Abstract
The interferon-inducible protein Z-DNA binding protein 1 (ZBP1, also known as DNA-dependent activator of IFN-regulatory factors (DAI) and DLM-1) was identified as a dsDNA sensor, which instigates innate immune responses. However, this classification has been disputed and whether ZBP1 functions as a pathogen sensor during an infection has remained unknown. Herein, we demonstrated ZBP1-mediated sensing of the influenza A virus (IAV) proteins NP and PB1, triggering cell death and inflammatory responses via the RIPK1–RIPK3–Caspase-8 axis. ZBP1 regulates NLRP3 inflammasome activation as well as induction of apoptosis, necroptosis and pyroptosis in IAV-infected cells. Importantly, ZBP1 deficiency protected mice from mortality during IAV infection owing to reduced inflammatory responses and epithelial damage. Overall, these findings indicate that ZBP1 is an innate immune sensor of IAV and highlight its importance in the pathogenesis of IAV infection.
Keywords: ZBP1, DAI, NLRP3, inflammasome, caspase-1, pyroptosis, necroptosis, apoptosis, influenza virus, IFNs, caspase-8, RIPK3
INTRODUCTION
The influenza virus infects millions of people annually and causes significant morbidity and up to half a million deaths (1). Type A Influenza virus (IAV) is also associated with epidemics and pandemics owing to high mutation rates and genetic reassortment. Pattern-recognition receptors, including Toll-like receptors (TLRs), RIG-I-like receptors (RLRs) and nucleotide and oligomerization domain, leucine-rich repeats-containing proteins (NLRs) have a central role in the recognition of IAV infection (2). Virus sensing by these receptors initiates an innate immune response aimed at controlling virus replication and eliminating the infectious virus.
Innate sensing of IAV triggers multiple intracellular signaling cascades that coordinately regulate induction of type I interferons (IFNs) and proinflammatory cytokines (2). In addition, virus sensing also induces cell death in order to destroy the replicative niche necessary for survival and propagation of these intracellular pathogens (3). While epithelial cell death facilitates control of virus replication by eliminating infected cells, uncontrolled cell death can exacerbate tissue injury and compromise lung function (4). Previous studies have demonstrated an inherent link between orchestration of various programmed cell death pathways and pathogenesis of IAV (5). Consistent with this, apoptosis in lung epithelial cells was shown to exacerbate pneumonia and mortality during IAV infection (6). Similarly, uncontrolled necroptosis in airway epithelial cells also increases morbidity and mortality during IAV infection (7). Unlike the detrimental effects of apoptosis and necroptosis, NLRP3 inflammasome activation leading to pyroptosis and release of proinflammatory cytokines IL-1β and IL-18 is protective during acute IAV infection (8, 9). Although all these cell death pathways are known to be activated during IAV infection, the innate immune sensors initiating cell death and the intracellular signaling cascades regulating this response are largely unknown and are defined in this study.
RESULTS
The IFN-inducible protein ZBP1 mediates cell death in response to IAV infection
To delineate the virus sensing pathways initiating cell death responses during IAV infection, cell death was assessed in primary murine BMDMs infected with Influenza virus A/Puerto Rico/8/34 (PR8; H1N1). BMDMs lacking the TLR adaptor proteins MyD88 or TRIF, or the RIG-I adaptor MAVS undergo cell death during infection with this mouse-adapted virus, probably due to functional redundancy of TLR and RIG-I pathways in IAV sensing in these cells (2) (fig. S1A and C). Furthermore, BMDMs and fibroblasts lacking the adaptor STING (central for DNA sensing), TNF receptor 1 (TNFR1), TNFR2 or the adaptor molecule TNFR1-associated death domain protein (TRADD) were also susceptible to IAV-induced cell death (Fig. 1A to D and fig. S1B and C). In contrast, cells lacking type I IFN receptor 1 (IFNAR1) or its downstream signaling proteins STAT1 and IRF9 were fully resistant to IAV-induced cell death (Fig. 1A to D and fig. S1D). Increased levels of M1 and NS1 proteins in IAV-infected Ifnar1−/− BMDMs confirmed proper virus entry and replication in these cells (fig. S1E). These results demonstrated that cell death during IAV infection is initiated through a pathway mediated by type I IFN signaling.
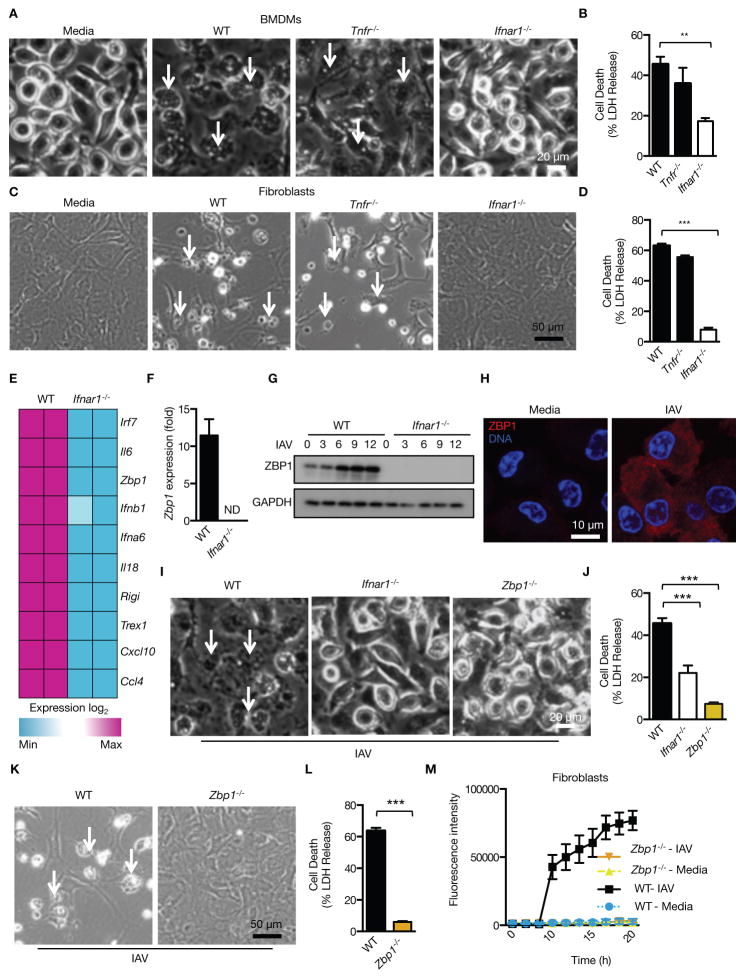
Figure 1. The IFN-inducible protein ZBP1 mediates cell death in response to IAV infection.
(A and B) Microscopic analysis and quantification of cell death by LDH release in BMDMs infected with IAV 16 h pi (p= 0.0131; n=4; two-tailed t test). (C and D) Microscopic analysis and quantification of cell death by LDH release in ear fibroblasts infected with IAV 16 h pi (p= 2.92096*10−6; n=4; two-tailed t test). (E) Microarray gene expression dataset enriched for nucleic acid sensing pathways with higher or lower expression in WT and Ifnar1−/− BMDMs 9 h pi with IAV (one experiment with two biological replicates per genotype). (F) Real-time quantitative RT-PCR analysis of Zbp1 expression in WT and Ifnar1−/− BMDMs 9 h pi with IAV (one experiment with two biological replicates per genotype). (G) Immunoblot analysis of ZBP1 and GAPDH (loading control) in unprimed BMDMs (0–12) h pi with IAV (n=3). (H) Immunostaining for ZBP1 in WT BMDMs infected with IAV for 16 h (n=2). (I and J) Microscopic analysis and quantification of cell death by LDH release in BMDMs infected with IAV 16 h pi (p= 0.0001; n=5; one-way ANOVA). (K and L) Microscopic analysis and quantification of cell death by LDH release in fibroblasts infected with IAV 16 h pi (p= 0.000460657; n=3; two-tailed t test). (M) Real time analysis of the kinetics of cell death in primary ear fibroblasts infected with IAV (MOI 10) (one experiment with three biological replicates per genotype).
Enrichment of the microarray gene expression dataset from IAV-infected WT and Ifnar1−/− BMDMs for nucleic acid sensing pathways followed by qRT-PCR revealed a significant reduction in the expression of a number of genes encoding proinflammatory cytokines and nucleic acid sensors in Ifnar1−/− BMDMs compared to WT BMDMs (Fig. 1E and F and fig. S2A). Interestingly, one of the most downregulated nucleic acid sensors in Ifnar1−/− BMDMs was the gene encoding Z–DNA binding protein 1 (ZBP1; also called DLM-1 and DAI) (Fig. 1E and F). Expression of ZBP1 was robustly upregulated in WT BMDMs infected with IAV, through a mechanism that required IFNAR1, STAT1 and IRF9 (Fig. 1G and H and fig. S2B). ZBP1 was initially identified as a cytosolic sensor of double-stranded (ds)DNA which drives type I IFN responses (10). However, this classification has been disputed after generation of Zbp1−/− mice (11–13). ZBP1 was of potential interest because of its unknown role in innate immune sensing and its possible role in the regulation of cell death (10–14).
The IFNAR-dependent cell death observed in IAV-infected cells prompted us to hypothesize that ZBP1 is an innate sensor of IAV driving cell death responses. Remarkably, Zbp1−/− BMDMs and fibroblasts were completely resistant to IAV-induced cell death (Fig. 1I to L). Real time analysis of ZBP1-dependent cell death in IAV-infected fibroblasts using IncuCyte and SYTOX green nucleic acid staining demonstrated that cell death was initiated at 8 to 10 h post infection (pi) which corresponds to one replication cycle of IAV (Fig. 1M). Comparable levels of the viral protein NS1 and IFNβ in Zbp1−/− BMDMs infected with IAV confirmed proper virus entry, replication and IFNβ secretion in these cells (fig. S2C and D). Together, these data demonstrate a critical role for ZBP1 in the regulation of cell death during IAV infection.
ZBP1 regulates NLRP3 inflammasome activation and proinflammatory cytokine production during IAV infection via the RIPK1–RIPK3–Caspase-8 axis
Distinct forms of programmed cell death, including pyroptosis, necroptosis and apoptosis, are initiated by context-specific stimuli encountered by the cell (15). Previous studies have demonstrated NLRP3 inflammasome-mediated activation of the pyroptosis-inducing cysteine protease, caspase-1, during IAV infection (16–19). While robust activation of caspase-1 was observed in WT BMDMs infected with IAV, this response was abrogated in Zbp1−/−, Ifnar1−/− and Nlrp3−/− BMDMs (Fig. 2A). Furthermore, the levels of the inflammasome-dependent cytokines, IL-1β and IL-18, were significantly reduced in Zbp1−/− and Ifnar1−/− cells compared to WT BMDMs (Fig. 2B to C), confirming a requirement for ZBP1 and type I IFN signaling in the activation of the NLRP3 inflammasome during IAV infection. ZBP1 was dispensable for the activation of caspase-1 and the release of IL-1β and IL-18 in response to the RNA virus vesicular stomatitis virus (VSV), the canonical NLRP3 activator LPS plus ATP, and to the non-canonical NLRP3 activators Escherichia coli and Citrobacter rodentium infection (Fig. 2D and fig. S3A to C). Notably, NLRP3 inflammasome activation during IAV infection did not require caspase-11 (Fig. 2E). These data identified a unique, type I IFN- and ZBP1-dependent pathway of NLRP3 inflammasome activation, which differ from the canonical and non-canonical NLRP3 inflammasome pathways.
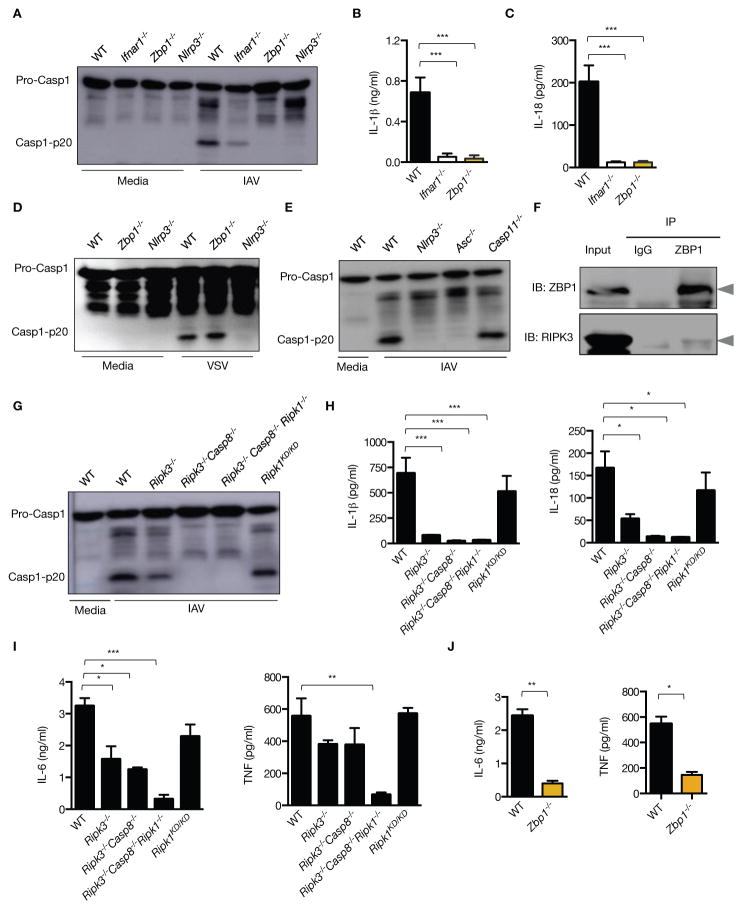
Figure 2. ZBP1 regulates NLRP3 inflammasome activation and proinflammatory cytokine production during IAV infection.
(A) Immunoblot analysis of pro-caspase-1 and caspase-1 subunit p20 in BMDMs 16 h pi with IAV (n=4)..(B and C) Levels of IL-1β and IL-18 in cell culture supernatants 16 h pi with IAV; (p= 0.0007 (IL-1β); p = 0.0015 (IL-18); n=3; one-way ANOVA)..(D and E) Immunoblot analysis of pro-caspase-1 and caspase-1 subunit p20 in BMDMs 16 h pi with VSV (Indiana strain) or IAV (n=3)..(F) Immunoprecipitation of ZBP1 from lysates of WT BMDMs infected with IAV for 16 h, and immunoblotted for ZBP1 and RIPK3 (n=3)..(G) Immunoblot analysis of pro-caspase-1 and caspase-1 subunit p20 in BMDMs 16 h pi with IAV (n=3)..(H) Levels of IL-1β and IL-18 in cell culture supernatants 16 h pi with IAV; (p=0.0003 (IL-1β); p=0.0033 (IL-18); n=3; one-way ANOVA)..(I and J) Levels of TNF and IL-6 in cell culture supernatants 16 h pi with IAV;.(I) (p=0.0003 (IL-6; one way ANOVA); p=0.04585299 (TNF; two-tailed t test) n=3);.(J) (p=0.00427068 (IL-6); p=0.01547619 (TNF;); n=3; two-tailed t test).
ZBP1 is dispensable for activation of the NLRC4 and AIM2 inflammasomes since infection by Salmonella enterica serovar Typhimurium, Francisella novicida and murine cytomegalovirus (MCMV), as well as transfection of poly(dA:dT) induced normal caspase-1 activation and secretion of IL-1β or IL-18 in Zbp1−/− BMDMs (fig. S3D to G). Although ZBP1 is a key regulator of IAV-induced NLRP3 inflammasome activation, ZBP1-dependent cell death during IAV infection occurred normally in Nlrp3−/−, Casp1−/− and Gsdmd−/− BMDMs (fig. S4A to D). These data suggest activation of other complimentary cell death pathways in IAV-infected cells.
ZBP1 contains two receptor-interacting protein homotypic interaction motif (RHIM) domains that can interact with other RHIM-containing proteins including the receptor interacting protein kinase-3 (RIPK3) (20, 21). Indeed, immunoprecipitation experiments revealed interaction of ZBP1 with RIPK3 in IAV-infected cells (Fig. 2F). Consistent with previous studies demonstrating RIPK3-mediated activation of the NLRP3 inflammasome (19, 22, 23), a substantial reduction in caspase-1 cleavage and the levels of IL-1β and IL-18 were observed in Ripk3−/− BMDMs infected with IAV (Fig. 2G and H). The residual activation of caspase-1 observed in Ripk3−/− BMDMs was abolished in Ripk3−/−Casp8−/− BMDMs (Fig. 2G). BMDMs lacking RIPK1 kinase activity (Ripk1KD/KD) showed normal activation of caspase-1 and release of IL-1β and IL-18 (Fig. 2G and H).
The ZBP1-RIPK1 complex transduces NFκB activation signals (20, 21). Consistent with this, secretion of proinflammatory cytokines, IL-6 and TNF was abrogated in Ripk3−/−Casp8−/−Ripk1−/− as well as Zbp1−/− BMDMs (Fig. 2I and J). A modest reduction in IL-6 and TNF production was observed in Ripk3−/− and Ripk3−/−Casp8−/− BMDMs (Fig. 2I). Regulation of IL-6 and TNF production is dependent on RIPK1 scaffolding function, but occurs independently of its kinase activity since the levels of these cytokines were comparable in WT and Ripk1KD/KD cells (Fig. 2I). Collectively, these data identified ZBP1 as an upstream regulator mediating NLRP3 inflammasome activation via the RIPK3–Caspase-8 axis and proinflammatory responses via RIPK1.
ZBP1 mediates RIPK3-dependent induction of apoptotic and necroptotic cell death pathways during IAV infection
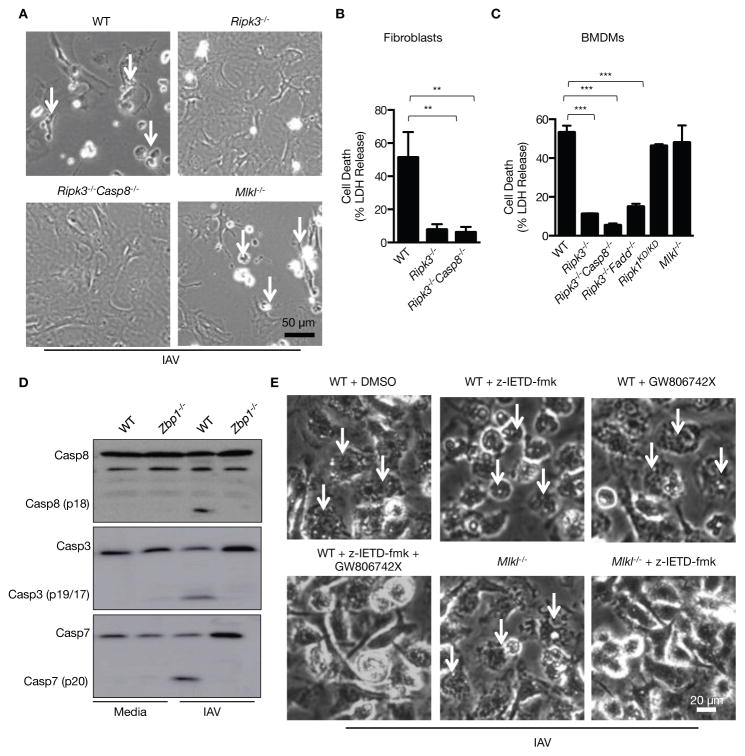
Previous studies identified RIPK3 as a critical regulator determining cell death via necroptotic or apoptotic pathways (24, 25). BMDMs and fibroblasts lacking RIPK3 or both RIPK3 and caspase-8 or RIPK3 and FADD were fully resistant to IAV-induced cell death, confirming a critical role of RIPK3 in mediating ZBP1-dependent cell death (Fig. 3A to C and fig. S5A). Necroptosis is executed by the RIPK3 substrate mixed lineage kinase like (MLKL) (26). However, cell death comparable to WT levels was observed in Mlkl−/− BMDMs and fibroblasts infected with IAV, demonstrating that MLKL is dispensable for ZBP1- and RIPK3-dependent cell death (Fig. 3A to C and fig. S5A). ZBP1 also promotes apoptosis during IAV infection since activation of caspase-8, caspase-3 and caspase-7 was abrogated in Zbp1−/− BMDMs compared to WT BMDMs infected with IAV (Fig. 3D). Robust activation of both caspase-1 and caspase-8 was observed in Mlkl−/− BMDMs infected with IAV and inhibition of these caspase activities using zIETD-FMK prevented IAV-induced cell death in Mlkl−/− BMDMs (Fig. 3E and fig. S5B to D). Activation of multiple, complementary cell death pathways in IAV-infected cells was further confirmed by simultaneous inhibition of apoptosis, pyroptosis and necroptosis in WT BMDMs with the caspase-8 inhibitor zIETD-FMK plus MLKL inhibitor GW806742X (27). Treatment with these inhibitors prevented WT BMDMs from undergoing cell death during IAV infection (Fig. 3E). Inhibition of either apoptosis or necroptosis in WT and Casp1−/− BMDMs was unable to block IAV-induced cell death (Fig. 3E and fig. S5D). These results collectively demonstrate parallel contribution of pyroptosis, necroptosis and apoptosis in the execution of IAV-induced cell death governed by ZBP1.
Figure 3. ZBP1 mediates RIPK3-dependent induction of apoptotic and necroptotic cell death pathways during IAV infection.
(A and B) Microscopic analysis and quantification of cell death by LDH release in fibroblasts infected with IAV 16 h pi; (p=0.0016; n=3; one-way ANOVA)..(C) Quantification of cell death by LDH release in BMDMs infected with IAV 16 h pi (p=0.0001; n=4; one-way ANOVA)..(D) Immunoblot analysis of the pro- and cleaved-forms of caspase-8, caspase-3 and caspase-7 in BMDMs 16 h pi with IAV (n=3)..(E) Microscopic analysis of BMDMs infected with IAV for 16 h in the absence or presence of inhibitors (n=3).
ZBP1 regulates cell death in response to both mouse-adapted and seasonal strains of IAV, but not in response to RNA viruses belonging to Paramyxoviriadae and Rhabdoviridae family
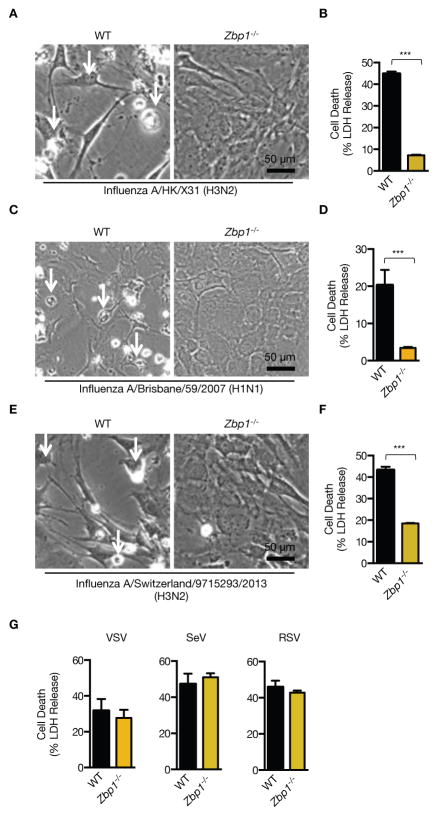
In addition to mouse adapted PR8 virus, ZBP1 broadly regulates cell death in response to IAV of different species- and strain-specificity since Zbp1−/− fibroblasts were protected from cell death during infection with mouse adapted Influenza A/X31 (H3N2) as well as non-mouse adapted seasonal strains Influenza A/Brisbane/59/2007 (H1N1) and A/Switzerland/9715293/2013 (H3N2) (Fig. 4A to F). Unlike different strains of IAV, cell death and inflammatory cytokine production occurred independently of ZBP1 in BMDMs infected with other negative–sense RNA viruses, VSV, Sendai virus (SeV) and respiratory syncytial virus (RSV) (Fig. 4G and fig. S6). Moreover, transfection of synthetic, IAV or mammalian-derived ssRNA species as well as the dsRNA ligand poly(I:C) and dsDNA ligand poly(dA:dT) induced comparable levels of cell death in WT and Zbp1−/− BMDMs (fig. S7A to C). Together, these data highlighted an IAV-specific role for ZBP1 in initiating cell death responses.
Figure 4. ZBP1 regulates cell death in response to both mouse-adapted and human strains of IAV, but not in response to RNA viruses belonging to Paramyxoviriadae and Rhabdoviridae family.
(A to F) Microscopic analysis and quantification of cell death by LDH release in fibroblasts infected with mouse adapted Influenza A/X31 (H3N2), Influenza A/Brisbane/59/2007 (H1N1) and A/Switzerland/9715293/2013 (H3N2) 16h pi (p= 4.5184*10−5 (X31); p=0.02523 (A/Brisbane); p=0.00671454 (A/Switzerland); n=4; two-tailed t test).(G) Quantification of cell death by LDH release in BMDMs infected with VSV (MOI 10), SeV (MOI 8) and RSV (MOI 10) 16 h pi (n=3).
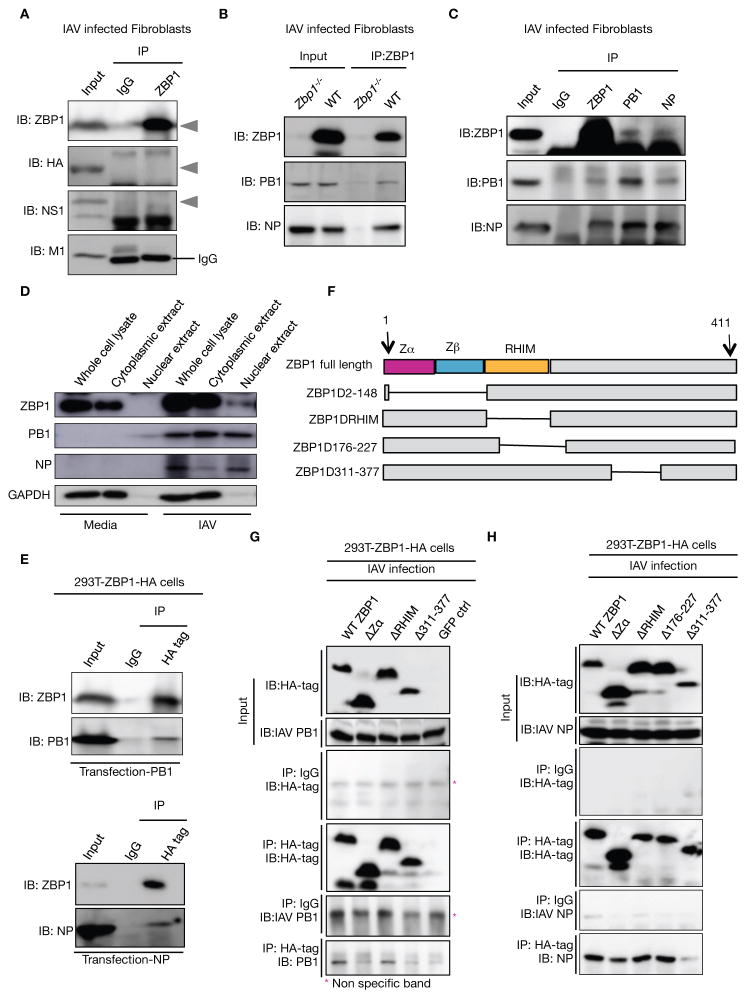
ZBP1 is a sensor of IAV nucleoprotein (NP) and polymerase subunit PB1
To investigate potential interaction of ZBP1 with IAV proteins, endogenous ZBP1 was immunoprecipitated from IAV-infected cells and probed for interacting IAV proteins. Immunoblotting for IAV M1, NS1 and HA proteins did not show any detectable levels of protein interaction (Fig. 5A). Interestingly, the IAV nucleoprotein (NP) and RNA polymerase subunit PB1 were co-precipitated from WT, but not Zbp1−/− fibroblasts infected with IAV (Fig. 5B). Conversely, immunoprecipitation of NP or PB1 from lysates of IAV-infected fibroblasts also showed co-precipitation of these proteins with ZBP1, confirming interaction of endogenous ZBP1 with NP and PB1 (Fig. 5C). ZBP1, PB1 and NP were observed in both nuclear and cytoplasmic fractions from infected cells at 8hr post infection indicating that ZBP1 interaction with viral proteins can occur in either or both of these compartments (Fig. 5D). Overexpression of PB1 or NP in ZBP1-expressing 293T cells also demonstrated interaction of these viral proteins with ZBP1 (Fig. 5E).
Figure 5. ZBP1 functions as a sensor of IAV infection by interacting with IAV PB1 and NP proteins.
(A) Immunoprecipitation of endogenous ZBP1 from lysates of WT BMDMs infected with IAV for 16 h, and immunoblotted for ZBP1, M1, NS1 and HA; (n= 4)..(B) Immunoprecipitation of endogenous ZBP1 from lysates of WT or Zbp1−/− fibroblasts infected with IAV for 16 h, and immunoblotted for ZBP1, PB1 and NP; (n= 3)..(C) Immunoprecipitation of ZBP1, PB1 and NP from lysates of WT fibroblasts infected with IAV for 16 h, and immunoblotted for ZBP1, PB1 and NP; (n= 3)..(D) Immunoblot analysis of ZBP1, PB1, NP and GAPDH from whole cell lysates, nuclear and cytoplasmic fractions of WT fibroblasts infected with IAV for 8 h; (n=3)..(E) Immunoprecipitation of HA-tagged ZBP1 from lysates of 239T cells expressing HA-tagged ZBP1 transfected with plasmids expressing PB1 or NP, and immunoblotted for ZBP1, PB1 and NP; (n= 2)..(F) Domain architecture of murine ZBP1 and schematics of the constructs used in this study..(G and H) Immunoprecipitation of HA-tagged ZBP1 from lysates of 239T cells expressing HA-tagged ZBP1 constructs infected with IAV for 16 h, and immunoblotted for the IAV proteins PB1, HA and NP, and HA tag; (n= 2).
In order to map the domains with which ZBP1 interacts with IAV proteins, various deletion mutants of ZBP1 with HA tag were generated and overexpressed in 293T cells (Fig. 5F). Efficient co-immunoprecipitation of PB1 or NP proteins with WT ZBP1 and ZBP1 lacking the RHIM, but not with ZBP1 lacking the C-terminal domain, demonstrated the importance of the C-terminal domain of ZBP1 for its interaction with IAV proteins (Fig. 5G and H). In addition to the C-terminal domain, the Zα domain of ZBP1 was also required for interaction with PB1 (Fig. 5G). Collectively, we identified ZBP1 as a sensor of influenza virus proteins that can trigger both cell death and inflammatory responses during infection.
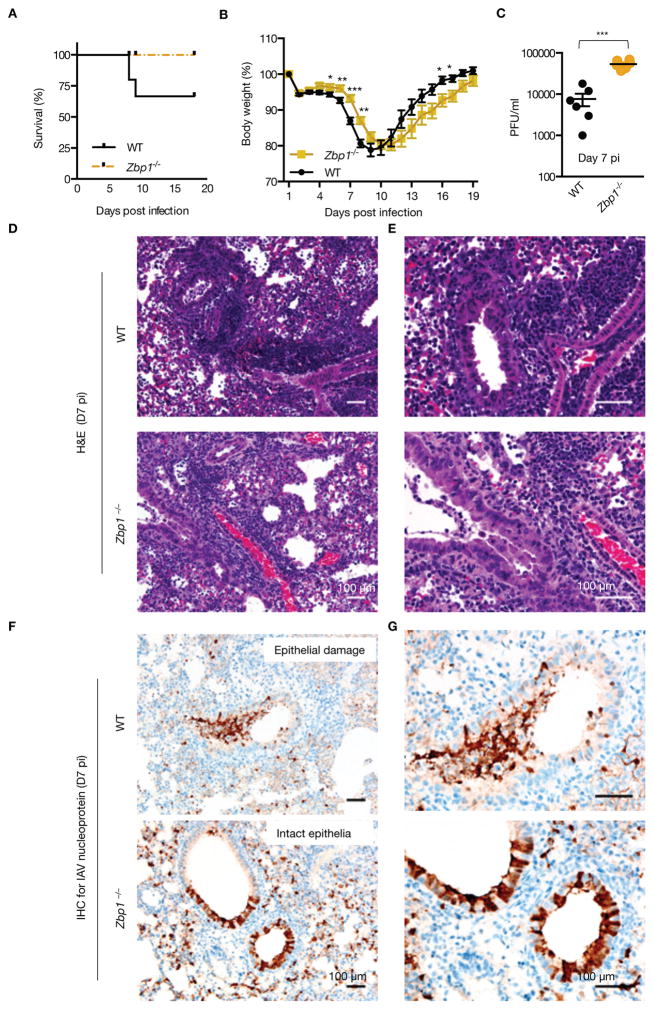
ZBP1 promotes inflammatory responses and epithelial damage during IAV infection in vivo
The physiological relevance of ZBP1 in regulating pathogenesis of IAV infection was assessed in WT and Zbp1−/− mice infected with approximately one LD50 of PR8 virus. Remarkably, Zbp1−/− mice were protected from mortality during IAV infection while 30% of WT mice succumbed to infection (Fig. 6A). Zbp1−/− mice also showed a significant reduction in weight loss and morbidity during the early phase of infection compared to WT mice (Fig. 6B). However, viral titers were significantly higher in Zbp1−/− mice at day 7 pi consistent with the notion that cell death destroys the niche necessary for virus replication (Fig. 6C). The defective viral clearance observed in Zbp1−/− mice led to increased weight loss at later stages of infection and these mice showed delayed recovery from infection compared to WT animals (Fig. 6B). In agreement with our in vitro findings, inflammatory responses as well as epithelial damage was markedly reduced in Zbp1−/− mice infected with IAV (Fig. 6D and E). While severe and extensive inflammatory responses characterized by diffuse intra-alveolar infiltrates of neutrophils and macrophages and perivascular accumulations of lymphocytes and granulocytes were observed in the lungs of WT mice, these responses were reduced in Zbp1−/− mice on day 7 pi (Fig. 6D and E). Collectively, these data demonstrated a critical role of ZBP1 in regulating pathogenesis and immunopathology during acute IAV infection by controlling both cell death and inflammatory responses.
Figure 6. ZBP1 promotes inflammatory responses and epithelial damage during IAV infection in vivo.
(A) Survival analysis of female WT and Zbp1−/− mice infected with 1000pfu (approximately one LD50) of IAV; (p=0.0819; n = 15 (WT) and 9 (Zbp1−/−) mice; Mantel-Cox test)..(B) Body weight (%) of WT and Zbp1−/−mice 0–18 d after infection compared to pre-infection body weight (set as 100%); (p=0.02743813 (D5); 0.00103121 (D6); 0.00081354 (D7); 0.05424982 (D14); 0.0231683 (D15); 0.02152296 (D16); WT (n = 21) and Zbp1−/− (n = 15); two-tailed t test)..(C) Lung viral titers in WT and Zbp1−/− mice infected with IAV for 7 days; (p=0.0022; n=6; Mann Whitney test). H &E staining (D and E; E is an inset of D) and immunohistochemistry analysis for IAV NP protein (F and G; G is an inset of F) of lungs from WT and Zbp1−/− mice infected with IAV for 7 days; (n=6).
DISCUSSION
Cell fate decisions are well integrated into antiviral immune responses during an infection and are critical for elimination of replicating viruses. Although cell death constitutes a major antiviral host defense mechanism during IAV infection, exaggerated responses often leads to severe clinical disease and mortality as exemplified by the substantial damage to the lungs and destruction of respiratory epithelium observed in autopsy samples from the 1918 influenza pandemic (28). Despite the importance of epithelial cell death in determining disease outcome during acute IAV infection, the host factors regulating cell death responses are less characterized.
The critical role of type I IFN signaling in potentiating IAV-induced apoptosis in MEFs via activation of the FADD–caspase-8 signaling axis has been reported (29). Induction of IFNAR1–RIPK3-dependent necroptosis was also demonstrated in both MEFs and macrophages when FADD–caspase-8 signaling was inactivated (30, 31). Although IFN Stimulated Gene Factor 3 (ISGF3) complex was recognized as a critical promoter of necroptosis, the IFN-stimulated gene mediating necroptosis and inflammatory responses in macrophages remained elusive (31). In addition to these in vitro studies, pathogenic potential of type I IFN signaling in mediating uncontrolled inflammatory response and epithelial cell death was also demonstrated during IAV infection in vivo (32). Our study has now identified ZBP1 as the IFN-inducible protein regulating both inflammatory responses and cell death during IAV infection in vitro and in vivo.
The RHIM domains of ZBP1 associate with RHIM domains of both RIPK1 and RIPK3 to mediate NFκB activation and cell death responses (14, 20, 21). Consistent with these findings, we observed a critical role for ZBP1–RIPK1 axis in mediating proinflammatory responses during IAV infection. Moreover, ZBP1 regulation of cell death in IAV-infected cells is mediated via RIPK3. Of note, while our manuscript was in revision Nogusa et al. reported RIPK3-dependent activation of parallel necroptotic and apoptotic pathways in IAV-infected cells (33). Although this study demonstrate the importance of RIPK3 in driving cell death during IAV-infection, the upstream receptors and signaling pathways regulating RIPK3 were not identified.
Despite being identified as a DNA sensor, it is still unclear whether ZBP1 functions as a pathogen sensor during an infection. Our study has now identified a role for ZBP1 in the recognition of an RNA virus, and linked it to both immune and cell death responses to clinically relevant IAV infection. Innate sensors of IAV have been well characterized, however all known IAV-receptors recognize viral RNA in infected cells (2). Notably, our study demonstrates ZBP1 as an innate sensor of IAV proteins regulating antiviral innate immune responses.
After our discovery of IAV-induced NLRP3 inflammasome activation almost a decade ago (16), multiple studies have investigated the molecular and cellular mechanisms regulating inflammasome assembly in response to IAV infection (17–19, 34–36). The importance of IAV M2 ion channel protein, RIG-I, type I IFN signaling, RIPK3 and RNaseL in mediating inflammasome activation during IAV infection have been demonstrated (18, 19, 34, 36). Our data demonstrating an IAV-specific role for IFNAR–ZBP1 axis in the regulation of NLRP3 inflammasome activation will help to reconcile these findings. While both RIG-I and RNaseL mediate type I IFN production necessary for induction of ZBP1, RIPK3 associates with ZBP1 to transduce downstream signals. The interconnected and complimentary nature of IAV-induced cell death pathways demonstrated in our study also helps to explain PB1-F2-mediated NLRP3 inflammasome activation since PB1-F2 is regarded as the viral effector mediating cell death in IAV-infected cells (35, 37). Therefore, the identification of ZBP1 as an IAV-specific sensor helps to further resolve the molecular mechanisms regulating NLRP3 inflammasome assembly during IAV infection.
The seemingly paradoxical observations of reduced mortality in Zbp1−/− mice in spite of increased viral titers are not unexpected, since previous studies in both humans and animal models reported exaggerated inflammatory response, substantial loss of pulmonary epithelia and acute lung injury as the major factors contributing to morbidity and mortality during pathogenic IAV infection (38, 39). An extensive systems analysis study also demonstrated elevated activation of inflammatory signaling networks as a signature that distinguishes lethal from sublethal IAV infections (40). Moreover, transcriptome analysis of lung samples from infected mice identified immune and cell death responses as the major factor distinguishing mild from highly pathogenic infections (41). Our data are in agreement with these studies demonstrating exaggerated immune response rather than direct viral damage as the key trigger potentiating mortality during IAV infection.
Although BMDMs and fibroblasts do not represent the major cell types infected during IAV infection, the in vitro data obtained from these cells are validated by in vivo infections. However, further studies are warranted to identify the host and viral effectors as well as the precise molecular mechanism by which ZBP1-dependent cell death is executed in IAV-infected cells. The relevance of human ZBP1 during IAV infection was also not assessed in our study. In vitro studies investigating the role of human ZBP1 during IAV infection are less feasible since most of the lung epithelial cell lines that support productive virus replication do not undergo IAV-induced cell death (37). Notably, proteomics analysis of human innate immunity interactome for type I IFN identified ZBP1 as one of the interacting proteins with antiviral activity (42). Moreover, human ZBP1 restricts replication of both human cytomegalovirus and herpes simplex-1 demonstrating functional importance of ZBP1 in antiviral responses (43, 44). Since cell death, inflammatory responses and virus replication are intricately associated with each other, various host factors modulating susceptibility to infection as well as dosage of infection will be critical in determining the role of ZBP1 in disease progression. Identification of genetic lesions in ZBP1 locus in patient populations will be of great value and further studies in this direction are necessary to determine the importance of ZBP1 in pathogenesis of IAV in humans. Nevertheless, the insights gained from this study improve our understanding about the mechanisms regulating pathogenesis and prognosis of acute influenza virus infection and may lead to improved disease intervention strategies for the prevention and treatment of IAV infection.
Materials and Methods
Study Design
Animal studies were conducted under protocols approved by the St. Jude Children’s Research Hospital on the Use and Care of Animals. Age- and sex-matched, 6- to 8-weeks old WT and Zbp1−/− mice bred at the Animal Research Center at SJCRH were used for in vivo experiments. Infected mice were monitored and body weights were recorded daily, and mice exhibiting severe signs of disease or more than 30% weight loss relative to pre-infection body weight were euthanized. Animals were euthanized at the indicated time points for lung harvest. Lung sections were processed at Veterinary Pathology Core at SJCRH and histopathological analysis was conducted by a pathologist blinded to experimental groups. No other blinding or randomization was performed.
Mice
Zbp1−/−, Ifnar1−/−, Stat1−/−, Irf9−/−, Aim2−/−, Nlrp3−/−, Nlrc4−/−, Casp1Null, Casp11−/−, Gsdmd−/−, Mavs−/−, Myd88−/−, Trif−/−, Tradd−/−, StingGt/Gt, Ripk3−/−, Ripk3−/−Casp8−/−, Ripk3−/−Casp8−/−Ripk1−/−, Ripk1KD/KD and Mlkl−/− mice have been described previously (13, 45–49). Tnfr−/− mice (Stock #003243) were purchased from The Jackson Laboratory.
Cell culture and stimulation
Cells were cultured overnight in antibiotic–free media before infection. The influenza A/Puerto Rico/8/34 virus (PR8) generated by an eight-plasmid reverse genetics system was propagated in allantoic cavity of 9- to 11-day old embryonated SPF chicken eggs and viral titers were enumerated by standard plaque assays. BMDMs (MOI 25) and fibroblasts (MOI 10) were infected with PR8 virus for 2 h. DMEM media containing 20% FBS was added after 2 h and samples were collected at indicated time points. For pharmacological inhibition, BMDMs were treated with 50μM Z-IETD-FMK (Millipore), 50 μM Z-VAD-FMK (Calbiochem) and/or 1μM GW806742X (SYNkinase) at the same time as IAV infection. RSV Line 19F was grown in HEp-2 cell line.
Cytokine measurement
IFNβ and IL-18 were measured using ELISA kits (BioLegend (IFNβ); eBioscience (IL-18)). All other cytokines were measured by multiplex ELISAs (Millipore).
Generation of ZBP1 overexpressing cells
pVSVg, pEQ-Pam3(-E) and pMIGII plasmids encoding the Zbp1 gene are transfected into 293T cells to generate retroviral stocks. Retroviral supernatants were harvested after 48 h of transfection and filtered through 0.4μm sterile filters. 293T cells were infected with the corresponding retroviral stocks in the presence of polybrene to generate cells stably expressing the respective ZBP1 proteins.
Co-immunoprecipitation
For immunoprecipitation, cell lysates were incubated with 3 μg of indicated primary antibodies on a rocking platform for 12–16h at 4°C. Protein A/G PLUS-Agarose (Santa Cruz) was added to the samples and incubated for another 2 h on the rocking platform. Agarose was centrifuged and washed 3 times with the lysis buffer. Immunoprecipitates were eluted in sample buffer after three washes in lysis buffer and then subjected to immunoblotting analysis.
Animal infection
WT and Zbp1−/− mice were anesthetized with 250 mg/kg Avertin followed by intranasal infection with 1000pfu (approximately one LD50) of PR8 virus in 30 μl PBS. Infected mice were observed over a period of 18 days for survival study. Lungs were harvested on day 7 pi and left lobe of the lungs was used for histopathological analysis. Formalin-preserved lungs were processed and embedded in paraffin according to standard procedures. Sections were stained with hematoxylin and eosin (H&E) or IAV nucleoprotein. Lung viral titers were enumerated by standard plaque assays after homogenizing lungs in 1 ml PBS using a bead mill homogenizer (Qiagen).
Statistical analysis
GraphPad Prism 6.0 software was used for data analysis. Statistical significance was determined by a t tests (two-tailed) or Mann Whitney test for two groups or one-way ANOVA for three or more groups; P < 0.05 was considered statistically significant where *P<0.05, **P<0.01, and ***P<0.001. Mean ± SEM of the data is presented.
Supplementary Material
Fig. S1: Cell death induced by IAV infection in BMDMs occurs independently of MyD88, MAVS, TRIF, STING and TRADD, but is dependent on the transcription factors STAT1 and IRF9.
Fig. S2: ZBP1 induced via IFN signaling regulates cell death in IAV-infected cells independently of virus replication and IFNβ production.
Fig. S3: ZBP1 is dispensable for activation of canonical and non-canonical NLRP3, NLRC4 and AIM2 inflammasomes.
Fig. S4: IAV-induced cell death is not prevented by the absence of the NLRP3, caspase-1 or gasdermin D.
Fig. S5: ZBP1 drives activation of complementary cell death pathways during IAV infection.
Fig. S6: ZBP1 is dispensable for proinflammatory cytokine production in response to other RNA viruses.
Fig. S7: ZBP1 is dispensable for cell death in response to transfected RNA and ds(DNA) ligands.
Table S1: Real-time qPCR primer sequences
Acknowledgments
We thank A. Burton and D. Horn for technical support, Dr. Ken J. Ishii (Osaka University) and Dr. Vishva M. Dixit (Genentech) for supplying mutant mice, Dr. P.G. Thomas (St. Jude) for MCMV Smith MSGV strain and IAV (X31), Dr. Richard Webby (St. Jude) for seasonal strains of IAV and plasmids encoding IAV proteins, Dr. Charles Russell (St. Jude) for SeV and Dr. Michael A. Whitt (UTHSS) for VSV. Confocal microscopy images were acquired at the St. Jude Cell & Tissue Imaging Center, which is supported by St. Jude Children’s Research Hospital and NCI P30 CA021765-35. We thank Dr. Prajwal Gurung for critical reading of the manuscript and members of the Kanneganti lab for their comments and suggestions.
Funding:
T.D.K is supported by the US National Institutes of Health (AI101935, AI124346, AR056296 and CA163507) and the American Lebanese Syrian Associated Charities; S.M.M is supported by the National health & Medical Research Council of Australia R.G. Menzies Early Career Fellowship.
Footnotes
Competing financial interests:
The authors declare no competing financial interests.
Data and material availability:
The microarray dataset was deposited under the accession code GSE77611.
Authors Contributions:
T.K. and T.D.K. conceptualized the study; T.K., R.K.S.M., K.S., S.M.M., R.K., D.P., G.N designed the methodology, performed the experiments and conducted the analysis; P.V. performed histopathological analysis; T.K. performed statistical analysis; T.K., S.M.M., and T.D.K. wrote the manuscript; T.D.K. acquired the funding and provided overall supervision.
References
- 1.WHO. Influenza (Seasonal) Fact sheet N0 211. 2009 [updated April 2009; cited 2012 June 12, 2012]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/index.html.
- 2.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nature reviews. Immunology. 2014;14:315–328. doi: 10.1038/nri3665. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunological reviews. 2015;265:130–142. doi: 10.1111/imr.12287. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders CJ, Vogel P, McClaren JL, Bajracharya R, Doherty PC, Thomas PG. Compromised respiratory function in lethal influenza infection is characterized by the depletion of type I alveolar epithelial cells beyond threshold levels. American journal of physiology Lung cellular and molecular physiology. 2013;304:L481–488. doi: 10.1152/ajplung.00343.2012. published online EpubApr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herold S, Ludwig S, Pleschka S, Wolff T. Apoptosis signaling in influenza virus propagation, innate host defense, and lung injury. Journal of leukocyte biology. 2012;92:75–82. doi: 10.1189/jlb.1011530. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 6.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, Steinmueller M, Lohmeyer J. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. American journal of respiratory and critical care medicine. 2011;183:1380–1390. doi: 10.1164/rccm.201009-1431OC. published online EpubMay 15. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigue-Gervais IG, Labbe K, Dagenais M, Dupaul-Chicoine J, Champagne C, Morizot A, Skeldon A, Brincks EL, Vidal SM, Griffith TS, Saleh M. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell host & microbe. 2014;15:23–35. doi: 10.1016/j.chom.2013.12.003. published online EpubJan 15. [DOI] [PubMed] [Google Scholar]
- 8.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. published online EpubApr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. published online EpubApr 17 (S1074-7613(09)00139-3 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. published online EpubJul 26. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz T, Behlke J, Lowenhaupt K, Heinemann U, Rich A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nature structural biology. 2001;8:761–765. doi: 10.1038/nsb0901-761. published online EpubSep. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Choi MK, Ban T, Yanai H, Negishi H, Lu Y, Tamura T, Takaoka A, Nishikura K, Taniguchi T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. published online EpubApr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. published online EpubFeb 7. [DOI] [PubMed] [Google Scholar]
- 14.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell host & microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. published online EpubMar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nature reviews. Immunology. 2016;16:7–21. doi: 10.1038/nri.2015.7. published online EpubJan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. The Journal of biological chemistry. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. published online EpubDec 1. [DOI] [PubMed] [Google Scholar]
- 17.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. published online EpubJan 16 (jem.20081667 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nature immunology. 2010;11:404–410. doi: 10.1038/ni.1861. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, Zhou R. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nature immunology. 2014;15:1126–1133. doi: 10.1038/ni.3015. published online EpubOct 19. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. Journal of immunology. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. published online EpubNov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO reports. 2009;10:916–922. doi: 10.1038/embor.2009.109. published online EpubAug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yabal M, Muller N, Adler H, Knies N, Gross CJ, Damgaard RB, Kanegane H, Ringelhan M, Kaufmann T, Heikenwalder M, Strasser A, Gross O, Ruland J, Peschel C, Gyrd-Hansen M, Jost PJ. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell reports. 2014;7:1796–1808. doi: 10.1016/j.celrep.2014.05.008. published online EpubJun 26. [DOI] [PubMed] [Google Scholar]
- 23.Moriwaki K, Bertin J, Gough PJ, Chan FK. A RIPK3-Caspase 8 Complex Mediates Atypical Pro-IL-1beta Processing. Journal of immunology. 2015;194:1938–1944. doi: 10.4049/jimmunol.1402167. published online EpubFeb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. published online EpubMar 21. [DOI] [PubMed] [Google Scholar]
- 25.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ. RIP3 induces apoptosis independent of pronecrotic kinase activity. Molecular cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. published online EpubNov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. published online EpubJan 20. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, Garnier JM, Dobson RC, Webb AI, Tripaydonis A, Babon JJ, Mulcair MD, Scanlon MJ, Alexander WS, Wilks AF, Czabotar PE, Lessene G, Murphy JM, Silke J. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:15072–15077. doi: 10.1073/pnas.1408987111. published online EpubOct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerging infectious diseases. 2006;12:15–22. doi: 10.3201/eid1201.050979. published online EpubJan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. Journal of virology. 2000;74:1513–1523. doi: 10.1128/JVI.74.3.1513-1523.2000. published online EpubFeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. published online EpubAug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, Gamero AM, Mossman KL, Sad S. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3206–3213. doi: 10.1073/pnas.1407068111. published online EpubAug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nature communications. 2014;5:3864. doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH, 3rd, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O, Verbist K, Gough PJ, Bertin J, Hartmann BM, Sealfon SC, Kaiser WJ, Mocarski ES, Lopez CB, Thomas PG, Oberst A, Green DR, Balachandran S. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell host & microbe. 2016 doi: 10.1016/j.chom.2016.05.011. published online EpubJun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pothlichet J, Meunier I, Davis BK, Ting JP, Skamene E, von Messling V, Vidal SM. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS pathogens. 2013;9:e1003256. doi: 10.1371/journal.ppat.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAuley JL, Tate MD, MacKenzie-Kludas CJ, Pinar A, Zeng W, Stutz A, Latz E, Brown LE, Mansell A. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS pathogens. 2013;9:e1003392. doi: 10.1371/journal.ppat.1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakrabarti A, Banerjee S, Franchi L, Loo YM, Gale M, Jr, Nunez G, Silverman RH. RNase L activates the NLRP3 inflammasome during viral infections. Cell host & microbe. 2015;17:466–477. doi: 10.1016/j.chom.2015.02.010. published online EpubApr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, Basta S, O’Neill R, Schickli J, Palese P, Henklein P, Bennink JR, Yewdell JW. A novel influenza A virus mitochondrial protein that induces cell death. Nature medicine. 2001;7:1306–1312. doi: 10.1038/nm1201-1306. published online EpubDec. [DOI] [PubMed] [Google Scholar]
- 38.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nature medicine. 2006;12:1203–1207. doi: 10.1038/nm1477. published online EpubOct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everitt AR, Clare S, Pertel T, John SP, Wash RS, Smith SE, Chin CR, Feeley EM, Sims JS, Adams DJ, Wise HM, Kane L, Goulding D, Digard P, Anttila V, Baillie JK, Walsh TS, Hume DA, Palotie A, Xue Y, Colonna V, Tyler-Smith C, Dunning J, Gordon SB, Gen II, Smyth RL, Openshaw PJ, Dougan G, Brass AL, Kellam P Investigators M. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484:519–523. doi: 10.1038/nature10921. published online EpubApr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154:197–212. doi: 10.1016/j.cell.2013.06.013. published online EpubJul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:578–581. doi: 10.1038/nature05181. published online EpubOct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S, Wang L, Berman M, Kong YY, Dorf ME. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity. 2011;35:426–440. doi: 10.1016/j.immuni.2011.06.014. published online EpubSep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pham TH, Kwon KM, Kim YE, Kim KK, Ahn JH. DNA sensing-independent inhibition of herpes simplex virus 1 replication by DAI/ZBP1. Journal of virology. 2013;87:3076–3086. doi: 10.1128/JVI.02860-12. published online EpubMar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. Journal of virology. 2010;84:585–598. doi: 10.1128/JVI.01748-09. published online EpubJan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. published online EpubMar 17 (nature09857 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. published online EpubMar 17 (nature09852 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. published online EpubSep 19. [DOI] [PubMed] [Google Scholar]
- 48.Chen NJ, Chio, Lin WJ, Duncan G, Chau H, Katz D, Huang HL, Pike KA, Hao Z, Su YW, Yamamoto K, de Pooter RF, Zuniga-Pflucker JC, Wakeham A, Yeh WC, Mak TW. Beyond tumor necrosis factor receptor: TRADD signaling in toll-like receptors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12429–12434. doi: 10.1073/pnas.0806585105. published online EpubAug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. published online EpubOct 29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Cell death induced by IAV infection in BMDMs occurs independently of MyD88, MAVS, TRIF, STING and TRADD, but is dependent on the transcription factors STAT1 and IRF9.
Fig. S2: ZBP1 induced via IFN signaling regulates cell death in IAV-infected cells independently of virus replication and IFNβ production.
Fig. S3: ZBP1 is dispensable for activation of canonical and non-canonical NLRP3, NLRC4 and AIM2 inflammasomes.
Fig. S4: IAV-induced cell death is not prevented by the absence of the NLRP3, caspase-1 or gasdermin D.
Fig. S5: ZBP1 drives activation of complementary cell death pathways during IAV infection.
Fig. S6: ZBP1 is dispensable for proinflammatory cytokine production in response to other RNA viruses.
Fig. S7: ZBP1 is dispensable for cell death in response to transfected RNA and ds(DNA) ligands.
Table S1: Real-time qPCR primer sequences