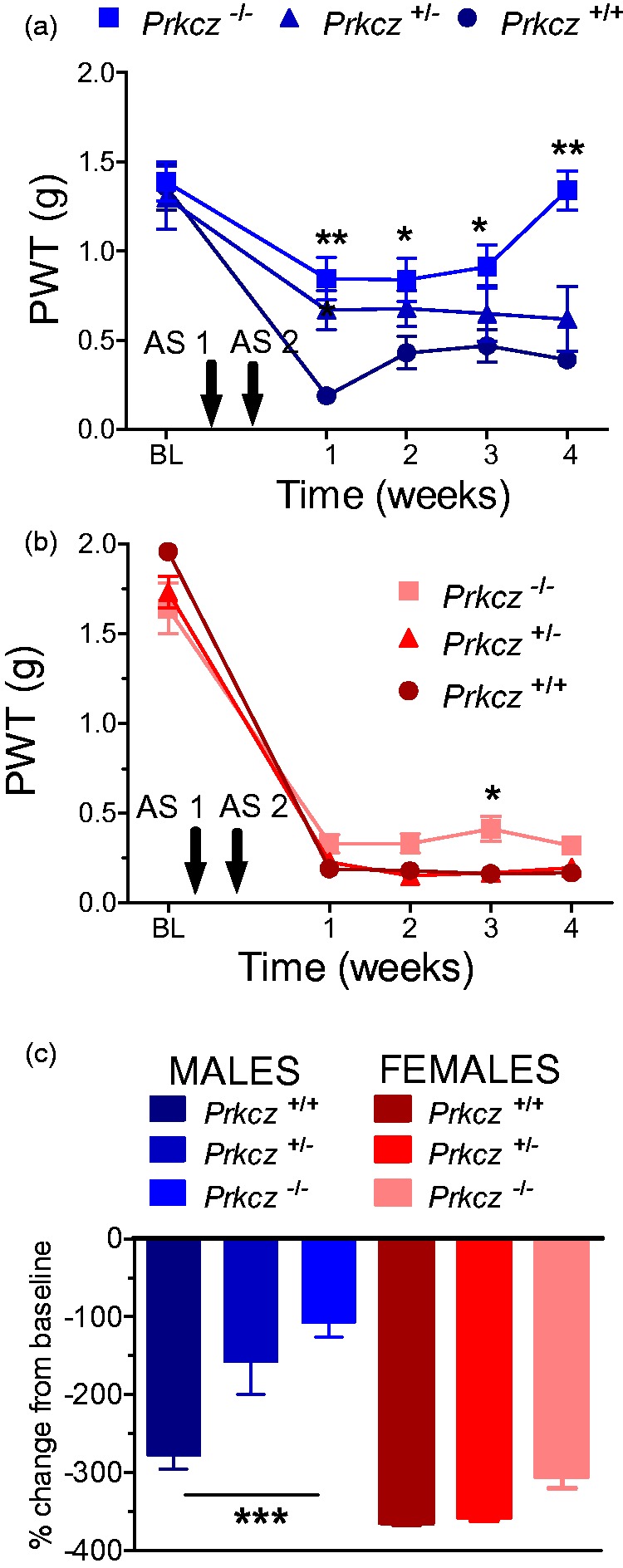
Sex differences after PKMζ genetic ablation in referred muscle pain. (a and b) Time course of development of mechanical allodynia in male transgenic mice after i.m. acidic saline (AS) injection. (a) Mutant (Prkcz−/−) male mice displayed significantly higher PWTs over a period of four weeks after acidic saline injection compared with their Prkcz+/+ and Prkcz+/− counterparts. Asterisks indicate differences between Prkcz−/− and Prkcz+/+ (*p < 0.05, **p < 0.01). Dagger indicates difference between Prkcz−/− and Prkcz+/− (†p < 0.05. (b) Prkcz−/− female mice only showed a small reduction in mechanical allodynia compared with wild-type at three weeks (c) Prkcz−/− male mice show a smaller change in PWTs from baseline than Prkcz+/+ males, while female PWTs do not change across genotype (***p < 0.001). (a–c) All of the data are presented as mean ± S.E.M. Males—blue shaded lines and bars; females—red/pink shaded lines and bars. Note. PKMζ = protein kinase M zeta; i.m. = intramuscular; ZIP = ζ-inhibitory peptide; PWTs = paw withdrawal thresholds.
Sex differences after PKMζ inhibition in rat formalin and muscle pain
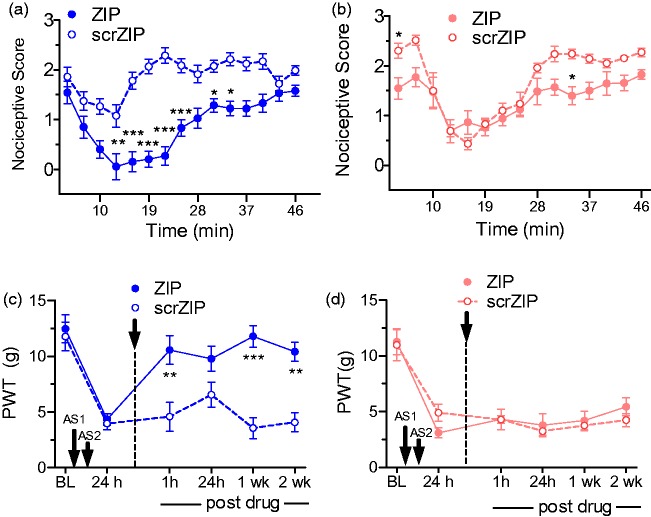
To parallel the sex difference studies in Prkcz−/− mice, additional experiments were performed in both male and female rats treated with ZIP. Similar to Prkcz−/− mice in other models, ZIP treatment produced less antinociception in the formalin test in female than in male rats (Sex × Drug treatment interaction, F14,345 = 22.89, p < 0.0001). When compared to the scrambled ZIP control group of the same sex, ZIP reduced nociceptive scores in males from 10 to 37 min post-formalin (Figure 9(a)), but only at the 4- and 33-min time periods post-formalin in females (Figure 9(b)). Pain after ZIP was also significantly less in males than in females at 7 and 10 min post-formalin administration. A similar pattern of results was seen in male and female rats treated with i.m. acidic saline. Thus, males, but not females, that received ZIP after the second of two i.m. acidic saline injections displayed a reduction of allodynia from 1 h to 2 weeks post-ZIP compared with scrambled ZIP (Sex × Drug interaction F1,21 = 26.772, p < 0.0001, Figure 9(c) and (d).
Figure 9.
Sex differences after PKMζ inhibition in formalin and muscle pain in rats. (a) Effects of ZIP or scrambled ZIP treatment on the response to 2% formalin in male (a) and female (b) rats. Relative to scrZIP, ZIP administration had a larger effect in male vs. female rats. For each sex, asterisks indicate a difference between ZIP- and scrZIP-treated groups (*p < 0.05, **p < 0.01, ***p < 0.001). ZIP treatment after the second of two intramuscular injections of acidic saline (AS) resulted in a significant increase in PWTs up to two weeks posttreatment in male rats (c), but was without comparable effect in female rats (d). Asterisks indicate a difference between ZIP- and scrZIP-treated groups (**p < 0.01, ***p < 0.001). (a–d) All of the data are presented as mean ± S.E.M. Males—blue shaded lines and bars; females—red/pink shaded lines. Note. PKMζ = protein kinase M zeta; ZIP = ζ-inhibitory peptide; scrZIP = scrambled ZIP; PWTs = paw withdrawal thresholds.
These findings suggest that PKMζ may either contribute more strongly to the maintenance of pain in male versus female rats, or in the case of transgenic mice, that a potential compensatory mechanism following constitutive knockout is more efficient in fulfilling the maintenance role in females as has been proposed for LTP.26
Discussion
This study provides novel insights into the role of PKMζ in models of persistent pain. First, we have shown that pharmacological inhibition of PKMζ using ZIP did not reverse CFA-induced ipsilateral thermal or mechanical allodynia or SNI-induced ipsilateral mechanical allodynia. Our results support the previous reports of weak or no effects of ZIP on CFA-induced hypersensitivity,6,8,27 suggesting PKMζ activity alone is not responsible for sustaining the underlying nociceptive sensitization. Our findings are also in line with previous studies conducted in rats with various nerve injuries showing PKMζ inhibition using ZIP did not alter ipsilateral hypersensitivity.6–8,28 Alternatively, the development of contralateral allodynia in SNI rats at 6 weeks after surgery was transiently reversed by spinal PKMζ inhibition. As evidence indicates that pain hypersensitivity after nerve injury depends heavily on hypersensitivity or enhanced inputs from damaged and undamaged afferents,29–31 we concluded that the inability to demonstrate a role for spinal PKMζ in the maintenance of ipsilateral neuropathic pain after SNI is masked by the overwhelming contribution of peripheral inputs. This conclusion is supported by our earlier demonstration that in rats with chronic postischemic pain, ZIP does not reduce allodynia at an early stage when peripheral pathology is evident but does reduce allodynia at a later stage when peripheral pathology has largely resolved.7
Importantly, we also showed that the pharmacological effects of ZIP in neuropathic rats were replicated in mice with genetic ablation of PKMζ. Thus, while PKC/Mζ knockout mice exhibited normal ipsilateral mechanical allodynia, they also showed significantly reduced contralateral allodynia four weeks post-nerve injury when compared with heterozygous and WT mice, suggesting PKMζ is required for the development of contralateral allodynia. Importantly, it has been shown that neuropathic contralateral allodynia is insensitive to doses of i.v. lidocaine that reverse ipsilateral allodynia, suggesting that contralateral allodynia depends on central sensitization.32
To discern the contribution of central neuroplasticity and ongoing peripheral inputs, we conducted formalin tests with increasing concentrations of formalin: 2%, 3.5%, and 5% in rats posttreated with ZIP. It has been established that the nociceptive responses to higher concentration formalin depend to a greater extend on peripheral inputs, as high, but not low, concentration formalin pain is reduced by anti-inflammatory agents.33 We reproduced our previous results7 showing PKMζ inhibition largely reversed second phase nociception after 2% formalin and importantly also showed considerably reduced analgesia in rats given 3.5% formalin, and no analgesia in rats given 5.0% formalin. These results are consistent with our earlier demonstration that preemptive spinal anesthesia is capable of reducing nociception induced by lower concentrations of formalin that do not produce plasma extravasation, but has no effects on higher concentrations which produce significant plasma extravasation.34 The results are also consistent with the report that posttreatment with ZIP to the periaqueductal gray area was unable to alleviate second phase nociceptive responses to 5.0% formalin.27 In contrast, spinal pretreatment with ZIP has been shown to reduce subsequent nociceptive responses to 5.0% formalin,8 although clearly the maintenance of nociceptive sensitization must be assessed using posttreatments.
Once again, we showed that the pharmacological effects of ZIP in formalin-treated rats were replicated in mice with genetic ablation of PKMζ. Thus, protein kinase M (PKM)/Cζ knockout substantially reduced second phase formalin pain at 1%, but the effect was considerably less pronounced at 2% formalin. Conversely, heterozygous and WT mice developed full second phase pain regardless of formalin concentration. These data support our hypothesis that increasing peripheral inputs masks the ability of ZIP to demonstrate a role for spinal PKMζ in the maintenance of nociceptive sensitization.
We further investigated whether PKMζ activity plays a role in the maintenance of long-lasting referred visceral pain. Earlier studies have shown that the remote allodynia that is expressed in the hind paw after i.c. capsaicin could be prevented by pretreatment with an inhibitor of ERK,35 implicating this protein kinase (PK) in the induction of the allodynia. Furthermore, plasma membrane-targeted trafficking of GluR1 associated with i.c. capsaicin was found to be blocked by pretreatment with an inhibitor of CaMKII,36 therefore also implicated in allodynia induction. However, studies here are the first to show that remote allodynia after i.c. capsaicin was reversed by posttreatment (1 h after capsaicin) as well as pretreatment with a PK inhibitor—in this case, an inhibitor of PKMζ, for which a single pre- or posttreatment completely prevented or reversed, respectively, remote allodynia for the 24 h duration of testing. This clearly implicates PKMζ in the maintenance of remote allodynia after visceral injury. These findings are consistent with our earlier finding that ZIP pretreatment or posttreatment alleviated secondary mechanical allodynia induced by i.pl. capsaicin injection.7 As in the case of neuropathic contralateral allodynia and low concentration formalin, the antiallodynic effects of pharmacological inhibition of PKMζ on i.c. capsaicin-induced allodynia were replicated by genetic ablation of PKMζ. However, in this model, we examined sex-dependent effects of PKM/Cζ knockout and showed that while PKM/Cζ knockout reduced i.c. capsaicin-induced allodynia in males, it had no effect in females.
As the remote allodynia induced by i.c. capsaicin is relatively short-lived (24 h), we used two i.c. capsaicin instillations to produce a more prolonged remote allodynia in the rat hind paw. This method replicated previous demonstrations of what has been called hyperalgesic priming, a term first used to describe the prolonged hyperalgesia induced by intradermal prostaglandin E2 in animals that were earlier primed by carrageenan injection.37 Here, two injections of i.c. capsaicin increased the length of remote hyperalgesia from 24 h after a single instillation up to six days after the second instillation. As in the case of a single i.c. capsaicin instillation, pretreatment or posttreatment with ZIP prevented or reversed, respectively, the allodynia for the duration of testing (six days) following a second i.c. capsaicin instillation. Our study is consistent with earlier studies which have shown that PKMζ inhibition reduces hyperalgesic priming elicited with hind paw IL-6, BDNF, or carrageenan injections followed by PGE2 injection or plantar incision or plantar incision followed by PGE2 injection.4,5,9 In these studies, however, PKMζ was inhibited prior to the priming stimulus or prior to the challenging stimulus. Thus, we present novel evidence that ZIP reversed established long-lasting allodynia when administered either prior to priming, as well as prior to, or following, the challenging stimulus.
Additional studies examined whether PKMζ activity plays a role in the maintenance of long-lasting referred muscle pain. Once again, earlier studies had shown that remote allodynia that is expressed in the hind paw after two injections of i.m. acetic saline could be reduced by pretreatment with an inhibitor of protein kinase A (PKA),37 but not by posttreatment with inhibitors of either PKA38 or PKC.39 When injected at either 24 h or one week post-i.m. acidic saline, the PKMζ inhibitor ZIP, but not the full-length PKC inhibitor NPC-15437, reversed the established mechanical allodynia. Importantly again, a single injection of ZIP at either of these posttreatment time points reversed allodynia for its duration (three to four weeks); thus strongly implicating PKMζ in the maintenance of remote allodynia after muscle injury. The lack of effect of the PKC inhibitor on remote allodynia after i.m. acetic saline supported our earlier effects demonstrating no contribution of PKC to the maintenance of nociceptive sensitization after cutaneous injury.7 Concordant with a reversal of i.m. acidic saline-induced remote allodynia following ZIP, the western blot data highlighted a decrease of pPKMζ, but not pPKCζ/λ, nor total PKM/Cζ/λ levels, compared to that produced by scrZIP. These data further indicate that a single injection of ZIP is sufficient for the disruption of PKMζ’s persistent activity. As in the case of allodynia after i.c. capsaicin, the anti-allodynic effects of pharmacological inhibition of PKMζ for i.m. acetic acid-induced allodynia were replicated by genetic ablation of PKMζ. However, once again, we found sex-dependent effects of PKM/Cζ knockout, showing that although PKM/Cζ knockout reduced i.m. acetic acid-induced allodynia in males, it had almost no effect in females.
Concerning sex differences, we generally show that female mice of all genotypes develop greater allodynia than their male counterparts—which is in line with previous reports on sex differences regarding pain behavior.40,41 However, the sex discrepancy in allodynia was most profound in Prkcz knockout mice—where females developed robust persistent allodynia, while their male counterparts did not. This finding suggests PKM/Cζ may contribute more strongly to the maintenance of pain and remote allodynia in male mice than in female mice, as has been shown for PKMζ’s role in long-term spatial memory.42 Alternatively, a potential compensatory mechanism which develops following constitutive knockout may be more efficient in fulfilling the maintenance role in female mice than in males, as has been shown the role of GluR1 and nitric oxide for hippocampal-dependent contextual conditioning.43 However, the former may be more likely, as our further studies on ZIP effects in both male and female rats indicated that PKMζ inhibition had greater effects in males than females both on nociception in the formalin test and on remote allodynia after either i.c. capsaicin or i.m. acetic acid.
Overall, our results indicate that, unlike findings with hippocampal LTP and memory maintenance, a consistency exists between the pharmacological antinociceptive effects of ZIP and the effects of genetic ablation, which implicates significant PKMζ-dependent mechanisms in the maintenance of long-term spinal nociceptive sensitization. Differences between models of referred pain versus inflammatory and neuropathic pain stress the importance of accounting for masking effects of afferent inputs on the modulation of central sensitization when assessing nociceptive maintenance. Importantly, there is also a differential contribution of PKMζ to pain maintenance between sexes. We propose that the maintenance of spinal nociceptive sensitization in females may rely more heavily on factors than PKMζ.
Acknowledgments
The authors wish to thank Robert O Messing for supplying the breeders for the null mice; Virginia Cornea, Stephanie Gregoire, and Magali Millecamps for assistance with surgical and behavioral testing training; and Flora Lopes for animal breeding and care.
Author Contributions
TJC and HN designed the study; HN, AL, SD, and AM conducted behavioral experiments; HM and HN performed protein assays; SG and HN bred and genotyped the mice; HN performed statistical analysis; HN, AL, and JVR prepared figures; HN, JVR, and TJC wrote the paper; TJC and US supervised experiments and edited the paper.
Declaration of Conflicting Interests
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Canadian Institutes of Health Research (MOP-53246 and MOP-119279), the Natural Sciences and Engineering Research Council (RGPIN/05605), and Louise and Alan Edwards Foundation to TJC. HN was supported by a studentship from the Louise and Alan Edwards Foundation.
References
- 1.Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci 2002; 22: 4196–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sluka KA, Willis WD. The effects of G-protein and protein kinase inhibitors on the behavioral responses of rats to intradermal injection of capsaicin. Pain 1997; 71: 165–178. [DOI] [PubMed] [Google Scholar]
- 3.Ji RR, Baba H, Brenner GJ, et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 4.An K, Zhen C, Liu ZH, et al. Spinal protein kinase Mζ contributes to the maintenance of peripheral inflammation-primed persistent nociceptive sensitization after plantar incision. Eur J Pain 2015; 19: 39–47. [DOI] [PubMed] [Google Scholar]
- 5.Melemedjian OK, Tillu DV, Asiedu MN, et al. BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol Pain 2013; 9: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King T, Qu C, Okun A, et al. Contribution of PKMζ-dependent and independent amplification to components of experimental neuropathic pain. Pain 2012; 153: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laferrière A, Pitcher MH, Haldane A, et al. PKMζ is essential for spinal plasticity underlying the maintenance of persistent pain. Mol Pain 2011; 7: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchand F, D’Mello R, Yip PK, et al. Specific involvement of atypical PKCζ/PKMζ in spinal persistent nociceptive processing following peripheral inflammation in rat. Mol Pain 2011; 7: 86–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asiedu MN, Tillu DV, Melemedjian OK, et al. Spinal protein kinase M ζ underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci 2011; 31: 6646–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AM, Kanter BR, Wang D, et al. Prkcz null mice show normal learning and memory. Nature 2013; 493: 416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volk LJ, Bachman JL, Johnson R, et al. PKM-ζ is not required for hippocampal synaptic plasticity, learning and memory. Nature 2013; 493: 420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology 2002; 123: 1686–1701. [DOI] [PubMed] [Google Scholar]
- 13.Yunus MB. Gender differences in fibromyalgia and other related syndromes. J Gend Specif Med 2002; 5: 42–47. [PubMed] [Google Scholar]
- 14.Sebastian V, Vergel T, Baig R, et al. PKMζ differentially utilized between sexes for remote long-term spatial memory. PLoS One 2013; 8: e81121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015; 18: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorge RE, LaCroix-Fralish ML, Tuttle AH, et al. Spinal cord toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011; 31: 15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet 1998; 1: 136–141. [DOI] [PubMed] [Google Scholar]
- 18.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 19.Sanoja R, Tortorici V, Fernandez C, et al. Role of RVM neuronsin capsaicin-evoked visceral nociception and referred hyperalgesia. Eur J Pain 2010; 14: 120.e1–120.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laird JM, Olivar T, Roza C, et al. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience 2000; 98: 345–352. [DOI] [PubMed] [Google Scholar]
- 21.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001; 24: 37–46. [DOI] [PubMed] [Google Scholar]
- 22.Sharma NK, Ryals JM, Liu H, et al. Acidic saline-induced primary and secondary mechanical hyperalgesia in mice. J Pain 2009; 10: 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 24.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 25.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977; 4: 161–174. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XC, Zhang YQ, Zhao ZQ. Involvement of nitric oxide in long-term potentiation of spinal nociceptive responses in rats. Neuroreport 2005; 16: 1197–1201. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Lu B, Yao J, et al. 2014) Intra-periaqueductal gray infusion of zeta inhibitory peptide attenuates pain-conditioned place avoidance in rats. Brain Res 2014; 1582: 55–63. [DOI] [PubMed] [Google Scholar]
- 28.Li XY, Ko HG, Chen T, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 29.Tal M, Eliav E. Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain 1996; 64: 511–518. [DOI] [PubMed] [Google Scholar]
- 30.Boada MD, Gutierrez S, Aschenbrenner CA, et al. Nerve injury induces a new profile of tactile and mechanical nociceptor input from undamaged peripheral afferents. J Neurophysiol 2015; 113: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith AK, O’Hara CL, Stucky CL. Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model. Mol Pain 2013; 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinnott CJ, Garfield JM, Strichartz GR. Differential efficacy of intravenous lidocaine in alleviating ipsilateral versus contralateral neuropathic pain in the rat. Pain 1999; 80: 521–531. [DOI] [PubMed] [Google Scholar]
- 33.Yashpal K, Coderre TJ. Influence of formalin concentration on the antinociceptive effects of anti-inflammatory drugs in the formalin test in rats: separate mechanisms underlying the nociceptive effects of low- and high-concentration formalin. Eur J Pain 1998; 2: 63–68. [DOI] [PubMed] [Google Scholar]
- 34.Yashpal K, Katz J, Coderre TJ. Effects of preemptive or postinjury intrathecal local anesthesia on persistent nociceptive responses in rats. Confounding influences of peripheral inflammation and the general anesthetic regimen. Anesthesiology 1996; 84: 1119–1128. [DOI] [PubMed] [Google Scholar]
- 35.Galan A, Cervero F, Laird JM. Extracellular signaling-regulated kinase-1 and -2 (ERK 1/2) mediate referred hyperalgesia in a murine model of visceral pain. Brain Res: Mol Brain Res 2003; 116: 126–134. [DOI] [PubMed] [Google Scholar]
- 36.Galan A, Laird JM, Cervero F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain 2004; 112: 315–323. [DOI] [PubMed] [Google Scholar]
- 37.Parada CA, Yeh JJ, Joseph EK, et al. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci 2003; 17: 1847–1852. [DOI] [PubMed] [Google Scholar]
- 38.Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci 2003; 23: 5437–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sluka KA, Audette KM. Activation of protein kinase C in the spinal cord produces mechanical hyperalgesia by activating glutamate receptors, but does not mediate chronic muscle-induced hyperalgesia. Mol Pain 2006; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aloisi AM, Albonetti ME, Carli G. Sex differences in the behavioural response to persistent pain in rats. Neurosci Lett 1994; 179: 79–82. [DOI] [PubMed] [Google Scholar]
- 41.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature Rev Neurosci 2012; 13: 859–866. [DOI] [PubMed] [Google Scholar]
- 42.Sebastian V, Vergel T, Baig R, Schrott LM, et al. PKMzeta differentially utilized between sexes for remote long-term spatial memory. PLoS One 2013; 8: e81121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dachtler J, Fox KD, Good MA. Gender specific requirement of GluR1 receptors in contextual conditioning but not spatial learning. Neurobiol Learn Mem 2011; 96: 461–467. [DOI] [PubMed] [Google Scholar]