This article provides a comprehensive overview of imaging of frostbite injuries, including radiography, digital subtraction angiography, multiphase bone scintigraphy, and SPECT/CT, with a focus on the full range of imaging findings, their clinical relevance, and how use of these modalities can help guide care of the frostbite patient.
Abstract
Frostbite is a localized cold thermal injury that results from tissue freezing. Frostbite injuries can have a substantial effect on long-term limb function and mobility if not promptly evaluated and treated. Imaging plays a critical role in initial evaluation of frostbite injuries and in monitoring response to treatment. A multimodality approach involving radiography, digital subtraction angiography (DSA), and/or multiphase bone scintigraphy with hybrid single photon emission computed tomography (SPECT)/computed tomography (CT) is often necessary for optimal guidance of frostbite care. Radiographs serve as an initial survey of the affected limb and may demonstrate characteristic findings, depending on the time course and severity of injury. DSA is used to evaluate perfusion of affected soft tissues and identify potential targets for therapeutic intervention. Angiography-directed thrombolysis plays an essential role in tissue preservation and salvage in deep frostbite injuries. Multiphase bone scintigraphy with technetium 99m–labeled diphosphonate provides valuable information regarding the status of tissue viability after initial treatment. The addition of SPECT/CT to multiphase bone scintigraphy enables precise anatomic localization of the level and depth of tissue necrosis before its appearance at physical examination and can help uncover subtle findings that may remain occult at scintigraphy alone. Multiphase bone scintigraphy with SPECT/CT is the modality of choice for prognostication and planning of definitive surgical care of affected limbs. Appropriate use of imaging to direct frostbite care can help limit the effects that these injuries have on limb function and mobility.
©RSNA, 2016
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Describe the role of diagnostic imaging in the management of frostbite.
■ Recognize characteristic findings of frostbite at radiography, conventional angiography, multiphase bone scintigraphy, and SPECT/CT.
■ Discuss how SPECT/CT can be used to demarcate the interface between viable and nonviable tissue.
Introduction
Frostbite is a localized cold thermal injury that results when tissues are exposed to temperatures below their freezing point (typically −0.55°C) for a prolonged period (1). Frostbite has historically been most frequently reported among military personnel and in countries with extreme environments (1–3), although the rise in popularity of winter sports has contributed to a substantial increase in civilian cases (4,5). Frostbite may also occur in the industrial setting from accidental exposure to refrigerant chemicals or dry ice, as well as in households from misuse of cold packs or fire extinguishers (6). The vast majority of frostbite occurs in the feet and hands (≥90%), but it is occasionally seen affecting the face (principally the nose, cheeks, and ears), perineum and buttocks (areas in direct contact with cold surfaces), and male genitalia (in runners and Nordic skiers) (5,7).
Alcohol consumption and psychiatric illness are the most common risk factors for frostbite injuries (2,8). Vehicular accidents and vehicular failure frequently result in accidental cold exposure in cold climates (9). Illicit drug use, homelessness, smoking, immobilizing traumatic injury, and systemic diseases such as peripheral vascular disease, diabetes mellitus, and Raynaud phenomenon also predispose individuals to frostbite (2,4). Frostbite is more likely to occur in males and in adults aged 30–49 years, presumably because of increased occupational exposure to cold and increased risk-taking behavior (1,6). Individuals of African American or Pacific Islander descent may be more susceptible to frostbite injuries than individuals with lighter skin tones (2,10).
Frostbite occurs in four interconnected progressive pathophysiologic phases that are dependent on the rate and duration of freezing, rate of rewarming, and anatomic extent of exposure (5). During the prefreeze phase, tissue cooling leads to local vasoconstriction and ischemia, with the resulting neuronal effects of hyperesthesia and paresthesia (11). During the freeze-thaw phase, intracellular ice crystals cause direct damage to cell membranes, while extracellular ice crystals alter oncotic pressures, leading to electrolyte shifts, intracellular dehydration, and cell death (12,13). The body initially responds to tissue freezing with alternating cycles of vasodilation and vasoconstriction (the “hunting reaction”), which lead to cycles of partial thawing and a prothrombotic microenvironment (4,5,12). During the vascular stasis phase, local vasoconstriction persists, and hypoxia and acidosis damage the endothelium and promote coagulation and interstitial edema (7,11). Finally, hypoxia, endothelial injury, and local thrombosis lead to the late ischemic phase, in which inflammatory mediators such as prostaglandins, thromboxanes, bradykinins, and histamine trigger additional vasoconstriction, platelet aggregation, and vessel thrombosis (7,11,12). Because these inflammatory mediators peak during rewarming, cycles of refreezing and rewarming can worsen the extent of tissue loss (4,7,11). Therefore, initial frostbite treatment is targeted at restoring perfusion to the affected limb(s) and limiting tissue loss after rewarming.
Cold injuries have a spectrum of clinical presentations that range from frostnip and pernio (chilblain) to frostbite. Frostnip is the earliest manifestation of cold injury and is characterized by temporary pallor and numbness without tissue freezing or loss (11,14). Pernio is a milder form of cold injury than frostbite, is caused by repetitive damp cold exposure at nonfreezing temperatures, and is characterized by pruritic and/or painful inflammatory lesions (14). Frostbite manifests with tissue freezing, discoloration, and complete anesthesia. Severe pain may occur with rewarming. Clinically, frostbite injuries are divided into tiers or degrees of injury that follow the classification scheme of thermal burn injuries (Table 1). However, this resemblance is superficial, and treatment differs significantly between the two types of injuries (16). Unlike burn injury, frostbite evolves slowly and causes substantial local ischemia in addition to direct cellular damage (17). Because frostbite lesions may appear similar early in their disease course and may change in appearance after rewarming, classification is best applied after rewarming is completed (2,11). The two-tier approach is most appropriate for use in the field, while the four-tier approach is generally applied in the hospital setting (11).
Table 1:
Two- and Four-Tier Classification of Frostbite According to Clinical Manifestations
Because frostbite injuries can have long-term implications for limb function and mobility, early evaluation and treatment are critical to outcome success. The primary role of imaging in frostbite injuries is to help define the precise severity, depth, and extent of tissue injury to better direct nonsurgical and surgical treatment. Imaging also plays an important role in monitoring response to frostbite treatment.
Radiography
Radiography is often the first imaging modality used to evaluate frostbite injuries. Radiographs primarily serve as an initial survey of the affected limb(s) and as an adjunct to the physical examination. In acute frostbite injuries, radiographs are useful for identifying occult traumatic injuries or radiopaque foreign bodies whose presence may be masked by frostbite-related anesthesia or cognitive impairment. In the later stages of injury, radiographs can be used to evaluate the progression of gross soft-tissue and bone changes and to examine for evidence of tissue or bone infection.
Radiographic findings of frostbite can be divided into three overlapping stages according to the time elapsed since the original injury (Table 2). In superficial injuries, initial radiographs may be normal or show mild soft-tissue swelling, with findings returning to normal days to weeks after healing occurs (18–20). With deeper injuries, initial radiographs may exhibit marked soft-tissue swelling with tissue distortion and atrophy in areas affected by gangrene and necrosis (Fig 1a). When the hand is affected, there may be relative sparing of the thumb due to protection from exposure when clenched in the palm (18). Weeks to months after the initial injury, osteopenia and periostitis may be observed in affected bone as an indirect sign of bone viability (18–20). After deep frostbite, involved bone can develop acroosteolysis, sclerotic foci at the ends of involved bone, early asymmetric osteoarthrosis, and/or periarticular erosions (Fig 1b) (19–21). In children, epiphyseal fragmentation and/or premature epiphyseal fusion with resulting chronic deformities have been reported to occur months to years after injury (12,22).
Table 2:
Radiographic Stages of Frostbite Injuries
Figure 1a.

Right toe frostbite in a 55-year-old man. (a) Initial dorsoplantar radiograph shows soft-tissue irregularities of the first, second, and fifth toes without underlying bone changes. (b) Dorsoplantar radiograph obtained 4 months later at follow-up shows progression of the first and second toe soft-tissue defects and a new periarticular erosion of the distal first proximal phalanx (black arrowhead). Note the interval healing of the soft tissue of the fifth toe, with new subtle osteopenia of the fifth distal phalanx (white arrowhead).
Figure 1b.
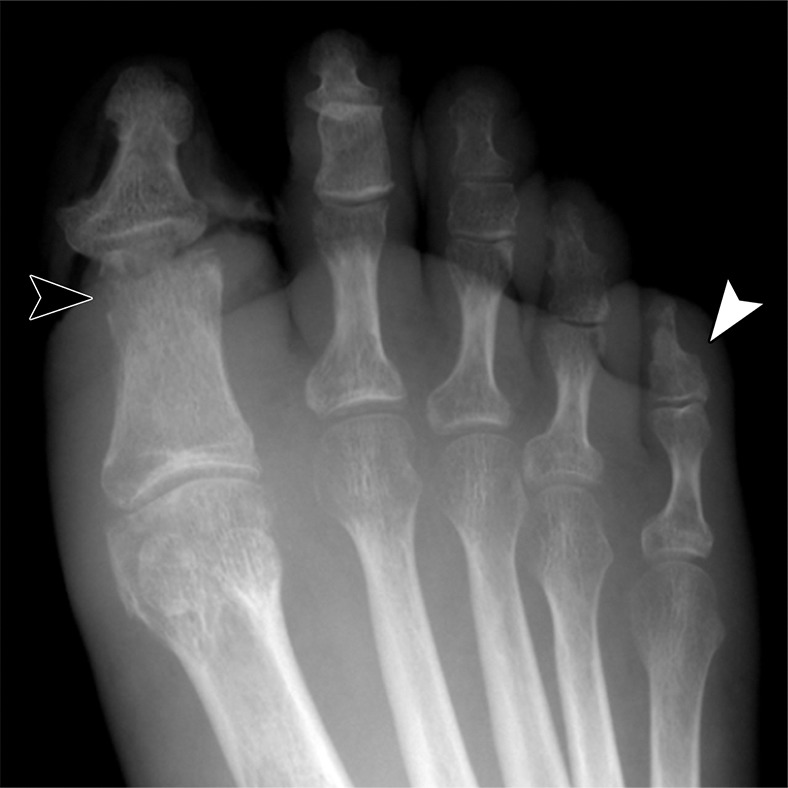
Right toe frostbite in a 55-year-old man. (a) Initial dorsoplantar radiograph shows soft-tissue irregularities of the first, second, and fifth toes without underlying bone changes. (b) Dorsoplantar radiograph obtained 4 months later at follow-up shows progression of the first and second toe soft-tissue defects and a new periarticular erosion of the distal first proximal phalanx (black arrowhead). Note the interval healing of the soft tissue of the fifth toe, with new subtle osteopenia of the fifth distal phalanx (white arrowhead).
Although several acute and chronic changes can be observed on radiographs obtained after frostbite injuries, the radiographic manifestations of frostbite are nonspecific. There may be discrepancies between the clinical presentation and the extent of radiographic findings at all stages (20).
Angiography
Digital subtraction angiography (DSA) is the diagnostic imaging modality of choice for patients who present within 24 hours of deep (third- or fourth- degree) frostbite injury and have suspected vascular compromise. The primary role of DSA is to help evaluate vessel patency, identify potential targets for thrombolysis, and monitor response to thrombolytic treatment. DSA can also provide limited information regarding perfusion of affected soft tissues for prognostication and surgical planning.
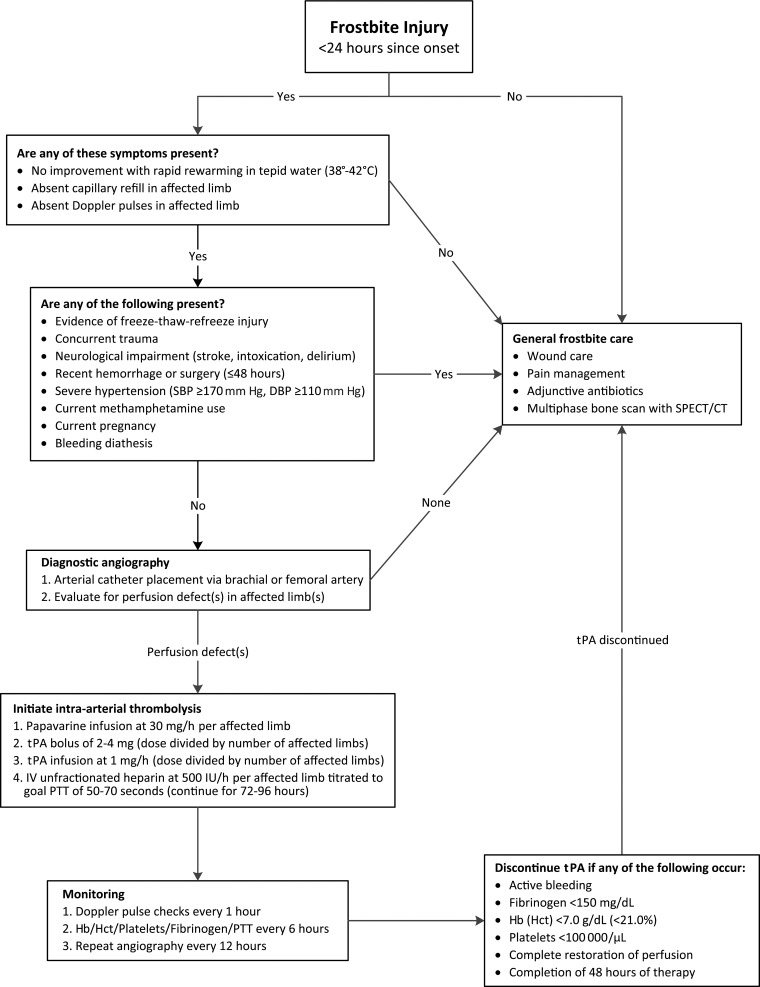
Early thrombolytic therapy can reverse the microvascular thrombosis induced by frostbite injuries, thereby reestablishing distal perfusion and limiting tissue loss (3,23). Although the use of intra-arterial thrombolysis for treatment of frostbite injuries is relatively new, early investigations into its benefits have thus far been promising (3,24–28). A retrospective comparative study by Bruen et al (29) demonstrated a significantly lower amputation rate of 10% among patients with severe frostbite who received intra-arterial tissue plasminogen activator (tPA), compared with an amputation rate of 41% in patients who did not. In the largest and most recent analysis to date, Gonzaga et al (30) reported a digit salvage rate of 68.6% in severe frostbite cases after administration of intra-arterial tPA, which increased to 98% for digits that demonstrated complete vascular reperfusion at final angiography. Simultaneous administration of a vasodilator and anticoagulation therapy are used as adjunctive treatments to reduce vasospasm, prevent new thrombi, and halt extension of existing clots (30). Thrombolysis is unlikely to benefit patients who present more than 24 hours after injury, use methamphetamines, or have evidence of multiple freeze-thaw-refreeze injuries (24,29,30). An algorithm for management of frostbite with angiography and thrombolytic therapy according to the available literature is provided (Fig 2).
Figure 2.
Diagram shows an algorithm for management of frostbite with thrombolytic therapy. CT = computed tomography, DBP = diastolic blood pressure, Hb = hemoglobin, Hct = hematocrit, IV = intravenous, PTT = partial thromboplastin time, SBP = systolic blood pressure, SPECT = single photon emission computed tomography. (Data are from references 24,28–31.)
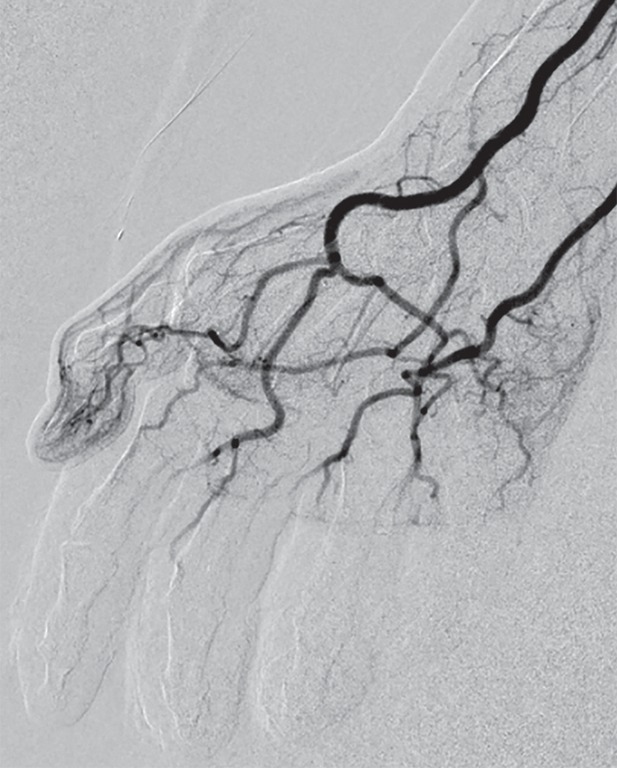
Catheters are typically inserted into a single brachial or femoral port, and the thrombolytic agent and vasodilator are infused coaxially into the involved limb(s) proximal to the antecubital or popliteal fossa to minimize local vasospasm and include proximal origins of outflow vessels (30). Initial DSA will demonstrate impaired perfusion with lack of distal blush in affected digits and/or abrupt cutoffs in vessels that are completely thrombosed (Fig 3). In more severe cases, occlusions in multiple proximal arteries may approximate the line of tissue demarcation demonstrated at physical examination (Fig 4). Initial DSA may also uncover areas of impaired perfusion that do not appear necrotic at physical examination (Fig 5). Sequential DSA is then performed at 12-hour increments for up to 48 hours to monitor response to treatment (1,28,29). Increments of 24 hours for a total of up to 72 hours have also been used (1,4,30). Subsequent DSA may demonstrate marked improvement in distal perfusion (Fig 6), although the persistence of perfusion abnormalities or even persistent occlusion in proximal vessels does not necessarily indicate complete tissue loss. The minimum of a partial response, as indicated by return of vascular flow beyond the next joint level for at least one digit, can be expected to occur in the vast majority of patients (30). Thrombolysis is terminated if perfusion is restored to all distal digits, improvement has not occurred at successive DSA, or complications of thrombolytic therapy develop. Anticoagulation therapy is continued after termination of thrombolysis to prevent recurrent thrombosis (30).
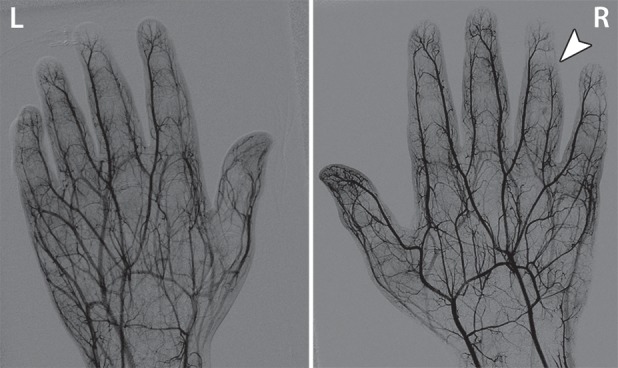
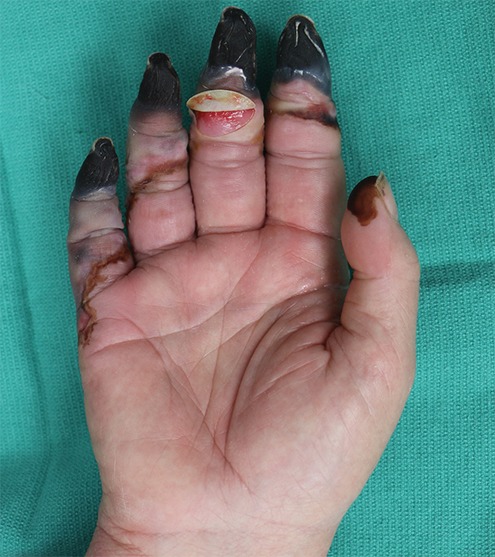
Figure 3a.
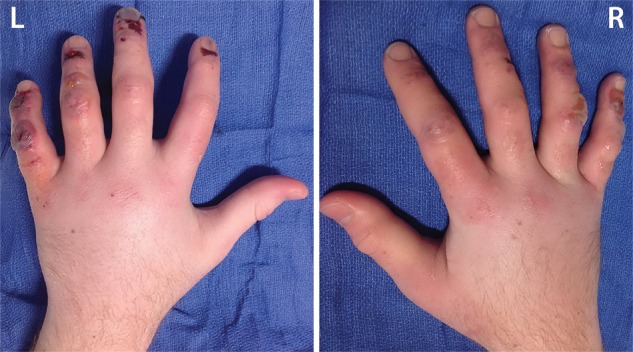
Third-degree frostbite of the hands in an 18-year-old man. (a) Dorsal photographs show bilateral digital edema with mixed clear and hemorrhagic blisters throughout both hands. (b) DSA images obtained at presentation show an abrupt occlusion in the right fourth digit ulnar proper digital artery at the level of the distal interphalangeal joint (arrowhead) and impaired perfusion of the tips of the right fourth digit and left third digit. (c) DSA images obtained after 12 hours of continuous intra-arterial tPA infusion via the ulnar arteries show improved distal perfusion bilaterally, with a short-segment persistent occlusion in the right fourth digit ulnar proper digital artery (arrowhead). The patient made a full recovery without further intervention.
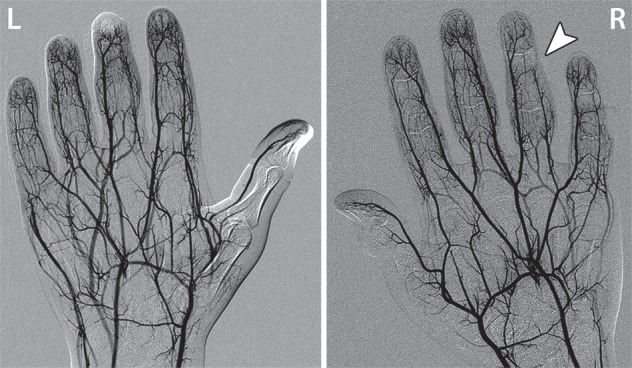
Figure 4a.

Fourth-degree frostbite of the left hand in a 45-year-old woman. (a) Dorsal photograph shows gangrene throughout the distal aspects of the second through fifth digits. Note the sparing of the thumb. (b–d) DSA images obtained at presentation (b) and after 12 (c) and 36 (d) hours of continuous intra-arterial tPA infusion via the ulnar artery show persistent occlusion of the second through fifth proper digital arteries distal to the metacarpophalangeal joints. Note the vasospasm of the ulnar artery at 12 hours (arrowhead in c), which complicates interpretation of the distal perfusion defects. The patient ultimately underwent amputation of the second through fifth digits through the mid proximal phalanges.
Figure 5a.
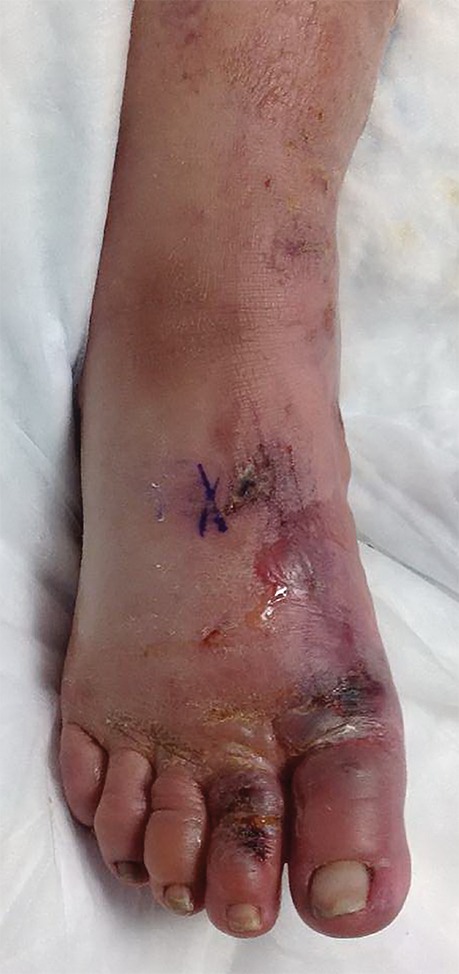
Third-degree frostbite of the right foot in a 45-year-old woman. (a) Dorsal photograph shows diffuse swelling of the midfoot and toes, with both clear and hemorrhagic blisters. (b) DSA image obtained at presentation shows occlusion of the first through fifth digital arteries. (c, d) DSA images obtained after 12 (c) and 36 (d) hours of continuous intra-arterial tPA infusion via the tibioperoneal trunk show restoration of perfusion in the first and second toes, with continued diminished perfusion of the distal third through fifth toes. The patient’s foot ultimately healed without surgical intervention.
Figure 6a.

Third-degree frostbite of the toes in a 22-year-old man. (a) Dorsal photographs show bilateral diffuse discoloration of the toes and hemorrhagic blisters. (b) DSA images obtained at presentation show absent perfusion beyond the distal interphalangeal joints bilaterally. (c) DSA images obtained after 24 hours of continuous intra-arterial tPA infusion via the popliteal arteries show near-complete resolution. (d–f) Multiphase technetium 99m (99mTc) labeled–methylene diphosphonate (MDP) bone scintigraphic images of the feet obtained 1 month later because of persistent superficial wounds show preserved uptake throughout both feet in the plantar blood flow phase at 35 seconds (with toe and heel markers) (d), soft-tissue phase (without markers) (e), and 4.5-hour delayed phase (without markers) (f). Note the nonuniform uptake on the delayed phase image, with areas of increased uptake within the heels, medial midfeet, and first toes that correspond to areas of hyperemia on the blood flow and soft-tissue phase images. (g) Fused SPECT/CT image shows preserved tracer uptake throughout both feet. The patient made a full recovery without tissue loss or surgery.
Figure 3b.
Third-degree frostbite of the hands in an 18-year-old man. (a) Dorsal photographs show bilateral digital edema with mixed clear and hemorrhagic blisters throughout both hands. (b) DSA images obtained at presentation show an abrupt occlusion in the right fourth digit ulnar proper digital artery at the level of the distal interphalangeal joint (arrowhead) and impaired perfusion of the tips of the right fourth digit and left third digit. (c) DSA images obtained after 12 hours of continuous intra-arterial tPA infusion via the ulnar arteries show improved distal perfusion bilaterally, with a short-segment persistent occlusion in the right fourth digit ulnar proper digital artery (arrowhead). The patient made a full recovery without further intervention.
Figure 3c.
Third-degree frostbite of the hands in an 18-year-old man. (a) Dorsal photographs show bilateral digital edema with mixed clear and hemorrhagic blisters throughout both hands. (b) DSA images obtained at presentation show an abrupt occlusion in the right fourth digit ulnar proper digital artery at the level of the distal interphalangeal joint (arrowhead) and impaired perfusion of the tips of the right fourth digit and left third digit. (c) DSA images obtained after 12 hours of continuous intra-arterial tPA infusion via the ulnar arteries show improved distal perfusion bilaterally, with a short-segment persistent occlusion in the right fourth digit ulnar proper digital artery (arrowhead). The patient made a full recovery without further intervention.
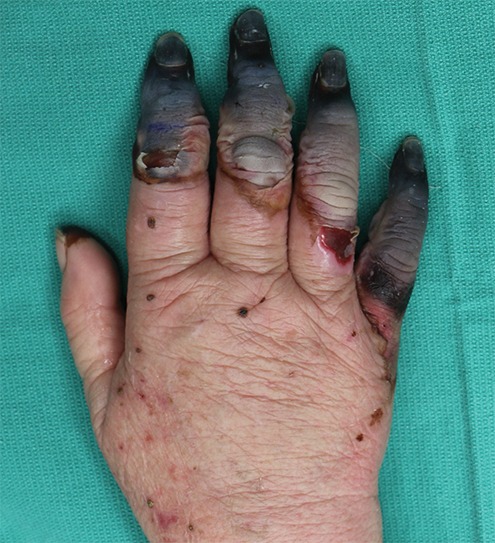
Figure 4b.
Fourth-degree frostbite of the left hand in a 45-year-old woman. (a) Dorsal photograph shows gangrene throughout the distal aspects of the second through fifth digits. Note the sparing of the thumb. (b–d) DSA images obtained at presentation (b) and after 12 (c) and 36 (d) hours of continuous intra-arterial tPA infusion via the ulnar artery show persistent occlusion of the second through fifth proper digital arteries distal to the metacarpophalangeal joints. Note the vasospasm of the ulnar artery at 12 hours (arrowhead in c), which complicates interpretation of the distal perfusion defects. The patient ultimately underwent amputation of the second through fifth digits through the mid proximal phalanges.
Figure 4c.

Fourth-degree frostbite of the left hand in a 45-year-old woman. (a) Dorsal photograph shows gangrene throughout the distal aspects of the second through fifth digits. Note the sparing of the thumb. (b–d) DSA images obtained at presentation (b) and after 12 (c) and 36 (d) hours of continuous intra-arterial tPA infusion via the ulnar artery show persistent occlusion of the second through fifth proper digital arteries distal to the metacarpophalangeal joints. Note the vasospasm of the ulnar artery at 12 hours (arrowhead in c), which complicates interpretation of the distal perfusion defects. The patient ultimately underwent amputation of the second through fifth digits through the mid proximal phalanges.
Figure 4d.
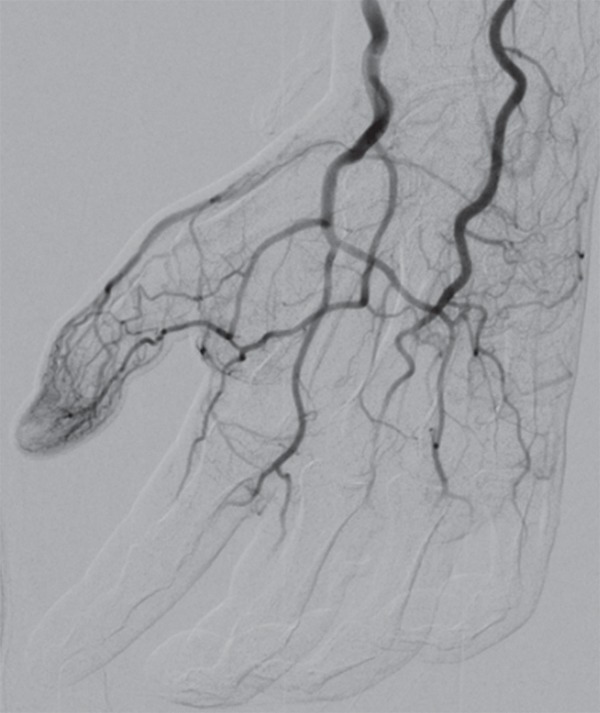
Fourth-degree frostbite of the left hand in a 45-year-old woman. (a) Dorsal photograph shows gangrene throughout the distal aspects of the second through fifth digits. Note the sparing of the thumb. (b–d) DSA images obtained at presentation (b) and after 12 (c) and 36 (d) hours of continuous intra-arterial tPA infusion via the ulnar artery show persistent occlusion of the second through fifth proper digital arteries distal to the metacarpophalangeal joints. Note the vasospasm of the ulnar artery at 12 hours (arrowhead in c), which complicates interpretation of the distal perfusion defects. The patient ultimately underwent amputation of the second through fifth digits through the mid proximal phalanges.
Figure 5b.

Third-degree frostbite of the right foot in a 45-year-old woman. (a) Dorsal photograph shows diffuse swelling of the midfoot and toes, with both clear and hemorrhagic blisters. (b) DSA image obtained at presentation shows occlusion of the first through fifth digital arteries. (c, d) DSA images obtained after 12 (c) and 36 (d) hours of continuous intra-arterial tPA infusion via the tibioperoneal trunk show restoration of perfusion in the first and second toes, with continued diminished perfusion of the distal third through fifth toes. The patient’s foot ultimately healed without surgical intervention.
Figure 5c.

Third-degree frostbite of the right foot in a 45-year-old woman. (a) Dorsal photograph shows diffuse swelling of the midfoot and toes, with both clear and hemorrhagic blisters. (b) DSA image obtained at presentation shows occlusion of the first through fifth digital arteries. (c, d) DSA images obtained after 12 (c) and 36 (d) hours of continuous intra-arterial tPA infusion via the tibioperoneal trunk show restoration of perfusion in the first and second toes, with continued diminished perfusion of the distal third through fifth toes. The patient’s foot ultimately healed without surgical intervention.
Figure 5d.
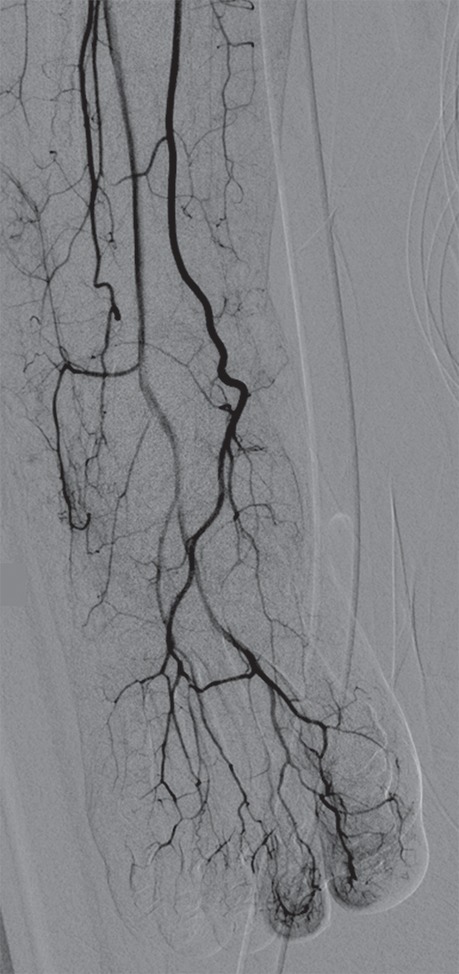
Third-degree frostbite of the right foot in a 45-year-old woman. (a) Dorsal photograph shows diffuse swelling of the midfoot and toes, with both clear and hemorrhagic blisters. (b) DSA image obtained at presentation shows occlusion of the first through fifth digital arteries. (c, d) DSA images obtained after 12 (c) and 36 (d) hours of continuous intra-arterial tPA infusion via the tibioperoneal trunk show restoration of perfusion in the first and second toes, with continued diminished perfusion of the distal third through fifth toes. The patient’s foot ultimately healed without surgical intervention.
Figure 6b.
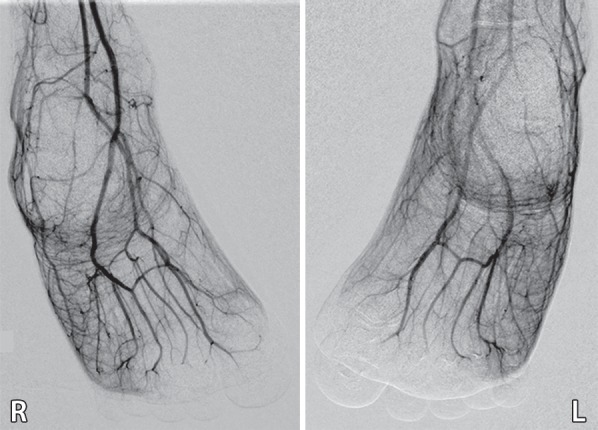
Third-degree frostbite of the toes in a 22-year-old man. (a) Dorsal photographs show bilateral diffuse discoloration of the toes and hemorrhagic blisters. (b) DSA images obtained at presentation show absent perfusion beyond the distal interphalangeal joints bilaterally. (c) DSA images obtained after 24 hours of continuous intra-arterial tPA infusion via the popliteal arteries show near-complete resolution. (d–f) Multiphase technetium 99m (99mTc) labeled–methylene diphosphonate (MDP) bone scintigraphic images of the feet obtained 1 month later because of persistent superficial wounds show preserved uptake throughout both feet in the plantar blood flow phase at 35 seconds (with toe and heel markers) (d), soft-tissue phase (without markers) (e), and 4.5-hour delayed phase (without markers) (f). Note the nonuniform uptake on the delayed phase image, with areas of increased uptake within the heels, medial midfeet, and first toes that correspond to areas of hyperemia on the blood flow and soft-tissue phase images. (g) Fused SPECT/CT image shows preserved tracer uptake throughout both feet. The patient made a full recovery without tissue loss or surgery.
Figure 6c.
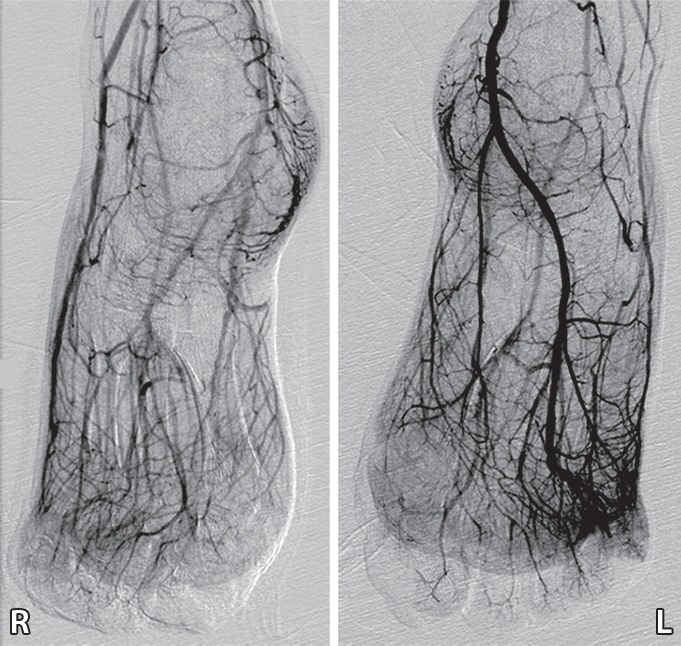
Third-degree frostbite of the toes in a 22-year-old man. (a) Dorsal photographs show bilateral diffuse discoloration of the toes and hemorrhagic blisters. (b) DSA images obtained at presentation show absent perfusion beyond the distal interphalangeal joints bilaterally. (c) DSA images obtained after 24 hours of continuous intra-arterial tPA infusion via the popliteal arteries show near-complete resolution. (d–f) Multiphase technetium 99m (99mTc) labeled–methylene diphosphonate (MDP) bone scintigraphic images of the feet obtained 1 month later because of persistent superficial wounds show preserved uptake throughout both feet in the plantar blood flow phase at 35 seconds (with toe and heel markers) (d), soft-tissue phase (without markers) (e), and 4.5-hour delayed phase (without markers) (f). Note the nonuniform uptake on the delayed phase image, with areas of increased uptake within the heels, medial midfeet, and first toes that correspond to areas of hyperemia on the blood flow and soft-tissue phase images. (g) Fused SPECT/CT image shows preserved tracer uptake throughout both feet. The patient made a full recovery without tissue loss or surgery.
Figure 6d.
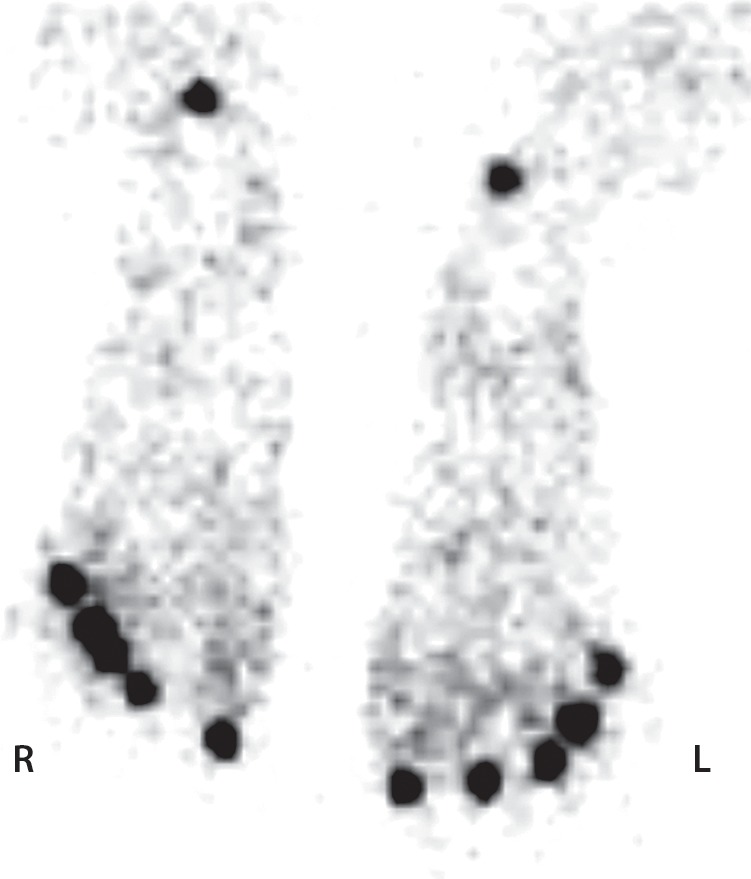
Third-degree frostbite of the toes in a 22-year-old man. (a) Dorsal photographs show bilateral diffuse discoloration of the toes and hemorrhagic blisters. (b) DSA images obtained at presentation show absent perfusion beyond the distal interphalangeal joints bilaterally. (c) DSA images obtained after 24 hours of continuous intra-arterial tPA infusion via the popliteal arteries show near-complete resolution. (d–f) Multiphase technetium 99m (99mTc) labeled–methylene diphosphonate (MDP) bone scintigraphic images of the feet obtained 1 month later because of persistent superficial wounds show preserved uptake throughout both feet in the plantar blood flow phase at 35 seconds (with toe and heel markers) (d), soft-tissue phase (without markers) (e), and 4.5-hour delayed phase (without markers) (f). Note the nonuniform uptake on the delayed phase image, with areas of increased uptake within the heels, medial midfeet, and first toes that correspond to areas of hyperemia on the blood flow and soft-tissue phase images. (g) Fused SPECT/CT image shows preserved tracer uptake throughout both feet. The patient made a full recovery without tissue loss or surgery.
Figure 6e.
Third-degree frostbite of the toes in a 22-year-old man. (a) Dorsal photographs show bilateral diffuse discoloration of the toes and hemorrhagic blisters. (b) DSA images obtained at presentation show absent perfusion beyond the distal interphalangeal joints bilaterally. (c) DSA images obtained after 24 hours of continuous intra-arterial tPA infusion via the popliteal arteries show near-complete resolution. (d–f) Multiphase technetium 99m (99mTc) labeled–methylene diphosphonate (MDP) bone scintigraphic images of the feet obtained 1 month later because of persistent superficial wounds show preserved uptake throughout both feet in the plantar blood flow phase at 35 seconds (with toe and heel markers) (d), soft-tissue phase (without markers) (e), and 4.5-hour delayed phase (without markers) (f). Note the nonuniform uptake on the delayed phase image, with areas of increased uptake within the heels, medial midfeet, and first toes that correspond to areas of hyperemia on the blood flow and soft-tissue phase images. (g) Fused SPECT/CT image shows preserved tracer uptake throughout both feet. The patient made a full recovery without tissue loss or surgery.
Figure 6f.
Third-degree frostbite of the toes in a 22-year-old man. (a) Dorsal photographs show bilateral diffuse discoloration of the toes and hemorrhagic blisters. (b) DSA images obtained at presentation show absent perfusion beyond the distal interphalangeal joints bilaterally. (c) DSA images obtained after 24 hours of continuous intra-arterial tPA infusion via the popliteal arteries show near-complete resolution. (d–f) Multiphase technetium 99m (99mTc) labeled–methylene diphosphonate (MDP) bone scintigraphic images of the feet obtained 1 month later because of persistent superficial wounds show preserved uptake throughout both feet in the plantar blood flow phase at 35 seconds (with toe and heel markers) (d), soft-tissue phase (without markers) (e), and 4.5-hour delayed phase (without markers) (f). Note the nonuniform uptake on the delayed phase image, with areas of increased uptake within the heels, medial midfeet, and first toes that correspond to areas of hyperemia on the blood flow and soft-tissue phase images. (g) Fused SPECT/CT image shows preserved tracer uptake throughout both feet. The patient made a full recovery without tissue loss or surgery.
Figure 6g.

Third-degree frostbite of the toes in a 22-year-old man. (a) Dorsal photographs show bilateral diffuse discoloration of the toes and hemorrhagic blisters. (b) DSA images obtained at presentation show absent perfusion beyond the distal interphalangeal joints bilaterally. (c) DSA images obtained after 24 hours of continuous intra-arterial tPA infusion via the popliteal arteries show near-complete resolution. (d–f) Multiphase technetium 99m (99mTc) labeled–methylene diphosphonate (MDP) bone scintigraphic images of the feet obtained 1 month later because of persistent superficial wounds show preserved uptake throughout both feet in the plantar blood flow phase at 35 seconds (with toe and heel markers) (d), soft-tissue phase (without markers) (e), and 4.5-hour delayed phase (without markers) (f). Note the nonuniform uptake on the delayed phase image, with areas of increased uptake within the heels, medial midfeet, and first toes that correspond to areas of hyperemia on the blood flow and soft-tissue phase images. (g) Fused SPECT/CT image shows preserved tracer uptake throughout both feet. The patient made a full recovery without tissue loss or surgery.
Magnetic resonance (MR) angiography has been reported as a noninvasive alternative to DSA for evaluating vessel patency in frostbite injuries (32,33), but unlike conventional angiography, MR angiography has no therapeutic potential. MR angiography may have limited clinical utility in helping to define persistently occluded vessels and demarcate the soft-tissue ischemic border in patients who present more than 24 hours after injury (32). The therapeutic relevance of this information beyond what can be provided at multiphase bone scintigraphy with SPECT/CT is uncertain.
Multiphase Bone Scintigraphy
Multiphase bone scintigraphy plays a key role in evaluating the depth of injury after initial nonsurgical and interventional treatment of frostbite injuries. Bone scintigraphy provides essential information regarding microvascular and bone perfusion and can demarcate the level of bone necrosis for surgical planning. For patients who undergo thrombolysis and are under consideration for surgery, this information can corroborate and enhance the macrovascular and soft-tissue information provided at angiography.
Multiphase bone scintigraphy is indicated in patients who present with second-, third-, or fourth-degree frostbite injuries (2,15). The scan should be performed 2–4 days after the original injury, or after angiography is completed in patients who undergo intra-arterial thrombolysis (34–38). A repeat scan at 7–10 days after injury can then be performed as warranted before surgical resection to evaluate interval change in areas of questionable uptake identified at the prior scan (34,35). By using this approach, the approximate level of amputation can be accurately predicted in up to 84% of cases at the initial scan, weeks before the demarcation of viable and nonviable tissue is clearly apparent at physical examination (34,36,39). Specificity increases at subsequent scanning as ongoing healing and necrosis resolve areas of questionable uptake (34,35). If feasible, débridement of blisters should be undertaken before scanning to limit false-positive results caused by pooling of tracer within blisters during the soft-tissue phase (34,35). However, the optimal management of frostbite-related blisters (especially hemorrhagic blisters) is controversial and is best left to the treatment team’s discretion (11). The first imaging study can provide all information necessary for surgical excision in patients with severe injuries who develop infection or sepsis or who cannot wait for subsequent imaging (34,35).
Although protocols vary among institutions, multiphase bone scintigraphy for frostbite is usually performed after intravenous administration of 740–1110 MBq (20–30 mCi) of 99mTc-labeled diphosphonates (MDP, hydroxymethylene diphosphonate, or pyrophosphate) (34–36,39–44). Technetium 99m–labeled pertechnetate has also been used with similar results but has been less widely investigated and does not provide critical information regarding bone viability (45,46). Planar blood flow phase images are first acquired at 1–3 frames per second for 60 seconds after injection, which provides information regarding macrovascular perfusion to the affected limb. Next, planar soft-tissue phase images (also commonly referred to as blood pool phase images) are acquired in 3–5-minute intervals for up to 10 minutes after tracer injection. Soft-tissue phase images enable evaluation of the soft tissues and microvascular perfusion. Delayed phase images, which demonstrate bone perfusion and viability, are then acquired 2–4 hours after tracer injection. For best results, the affected limb should be flat while in contact with the detector, although limited mobility due to extensive injury may make acquisition in ideal imaging planes difficult. Cobalt markers applied to the tips of the phalanges and the heels of the feet can improve localization of findings (34). Use of a gamma camera equipped with a low-energy high-resolution collimator will aid acquisition of the highest-resolution images (47).
Technetium 99m–labeled diphosphonates bind to bone through physicochemical adsorption (chemisorption) to the hydroxyapatite structure of bone tissue (47–49). The tracer is located primarily within the blood and vascular compartment during the blood flow phase (49–51). Soft-tissue phase images are obtained during the major shift of tracer from the blood to the extracellular space by passive diffusion through the capillaries (49–52). Delayed phase images are then acquired after sufficient tracer has reached its target and is bound to viable bone (49–51). It is important to recognize that regional blood flow is a prerequisite for tracer uptake in bone (53). This accounts for the lack of osseous radiopharmaceutical tracer uptake on delayed phase images in frostbite injuries (54). Patients with peripheral vascular disease or low cardiac output may exhibit diminished osseous uptake on initial delayed phase images (50,51). In these cases, late delayed phase images obtained 18–24 hours after tracer injection may better elicit the osseous findings, after adequate uptake in bone and sufficient clearance from soft tissues has occurred (51,55).
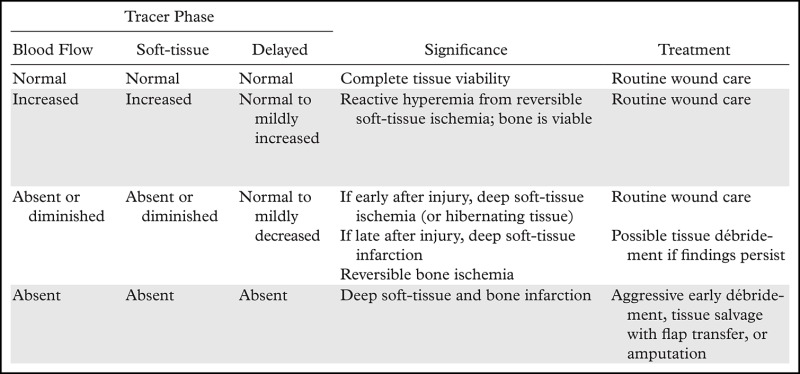
Various uptake patterns may be present at multiphase bone scintigraphy after frostbite injuries, depending on the depth and extent of injury (Table 3). Normal uptake and distribution of tracer on blood flow, soft-tissue, and delayed phase images can be seen if the injured tissue remains completely viable or after a complete response to thrombolytic therapy (56). Increased activity on blood flow and soft-tissue phase images and normal to mildly increased uptake of tracer on delayed phase images are indicative of reactive hyperemia and inflammation from superficial tissue ischemia without infarction (Fig 6) (36,40,43). Absent or diminished tracer on blood flow and soft-tissue phase images and visible but possibly diminished uptake in underlying bone on delayed phase images are indicative of tissue ischemia or hibernating tissue but do not necessarily indicate irreversible soft-tissue loss if seen early in the treatment course (36,46,56). Reactive hyperemia may be observed proximal to areas of absent soft-tissue phase uptake (36). Finally, absence of uptake in all phases indicates deep-tissue and bone necrosis and necessitates definitive surgical treatment (Fig 7) (36,56). Increased tracer uptake on delayed phase images indicates rebound hyperemia and increased bone turnover and may be seen in a periarticular distribution similar to that seen in complex regional pain syndrome (Fig 7d) (41). Delayed phase images are the most important for determining the ultimate extent of frostbite injury and guiding surgical management (36).
Table 3:
General Patterns of Tracer Uptake at Multiphase Bone Scintigraphy of Frostbite Injuries
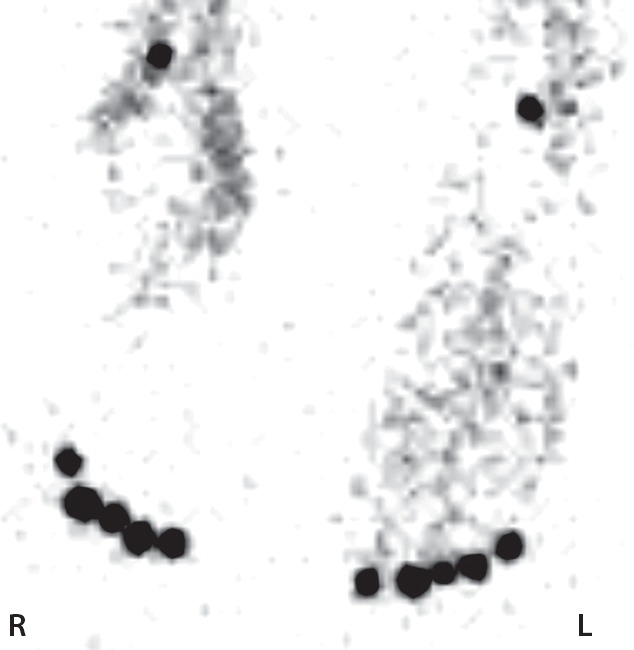
Figure 7a.
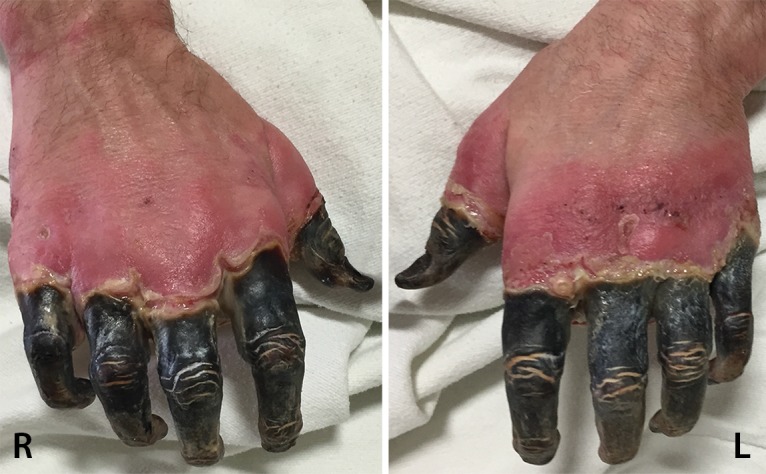
Fourth-degree frostbite of the hands in a 55-year-old man. (a) Dorsal photographs show gangrene involving all digits of both hands. (b–d) Multiphase 99mTc-MDP bone scintigraphic images of the hands show absent tracer uptake throughout the bilateral phalanges in the palmar blood flow phase at 80 seconds (with markers delineating the distal aspects of the fingers) (b), palmar soft-tissue phase (without markers) (c), and 4-hour delayed phase (with markers) (d). Note the increased tracer uptake at the metacarpophalangeal joints proximal to the level of necrosis on the delayed phase image. (e, f) Fused SPECT/CT images of the right (e) and left (f) hands show absent tracer uptake distal to the metacarpophalangeal joints bilaterally, with the exception of increased uptake in the left second and third proximal phalanges. SPECT/CT enables delineation of the precise level of tracer cutoff. The patient ultimately underwent amputation of the bilateral thumbs at the level of the mid proximal phalanges and total amputation of the other fingers.
Figure 7d.
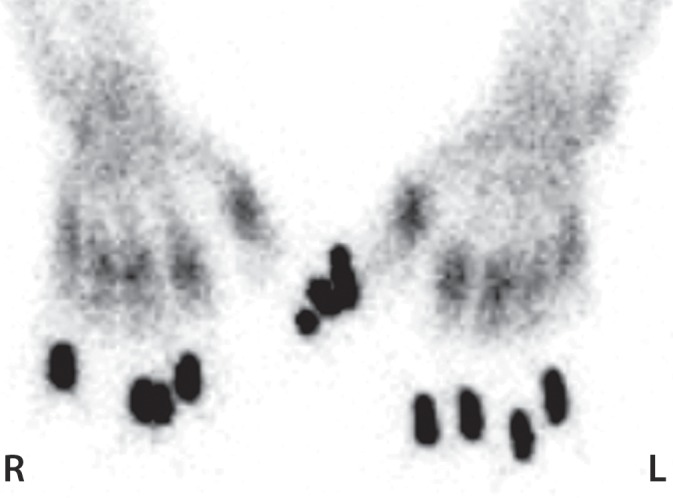
Fourth-degree frostbite of the hands in a 55-year-old man. (a) Dorsal photographs show gangrene involving all digits of both hands. (b–d) Multiphase 99mTc-MDP bone scintigraphic images of the hands show absent tracer uptake throughout the bilateral phalanges in the palmar blood flow phase at 80 seconds (with markers delineating the distal aspects of the fingers) (b), palmar soft-tissue phase (without markers) (c), and 4-hour delayed phase (with markers) (d). Note the increased tracer uptake at the metacarpophalangeal joints proximal to the level of necrosis on the delayed phase image. (e, f) Fused SPECT/CT images of the right (e) and left (f) hands show absent tracer uptake distal to the metacarpophalangeal joints bilaterally, with the exception of increased uptake in the left second and third proximal phalanges. SPECT/CT enables delineation of the precise level of tracer cutoff. The patient ultimately underwent amputation of the bilateral thumbs at the level of the mid proximal phalanges and total amputation of the other fingers.
Figure 7b.

Fourth-degree frostbite of the hands in a 55-year-old man. (a) Dorsal photographs show gangrene involving all digits of both hands. (b–d) Multiphase 99mTc-MDP bone scintigraphic images of the hands show absent tracer uptake throughout the bilateral phalanges in the palmar blood flow phase at 80 seconds (with markers delineating the distal aspects of the fingers) (b), palmar soft-tissue phase (without markers) (c), and 4-hour delayed phase (with markers) (d). Note the increased tracer uptake at the metacarpophalangeal joints proximal to the level of necrosis on the delayed phase image. (e, f) Fused SPECT/CT images of the right (e) and left (f) hands show absent tracer uptake distal to the metacarpophalangeal joints bilaterally, with the exception of increased uptake in the left second and third proximal phalanges. SPECT/CT enables delineation of the precise level of tracer cutoff. The patient ultimately underwent amputation of the bilateral thumbs at the level of the mid proximal phalanges and total amputation of the other fingers.
Figure 7c.

Fourth-degree frostbite of the hands in a 55-year-old man. (a) Dorsal photographs show gangrene involving all digits of both hands. (b–d) Multiphase 99mTc-MDP bone scintigraphic images of the hands show absent tracer uptake throughout the bilateral phalanges in the palmar blood flow phase at 80 seconds (with markers delineating the distal aspects of the fingers) (b), palmar soft-tissue phase (without markers) (c), and 4-hour delayed phase (with markers) (d). Note the increased tracer uptake at the metacarpophalangeal joints proximal to the level of necrosis on the delayed phase image. (e, f) Fused SPECT/CT images of the right (e) and left (f) hands show absent tracer uptake distal to the metacarpophalangeal joints bilaterally, with the exception of increased uptake in the left second and third proximal phalanges. SPECT/CT enables delineation of the precise level of tracer cutoff. The patient ultimately underwent amputation of the bilateral thumbs at the level of the mid proximal phalanges and total amputation of the other fingers.
Figure 7e.
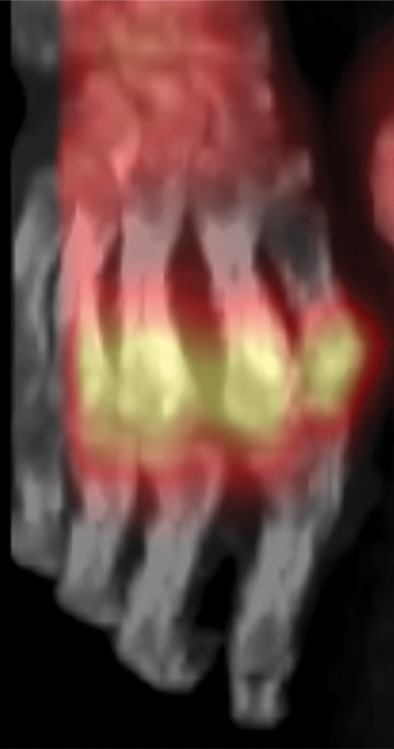
Fourth-degree frostbite of the hands in a 55-year-old man. (a) Dorsal photographs show gangrene involving all digits of both hands. (b–d) Multiphase 99mTc-MDP bone scintigraphic images of the hands show absent tracer uptake throughout the bilateral phalanges in the palmar blood flow phase at 80 seconds (with markers delineating the distal aspects of the fingers) (b), palmar soft-tissue phase (without markers) (c), and 4-hour delayed phase (with markers) (d). Note the increased tracer uptake at the metacarpophalangeal joints proximal to the level of necrosis on the delayed phase image. (e, f) Fused SPECT/CT images of the right (e) and left (f) hands show absent tracer uptake distal to the metacarpophalangeal joints bilaterally, with the exception of increased uptake in the left second and third proximal phalanges. SPECT/CT enables delineation of the precise level of tracer cutoff. The patient ultimately underwent amputation of the bilateral thumbs at the level of the mid proximal phalanges and total amputation of the other fingers.
Figure 7f.
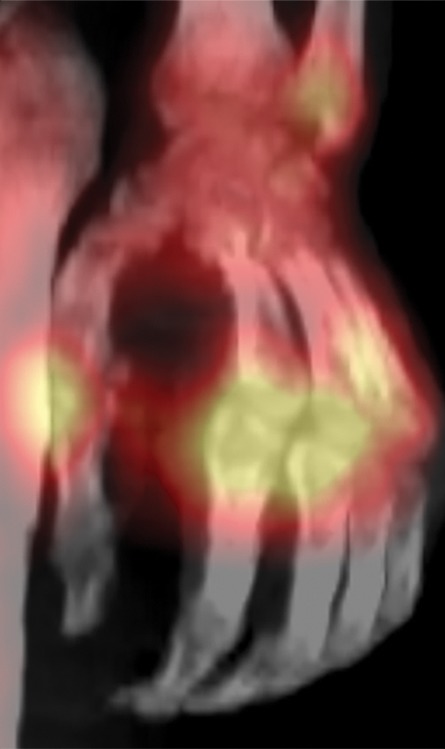
Fourth-degree frostbite of the hands in a 55-year-old man. (a) Dorsal photographs show gangrene involving all digits of both hands. (b–d) Multiphase 99mTc-MDP bone scintigraphic images of the hands show absent tracer uptake throughout the bilateral phalanges in the palmar blood flow phase at 80 seconds (with markers delineating the distal aspects of the fingers) (b), palmar soft-tissue phase (without markers) (c), and 4-hour delayed phase (with markers) (d). Note the increased tracer uptake at the metacarpophalangeal joints proximal to the level of necrosis on the delayed phase image. (e, f) Fused SPECT/CT images of the right (e) and left (f) hands show absent tracer uptake distal to the metacarpophalangeal joints bilaterally, with the exception of increased uptake in the left second and third proximal phalanges. SPECT/CT enables delineation of the precise level of tracer cutoff. The patient ultimately underwent amputation of the bilateral thumbs at the level of the mid proximal phalanges and total amputation of the other fingers.
SPECT/CT
SPECT/CT is a newer technology that combines the functional information from scintigraphy regarding bone perfusion and uptake with the anatomic information derived from CT. The addition of SPECT/CT to multiphase bone scintigraphy greatly improves surgical planning for deep frostbite injuries by enabling precise anatomic localization of functional information (31,57). SPECT/CT is performed immediately after acquisition of delayed phase images during bone scintigraphy. At our institution, the affected limbs are secured with towels and wraps before scanning to minimize potential movement during acquisition and subsequent image misregistration. The feet are typically positioned in plantar flexion, while the hands are positioned with the fingers extended and are spread either adjacent to the hips or above the head, depending on patient mobility and comfort. Images are acquired with a hybrid gamma camera with in-line CT capabilities (Symbia T6; Siemens Medical Solutions, Malvern, Pa). SPECT is performed using a 64-step (20 seconds per stop) 360° noncircular orbit with reconstruction in a 128 × 128 matrix using a three-dimensional ordered-subset expectation maximization (OSEM) algorithm. Low-dose nonenhanced CT is then performed, with images reconstructed at a section thickness of 5 mm in a 256 × 256 matrix. A CT-based attenuation correction algorithm is applied. Coregistered planar and three-dimensional images are viewed side by side by using a localizer tool or are viewed in graded blended fusion mode with proprietary software (MedView; MedImage, Canton, Mich). The patterns and significance of tracer uptake at SPECT/CT correlate with those observed at delayed phase bone scintigraphy (Table 3).
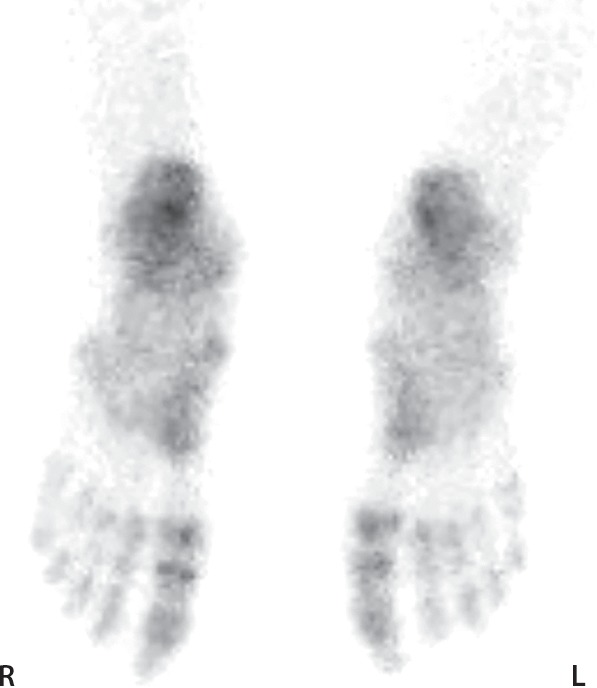
SPECT/CT can be used to define the exact level of bone necrosis in frostbite injuries (Figs 7, 8), which is critical for surgical planning and limiting surgery-related tissue loss. The status of the small digits and the tips of the distal phalanges, which is often difficult to assess at multiphase bone scintigraphy (34,36), is easily evaluated at SPECT/CT. SPECT/CT may also help uncover subtle findings that can be missed at scintigraphy alone, leading to a more accurate diagnosis (Fig 9). Clear demarcation of the level of tissue loss occurs at SPECT/CT well before its appearance at physical examination (Fig 10). This enables earlier surgical planning and intervention and in turn may contribute to lower risk for superinfection or sepsis, shorter hospitalization, and earlier rehabilitation (15,34). Use of SPECT/CT during initial multiphase bone scintigraphy may minimize the necessity of performing follow-up examinations because fewer ambiguities arise regarding the status of affected areas. When follow-up scans are warranted, areas of absent osseous tracer uptake initially observed do not regain viability on subsequent images (Fig 11). Multiphase bone scintigraphy with SPECT/CT may also be used to evaluate response to treatment in cases of questionable tissue viability (Fig 6).
Figure 8a.
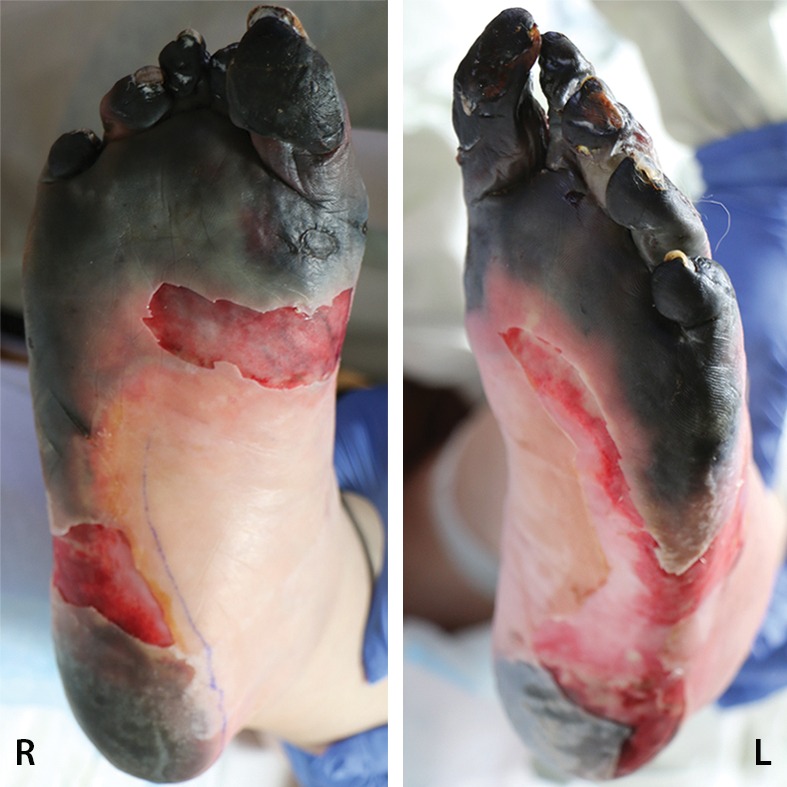
Fourth-degree frostbite of the feet in a 65-year-old woman. (a) Plantar photographs show gangrene of the bilateral toes and superficial necrosis of the bilateral midfeet and heels. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe markers (arrowheads) show absent tracer uptake distal to the midfoot bilaterally in the plantar blood flow phase at 30 seconds (b), soft-tissue phase (c), and 4-hour delayed phase (d). Note that the level of deep tissue injury is greater than what might have been predicted at physical examination. (e, f) Fused SPECT/CT images show absent tracer uptake at the level of the mid metatarsals in the right foot (e) and just proximal to the metatarsophalangeal joints in the left foot (f). The patient subsequently underwent bilateral amputation of the feet, with full-thickness graft reconstruction at the same levels.
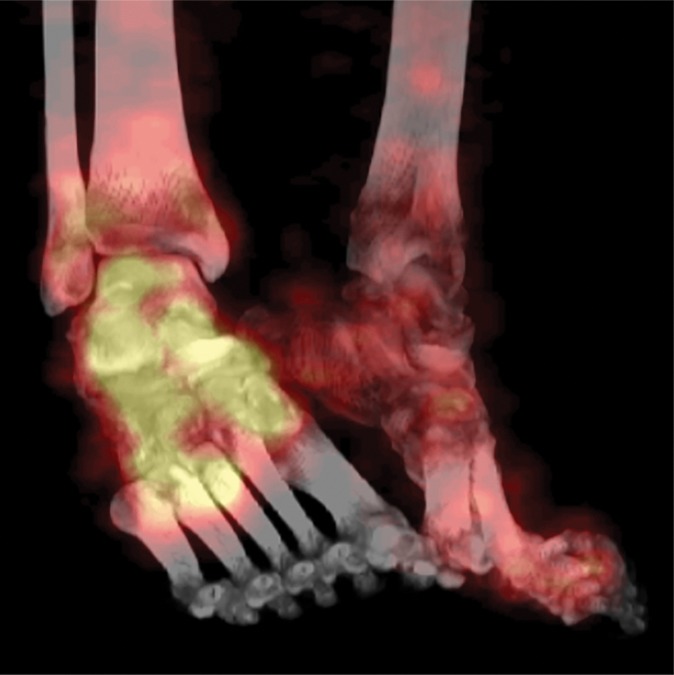
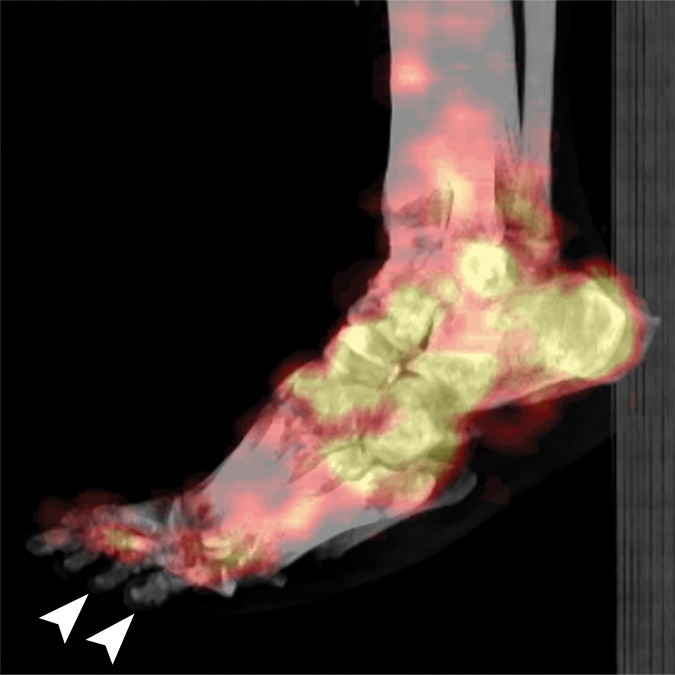
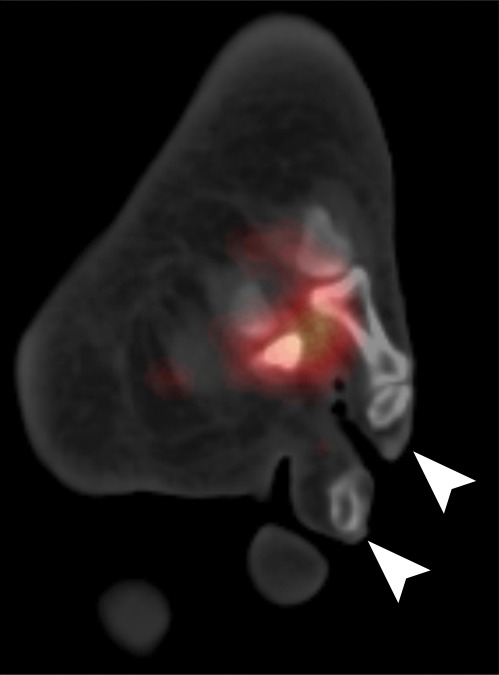
Figure 9a.
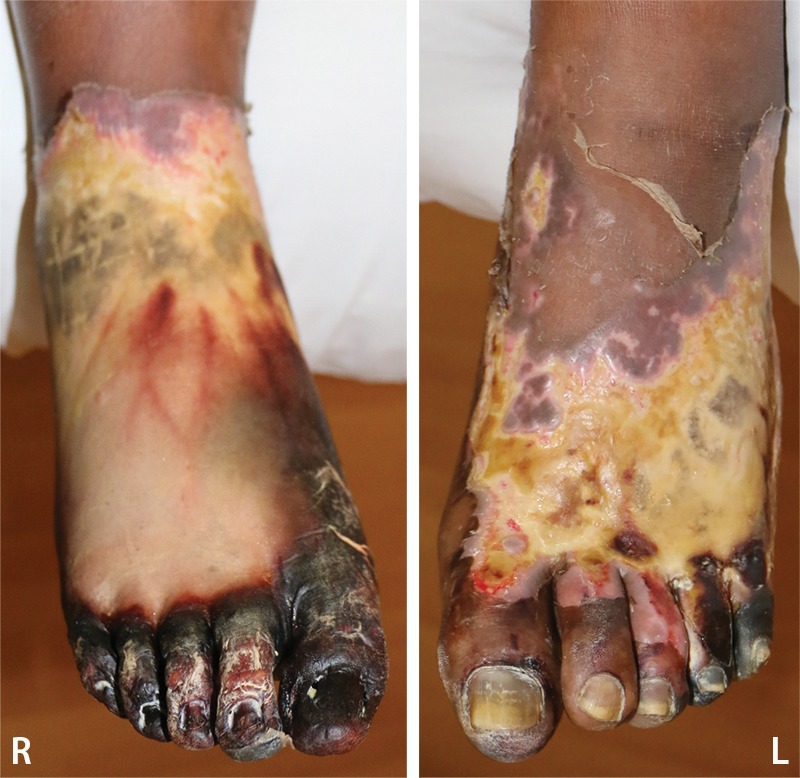
Fourth-degree frostbite of the feet in a 46-year-old man with a history of congestive heart failure. (a) Dorsal photographs show bilateral toe gangrene, which is more extensive in the right foot. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe and heel markers show absent tracer uptake beyond the right midfoot in the plantar blood flow phase at 100 seconds (b), soft-tissue phase (c), and 5.5-hour delayed phase (d). Note absent tracer uptake in the left fourth and fifth toes (arrowhead in c) on the soft-tissue phase image. Also note the lack of osseous uptake on the delayed phase image in d, a finding attributed to a combination of impaired local delivery of tracer and the patient’s low cardiac output. Late delayed phase images should be obtained 18–24 hours after tracer injection if osseous uptake is absent on initial delayed phase images. (e) Fused SPECT/CT image of the feet obtained 24 hours after tracer administration delineates the level of tracer cutoff in the right foot as just distal to the tarsometatarsal joints. (f, g) Three-dimensional lateral (f) and two-dimensional dorsoplantar (g) SPECT/CT images show that tracer uptake is also absent in the left fourth and fifth toes (arrowheads). The patient subsequently underwent a right below-the-knee amputation and left fourth and fifth toe amputations.
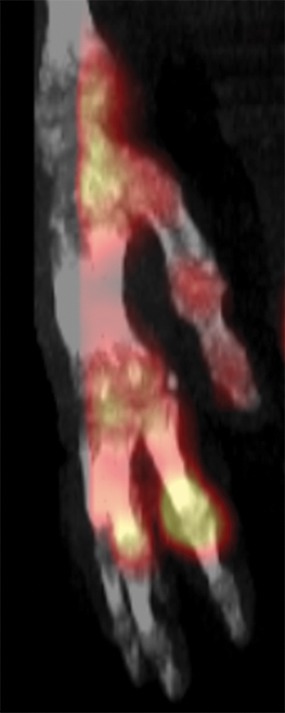
Figure 10a.
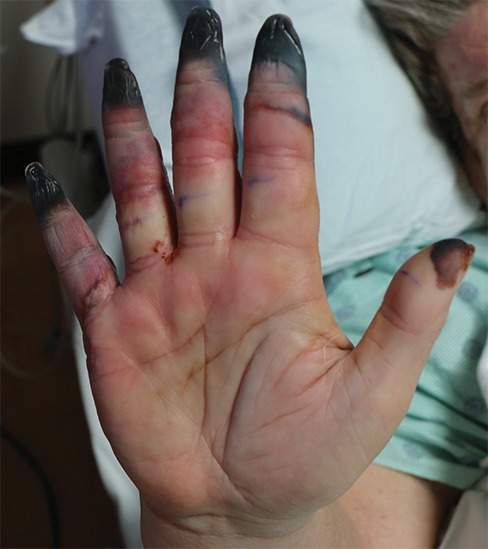
Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
Figure 11a.
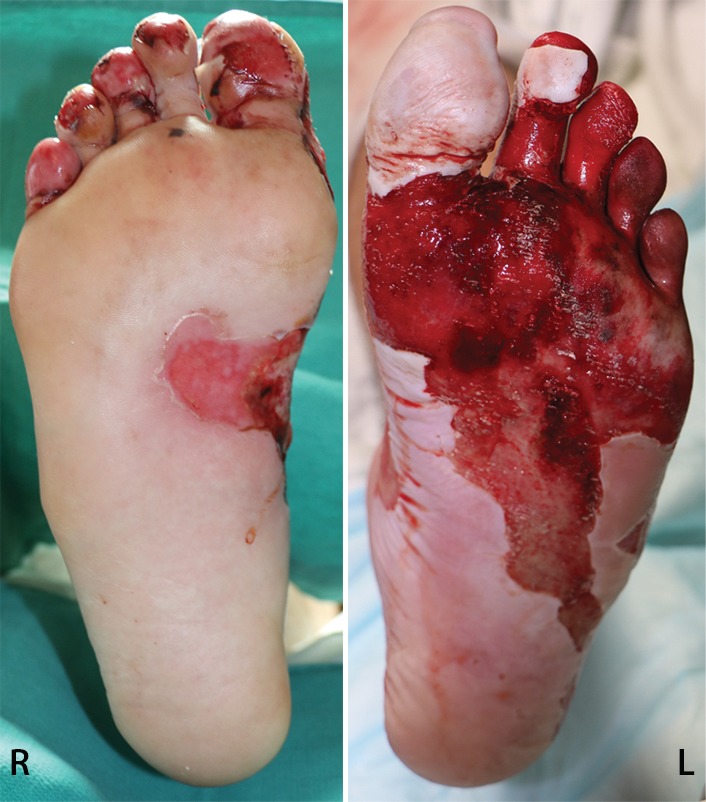
Third-degree frostbite of the right foot and fourth-degree frostbite of the left foot in a 39-year-old man. (a) Plantar photographs obtained after blister aspiration and débridement show large areas of freshly débrided tissue involving the toes and plantar surfaces of both feet. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show an absence of tracer uptake in the distal left foot in the plantar blood flow phase at 100 seconds (with toe markers) (b), soft-tissue phase (with toe markers) (c), and 6-hour delayed phase (without markers) (d). (e) Fused SPECT/CT image clearly demonstrates the level of tracer cutoff at the metatarsophalangeal joints in the left second through fifth toes. The patient underwent amputation of the left second through fifth toes but was unwilling to have his left first toe removed at that time. (f) Repeat fused SPECT/CT image obtained 8 days later shows absent tracer uptake within the left first toe (arrowhead) at the identical level to that seen at previous SPECT/CT.
Figure 8b.
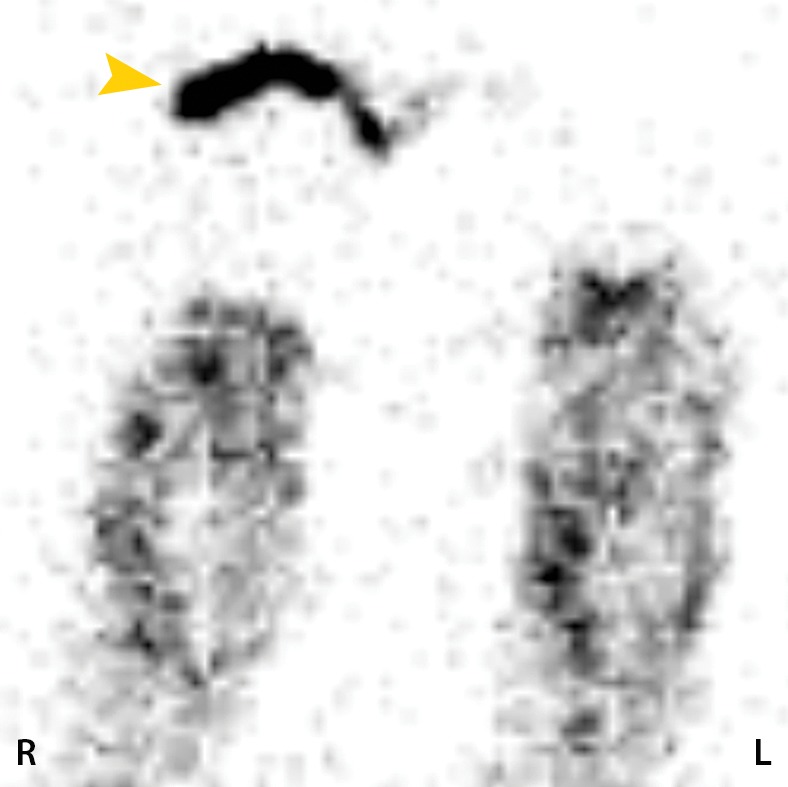
Fourth-degree frostbite of the feet in a 65-year-old woman. (a) Plantar photographs show gangrene of the bilateral toes and superficial necrosis of the bilateral midfeet and heels. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe markers (arrowheads) show absent tracer uptake distal to the midfoot bilaterally in the plantar blood flow phase at 30 seconds (b), soft-tissue phase (c), and 4-hour delayed phase (d). Note that the level of deep tissue injury is greater than what might have been predicted at physical examination. (e, f) Fused SPECT/CT images show absent tracer uptake at the level of the mid metatarsals in the right foot (e) and just proximal to the metatarsophalangeal joints in the left foot (f). The patient subsequently underwent bilateral amputation of the feet, with full-thickness graft reconstruction at the same levels.
Figure 8c.

Fourth-degree frostbite of the feet in a 65-year-old woman. (a) Plantar photographs show gangrene of the bilateral toes and superficial necrosis of the bilateral midfeet and heels. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe markers (arrowheads) show absent tracer uptake distal to the midfoot bilaterally in the plantar blood flow phase at 30 seconds (b), soft-tissue phase (c), and 4-hour delayed phase (d). Note that the level of deep tissue injury is greater than what might have been predicted at physical examination. (e, f) Fused SPECT/CT images show absent tracer uptake at the level of the mid metatarsals in the right foot (e) and just proximal to the metatarsophalangeal joints in the left foot (f). The patient subsequently underwent bilateral amputation of the feet, with full-thickness graft reconstruction at the same levels.
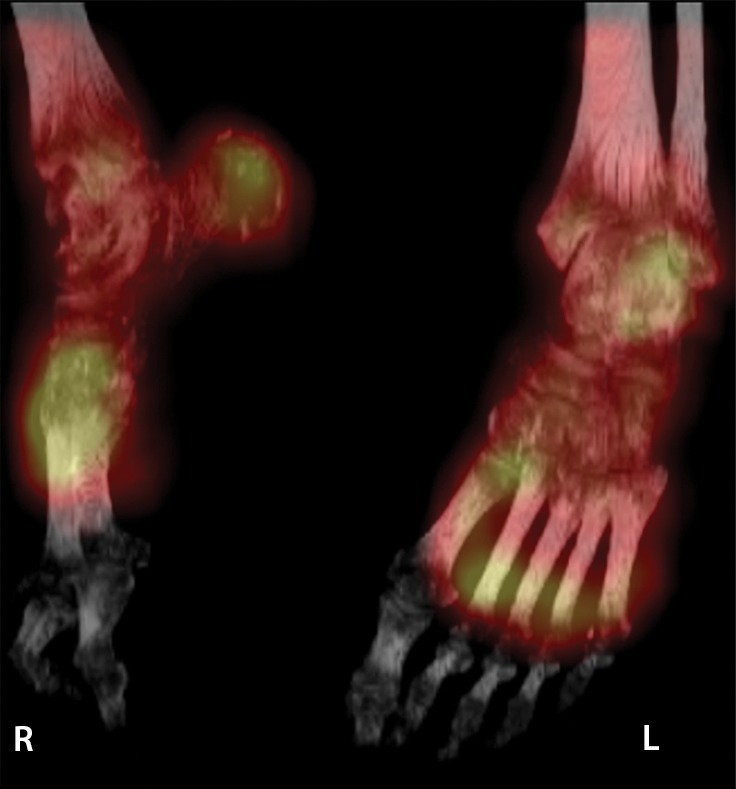
Figure 8d.
Fourth-degree frostbite of the feet in a 65-year-old woman. (a) Plantar photographs show gangrene of the bilateral toes and superficial necrosis of the bilateral midfeet and heels. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe markers (arrowheads) show absent tracer uptake distal to the midfoot bilaterally in the plantar blood flow phase at 30 seconds (b), soft-tissue phase (c), and 4-hour delayed phase (d). Note that the level of deep tissue injury is greater than what might have been predicted at physical examination. (e, f) Fused SPECT/CT images show absent tracer uptake at the level of the mid metatarsals in the right foot (e) and just proximal to the metatarsophalangeal joints in the left foot (f). The patient subsequently underwent bilateral amputation of the feet, with full-thickness graft reconstruction at the same levels.
Figure 8e.

Fourth-degree frostbite of the feet in a 65-year-old woman. (a) Plantar photographs show gangrene of the bilateral toes and superficial necrosis of the bilateral midfeet and heels. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe markers (arrowheads) show absent tracer uptake distal to the midfoot bilaterally in the plantar blood flow phase at 30 seconds (b), soft-tissue phase (c), and 4-hour delayed phase (d). Note that the level of deep tissue injury is greater than what might have been predicted at physical examination. (e, f) Fused SPECT/CT images show absent tracer uptake at the level of the mid metatarsals in the right foot (e) and just proximal to the metatarsophalangeal joints in the left foot (f). The patient subsequently underwent bilateral amputation of the feet, with full-thickness graft reconstruction at the same levels.
Figure 8f.
Fourth-degree frostbite of the feet in a 65-year-old woman. (a) Plantar photographs show gangrene of the bilateral toes and superficial necrosis of the bilateral midfeet and heels. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe markers (arrowheads) show absent tracer uptake distal to the midfoot bilaterally in the plantar blood flow phase at 30 seconds (b), soft-tissue phase (c), and 4-hour delayed phase (d). Note that the level of deep tissue injury is greater than what might have been predicted at physical examination. (e, f) Fused SPECT/CT images show absent tracer uptake at the level of the mid metatarsals in the right foot (e) and just proximal to the metatarsophalangeal joints in the left foot (f). The patient subsequently underwent bilateral amputation of the feet, with full-thickness graft reconstruction at the same levels.
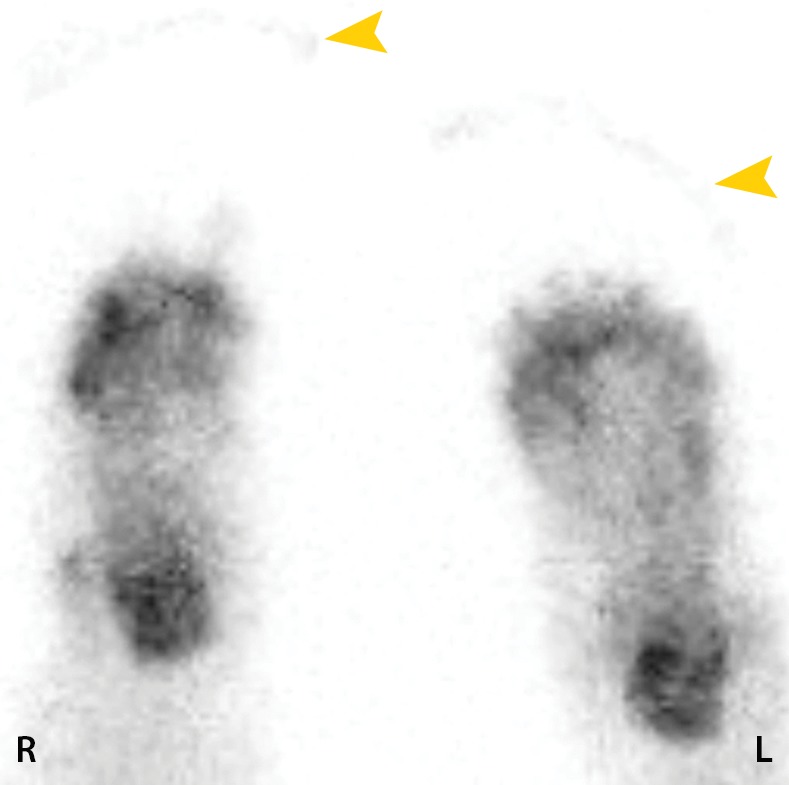
Figure 9b.
Fourth-degree frostbite of the feet in a 46-year-old man with a history of congestive heart failure. (a) Dorsal photographs show bilateral toe gangrene, which is more extensive in the right foot. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe and heel markers show absent tracer uptake beyond the right midfoot in the plantar blood flow phase at 100 seconds (b), soft-tissue phase (c), and 5.5-hour delayed phase (d). Note absent tracer uptake in the left fourth and fifth toes (arrowhead in c) on the soft-tissue phase image. Also note the lack of osseous uptake on the delayed phase image in d, a finding attributed to a combination of impaired local delivery of tracer and the patient’s low cardiac output. Late delayed phase images should be obtained 18–24 hours after tracer injection if osseous uptake is absent on initial delayed phase images. (e) Fused SPECT/CT image of the feet obtained 24 hours after tracer administration delineates the level of tracer cutoff in the right foot as just distal to the tarsometatarsal joints. (f, g) Three-dimensional lateral (f) and two-dimensional dorsoplantar (g) SPECT/CT images show that tracer uptake is also absent in the left fourth and fifth toes (arrowheads). The patient subsequently underwent a right below-the-knee amputation and left fourth and fifth toe amputations.
Figure 9c.

Fourth-degree frostbite of the feet in a 46-year-old man with a history of congestive heart failure. (a) Dorsal photographs show bilateral toe gangrene, which is more extensive in the right foot. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe and heel markers show absent tracer uptake beyond the right midfoot in the plantar blood flow phase at 100 seconds (b), soft-tissue phase (c), and 5.5-hour delayed phase (d). Note absent tracer uptake in the left fourth and fifth toes (arrowhead in c) on the soft-tissue phase image. Also note the lack of osseous uptake on the delayed phase image in d, a finding attributed to a combination of impaired local delivery of tracer and the patient’s low cardiac output. Late delayed phase images should be obtained 18–24 hours after tracer injection if osseous uptake is absent on initial delayed phase images. (e) Fused SPECT/CT image of the feet obtained 24 hours after tracer administration delineates the level of tracer cutoff in the right foot as just distal to the tarsometatarsal joints. (f, g) Three-dimensional lateral (f) and two-dimensional dorsoplantar (g) SPECT/CT images show that tracer uptake is also absent in the left fourth and fifth toes (arrowheads). The patient subsequently underwent a right below-the-knee amputation and left fourth and fifth toe amputations.
Figure 9d.
Fourth-degree frostbite of the feet in a 46-year-old man with a history of congestive heart failure. (a) Dorsal photographs show bilateral toe gangrene, which is more extensive in the right foot. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe and heel markers show absent tracer uptake beyond the right midfoot in the plantar blood flow phase at 100 seconds (b), soft-tissue phase (c), and 5.5-hour delayed phase (d). Note absent tracer uptake in the left fourth and fifth toes (arrowhead in c) on the soft-tissue phase image. Also note the lack of osseous uptake on the delayed phase image in d, a finding attributed to a combination of impaired local delivery of tracer and the patient’s low cardiac output. Late delayed phase images should be obtained 18–24 hours after tracer injection if osseous uptake is absent on initial delayed phase images. (e) Fused SPECT/CT image of the feet obtained 24 hours after tracer administration delineates the level of tracer cutoff in the right foot as just distal to the tarsometatarsal joints. (f, g) Three-dimensional lateral (f) and two-dimensional dorsoplantar (g) SPECT/CT images show that tracer uptake is also absent in the left fourth and fifth toes (arrowheads). The patient subsequently underwent a right below-the-knee amputation and left fourth and fifth toe amputations.
Figure 9e.
Fourth-degree frostbite of the feet in a 46-year-old man with a history of congestive heart failure. (a) Dorsal photographs show bilateral toe gangrene, which is more extensive in the right foot. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe and heel markers show absent tracer uptake beyond the right midfoot in the plantar blood flow phase at 100 seconds (b), soft-tissue phase (c), and 5.5-hour delayed phase (d). Note absent tracer uptake in the left fourth and fifth toes (arrowhead in c) on the soft-tissue phase image. Also note the lack of osseous uptake on the delayed phase image in d, a finding attributed to a combination of impaired local delivery of tracer and the patient’s low cardiac output. Late delayed phase images should be obtained 18–24 hours after tracer injection if osseous uptake is absent on initial delayed phase images. (e) Fused SPECT/CT image of the feet obtained 24 hours after tracer administration delineates the level of tracer cutoff in the right foot as just distal to the tarsometatarsal joints. (f, g) Three-dimensional lateral (f) and two-dimensional dorsoplantar (g) SPECT/CT images show that tracer uptake is also absent in the left fourth and fifth toes (arrowheads). The patient subsequently underwent a right below-the-knee amputation and left fourth and fifth toe amputations.
Figure 9f.
Fourth-degree frostbite of the feet in a 46-year-old man with a history of congestive heart failure. (a) Dorsal photographs show bilateral toe gangrene, which is more extensive in the right foot. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe and heel markers show absent tracer uptake beyond the right midfoot in the plantar blood flow phase at 100 seconds (b), soft-tissue phase (c), and 5.5-hour delayed phase (d). Note absent tracer uptake in the left fourth and fifth toes (arrowhead in c) on the soft-tissue phase image. Also note the lack of osseous uptake on the delayed phase image in d, a finding attributed to a combination of impaired local delivery of tracer and the patient’s low cardiac output. Late delayed phase images should be obtained 18–24 hours after tracer injection if osseous uptake is absent on initial delayed phase images. (e) Fused SPECT/CT image of the feet obtained 24 hours after tracer administration delineates the level of tracer cutoff in the right foot as just distal to the tarsometatarsal joints. (f, g) Three-dimensional lateral (f) and two-dimensional dorsoplantar (g) SPECT/CT images show that tracer uptake is also absent in the left fourth and fifth toes (arrowheads). The patient subsequently underwent a right below-the-knee amputation and left fourth and fifth toe amputations.
Figure 9g.
Fourth-degree frostbite of the feet in a 46-year-old man with a history of congestive heart failure. (a) Dorsal photographs show bilateral toe gangrene, which is more extensive in the right foot. (b–d) Multiphase 99mTc-MDP bone scintigraphic images with toe and heel markers show absent tracer uptake beyond the right midfoot in the plantar blood flow phase at 100 seconds (b), soft-tissue phase (c), and 5.5-hour delayed phase (d). Note absent tracer uptake in the left fourth and fifth toes (arrowhead in c) on the soft-tissue phase image. Also note the lack of osseous uptake on the delayed phase image in d, a finding attributed to a combination of impaired local delivery of tracer and the patient’s low cardiac output. Late delayed phase images should be obtained 18–24 hours after tracer injection if osseous uptake is absent on initial delayed phase images. (e) Fused SPECT/CT image of the feet obtained 24 hours after tracer administration delineates the level of tracer cutoff in the right foot as just distal to the tarsometatarsal joints. (f, g) Three-dimensional lateral (f) and two-dimensional dorsoplantar (g) SPECT/CT images show that tracer uptake is also absent in the left fourth and fifth toes (arrowheads). The patient subsequently underwent a right below-the-knee amputation and left fourth and fifth toe amputations.
Figure 10b.

Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
Figure 10c.

Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
Figure 10d.

Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
Figure 10e.
Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
Figure 10f.

Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
Figure 10g.
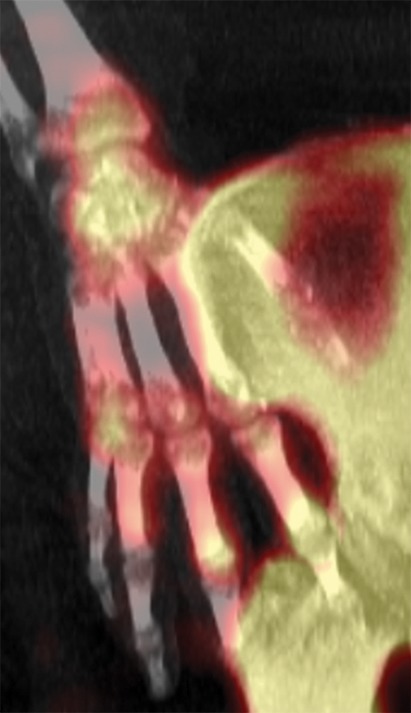
Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
Figure 10h.
Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
Figure 10i.
Fourth-degree frostbite of the right hand in a 65-year-old woman. (a) Palmar photograph obtained at bone scintigraphy shows tip gangrene of all digits. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show absent tracer uptake within the distal second, third, and fourth digits and absent tracer uptake distal to the fifth digit metacarpophalangeal joint in the palmar blood flow phase at 120 seconds (b), soft-tissue phase (c), and 3.5-hour delayed phase (d). Note the preservation of tracer uptake throughout the thumb. (e–g) Fused SPECT/CT images of the right hand (obtained in different projections) show the exact level of bone necrosis within the right second, third, fourth, and fifth digits, with preserved uptake throughout the thumb. (h, i) Palmar (h) and dorsal (i) photographs obtained 1 week after bone scintigraphy demonstrate the level of tissue loss previously defined at SPECT/CT.
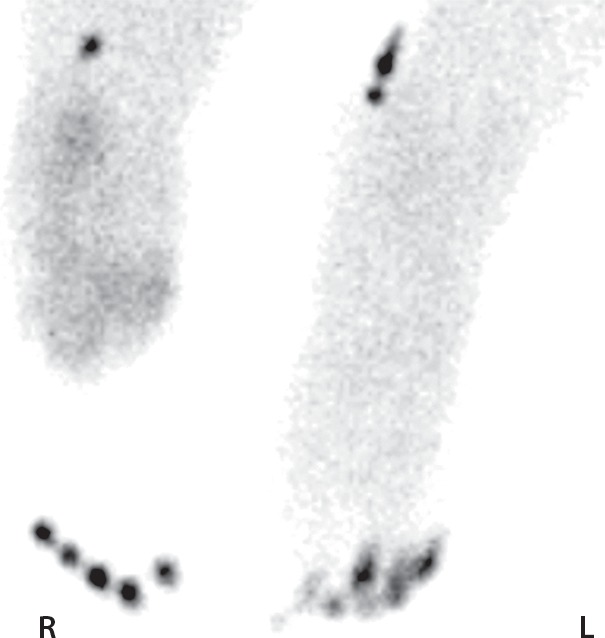
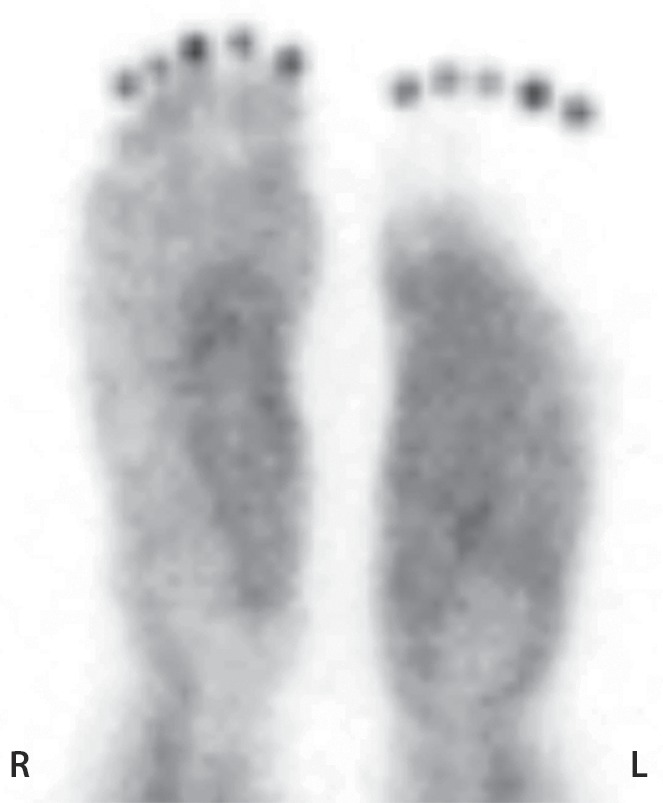
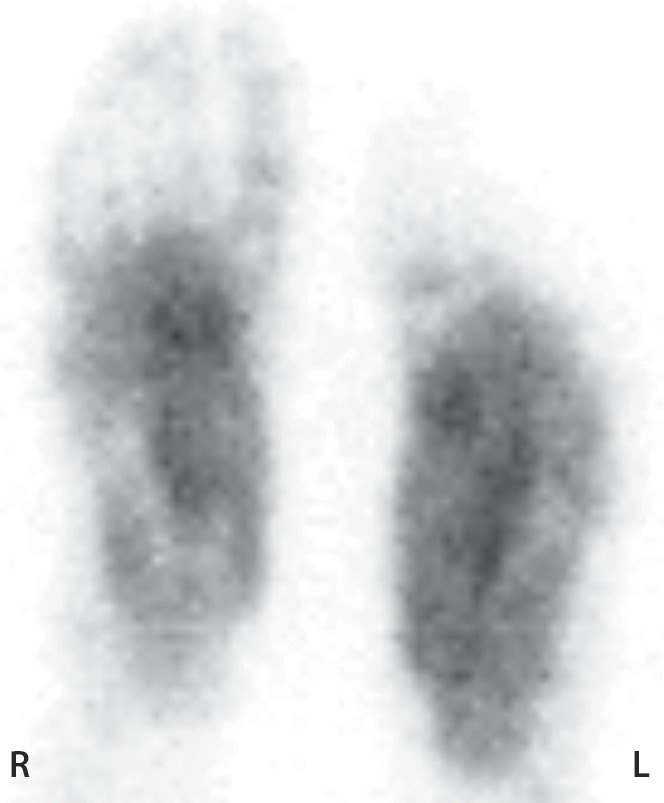
Figure 11b.
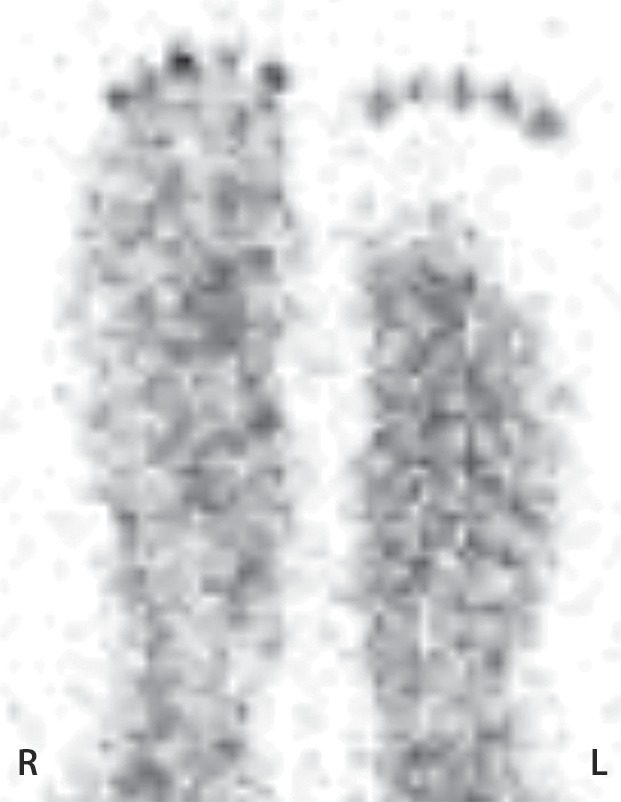
Third-degree frostbite of the right foot and fourth-degree frostbite of the left foot in a 39-year-old man. (a) Plantar photographs obtained after blister aspiration and débridement show large areas of freshly débrided tissue involving the toes and plantar surfaces of both feet. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show an absence of tracer uptake in the distal left foot in the plantar blood flow phase at 100 seconds (with toe markers) (b), soft-tissue phase (with toe markers) (c), and 6-hour delayed phase (without markers) (d). (e) Fused SPECT/CT image clearly demonstrates the level of tracer cutoff at the metatarsophalangeal joints in the left second through fifth toes. The patient underwent amputation of the left second through fifth toes but was unwilling to have his left first toe removed at that time. (f) Repeat fused SPECT/CT image obtained 8 days later shows absent tracer uptake within the left first toe (arrowhead) at the identical level to that seen at previous SPECT/CT.
Figure 11c.
Third-degree frostbite of the right foot and fourth-degree frostbite of the left foot in a 39-year-old man. (a) Plantar photographs obtained after blister aspiration and débridement show large areas of freshly débrided tissue involving the toes and plantar surfaces of both feet. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show an absence of tracer uptake in the distal left foot in the plantar blood flow phase at 100 seconds (with toe markers) (b), soft-tissue phase (with toe markers) (c), and 6-hour delayed phase (without markers) (d). (e) Fused SPECT/CT image clearly demonstrates the level of tracer cutoff at the metatarsophalangeal joints in the left second through fifth toes. The patient underwent amputation of the left second through fifth toes but was unwilling to have his left first toe removed at that time. (f) Repeat fused SPECT/CT image obtained 8 days later shows absent tracer uptake within the left first toe (arrowhead) at the identical level to that seen at previous SPECT/CT.
Figure 11d.
Third-degree frostbite of the right foot and fourth-degree frostbite of the left foot in a 39-year-old man. (a) Plantar photographs obtained after blister aspiration and débridement show large areas of freshly débrided tissue involving the toes and plantar surfaces of both feet. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show an absence of tracer uptake in the distal left foot in the plantar blood flow phase at 100 seconds (with toe markers) (b), soft-tissue phase (with toe markers) (c), and 6-hour delayed phase (without markers) (d). (e) Fused SPECT/CT image clearly demonstrates the level of tracer cutoff at the metatarsophalangeal joints in the left second through fifth toes. The patient underwent amputation of the left second through fifth toes but was unwilling to have his left first toe removed at that time. (f) Repeat fused SPECT/CT image obtained 8 days later shows absent tracer uptake within the left first toe (arrowhead) at the identical level to that seen at previous SPECT/CT.
Figure 11e.

Third-degree frostbite of the right foot and fourth-degree frostbite of the left foot in a 39-year-old man. (a) Plantar photographs obtained after blister aspiration and débridement show large areas of freshly débrided tissue involving the toes and plantar surfaces of both feet. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show an absence of tracer uptake in the distal left foot in the plantar blood flow phase at 100 seconds (with toe markers) (b), soft-tissue phase (with toe markers) (c), and 6-hour delayed phase (without markers) (d). (e) Fused SPECT/CT image clearly demonstrates the level of tracer cutoff at the metatarsophalangeal joints in the left second through fifth toes. The patient underwent amputation of the left second through fifth toes but was unwilling to have his left first toe removed at that time. (f) Repeat fused SPECT/CT image obtained 8 days later shows absent tracer uptake within the left first toe (arrowhead) at the identical level to that seen at previous SPECT/CT.
Figure 11f.
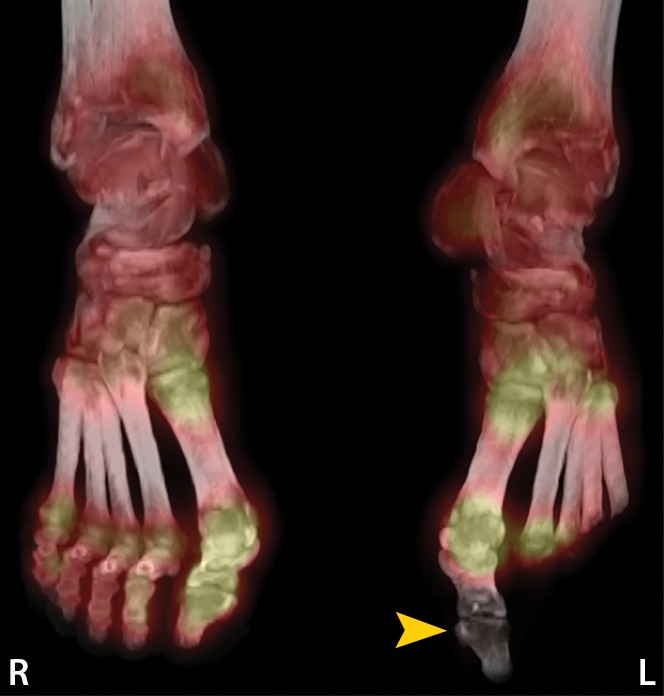
Third-degree frostbite of the right foot and fourth-degree frostbite of the left foot in a 39-year-old man. (a) Plantar photographs obtained after blister aspiration and débridement show large areas of freshly débrided tissue involving the toes and plantar surfaces of both feet. (b–d) Multiphase 99mTc-MDP bone scintigraphic images show an absence of tracer uptake in the distal left foot in the plantar blood flow phase at 100 seconds (with toe markers) (b), soft-tissue phase (with toe markers) (c), and 6-hour delayed phase (without markers) (d). (e) Fused SPECT/CT image clearly demonstrates the level of tracer cutoff at the metatarsophalangeal joints in the left second through fifth toes. The patient underwent amputation of the left second through fifth toes but was unwilling to have his left first toe removed at that time. (f) Repeat fused SPECT/CT image obtained 8 days later shows absent tracer uptake within the left first toe (arrowhead) at the identical level to that seen at previous SPECT/CT.
Surgical options after multiphase bone scintigraphy with SPECT/CT include early amputation or limb salvage with débridement and flap transfer (31,34,56). The combination of the soft-tissue information provided at multiphase bone scintigraphy and the detailed and anatomically localized osseous information provided at SPECT/CT guides the treatment team toward the surgical approach that will enable the minimal amount of tissue loss and maximal functional capacity after recovery.
Conclusion
Frostbite injuries can result in substantial long-term functional morbidity if not promptly evaluated and treated. It is impossible to assess the extent and depth of frostbite-induced tissue loss at clinical examination alone. Therefore, imaging plays a critical role in assessing frostbite injuries and guiding appropriate nonsurgical and surgical care. Although radiography and angiography-directed thrombolysis are important in initial evaluation and treatment of frostbite injuries, multiphase bone scintigraphy with SPECT/CT is the modality of choice for prognostication and planning of definitive surgical care.
Acknowledgments
Acknowledgment
The authors thank Vanessa Allen for her assistance with preparing the figures for publication.
Recipient of a Cum Laude award for an education exhibit at the 2015 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
B.L. supported by the Plastic Surgery Foundation (National Endowment Award), American Association of Plastic Surgeons (academic scholarship), National Institute of General Medical Sciences (K08GM109105-0), American Association for the Surgery of Trauma (Research & Education Foundation Scholarship), and Association for Academic Surgery Foundation (Roslyn Award).
Abbreviations:
- DSA
- digital subtraction angiography
- MDP
- methylene diphosphonate
- tPA
- tissue plasminogen activator
References
- 1.Handford C, Buxton P, Russell K, et al. Frostbite: a practical approach to hospital management. Extrem Physiol Med 2014;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingram BJ, Raymond TJ. Recognition and treatment of freezing and nonfreezing cold injuries. Curr Sports Med Rep 2013;12(2):125–130. [DOI] [PubMed] [Google Scholar]
- 3.Gross EA, Moore JC. Using thrombolytics in frostbite injury. J Emerg Trauma Shock 2012;5(3):267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallam MJ, Cubison T, Dheansa B, Imray C. Managing frostbite. BMJ 2010;341:c5864. [DOI] [PubMed] [Google Scholar]
- 5.Imray C, Grieve A, Dhillon S; Caudwell Xtreme Everest Research Group. Cold damage to the extremities: frostbite and non-freezing cold injuries. Postgrad Med J 2009;85(1007):481–488. [DOI] [PubMed] [Google Scholar]
- 6.Johnson AR, Jensen HL, Peltier G, DelaCruz E. Efficacy of intravenous tissue plasminogen activator in frostbite patients and presentation of a treatment protocol for frostbite patients. Foot Ankle Spec 2011;4(6):344–348. [DOI] [PubMed] [Google Scholar]
- 7.Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract 1998;11(1):34–40. [DOI] [PubMed] [Google Scholar]
- 8.Valnicek SM, Chasmar LR, Clapson JB. Frostbite in the prairies: a 12-year review. Plast Reconstr Surg 1993;92(4): 633–641. [DOI] [PubMed] [Google Scholar]
- 9.Hutchison RL. Frostbite of the hand. J Hand Surg Am 2014;39(9):1863–1868. [DOI] [PubMed] [Google Scholar]
- 10.Burgess JE, Macfarlane F. Retrospective analysis of the ethnic origins of male British army soldiers with peripheral cold weather injury. J R Army Med Corps 2009;155(1):11–15. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh SE, Opacic M, Freer L, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite: 2014 update. Wilderness Environ Med 2014;25(4 suppl):S43–S54. [DOI] [PubMed] [Google Scholar]
- 12.Murphy JV, Banwell PE, Roberts AH, McGrouther DA. Frostbite: pathogenesis and treatment. J Trauma 2000;48(1):171–178. [DOI] [PubMed] [Google Scholar]
- 13.Salimi Z, Wolverson MK, Herbold DR, Vas W. Frostbite: experimental assessment of tissue damage using Tc-99m pyrophosphate—work in progress. Radiology 1986;161(1): 227–231. [DOI] [PubMed] [Google Scholar]
- 14.Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg 2014;51(10):417–431. [DOI] [PubMed] [Google Scholar]
- 15.Cauchy E, Chetaille E, Marchand V, Marsigny B. Retrospective study of 70 cases of severe frostbite lesions: a proposed new classification scheme. Wilderness Environ Med 2001;12(4):248–255. [DOI] [PubMed] [Google Scholar]
- 16.Zafren K. Frostbite: prevention and initial management. High Alt Med Biol 2013;14(1):9–12. [DOI] [PubMed] [Google Scholar]
- 17.Woo EK, Lee JW, Hur GY, et al. Proposed treatment protocol for frostbite: a retrospective analysis of 17 cases based on a 3-year single-institution experience. Arch Plast Surg 2013;40(5):510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemp SS, Dalinka MK, Schumacher HR. Acro-osteolysis: etiologic and radiological considerations. JAMA 1986;255(15): 2058–2061. [DOI] [PubMed] [Google Scholar]
- 19.Tishler JM. The soft-tissue and bone changes in frostbite injuries. Radiology 1972;102(3):511–513. [DOI] [PubMed] [Google Scholar]
- 20.Vinson HA, Schatzki R. Roentgenologic bone changes encountered in frostbite, Korea 1950–51. Radiology 1954;63(5): 685–695. [DOI] [PubMed] [Google Scholar]
- 21.Ellis R, Short JG, Simonds BD. Unilateral osteoarthritis of the distal interphalangeal joints following frostbite: a case report. Radiology 1969;93(4):857–858. [DOI] [PubMed] [Google Scholar]
- 22.Selke AC, Jr. Destruction of phalangeal epiphyses by frostbite. Radiology 1969;93(4):859–860. [DOI] [PubMed] [Google Scholar]
- 23.Ouriel K, Kandarpa K. Safety of thrombolytic therapy with urokinase or recombinant tissue plasminogen activator for peripheral arterial occlusion: a comprehensive compilation of published work. J Endovasc Ther 2004;11(4):436–446. [DOI] [PubMed] [Google Scholar]
- 24.Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma 2005;59(6):1350–1354; discussion 1354–1355. [DOI] [PubMed] [Google Scholar]
- 25.Saemi AM, Johnson JM, Morris CS. Treatment of bilateral hand frostbite using transcatheter arterial thrombolysis after papaverine infusion. Cardiovasc Intervent Radiol 2009;32(6):1280–1283. [DOI] [PubMed] [Google Scholar]
- 26.Sheridan RL, Goldstein MA, Stoddard FJ, Jr, Walker TG. Case records of the Massachusetts General Hospital: case 41-2009—a 16-year-old boy with hypothermia and frostbite. N Engl J Med 2009;361(27):2654–2662. [DOI] [PubMed] [Google Scholar]
- 27.Wagner C, Pannucci CJ. Thrombolytic therapy in the acute management of frostbite injuries. Air Med J 2011;30(1): 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim AE, Goverman J, Sarhane KA, Donofrio J, Walker TG, Fagan SP. The emerging role of tissue plasminogen activator in the management of severe frostbite. J Burn Care Res 2015;36(2):e62–e66. [DOI] [PubMed] [Google Scholar]
- 29.Bruen KJ, Ballard JR, Morris SE, Cochran A, Edelman LS, Saffle JR. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg 2007;142(6):546–551; discussion 551–553. [DOI] [PubMed] [Google Scholar]
- 30.Gonzaga T, Jenabzadeh K, Anderson CP, Mohr WJ, Endorf FW, Ahrenholz DH. Use of intraarterial thrombolytic therapy for acute treatment of frostbite in 62 patients with review of thrombolytic therapy in frostbite. J Burn Care Res doi:10.1097/BCR.0000000000000245. Published online April 24, 2015. [DOI] [PMC free article] [PubMed]
- 31.Kraft CT, Millet JD, Agarwal S, et al. SPECT/CT in the evaluation of frostbite. J Burn Care Res doi:10.1097/BCR.0000000000000359. Published online June 10, 2016.
- 32.Barker JR, Haws MJ, Brown RE, Kucan JO, Moore WD. Magnetic resonance imaging of severe frostbite injuries. Ann Plast Surg 1997;38(3):275–279. [DOI] [PubMed] [Google Scholar]
- 33.Raman SR, Jamil Z, Cosgrove J. Magnetic resonance angiography unmasks frostbite injury. Emerg Med J 2011;28(5):450. [DOI] [PubMed] [Google Scholar]
- 34.Cauchy E, Marsigny B, Allamel G, Verhellen R, Chetaille E. The value of technetium 99 scintigraphy in the prognosis of amputation in severe frostbite injuries of the extremities: a retrospective study of 92 severe frostbite injuries. J Hand Surg Am 2000;25(5):969–978. [DOI] [PubMed] [Google Scholar]
- 35.Cauchy E, Chetaille E, Lefevre M, Kerelou E, Marsigny B. The role of bone scanning in severe frostbite of the extremities: a retrospective study of 88 cases. Eur J Nucl Med 2000;27(5):497–502. [DOI] [PubMed] [Google Scholar]
- 36.Mehta RC, Wilson MA. Frostbite injury: prediction of tissue viability with triple-phase bone scanning. Radiology 1989;170(2):511–514. [DOI] [PubMed] [Google Scholar]
- 37.Zavadovskaia VD, Borodulin VG. Gamma scintigraphy in deep frostbite [in Russian]. Med Radiol (Mosk) 1981;26(2): 8–10. [PubMed] [Google Scholar]
- 38.Ikawa G, dos Santos PA, Yamaguchi KT, Stroh-Recor C, Ibello R. Frostbite and bone scanning: the use of 99m-labeled phosphates in demarcating the line of viability in frostbite victims. Orthopedics 1986;9(9):1257–1261. [DOI] [PubMed] [Google Scholar]
- 39.Santapau A, Razola P, Tardin L, Andrés A, Prats E, Banzo J. The role of bone scanning in severe frostbite of the feet in a mountaineer. Rev Esp Med Nucl Imagen Mol 2013;32(2):113–114. [DOI] [PubMed] [Google Scholar]
- 40.Affleck DG, Edelman L, Morris SE, Saffle JR. Assessment of tissue viability in complex extremity injuries: utility of the pyrophosphate nuclear scan. J Trauma 2001;50(2): 263–269. [DOI] [PubMed] [Google Scholar]
- 41.Kenney A, 3rd, Vyas P. Frostbite injury: appearance on three-phase bone scan. Clin Nucl Med 1998;23(3):188. [DOI] [PubMed] [Google Scholar]
- 42.Lisbona R, Rosenthall L. Assessment of bone viability by scintiscanning in frostbite injuries. J Trauma 1976;16(12): 989–992. [DOI] [PubMed] [Google Scholar]
- 43.Shih WJ, Riley C, Magoun S, Ryo UY. Intense bone imaging agent uptake in the soft tissues of the lower legs and feet relating to ischemia and cold exposure. Eur J Nucl Med 1988;14(7-8):419–421. [DOI] [PubMed] [Google Scholar]
- 44.Purdue GF, Lewis SA, Hunt JL. Pyrophosphate scanning in early frostbite injury. Am Surg 1983;49(11):619–620. [PubMed] [Google Scholar]
- 45.Salimi Z, Vas W, Tang-Barton P, Eachempati RG, Morris L, Carron M. Assessment of tissue viability in frostbite by 99mTc pertechnetate scintigraphy. AJR Am J Roentgenol 1984;142(2):415–419. [DOI] [PubMed] [Google Scholar]
- 46.Bhatnagar A, Sarker BB, Sawroop K, Chopra MK, Sinha N, Kashyap R. Diagnosis, characterisation and evaluation of treatment response of frostbite using pertechnetate scintigraphy: a prospective study. Eur J Nucl Med Mol Imaging 2002;29(2):170–175. [DOI] [PubMed] [Google Scholar]
- 47.Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ. Radionuclide bone imaging: an illustrative review. RadioGraphics 2003;23(2):341–358. [DOI] [PubMed] [Google Scholar]
- 48.Zuckier LS, Freeman LM. Nonosseous, nonurologic uptake on bone scintigraphy: atlas and analysis. Semin Nucl Med 2010;40(4):242–256. [DOI] [PubMed] [Google Scholar]
- 49.Wong KK, Piert M. Dynamic bone imaging with 99mTc-labeled diphosphonates and 18F-NaF: mechanisms and applications. J Nucl Med 2013;54(4):590–599. [DOI] [PubMed] [Google Scholar]
- 50.Charkes ND, Makler PT, Jr. Studies in skeletal tracer kinetics. V. Computer-simulated Tc-99m (Sn) MDP bone-scan changes in some systemic disorders: concise communication. J Nucl Med 1981;22(7):601–605. [PubMed] [Google Scholar]
- 51.Charkes ND. Skeletal blood flow: implications for bone-scan interpretation. J Nucl Med 1980;21(1):91–98. [PubMed] [Google Scholar]
- 52.Blake GM, Park-Holohan SJ, Fogelman I. Quantitative studies of bone in postmenopausal women using 18F-fluoride and 99mTc-methylene diphosphonate. J Nucl Med 2002;43(3):338–345. [PubMed] [Google Scholar]
- 53.Charkes ND. Mechanisms of skeletal tracer uptake. J Nucl Med 1979;20(7):794–795. [PubMed] [Google Scholar]
- 54.Peller PJ, Ho VB, Kransdorf MJ. Extraosseous Tc-99m MDP uptake: a pathophysiologic approach. RadioGraphics 1993;13(4):715–734. [DOI] [PubMed] [Google Scholar]
- 55.Alazraki N, Dries D, Datz F, Lawrence P, Greenberg E, Taylor A, Jr. Value of a 24-hour image (four-phase bone scan) in assessing osteomyelitis in patients with peripheral vascular disease. J Nucl Med 1985;26(7):711–717. [PubMed] [Google Scholar]
- 56.Greenwald D, Cooper B, Gottlieb L. An algorithm for early aggressive treatment of frostbite with limb salvage directed by triple-phase scanning. Plast Reconstr Surg 1998;102(4): 1069–1074. [DOI] [PubMed] [Google Scholar]
- 57.Buck AK, Nekolla S, Ziegler S, et al. SPECT/CT. J Nucl Med 2008;49(8):1305–1319. [DOI] [PubMed] [Google Scholar]