Our study adds to the growing body of evidence that a season of play in a contact sport can result in brain MR imaging changes, even in the absence of concussion.
Abstract
Purpose
To examine the effects of subconcussive impacts resulting from a single season of youth (age range, 8–13 years) football on changes in specific white matter (WM) tracts as detected with diffusion-tensor imaging in the absence of clinically diagnosed concussions.
Materials and Methods
Head impact data were recorded by using the Head Impact Telemetry system and quantified as the combined-probability risk-weighted cumulative exposure (RWECP). Twenty-five male participants were evaluated for seasonal fractional anisotropy (FA) changes in specific WM tracts: the inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus, and superior longitudinal fasciculus (SLF). Fiber tracts were segmented into a central core and two fiber terminals. The relationship between seasonal FA change in the whole fiber, central core, and the fiber terminals with RWECP was also investigated. Linear regression analysis was conducted to determine the association between RWECP and change in fiber tract FA during the season.
Results
There were statistically significant linear relationships between RWEcp and decreased FA in the whole (R2 = 0.433; P = .003), core (R2 = 0.3649; P = .007), and terminals (R2 = 0.5666; P < .001) of left IFOF. A trend toward statistical significance (P = .08) in right SLF was observed. A statistically significant correlation between decrease in FA of the right SLF terminal and RWECP was also observed (R2 = 0.2893; P = .028).
Conclusion
This study found a statistically significant relationship between head impact exposure and change of FA value of whole, core, and terminals of left IFOF and right SLF’s terminals where WM and gray matter intersect, in the absence of a clinically diagnosed concussion.
© RSNA, 2016
American football has a high rate of traumatic brain injury (TBI) among contact team sports in the United States (1–3). It is estimated that there are more than 6 000 000 athletes who play football in the United States. The vast majority of these players participate at the youth and high school level. Nearly 3 000 000 youth school players participate in tackle football programs across the United States (4).
Concussions can result from a broad range of impact magnitudes, and there is little correlation between impact magnitude, severity of patient reported symptoms, and clinical outcome (5–7). A growing body of evidence suggests that repetitive subconcussive head trauma can also result in neurocognitive deficits (8,9). A generally accepted hypothesis is that microstructural white matter (WM) damage and axonal injury is responsible for the neurologic dysfunction that is associated with TBI (10–13).
To measure the underlying biomechanical response and head impact exposure for each player during the football season, an effective technique is required. The number and magnitude of head impacts that football players experience is highly individualized, and some players experience higher exposure during a season than others (14). The Head Impact Telemetry (HIT) system (Simbex, Lebanon, NH) was developed to assist understanding of the relationship between mild TBI and head kinematics. This system is placed at the sidelines and collects data in real time from helmet-embedded sensors. Data gathered includes the number, magnitude, and direction of impacts in both linear and rotational acceleration that can be investigated as biomechanical risk factors (15,16).
Specific fiber tracing shows the pathways passing through regions of interest in the brain that are affected by head impact exposure. This technique can reconstruct intrahemispheric WM bundles that are sensitive to mild TBI (17). Several mild TBI studies (18–20) demonstrated changes in specific tracts, mostly in the forceps minor or major of the corpus callosum, inferior fronto-occipital fasciculus (IFOF), superior longitudinal fasciculus (SLF), and inferior longitudinal fasciculus (ILF). Kraus et al (21) demonstrated reduced WM integrity in specific tracts in the IFOF, SLF, ILF, and corpus callosum in patients with chronic TBI.
Youth age and adolescence is an important time of development that is identified by both immature and mature brain processes (22), with myelination continuing to progress through adolescence (22,23). Several diffusion-tensor imaging studies (24–29) investigated WM tract development in adolescence. Studies by Schmithorst et al (24), Barnea-Goraly et al (25), and Ashtari et al (26) illustrated continued maturation in association and projection tracts during adolescence. Asato et al (28) showed that the tracts that are related to complex behavior determine continued development through adolescence. Maturation by adolescence was previously reported (30,31) in the corticospinal tract, frontal portions of the corona radiate, and in several key long association tracts, including the IFOF, ILF, and SLF.
Studying specific fiber tracts within the brain, especially those of interest in mild TBI, will assist in detecting the location of traumatic axonal injury as it relates to subconcussive head impact exposure. The purpose of this study is to examine the effects of subconcussive impacts resulting from a single season of youth (age range, 8–13 years) football on changes in specific WM tracts as detected with diffusion-tensor imaging in the absence of clinically diagnosed concussions. This is one of just a few studies that investigated the changes in terminal regions of tracts where the gray-white junction is located because of mild TBI. Our hypothesis is that increased head impact exposure over the course of a single football season is associated with decreased fractional anistropy (FA) values of specific cerebral WM tracts, including ILF, SLF, and IFOF.
Materials and Methods
Participants
All research procedures were approved by the Wake Forest School of Medicine institutional review board committee. This study was Health Insurance Portability and Accountability Act–compliant, with written informed parental consent and assent from the participants. Participants were recruited from a local youth football league that participated in the 2012 or 2013 season via phone, e-mail, and in-person meetings. We included all youth football players (age range, 8–13 years) who participated in the practices and games in one season and used the biomechanical instrumentations during the season. During the 2012 football season, athlete concussions were reported on the basis of a player, parent, and coach reporting a suspected concussion. During the 2013 football season, a certified athletic trainer was present during all games and practices and evaluated players suspected of having concussions (32). Players identified with symptoms of a concussion were then evaluated by a sports-medicine physician experienced in the clinical diagnosis and treatment of concussions. We collected 40 pairs of magnetic resonance (MR) image sets for 2012 and 2013 that had the HIT system data. There were nine participants who played in both seasons. We excluded the repeated season from the analysis (n = 9). Participants were excluded if they had a previous concussion (n = 4) or acquired a clinically diagnosed concussion during the season (n = 2). This resulted in 25 unique male participants (age range, 8–13 years; mean age, 11.72 years ± 1.05 [standard deviation]) from the two seasons with complete biomechanical data and pre- and postseason diffusion-tensor imaging. All games and practices were video recorded and reviewed to confirm the accuracy of the impacts.
HIT System Data Collection
All players used football helmets (Riddell Revolution Speed, Rosemont, Ill) that contained MxEncoders that fit into the existing gap between padding in the helmet. All head impact data were verified by video to ensure that the head impacts were the result of the helmet being worn by the athlete. The HIT system is well established and data were processed in concordance with previous methods (33,34). The biomechanical data acquired from the HIT system (35) were aggregated to generate a risk-weighted cumulative exposure (RWE) for each participant (33,36,37). RWE represents the cumulative exposure to subconcussive head impacts over the course of a season, and it is on the basis of the computed risk associated with each head impact measured in terms of linear and rotational acceleration. In this study, the combined-probability RWE (RWECP), which is on the basis of the risk associated with the linear and rotational components of each impact, was computed for each athlete (37). The risk for each respective head impact for a single player was computed and summed to generate the RWECP for the season.
MR Imaging Acquisition
MR imaging data were acquired in accordance with the National Institute of Neurological Disorders and Stroke Common Data Elements advanced protocol recommendations. The images were acquired on a 3-T MR imager (Skyra; Siemens Healthcare, Erlangen, Germany) by using a high-resolution 32-channel head and neck coil (Siemens Healthcare). T1-weighted images were obtained for anatomic correlation by using a three-dimensional volumetric magnetization-prepared rapid gradient-echo sequence with isotropic resolution of 0.9 mm3 (repetition time msec/echo time msec, 1900/2.93; inversion time, 900 msec; flip angle, 9°; 176 sections). Diffusion-tensor imaging sequences were acquired by using a two-dimensional single-shot echo-planar imaging sequence (10 500/99; flip angle, 90°; spatial resolution, 2.2 × 2.2 mm; section thickness, 3 mm; 54 sections; 10 volumes of b = 0 sec/mm2; 30 diffusion directions, with 15 directions of b = 1000 sec/mm2 and 15 directions of b = 2000 sec/mm2). MR imaging data for all participants were obtained before the beginning of the season and after the end of the season.
Fiber Tracking and Fiber Parcellation
Fiber tracking was conducted by using the automated fiber quantification software package (AFQ; http://web.stanford.edu/group/vista/cgi-bin/wiki/index.php/AFQ) via the following steps (38): (a) Whole-brain tractography was performed by using the deterministic streamlines tracking algorithm; (b) fiber tract segmentation was performed by using the two-way point region-of-interest procedure, where each fiber that passes through both regions of interest is a candidate for a specific fiber group; and (c) fiber tract refinement was performed via comparison of each fiber to a fiber tract probability map. ILF and SLF, forceps minors and majors of corpus callosum, and the IFOF are among the most affected WM tracts in mild TBI (18–20,39). We extracted and quantified intrahemispheric association fibers of the ILF and SLF, forceps minors and majors of corpus callosum, and the IFOF. FA measurements across 100 equidistant nodes of the fiber were used to calculate the mean FA within the fiber (38).
To investigate the changes in different parts of fibers, each fiber was partitioned into three parts after fiber extraction. Because it is challenging to determine the exact location of WM tract linkages in the gray matter region (40,41), the volumes that contained the first and the last 10th percentile of equidistant nodes along the fiber were designated as WM fiber terminals. The volumes that contained the centric 40 nodes along the fiber were designated as the fiber core. The terminals located in the overlapping areas of WM and gray matter may have varied between participants (40,42), however, the core of the fiber had less interparticipant variability and their spatial locations remained stationary (19,40).
Statistical Analysis
Percent change of FA was computed as 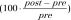 per tract for each participant during the season, where post = postseason FA and pre = preseason FA. Linear regression analysis was conducted by using age and time between the examinations as covariates to determine the associations and correlations between RWECP and change of FA in the fiber tracts during the season. The Mahalanobis distance method was used to remove outliers from each regression (43). P values less than .05 indicated statistical significance. All statistical calculations were performed by using software (JMP Pro version 11.2.0; SAS Institute, Cary, NC).
per tract for each participant during the season, where post = postseason FA and pre = preseason FA. Linear regression analysis was conducted by using age and time between the examinations as covariates to determine the associations and correlations between RWECP and change of FA in the fiber tracts during the season. The Mahalanobis distance method was used to remove outliers from each regression (43). P values less than .05 indicated statistical significance. All statistical calculations were performed by using software (JMP Pro version 11.2.0; SAS Institute, Cary, NC).
Results
Whole Fiber Analysis
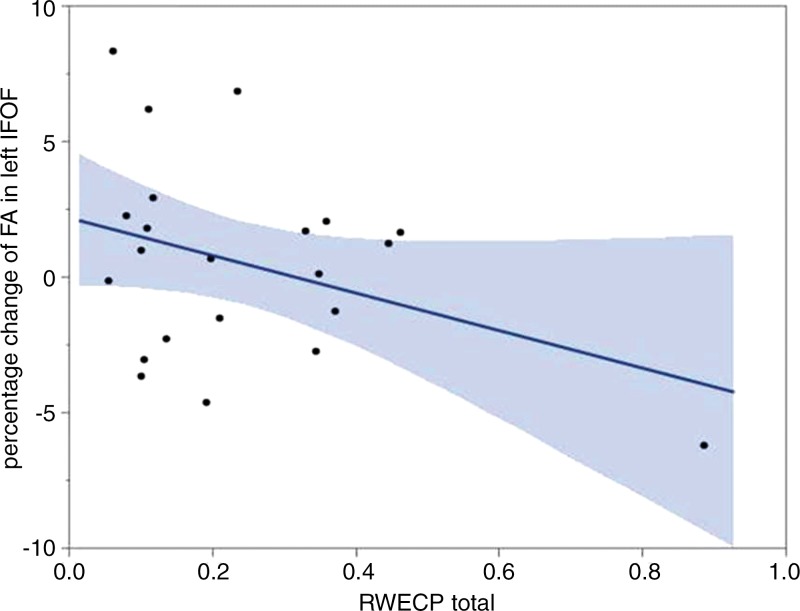
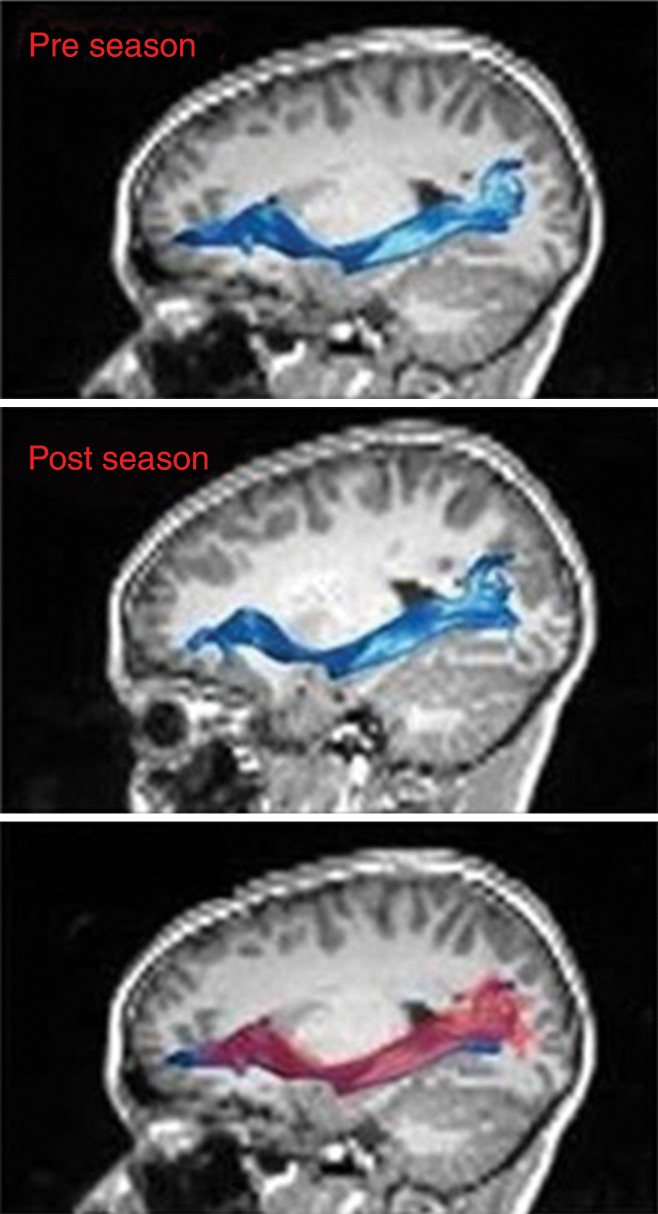
There was a statistically significant linear relationship between RWECP and change of FA (decreased) in the left IFOF (n = 22; R2 = 0.4334; P = .003). Figure 1a shows the association between changes of FA of the left IFOF with respect to RWECP. Figure 1b shows the left IFOF tract, overlaid on the structural T1-weighted image, before the beginning of the season and after the end of the season. There was a statistically significant linear relationship between RWECP and decreased FA in the right SLF (n = 22; R2 = 0.2996; P = .042). No statistically significant association between total RWECP and FA changes in the forceps minor or major of the corpus callosum or ILF were observed.
Figure 1a:
(a) Linear regression plot depicts the relationship between percent change of FA of the left IFOF and cumulative exposure. The blue shaded area indicates the estimated confidence interval region around the true regression line. RWECP = RWECP. (b) MR images of left IFOF (top) before and (middle) after the playing season, and (bottom) the overlay. In the overlay (bottom), the red region is after the season and the blue region is before the season.
Figure 1b:
(a) Linear regression plot depicts the relationship between percent change of FA of the left IFOF and cumulative exposure. The blue shaded area indicates the estimated confidence interval region around the true regression line. RWECP = RWECP. (b) MR images of left IFOF (top) before and (middle) after the playing season, and (bottom) the overlay. In the overlay (bottom), the red region is after the season and the blue region is before the season.
Fiber Core Analysis
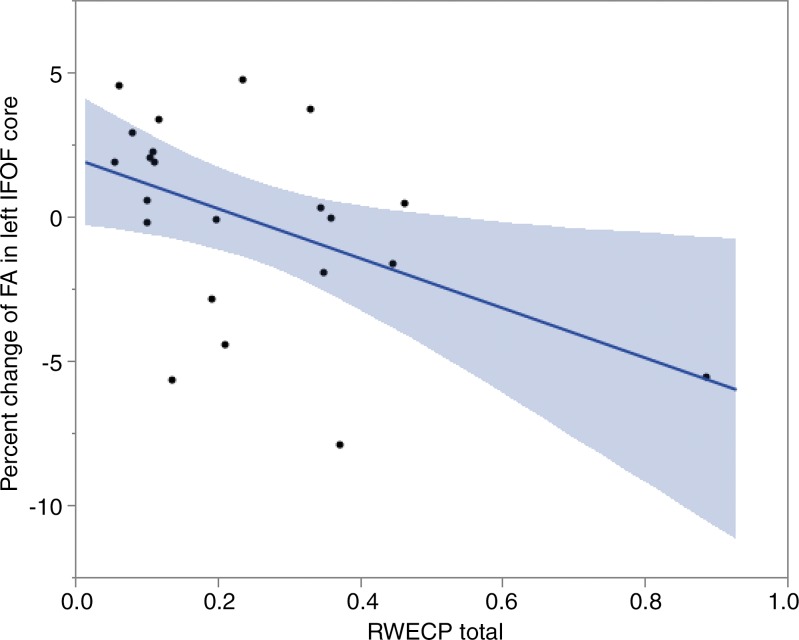
A statistically significant correlation between change of FA in left IFOF’s core and RWECP was demonstrated (n = 22; R2 = 0.3649l; P = .007). Figure 2 shows the association between changes of FA in core part of left IFOF with respect to RWECP. No statistically significant associations between total RWECP and FA changes in the forceps minor or major of the corpus callosum, ILF, or SLF cores were observed.
Figure 2:
Linear regression plot depicts the relationship between percent change of FA of the core part of left IFOF and cumulative exposure. The blue shaded area indicates the estimated confidence interval region around the true regression line. RWECP = RWECP.
Fiber Terminal Analysis
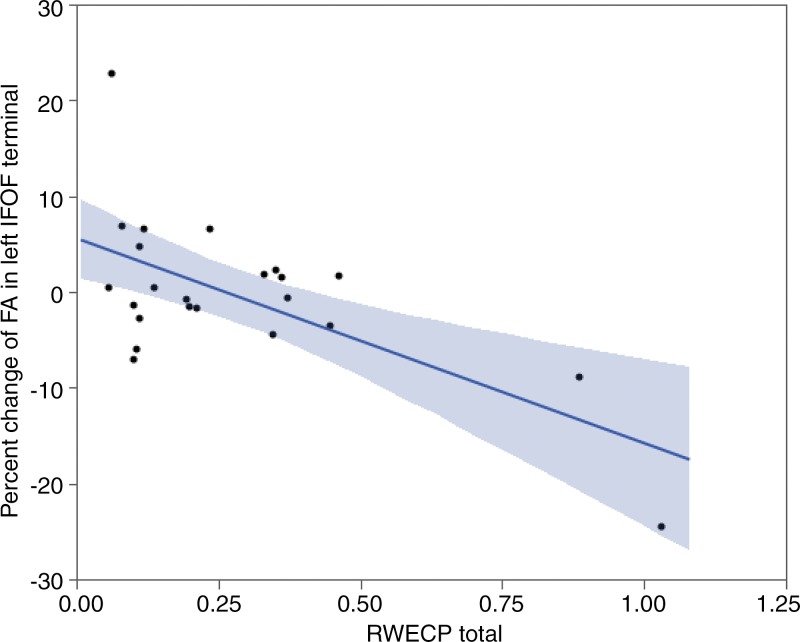
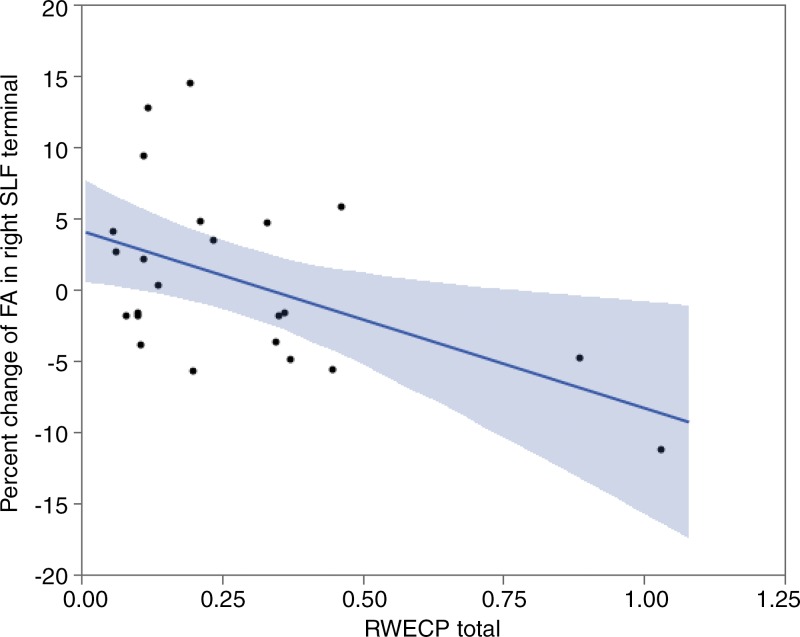
We observed a statistically significant correlation between decrease of FA of the left IFOF’s terminal and RWECP (n = 23; R2 = 0.5666; P < .001). Decrease of FA of the right SLF’s terminals demonstrated a statistically significant association with RWECP (n = 23; R2 = 0.2893; P = .028). Figure 3 and Figure 4 show the association between changes of FA in terminal parts of the left IFOF and right SLF with respect to RWECP, respectively. No statistically significant association was found between total RWECP and FA changes in the forceps minor and major of the corpus callosum (R2= 0.0304; P = .38), and ILF was observed (R2 = 0.1080; P = .11).
Figure 3:
Linear regression plot depicts the relationship between percent change of FA of the terminal part of left IFOF and cumulative exposure. The blue shaded area indicates the estimated confidence interval region around the true regression line. RWECP = RWECP.
Figure 4:
Linear regression plot depicts the relationship between percent change of FA of the terminal part of right SLF and cumulative exposure. The blue shaded area indicates the estimated confidence interval region around the true regression line. RWECP = RWECP.
As shown in Figures 1–4, changes of FA in different participants occurred in different directions over a single season of football. However, cumulative head impact exposure (RWECP) was associated with more decreased FA for those who had FA reduction and less increased FA for those who had FA increase over the single season of football.
Discussion
The statistically significant relationship between RWECP and change of FA in the left IFOF suggests that an increase in subconcussive head impact exposure may have an effect on WM integrity in youth athletes, even in the absence of a clinically diagnosed concussion. Percent change of FA of whole left IFOF and terminals of left IFOF and right SLF were significantly related to head impact exposure, however no significant relationship was observed in the forceps minors or majors of the corpus callosum. The results of this study suggest that subconcussive impacts can result in changes in the WM microstructure of the IFOF and SLF fiber bundles.
Similar to our current work, several previous studies of mild TBI and sports-related head injury demonstrated changes in these same specific tracts, including the IFOF, SLF, and ILF (18–20). Kraus et al (21) showed reduced WM integrity in the IFOF, SLF, ILF, and corpus callosum in patients with chronic TBI. Bendlin et al (18) demonstrated decreased FA and increased mean diffusivity in several major fiber bundles, including the SLF, IFOF, and corpus callosum. Chamard et al (19) studied sport-related concussion in female athletes, and detected a large change in the IFOF, SLF, ILF, uncinate fasciculus, and corpus callosum of athletes with concussion. Gajawelli et al (20) found change of FA in the brain of athletes who play contact sports compared with athletes who do not play contact sports. These changes involved specific tracts that included the IFOF and corpus callosum. Our findings are in agreement with previous studies and indicate there is an association between cumulative head impact exposure and microstructural changes in specific tracts.
In previous studies (19,44,45,46), changes in WM integrity of the whole IFOF were observed after concussive and subconcussive exposure, which is in agreement with our findings. Specifically, abnormal findings in the left IFOF were observed in two studies (19,45) of athletes who exhibited prolonged symptoms after concussion. Abnormal findings in the IFOF were also found in contact-sports–related athletes without a history of concussion compared with healthy control patients (46). Our study shows changes in WM-specific tracts that are associated with RWECP in the absence of a clinical diagnosis of concussion. These changes and abnormal findings in the whole left IFOF, terminals, and core part of IFOF suggests that subconcussive impacts can also contribute to alterations of varied segments of long-range WM tracts. Preliminary hypotheses suggest that IFOF and SLF may be preferentially susceptible to concussive and subconcussive forces because of the high degree of crossing and merging of WM fibers in these regions (45,47).
Mechanical properties of tissue vary throughout the brain. Studies illustrated differences in mechanical properties between WM and gray matter, although the exact property differences have yet to be fully established (48–50). It is also challenging to determine the exact location of WM tract links in the gray matter region (40,41). Budday et al (48) concluded that WM is stiffer and more viscous than gray matter, and thus responds more slowly to impact. That study also demonstrated that there were more regional variations within WM in response to indentation force tests, which thus supported the idea that WM microstructural architecture is heterogeneous (48). Moreover, cerebral microbleeds from TBI often occur at the cortical gray matter and WM interface (51,52). We can reasonably speculate that WM mechanical properties would not be entirely uniform throughout the WM fiber tracts. This has some clinical implications for how the terminal fiber tracts respond to TBI compared with the core part of the fiber tracts. In this study, the observed alterations in the SLF are in agreement with current head impact literature (18,45,47) that demonstrates changes in the FA in the inferior and superior longitudinal fasciculus. Bendlin et al (18) showed decreased FA in the SLF of patients with TBI compared with healthy control patients. Changes in the SLF’s terminals are in the gray matter–WM junction. The structure of gray matter differs from WM both in density and rigidity (53). This dichotomy of structure results in different responses to rotation and acceleration; therefore, forces acting at the borders of these tissues can contribute to the mechanism of subconcussive-induced injuries and subsequently explain changes in the IFOF and SLF terminals.
Our study had limitations. Findings related to the changes in WM fiber terminals may have been affected by intersubject variability at the terminal projections of the tracts adjacent to the cerebral cortex (40,42). The parcellation of fiber tracts was on the basis of the spatial location of nodes along the fibers, which made identification of the exact boundary of the core and terminals of the fibers difficult. Also, the inclusion of only male participants limits inferences about the generalizability of this study. We do not know what the functional associations of these findings are and whether there is any long-term implication. Finally, our study’s findings and interpretations are on the basis of a single season of youth football, which makes inferences about the effects of accumulating subconcussive-induced deficits and the long-term outcomes of these deficits difficult. Future studies should include both male and female participants and larger sample sizes with longitudinal study designs.
In conclusion, the relationship between traumatic axonal injury and alterations of the whole structural WM network was investigated. We demonstrated a statistically significant relationship between head impact exposure in a single season of youth football and change of FA value of the left IFOF in the absence of clinically diagnosed concussions. Additionally, we found a statistically significant relationship between head impact and change of FA value of the right SLF terminals where WM and gray matter intersect. This suggests that there are regional variations within WM in response to head impact exposure, especially near the terminals where WM and gray matter link. This study adds to the growing body of evidence that a season of play in a contact sport can result in brain changes at MR imaging, even in the absence of concussion.
Advances in Knowledge
■ Our study demonstrated a statistically significant relationship between head impact exposure in a single season of youth football and change in fractional anisotropy (FA) value of the inferior fronto-occipital fasciculus in the absence of clinically diagnosed concussions (R2 = 0.4334; P = .003).
■ Our study demonstrated a significant relationship between head impact exposure and change of FA of the superior longitudinal fasciculus terminals where white matter and gray matter meet (P = .0283, R2 = 0.2893).
Implication for Patient Care
■ This work provides a better understanding of the effects of subconcussive head impacts on the brain of youth football players (age range, 8–13 years).
Acknowledgments
Acknowledgments
The authors thank Meghan Johnston and all those who contributed to the study development. The authors also thank the Childress Institute for Pediatric Trauma at Wake Forest Baptist Medical Center for supporting this study.
Received March 17, 2016; revision requested April 26; revision received July 8; accepted July 25; final version accepted August 25.
Supported by National Institutes of Health (grant no. R01NS082453 NINDS and R03NS088082 NINDS).
Disclosures of Conflicts of Interest: N.B. disclosed no relevant relationships. D.S. disclosed no relevant relationships. S.R. disclosed no relevant relationships. E.M.D. disclosed no relevant relationships. J.E.U. disclosed no relevant relationships. B.W. disclosed no relevant relationships. Y.J. disclosed no relevant relationships. C.G.V. disclosed no relevant relationships. G.A.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author disclosed coauthorship on article about Multimodal Assessment of Cognition & Symptoms cognitive test and the Postconcussion Symptom Inventory symptom scales. Other relationships: disclosed no relevant relationships. J.D.S. disclosed no relevant relationships. C.T.W. disclosed no relevant relationships. J.A.M. disclosed no relevant relationships.
Abbreviations:
- FA
- fractional anisotropy
- HIT
- Head Impact Telemetry
- IFOF
- inferior fronto-occipital fasciculus
- ILF
- inferior longitudinal fasciculus
- RWE
- risk-weighted cumulative exposure
- RWECP
- combined-probability RWE
- SLF
- superior longitudinal fasciculus
- TBI
- traumatic brain injury
- WM
- white matter
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21(5):375–378. [DOI] [PubMed] [Google Scholar]
- 2.Bachynski KE, Goldberg DS. Youth sports & public health: framing risks of mild traumatic brain injury in american football and ice hockey. J Law Med Ethics 2014;42(3):323–333. [DOI] [PubMed] [Google Scholar]
- 3.Nonfatal Traumatic Brain Injuries Related to Sports and Recreation Activities Among Persons Aged ≤19 Years—United States, 2001–2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6039a1.htm. Accessed February 3, 2015. [PubMed]
- 4.Kontos AP, Elbin RJ, Fazio-Sumrock VC, et al. Incidence of sports-related concussion among youth football players aged 8-12 years. J Pediatr 2013;163(3):717–720. [DOI] [PubMed] [Google Scholar]
- 5.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 6.Broglio SP, Eckner JT, Surma T, Kutcher JS. Post-concussion cognitive declines and symptomatology are not related to concussion biomechanics in high school football players. J Neurotrauma 2011;28(10):2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckwith JG, Greenwald RM, Chu JJ, et al. Timing of concussion diagnosis is related to head impact exposure prior to injury. Med Sci Sports Exerc 2013;45(4):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong KL, Zhu YS, Zhang WG. Diffusion tensor imaging and magnetic resonance spectroscopy in traumatic brain injury: a review of recent literature. Brain Imaging Behav 2014;8(4):487–496. [DOI] [PubMed] [Google Scholar]
- 9.McAllister TW, Ford JC, Flashman LA, et al. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology 2014;82(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010;74(8):643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voelbel GT, Genova HM, Chiaravalotti ND, Hoptman MJ. Diffusion tensor imaging of traumatic brain injury review: implications for neurorehabilitation. NeuroRehabilitation 2012;31(3):281–293. [DOI] [PubMed] [Google Scholar]
- 12.Borich M, Makan N, Boyd L, Virji-Babul N. Combining whole-brain voxel-wise analysis with in vivo tractography of diffusion behavior after sports-related concussion in adolescents: a preliminary report. J Neurotrauma 2013;30(14):1243–1249. [DOI] [PubMed] [Google Scholar]
- 13.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24(9):1447–1459. [DOI] [PubMed] [Google Scholar]
- 14.Martini D, Eckner J, Kutcher J, Broglio SP. Subconcussive head impact biomechanics: comparing differing offensive schemes. Med Sci Sports Exerc 2013;45(4):755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobb BR, Urban JE, Davenport EM, et al. Head impact exposure in youth football: elementary school ages 9-12 years and the effect of practice structure. Ann Biomed Eng 2013;41(12):2463–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funk JR, Rowson S, Daniel RW, Duma SM. Validation of concussion risk curves for collegiate football players derived from HITS data. Ann Biomed Eng 2012;40(1):79–89. [DOI] [PubMed] [Google Scholar]
- 17.Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol 2008;29(5):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendlin BB, Ries ML, Lazar M, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage 2008;42(2):503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamard E, Lassonde M, Henry L, et al. Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain Inj 2013;27(9):1038–1046. [DOI] [PubMed] [Google Scholar]
- 20.Gajawelli N, Lao Y, Apuzzo ML, et al. Neuroimaging changes in the brain in contact versus noncontact sport athletes using diffusion tensor imaging. World Neurosurg 2013;80(6):824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007;130(Pt 10):2508–2519. [DOI] [PubMed] [Google Scholar]
- 22.Houston SM, Herting MM, Sowell ER. The neurobiology of childhood structural brain development: conception through adulthood. Curr Top Behav Neurosci 2014;16:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia 1990;28(6):517–527. [DOI] [PubMed] [Google Scholar]
- 24.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology 2002;222(1):212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry 2004;55(3):323–326. [DOI] [PubMed] [Google Scholar]
- 26.Ashtari M, Cervellione KL, Hasan KM, et al. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage 2007;35(2):501–510. [DOI] [PubMed] [Google Scholar]
- 27.Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex 2010;20(9):2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giorgio A, Watkins KE, Chadwick M, et al. Longitudinal changes in grey and white matter during adolescence. Neuroimage 2010;49(1):94–103. [DOI] [PubMed] [Google Scholar]
- 29.Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 2007;17(12):2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas C, Humphreys K, Jung KJ, Minshew N, Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: a DTI tractography study. Cortex 2011;47(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters BD, Szeszko PR, Radua J, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull 2012;38(6):1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putukian M, Echemendia R, Dettwiler-Danspeckgruber A, et al. Prospective clinical assessment using Sideline Concussion Assessment Tool-2 testing in the evaluation of sport-related concussion in college athletes. Clin J Sport Med 2015;25(1):36–42. [DOI] [PubMed] [Google Scholar]
- 33.Urban JE, Davenport EM, Golman AJ, et al. Head impact exposure in youth football: high school ages 14 to 18 years and cumulative impact analysis. Ann Biomed Eng 2013;41(12):2474–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davenport EM, Whitlow CT, Urban JE, et al. Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J Neurotrauma 2014;31(19):1617–1624 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crisco JJ, Chu JJ, Greenwald RM. An algorithm for estimating acceleration magnitude and impact location using multiple nonorthogonal single-axis accelerometers. J Biomech Eng 2004;126(6):849–854. [DOI] [PubMed] [Google Scholar]
- 36.Rowson S, Duma SM. Development of the STAR evaluation system for football helmets: integrating player head impact exposure and risk of concussion. Ann Biomed Eng 2011;39(8):2130–2140. [DOI] [PubMed] [Google Scholar]
- 37.Rowson S, Duma SM. Brain injury prediction: assessing the combined probability of concussion using linear and rotational head acceleration. Ann Biomed Eng 2013;41(5):873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One 2012;7(11):e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messé A, Caplain S, Paradot G, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp 2011;32(6):999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciccarelli O, Toosy AT, Parker GJ, et al. Diffusion tractography based group mapping of major white-matter pathways in the human brain. Neuroimage 2003;19(4):1545–1555. [DOI] [PubMed] [Google Scholar]
- 41.Tozer DJ, Chard DT, Bodini B, et al. Linking white matter tracts to associated cortical grey matter: a tract extension methodology. Neuroimage 2012;59(4):3094–3102. [DOI] [PubMed] [Google Scholar]
- 42.Holl N, Noblet V, Rodrigo S, et al. Temporal lobe association fiber tractography as compared to histology and dissection. Surg Radiol Anat 2011;33(8):713–722. [DOI] [PubMed] [Google Scholar]
- 43.De Maesschalck R, Jouan-Rimbaud D, Massart DL. The Mahalanobis distance. Chemometr Intell Lab Syst 2000;50(1):1–18. [Google Scholar]
- 44.Yuan W, Holland SK, Schmithorst VJ, et al. Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR Am J Neuroradiol 2007;28(10):1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma 2011;28(2):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koerte IK, Ertl-Wagner B, Reiser M, Zafonte R, Shenton ME. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 2012;308(18):1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murugavel M, Cubon V, Putukian M, et al. A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports-related concussion. J Neurotrauma 2014;31(22):1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Budday S, Nay R, de Rooij R, et al. Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed Mater 2015;46:318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McArdle CB, Richardson CJ, Nicholas DA, Mirfakhraee M, Hayden CK, Amparo EG. Developmental features of the neonatal brain: MR imaging. Part I. Gray-white matter differentiation and myelination. Radiology 1987;162(1 Pt 1):223–229. [DOI] [PubMed] [Google Scholar]
- 50.Christ AF, Franze K, Gautier H, et al. Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy. J Biomech 2010;43(15):2986–2992. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Kou Z, Tian Y. Diffuse axonal injury after traumatic cerebral microbleeds: an evaluation of imaging techniques. Neural Regen Res 2014;9(12):1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabu S, Jaffer H, Petro M, et al. Blast-associated shock waves result in increased brain vascular leakage and elevated ROS levels in a rat model of traumatic brain injury. PLoS One 2015;10(5):e0127971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Held K, Rota Kops E, Krause BJ, Wells WM, 3rd, Kikinis R, Müller-Gärtner HW. Markov random field segmentation of brain MR images. IEEE Trans Med Imaging 1997;16(6):878–886. [DOI] [PubMed] [Google Scholar]