Abstract
Xenorhabdus is a bacterial symbiont of entomopathogenic Steinernema nematodes and is pathogenic for insects. Its life cycle involves a stage inside the insect cadaver, in which it competes for environmental resources with microorganisms from soil and the insect gut. Xenorhabdus is, thus, a useful model for identifying new interbacterial competition systems. For the first time, in an entomopathogenic bacterium, Xenorhabdus doucetiae strain FRM16, we identified a cdi-like locus. The cdi loci encode contact-dependent inhibition (CDI) systems composed of proteins from the two–partner secretion (TPS) family. CdiB is the outer membrane protein and CdiA is the toxic exoprotein. An immunity protein, CdiI, protects bacteria against inhibition. We describe here the growth inhibition effect of the toxic C-terminus of CdiA from X. doucetiae FRM16, CdiA-CTFRM16, following its production in closely and distantly related enterobacterial species. CdiA-CTFRM16 displayed Mg2+-dependent DNase activity, in vitro. CdiA-CTFRM16-mediated growth inhibition was specifically neutralized by CdiIFRM16. Moreover, the cdi FRM16 locus encodes an ortholog of toxin-activating proteins C that we named CdiCFRM16. In addition to E. coli, the cdiBCAI-type locus was found to be widespread in environmental bacteria interacting with insects, plants, rhizospheres and soils. Phylogenetic tree comparisons for CdiB, CdiA and CdiC suggested that the genes encoding these proteins had co-evolved. By contrast, the considerable variability of CdiI protein sequences suggests that the cdiI gene is an independent evolutionary unit. These findings further characterize the sparsely described cdiBCAI-type locus.
Introduction
Xenorhabdus, a member of the Enterobacteriaceae family, is a natural symbiont of entomopathogenic Steinernema spp. nematodes living in the soil [1, 2]. The bacterium-nematode symbiosis is used for the biological control of various groups of insects [3], but the genus Xenorhabdus is also a pertinent and tractable model for investigating processes of antagonism between bacteria [4]. Indeed, the complex lifecycle of this bacterium involves an alternation between ecological niches: the nematode gut, the hemocoel of the living insect and the insect cadaver. Xenorhabdus proliferation in the insect cadaver is dependent on the ability of the bacterium to kill other microorganisms living in the insect gut, on the nematode cuticle or in soils, which would otherwise compete for resources.
Xenorhabdus produces a broad diversity of bioactive secreted metabolites with antimicrobial activity [5]. For example, xenocoumacins [6–8], xenortides [9], xenematides [10] and PAX-peptides [11, 12], which target a broad spectrum of microorganisms, are synthesized by the non-ribosomal peptide synthetase (NRPS) enzymes and polyketide synthase (PKS) enzymes of Xenorhabdus strains. Antagonism with other Xenorhabdus or closely related bacterial genera may also be mediated by ribosomal-encoded bacteriocins [13, 14]. Xenorhabdus produces phage-derived bacteriocins [15, 16] and colicin E3-type killer proteins [17].
New bacterial antagonism systems requiring direct cell-to-cell contact for toxin delivery between Gram-negative bacteria have been described in the last decade. Bacterial type 6 secretion systems (T6SS) were first described in Vibrio cholerae [18]. In several bacterial taxa, the T6SS injects toxic effectors into competing bacteria to inhibit their growth [19–21]. Xenorhabdus genomes harbor several clusters of genes encoding putative T6SS [22, 23], but the functional role of the products of these genes has yet to be demonstrated. Contact-dependent growth inhibition (CDI) systems have been shown to mediate interbacterial competition in a contact-dependent manner. CDI involves a two-partner secretion (TPS) system, a member of the type 5 secretion system found in Gram-negative bacteria [24–26]. CDI systems are composed of two proteins encoded in a same genomic cluster: CdiB, an outer membrane β-barrel protein and CdiA, a large and secreted exoprotein. The growth inhibition activity resides within the carboxy-terminal region of CdiA of ~ 300 amino acids (CdiA-CT). The CDI systems display the typical features of the large growing family of polymorphic toxin systems: a protein, CdiI, encoded immediately downstream of the CdiA-encoding gene, protects CDI+ bacterial cells from growth inhibition, each CdiA-CT toxin is specifically neutralized by its cognate CdiI protein and the CdiA-CT toxin domains and the CdiI immunity proteins are highly polymorphic [27]. The CDI toxin/immunity complex therefore serves as a basis for self/non-self discrimination during inter-strain competition. Furthermore, it was recently shown to participate to the stabilization of mobile genetic elements in bacterial cells [28]. Clusters of genes encoding putative CDI systems are mainly found throughout the Proteobacteria [27, 29]. Two different cdi loci have been characterized functionally: the “E. coli-type”, which displays the typical cdiBAI organization and is widespread in the Enterobacteriaceae [27, 30] and in the genus Pseudomonas [31], and the “Burkholderia-type”, with its typical bcpAIOB organization [32, 33].
We describe here a functional cdi locus in the entomopathogenic bacterium Xenorhadbus doucetiae strain FRM16. This locus has an atypical gene organisation, cdiBCAI, and is widespread in Gram-negative bacteria interacting with insects or plants, or inhabiting soils.
Materials and Methods
Bacterial strains, plasmids and primers
All the bacterial strains used in this study are presented in S1 Table. Bacteria were routinely grown in Luria–Bertani (LB) medium at 28°C (Xenorhabdus strains) or 37°C (E. coli strains). Kanamycin (20 mg.L-1) and ampicillin (150 mg.L-1) were added to the medium as required. Ptet promoters were induced by adding anhydrotetracycline (aTc) at a final concentration of 0.1 mg. L-1. The primers (Eurogentec) and plasmids used in this study are described in S1 Table.
Plasmid construction for toxicity assays
The CdiA-CTFRM16 fragment was amplified by PCR with the primer pairs F_EcorI_Cter_tpsA_III/ R_sal1_tpsA_III. The PCR product was digested with EcoRI and SalI and inserted into the corresponding sites of pGJ907 to yield pGJ907_CdiA-CTFRM16. Constructs were checked by sequencing (MWG-Eurofins, Germany). Plasmids pGJ907 and pGJ907_ CdiA-CTFRM16 were introduced into E. coli strain EPI400 (Epicentre, France) or E. coli strain WM3064 by transformation. The WM3064 transformants were used to transfer plasmids pGJ907 and pGJ907_ CdiA-CTFRM16 inside Xenorhabdus bovienii strain CS03 by conjugative mating (according to the protocol described by [34]) to construct the recombinant strain Xb_CS03_ PGJ907 and Xb_CS03_ PGJ907_ CdiA-CTFRM16.
XDD1_1118 and XDD1_1120 were amplified by PCR with the primer pairs XD1118_EcoR1_F/ XD1118_sal1_R and XD1120_EcoR1_F/ XD1120_sal1_R, respectively. The PCR products were digested with EcoRI and SalI and inserted into the corresponding sites of pUC18 to yield plasmids pUC18_XD1118 and pUC18_XD1120. Competent E. coli EPI400 cells harbouring pGJ907_ CdiA-CTFRM16 (see above) were then transformed with pUC18_XD1118 and pUC18_XD1120, to yield the recombinant strains E. coli_ PGJ907_ CdiA-CTFRM16 / pUC18_XD118 and E. coli_ PGJ907_ CdiA-CTFRM16 / pUC18_XD1120. As controls, plasmids pUC18 and pGJ907 were used to construct the recombinant strains E. coli_ PGJ907_ CdiA-CTFRM16 / pUC18 and E. coli_ PGJ907 / pUC18.
Plasmid construction for protein overproduction in E. coli BL21
The cdiA-CTFRM16_XDD1_1120 fragment and the XDD1_1120 gene were amplified by PCR with the primer pairs F_EcorI_Cter_tpsA_III/ XD_1120_sal1R_bis and XD1120_EcoR1_F/ XD_1120_sal1R_bis, respectively. The PCR products were digested with EcoRI and SalI and inserted into the corresponding sites of pET28b to yield pET28b_CdiA-CTFRM16_CdiI-His6 and pET28b_CdiI-His6.
Plasmids pET28b _CdiA-CTFRM16_CdiI-His6 and pET28b_CdiI-His6 were introduced into E. coli strain BL21 by transformation.
Toxicity assays with CdiA-CTFRM16
E. coli strain EPI400 cells carrying pGJ907 (empty vector control), pGJ907_CdiA-CTFRM16 were cultured overnight in LB medium supplemented with kanamycin. The cultures were then diluted 1/500 in fresh LB supplemented with kanamycin and 200 μL of the resulting suspensions was used to inoculate 96-well plates (Greiner). The plates were incubated at 28°C, with orbital shaking, in an Infinite M200 microplate reader (Tecan). Absorbance at 600 nm was measured every 30 minutes. When the OD600 reached 0.15 to 0.2, aTc was (final concentration = 100 ng.mL-1) or was not (controls) added to the medium to induce CdiA-CTFRM16 expression under the control of the Ptet promoter. Growth was evaluated for ~10 hours. All experiments were performed in triplicate. We used the same protocol for Xenorhabdus growth inhibition assays.
Modulation of growth inhibition
Recombinant EPI400 E. coli_ PGJ907_ CdiA-CTFRM16 / pUC18, E. coli_ PGJ907_ CdiA-CTFRM16 / pUC18_XD118 and E. coli_ PGJ907_ CdiA-CTFRM16 / pUC18_XD1120 cells were used to inoculate 96-well plates in LB medium supplemented with IPTG (0.2 mM final), and were incubated at 28°C, as described above. We added aTc to the medium when the OD600 reached 0.15 to 0.2, to induce CdiA-CTFRM16 expression under the control of the Ptet promoter. Growth was assessed for ~10 hours. E. coli strain EPI400 cells carrying pGJ907 and pUC18 were used as empty vectors control. All experiments were performed in triplicate.
Protein purification
The purified CdiA-CTFRM16 was obtained from E. coli strain BL21 carrying pET28b_cdiA-CTFRM16_cdiIFRM16. CdiA-CTFRM16/CdiIFRM16-His6 complexes were first overproduced and purified under non denaturing conditions in reaction buffer (20 mM sodium phosphate pH 7.0, 150 mM NaCl, 10 mM β-mercaptoethanol) as previously described [27]. The complex was denatured in reaction buffer containing 6 M guanidine-HCl, and the CdiA-CTFRM16 protein was isolated from CdiIFRM16-His6 by Ni2+-affinity chromatography. Purified CdiA-CTFRM16 and CdiIFRM16-His6 proteins were refolded by dialysis against reaction buffer.
As a control, purified CdiIFRM16-His6 alone was obtained from E. coli strain BL21 carrying pET28b_cdiIFRM16 and purified by Ni2+-affinity chromatography, as described above (without the denaturation step).
Purified proteins were analyzed by SDS-PAGE on a 15% polyacrylamide gel in the presence of SDS as previously described [35]. The samples, 5 μg of purified fraction, were run under reducing conditions, reduction being achieved by treating (3 min, 100°C) the samples with a solution containing β-2-mercaptoethanol (0.5% final concentration). The molecular mass was determined by the use of Page RulerTM plus prestained protein ladder (Thermo Scientific, USA). Gels were stained with Coomassie brilliant blue.
Pull down experiment
Purified CdiA-CTFRM16 (5 μM) was incubated with CdiIFRM16-His6 (5 μM) for 30 min at room temperature and an aliquot was removed for analysis by SDS-PAGE. 500 μL of Ni2+-NTA resin, previously washed four times with binding buffer (20 mM sodium phosphate pH 7.0 containing 150 mM sodium chloride and 10 mM β-mercaptoethanol), was then added and incubated for 1 h 30 at 4°C. The resin was then collected by centrifugation and the supernatant (unbound proteins) was collected for analysis by SDS-PAGE. Ni2+-NTA was washed with binding buffer three times, and the bound proteins were eluted in elution buffer (20 mM sodium phosphate pH 7.0 containing 150 mM sodium chloride, 10 mM β-mercaptoethanol and 250 mM imidazole). Samples were finally analysed by SDS-PAGE and the gel was stained with Coomassie blue as described above.
Nuclease assays
The activity of purified CdiA-CTFRM16 was assayed in vitro on genomic DNA from Xd_FRM16. CdiA-CTFRM16 (final concentration of 1 μM) was incubated with 2 μg of DNA in 50 μL of sterilized water supplemented with 2 mM MgCl2 or CaCl2 for 1 h at 37°C. Where indicated, purified CdiIFRM16-His6 protein from pET28b_cdiIFRM16 (immunity protein purified alone without CdiA-CT) was included at different final concentrations and allowed to bind CdiA-CTFRM16 for 30 minutes at room temperature before the addition of substrate DNA. Reactions were quenched with EDTA, and the reaction mixture was subjected to electrophoresis in a 1% agarose gel stained with ethidium bromide.
RNA isolation and RT-PCR analysis
X. doucetiae FRM16 in LB broth was incubated at 28°C (100 mL) with horizontal shaking. Three samples were collected at different incubation times: i) early exponential growth phase (OD at 600 nm = 0.75); ii) end of the exponential growth phase (OD at 600 nm = 1.70); iii) stationary phase (OD at 600 nm = 3.70).
At each sampling time, total RNA was extracted with the RNeasy Protect Bacteria miniprep kit (from Qiagen), including incubation with DNase I, according to the manufacturer’s instructions. RNA concentration was determined by measuring absorbance at 260 nm. For each RNA preparation, we assessed DNA contamination by carrying out a control PCR targeting the 16S ribosomal RNA gene, by using Xenorhabdus-specific 16S primers (see S1 Table). The quantity and quality of total and messenger RNA, respectively, were assessed with a NanoDrop 2000 spectrophotometer (Thermo Scientific) and an Agilent 2100 Bioanalyzer with the RNA 6000 Nano LabChip kit (Agilent). Total RNA (2 μg) was reverse-transcribed with Super Script IV Reverse Transcriptase (Invitrogen) and random hexamers (100 ng.μL−1, Applied Biosystems) according to the manufacturer’s instructions. The primers used to amplify cdi genes and cdi gene junctions are listed in S1 Table. All PCRs on cDNA were performed with GoTaq DNA polymerase (Promega) or iProof DNA Polymerase (BioRad), in accordance with the manufacturer’s recommendations. The final PCR products were subjected to electrophoresis in 1% agarose gels in TAE, alongside the 1 kb DNA Ladder Plus (Euromedex).
Genomic analysis
We used the Genoscope microscopy platform (http://www.genoscope.cns.fr/agc/microscope/home/) to identify the tpsAB loci present in available Xenorhabdus and Photorhabdus complete genome sequences, by searching for protein sequences with the conserved NPNGI and NPNL motifs; the N-terminal TPS domain is a hallmark of TpsA proteins.
We used BlastP with default parameters to search for orthologs of the CdiB/CdiC/CdiA/CdiI proteins in other bacteria present in the NCBI non-redundant protein database.
Phylogenetic analysis
A sequence alignment (Muscle) was generated and phylogenetic analysis methods (maximum-likelihood analysis) were used with the Seaview Platform (http://doua.prabi.fr/software/seaview), as described elsewhere [23].
The strains used in phylogenetic analysis and their accession numbers are shown in S2 Table.
Results
Xd FRM16 harbors a new cdi locus
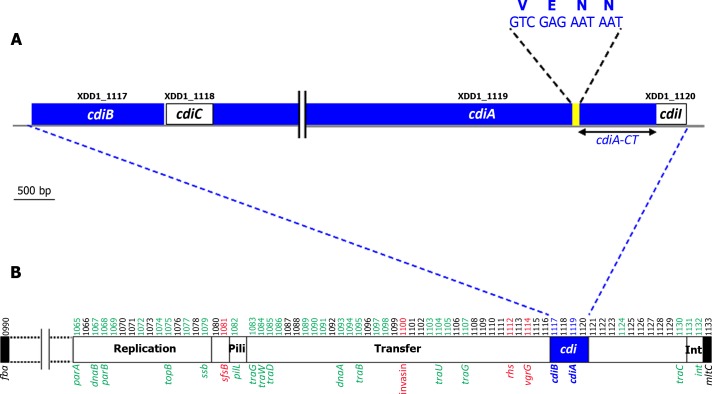
A putative cdi locus was identified in the genome of Xenorhabdus doucetiae strain FRM16 (Xd_FRM16). It contains four ORFs, XDD1_1117 to XDD1_1120 (Fig 1A). XDD1_1117 encodes a homolog of the outer membrane protein CdiB (29% and 36% identity to the CdiB of E. coli 536 and Burkholderia thailandensis E264, respectively). XDD1_1118 encodes a homolog of acyltransferase proteins generally described as toxin-activating protein C (34% identity to HlyC of E. coli strain PM152 [36]). For this reason, we named the ORF XDD1_1118 CdiCFRM16. XDD1_1119 is similar to CdiA (36% and 40% identity to the sequences of CdiA of E. coli 536 and B. thailandensis E264, respectively) and encodes the VENN motif separating the conserved N-terminus (~ 4 100 aa) from the variable C-terminus (~ 300 aa) in many CdiA proteins (Fig 1A). This carboxy-terminal region is referred to below as CdiA-CTFRM16. Finally, XDD1_1120 encodes a putative protein of unknown function. Its location just after the cdiA gene suggests that it may encode the CdiI immunity protein.
Fig 1.
The cdiBCAI locus of Xenorhabdus doucetiae FRM16 A. Genetic organization of the cdiBCAI locus Boxes represent genes. Gene labels are shown above the boxes. The putative cdiA and cdiB genes are shown in blue. The cdiA-CT region is indicated. The location of the nucleotide sequence encoding the VENN motif is indicated in yellow. B. The cdiBCAI locus is located in an integrative conjugative element (ICE). The cdi locus is shown in blue. The conserved genes of the ICE, as defined in a previous study [40], are highlighted in green. Notable cargo genes (e.g. potentially involved in host interactions) are highlighted in red. The label numbers of the genes are indicated above the locus. The ICE is embedded in a large genomic island inserted between the fba and mltC genes (black boxes). The genetic content of the genomic island is described in S3 Table.
The cdi locus of Xd_FRM16 is located within a previously described integrative and conjugative element (ICE) [23] (Fig 1B) harbouring the remnant of a pilus synthesis locus (Fig 1B), but it contains the essential machinery for mobilization [23]. The ICE is embedded in a large genomic island (GI) inserted between the fba and mltC genes of the core genome (Fig 1B and S3 Table for genetic content). This GI contains several genes or gene clusters potentially involved in host interactions: i) the mcf gene, which encodes a toxin active against caterpillars [37], ii) a paa-like locus encoding enzymes of the phenylacetic acid catabolic pathway required for the oral pathogenicity of Burkholderia cenocepacia in Caenorhabditis elegans [38], iii) an iol-like locus encoding enzymes involved in myoinositol catabolism, the products of which have been implicated in the pathogenicity of the human fungal pathogen Cryptococcus neoformans [39].
Growth inhibition function of the CdiA-CTFRM16 fragment and identification of the CdiI protein
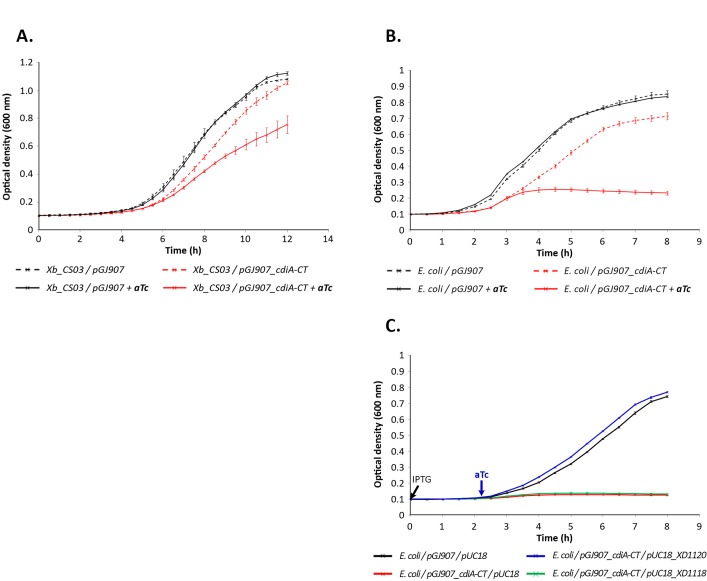
The region encoding the CdiA-CTFRM16 polypeptide (residues 4167 to 4436) was inserted into plasmid pGJ907 under the control of the Ptet promoter, which is inducible by adding aTc (anhydrotetracycline) to the culture medium [41]. When introduced into Xenorhabdus bovienii strain CS03, which does not possess the cdi locus [42], the resulting plasmid, pGJ907_cdiA-CTFRM16, conferred a growth inhibition phenotype to the bacterium cultured in LB broth, upon aTc induction (Fig 2A). Similar results were obtained with Escherichia coli EPI400 (Fig 2B). The CdiA-CTFRM16 fragment therefore confers a capacity to inhibit the growth of both closely related (X. bovienii) and more distantly related (E. coli) species.
Fig 2. CdiA-CTFRM16 inhibits cell growth when expressed in E. coli and Xenorhabdus bovienii.
A. CdiA-CTFRM16–mediated growth inhibition in X. bovienii strain CS03 growth. X. bovienii CS03 carrying pGJ907 (black lines), or pGJ907_cdiA-CTFRM16 (red lines) was grown at 28°C in LB broth supplemented with kanamycin (dotted curves), or LB broth supplemented with kanamycin plus aTc (continuous curves). B. CdiA-CTFRM16–mediated growth inhibition in E. coli EPI400. E. coli EPI400 carrying pGJ907 (black lines) or pGJ907_cdiA-CTFRM16 (red lines) was grown at 37°C in LB broth supplemented with kanamycin (dotted curves), or LB broth supplemented with kanamycin plus aTc (continuous curves). C. XDD1-1120 confers immunity to CdiA-CTFRM16 toxicity in E. coli EPI400. E. coli carrying pGJ907 and pUC18 (black line), pGJ907_cdiA-CTFRM16 and pUC18 (red line), pGJ907_cdiA-CTFRM16 and pUC18_ XD1120 (blue line), pGJ907_cdiA-CTFRM16 and pUC18_ XD1118 (green line) were grown at 37°C in LB broth supplemented with kanamycin. The times when IPTG and aTc were added at the culture are indicated by an arrow. In each panel, optical density at 600 nm was recorded every 30 minutes. When required, aTc was added 2 hours after the start of culture (OD600 nm~0.15). The results shown are the mean and standard deviation of three experiments.
This growth inhibition was completely blocked in E. coli/pGJ907_cdiA-CTFRM16 following co-expression of the XDD1_1120 gene within pUC18, under the control of the Plac promoter (Fig 2C). These results confirm that XDD1_1120 confers immunity to CdiA-CTFRM16 mediated growth inhibition. We therefore named the XDD1_1120-encoded protein CdiIFRM16. By contrast, the expression of cdiCFRM16gene (XDD1_1118) does not display significant consequence on growth inhibition caused by cdiA-CTFRM16 (Fig 2C).
CdiA-CTFRM16 displays DNAse activity
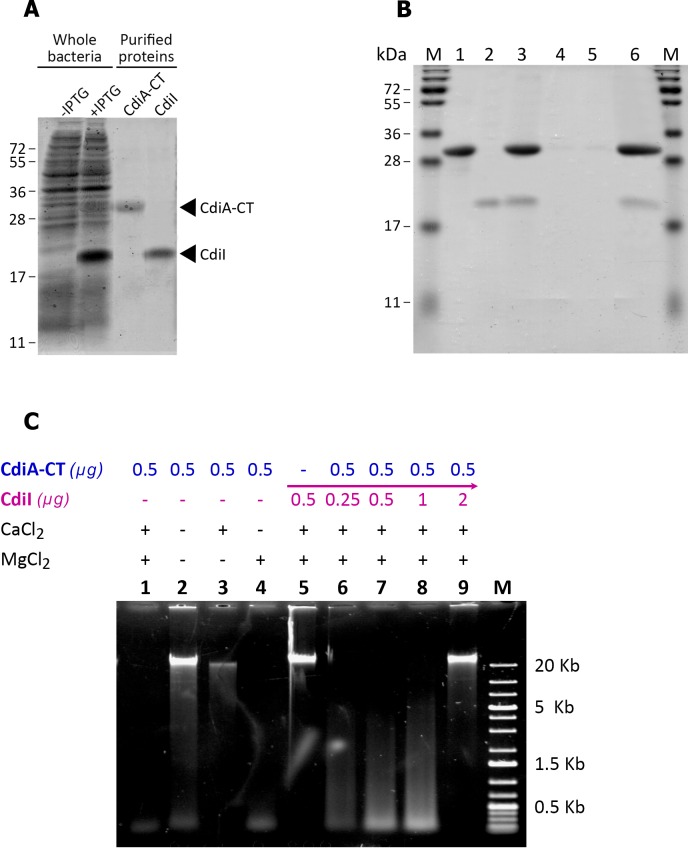
The most closely related ortholog of CdiA-CTFRM16 is CdiA-CT from Dickeya dadantii 3937 (67% amino-acid identity), an Mg2+-dependent DNase [27]. We assessed the DNase activity of CdiA-CTFRM16, by using the pET28b vector to overproduce (i) the CdiA-CTFRM16 fragment and the immunity protein CdiIFRM16 (Fig 3A) or (ii) the immunity protein CdiIFRM16 alone. His6-tagged CdiIFRM16 was purified by Ni-NTA chromatography under denaturing conditions. The CdiA-CTFRM16 toxin was purified together with its cognate His6-tagged CdiIFRM16 immunity protein, to prevent autotoxicity in the E. coli strain overproducing it. It was then separated from the immunity protein by SDS-PAGE under denaturing conditions. The purified CdiA-CTFRM16 and CdiIFRM16 polypeptides had observed sizes of 30 and 18 kDa, respectively (Fig 3A), consistent with the predicted sizes of the recombinant products. Purified recombinant protein yield was ~500 mg.L-1 for CdiA-CTFRM16 and ~100 mg.L-1 for CdiIFRM16. We underwent pull-down experiment to confirm that the over-produced CdiIFRM16 immunity protein purified alone binds specifically to cognate over-produced CdiA-CTFRM16 protein (Fig 3B).
Fig 3.
In vitro analysis of CdiA-CTFRM16/CdiIFRM16 activities A. SDS-polyacrylamide gel electrophoresis of the purified recombinant CdiA-CTFRM16 and CdiIFRM16 proteins. Electrophoretic separation, by SDS-PAGE (15% acrylamide), of proteins after the culture in LB broth of E. coli/pET28b_ cdiA-CTFRM16_ XD1120 with or without IPTG induction (whole bacteria), or after purification by Ni2+-affinity chromatography (purified proteins). Proteins were visualized by Coomassie Blue staining. CdiA-CTFRM16 and CdiIFRM16 had apparent sizes of 30 kDa and 18 kDa, respectively. B. CdiIFRM16 binds specifically to its cognate CdiA-CT. Purified CdiA-CTFRM16 (lane 1) and CdiIFRM16-His6 purified alone (lane 2) were incubated in equimolar mixture (lane 3), and subjected to Ni2+-affinity chromatography to bind the CdiA-CTFRM16-CdiI FRM16-His6 complex. The complex was washed twice to liberate the unbound protein fractions (lanes 4 and 5, respectively). The bound protein fraction (lane 6) was eluted using imidazole. All fractions were run on SDS-polyacrylamide gels and stained with Coomassie blue. M, protein marker (PageRuler plus prestained protein ladder, Thermo scientific). C. CdiA-CTFRM16 has DNAse activity CdiA-CTFRM16 was assayed for DNase activity with bacterial genomic DNA from Xd_FRM16. DNA was visualized by electrophoresis in a 0.7% agarose gel. Lanes 1 to 4: The purified CdiA-CTFRM16 protein and genomic DNA were incubated for 1 hour at 37°C with or without CaCl2 (2 mM) and MgCl2 (2 mM). Lane 5: The CdiIFRM16 protein purified alone and genomic DNA were incubated for 1 hour at 37°C with CaCl2 (2 mM) and MgCl2 (2 mM). Lanes 6 to 9: The purified CdiA-CTFRM16 and CdiIFRM16 proteins were first incubated for 30 minutes at 37°C in the presence of various concentrations of CdiIFRM16. Genomic DNA was then added and the mixture was incubated for 1 hour at 37°C with CaCl2 (2 mM) and MgCl2 (2 mM). Lane M: 1-kb DNA ladder (Eurogentec).
We then assessed the nuclease activity of the purified CdiA-CTFRM16 fragment in vitro (Fig 3C). Xd_FRM16 genomic DNA was completely degraded following incubation with 0.5 μg of purified CdiA-CTFRM16, in the presence of the bivalent cations Mg2+ and Ca2+, or Mg2+ alone (lanes 1 and 4, Fig 3C). CdiA-CTFRM16 also displayed DNase activity against genomic DNA from different Xenorhabdus and Photorhabdus species, supercoiled plasmid DNA and eukaryotic genomic DNA (data not shown). In the absence of Mg2+ ions, little or no nuclease activity was observed in the absence or presence of Ca2+ cations (lanes 2 and 3, Fig 3C). The CdiIFRM16 polypeptide had no DNase activity (lane 5, Fig 3C). We then evaluated the impact on CdiA-CTFRM16 DNase activity of increasing concentrations of CdiIFRM16 by pre-incubation of the two polypeptides. Protection against DNase activity was observed for a CdiA-CTFRM16 / CdiIFRM16 ratio greater than two (lanes 6 to lanes 9, Fig 3C). Thus, CdiA-CTFRM16 displays Mg2+-dependent DNase activity that is neutralized by its cognate CdiI FRM16 immunity protein.
cdiB, cdiC, cdiA and cdiI belong to the same transcriptional unit
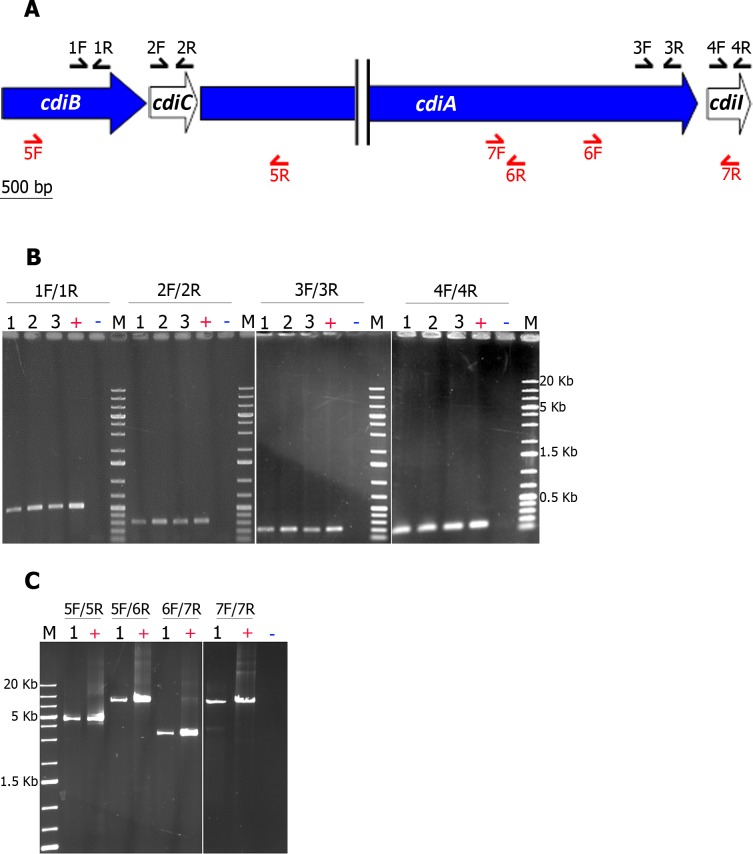
We investigated whether the cdi locus could be considered to function as an operon, by mapping the RNAs of the cdi genes by PCR (see Fig 4A for location of the primers). We checked that RNA is free of DNA by absence of 16S rDNA gene amplification (data not shown). We then showed that the four genes were transcribed at different time points during bacterial growth in LB (Fig 4B). We finally mapped the cdiFRM16 cDNA with primers allowing the amplification of nucleotides 400 of cdiB through 2779 of cdiA (4641-bp fragment flanked by the primers 5F and 5R) or of nucleotides 400 of cdiB through 6568 of cdiA (8430-bp fragment flanked by the primers 5F and 6R), and the amplification of nucleotides 10184 of cdiA through 287 of cdiI (3430-bp fragment flanked by the primers 6F and 7R) or of nucleotides 5625 of cdiA through 287 of cdiI (7988-bp fragment flanked by the primers 7F and 7R) (Fig 4C). The cdiB-cdiC-cdiA and cdiA-cdiI genes were respectively co-transcribed, consistent with an operon structure for the cdiFRM16 locus.
Fig 4. Xd_FRM16 cdiC, cdiB and cdiA are transcribed as a single transcription unit.
A. Position of the primers used in RT-PCR analyses. The cdi genes are indicated by large arrows, the size of which is proportional to gene length. The positions of the primers are indicated by thin black arrows for internal PCRs and thin red arrows for junction PCRs (see S1 Table for a description of the primers). B. cdiB, cdiC, cdiA and cdiI were transcribed at different time points during bacterial growth in LB broth. cDNAs were synthesized from total RNA extracted from cultures of X. doucetiae FRM16 in LB broth at different incubation times: 1 (OD at 600 nm = 0.75), 2 (OD at 600 nm = 1.70), 3 (OD at 600 nm = 3.70). 50 ng of cDNA were then amplified with specific primers targeting the four genes of the X. doucetiae FRM16 cdi locus (see panel A and S1 Table). +, ~70 ng of genomic DNA from X. doucetiae FRM16; -, 1 μL of water; M, 5 μL of 1 kb DNA Ladder Plus (Euromedex). The PCR products were subjected to electrophoresis in 1% agarose gels. C. cdiB, cdiC, cdiA and cdiI genes form a single operon. Specific primers allowing the mapping of the cdiB-cdiC-cdiA and the cdiA-cdiI junctions were used (see panel A and S1 Table). ~50 ng of cDNA of the condition 1 (panel B) were used. +, ~70 ng of genomic DNA from X. doucetiae FRM16; -, 1 μL of water; M, 5 μL of 1 kb DNA Ladder Plus (Euromedex).
The cdiBCAI locus is widespread in environmental bacteria interacting with insects, plants, rhizospheres and soils
We investigated the frequency of occurrence of the cdiBCAI-type locus in entomopathogenic bacteria, by searching for orthologs of genes encoding the CdiA, CdiB, CdiC and CdiI proteins in 41 Xenorhabdus and Photorhabdus available genomes. cdi-like loci are frequently redundant in all Photorhabdus genomes. However, a cdiBCAI-type locus was detected only in the P. luminescens BA1 genome. By contrast, cdi-like loci are present in only ~30% of Xenorhabdus genomes, but a majority of Xenorhabdus cdi-like loci were of cdiBCAI type. The cdiBCAI-type loci encountered in the genomes of Xenorhabdus and P. luminescens BA1 are described in Table 1.
Table 1. Inventory of cdiBCAI-type loci in Xenorhabdus and Photorhabdus genomes.
| Organism | Putative CdiB proteins | Putative CdiC proteins | Putative CdiA proteins | Putative CdiI proteins | |||||
|---|---|---|---|---|---|---|---|---|---|
| Label | Length (aa) | Label | Length (aa) | Label | Length (aa) | Motifs and domains | Label | Length (aa) | |
| X. doucetiae FRM161 | XDD1_1117 | 567 | XDD1_1118 | 179 | XDD1_1119 | 4436 | Haem-FhaB-VENN | XDD1_1120 | 153 |
| X. bovienii SS-20042 | XBJ1_1975 | 453 | XBJ1_1976 | 179 | XBJ1_1977a/XBJ1_1979a | 642/2663 | FhaB-DUF638-VENN | Absent | / |
| X. szentirmaii DSM163383 | / | / | Absent | / | XSR1v1_770001 | 2539 | Haem-FhaB-VENN | ND | / |
| XSR1v1_900001 | 565 | XSR1v1_900002 | 180 | XSR1v1_900003a | 438 | / | ND | / | |
| X. cabanillasii JM264 | XCR1v1_1000008 | 412 | XCR1v1_1000009 | 179 | XCR1v1_1000010a | 779 | Haem-FhaB-VENN | ND | / |
| XCR1v1_1970003 | 488 | XCR1v1_1970002 | 179 | / | / | / | ND | / | |
| XCR1v1_1510010 | 3226 | Haem-FhaB-VENN | ND | / | |||||
| Xenorhabdus sp. GDc3285 | LGYQ01_v1_1280018 | 565 | LGYQ01_v1_1280017 | 179 | LGYQ01_v1_1280016 | 4428 | Haem-FhaB-IESN | ND | / |
| X. griffiniae BMMCBg6 | LDNM01_v1_250020 | 565 | LDNM01_v1_250019 | 179 | LDNM01_v1_250018 | 4428 | Haem-FhaB-IESN | LDNM01_v1_250017 | 74 |
| Xenorhabdus sp. NBAII XenSa047 | JTHK01_v1_820003 | 566 | JTHK01_v1_820004 | 179 | JTHK01_v1_820005a | 2608 | Haem | ND | / |
| P. luminescens BA18 | JFGV01_v1_310043 | 570 | JFGV01_v1_310044 | 179 | JFGV01_v1_310045a | 3216 | Haem-FhaB | ND | / |
a The genes are fragmented (pseudogenes)
Accession numbers
1NZ_FO704550.1 and NZ_FO704549.1 (pl.)
5 LGYQ0000000
8PRJNA217861
We then expanded our biodiversity survey by searching for proteins orthologous to CdiCFRM16, the hallmark of the cdiBCAI-type locus, in the NCBI public database. CdiCFRM16 orthologs are systematically encoded inside cdi-like loci (S4 Table). Most of the CdiC orthologs were associated with (i) entomopathogenic bacteria, such as Xenorhabdus and Serratia, (ii) bacteria interacting with plants, such as Pseudomonas syringae and D. dadantii or (iii) soil and rhizosphere bacteria, such as Pseudomonas fluorescens, iv) and numerous pathogenic enterobacteria, e.g. E. coli strains.
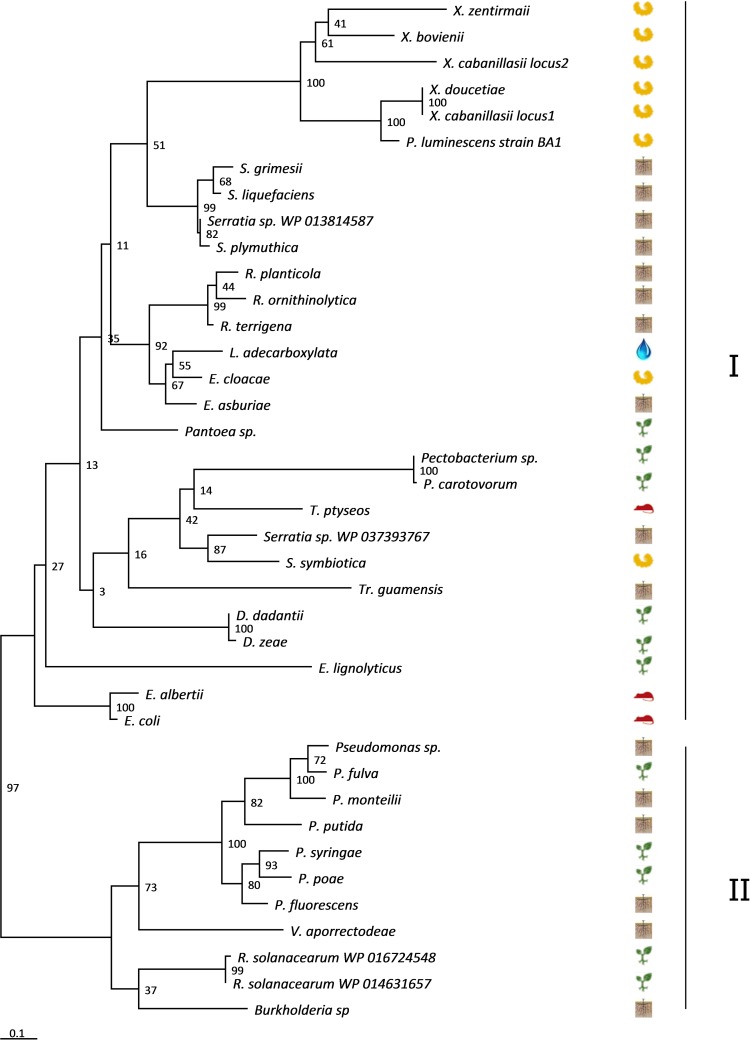
Phylogenetic analysis of a representative set of bacterial species clustered the CdiC sequences in two clades (Fig 5). Clade I contained CdiC sequences from Enterobacteriaceae, including environmental strains such as insects pathogens (Xenorhabdus strains, P. luminescens strain BA1 and Serratia) and plant pathogens (Dickeya, Pantoea and Pectobacterium), whereas clade II contained CdiC sequences from environmental proteobacteria of the Pseudomonadaceae and Burkholderiaceae families. Some features suggested probable horizontal genetic transfers of the cdiC gene between closely related species. For example, the CdiC P. luminescens BA1 sequence clustered with CdiCFRM16 (Fig 5). Moreover, the CdiC proteins of X. doucetiae and X. cabanillasii (locus 1) were 100% identical, despite the distant relationship of these two species within the genus Xenorhabdus.
Fig 5. Phylogenetic analysis of CdiC FRM16 orthologous sequences.
Phylogenetic trees were constructed by the maximum likelihood (ML) method, with bootstrap values indicated at the nodes. The branch length scale bar below the phylogenetic tree reflects the numbers of amino-acid substitutions per site. The protein sequences are split into two clades, I and II, indicated at the right of the tree. The ecological niches are symbolized by pictograms (insects, rhizosphere, water, plants, and vertebrates). Accession numbers of the sequences are indicated in S2 Table.
Evolutionary history of cdiBCAI genes
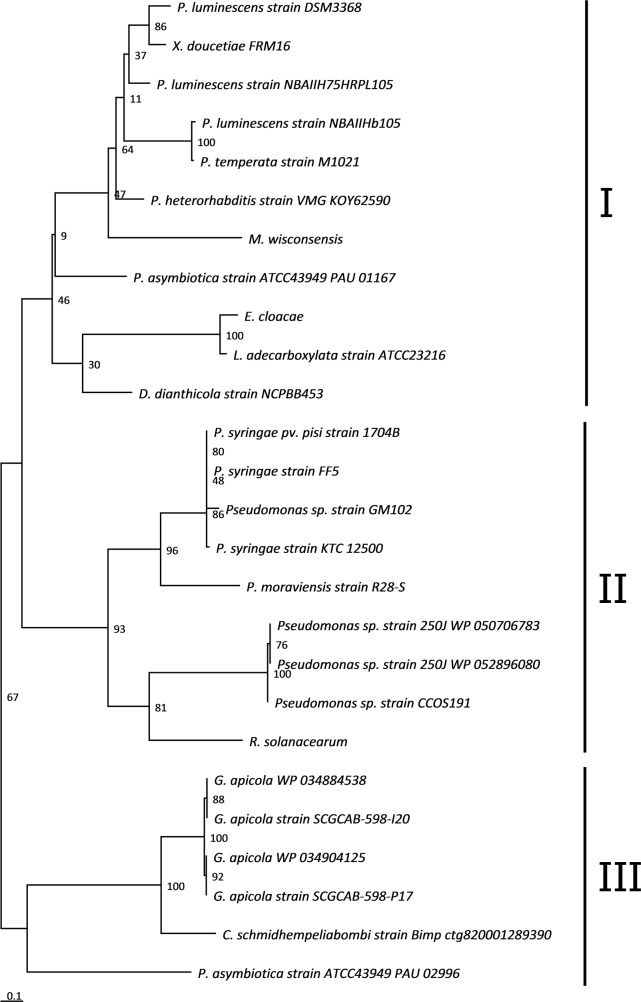
We analyzed the co-evolution of the Cdi proteins by comparing their phylogeny. As previously described for CdiC, the sequences of the CdiB, CdiA and CdiI proteins were retrieved by BlastP searches of the NCBI public database with CdiAFRM16, CdiBFRM16 and CdiIFRM16 as queries (S4 Table). The phylogenetic trees for the CdiB, CdiA and CdiC proteins were congruent, suggesting co-evolution of the three genes (S1 Fig). The CdiB and CdiA sequences from “E. coli-type” and “Burkholderia-type” cdi loci, used as references, branched outside these trees, confirming that the cdiBCAI-type locus was phylogenetically distinct from these previously characterized CDI systems. By contrast, the phylogenetic tree of CdiI (Fig 6) differed from those for CdiB, CdiA and CdiC and from bacterial species phylogeny.
Fig 6. Phylogenetic analysis of CdiIFRM16 orthologous sequences.
Phylogenetic trees were constructed by the maximum likelihood (ML) method, with bootstrap values indicated at the nodes. The branch length scale bar below the phylogenetic tree reflects the numbers of amino-acid substitutions per site. The protein sequences are split into three clades, I, II and III, indicated at the right of the tree. Accession numbers of the sequences are indicated in S2 Table.
Finally, we found no genes encoding orthologs of CdiIFRM16 in the other Xenorhabdus genomes. The closest CdiIFRM16 orthologs were encoded by genes in the Photorhabdus genus (P. asymbiotica ATCC43949, P. heterorhabditis VMG, Photorhabdus temperata M1021 and numerous strains of P. luminescens), in Pseudomonas syringae strains, and, more surprisingly, in a few strains of Gilliamella apicola, a gut symbiont of honey bees (Fig 6).
Discussion
CDI systems may play a key role in competition strategies, by delivering toxins that kill neighbouring bacteria, thereby eliminating bacterial competitors. Known CDI systems are encoded by two different classes of genomic loci: those of the ‘‘E. coli-type” generally found in Enterobacteriaceae [27], and those of the ‘‘Burkholderia-type” found in the genera Burkholderia, Cupriavidus, Ralstonia [32, 33]. In this study, we used Xenorhabdus genomic resources to identify a new type of cdi locus, the cdiBCAI-type locus, in the Xenorhabdus doucetiae FRM16 genome. This locus is characterized by the presence of an additional ORF, located between cdiB and cdiA that we named cdiC, due to the orthology of its product with toxin-activating proteins C. Although the presence of such an accessory gene in cdi loci has been reported in some Gram-negative strains [43], it was never exhaustively investigated. A more exhaustive search of cdiBCAI-type loci in a bacterial database showed that the cdiBCAI type locus was frequently present in Xenorhabdus genomes (~ 30% of the sequenced genomes) and many environmental bacteria interacting with insects, plants, rhizosphere and soil. Previous studies have suggested that cdi loci probably undergo horizontal gene transfer (HGT) between bacteria, because they are often present in genomic and pathogenicity islands [26, 28]. The Xd_FRM16 cdi locus is the first example found to be located within an ICE, a class of mobile genetic elements known to mediate HGT [44, 45].
The CdiA toxin module of X. doucetiae FRM16, CdiA-CTFRM16, has many features in common with other described CdiA-CT. It is flanked at its C-terminal end by a VENN motif, and it is toxic when produced within the cells of closely or more distantly related bacteria [26]. CdiA-CTFRM16 is an Mg2+-dependent DNase, as shown for its ortholog CdiA-CT in D. dadantii strain 3937 [27]. Several other CdiA-CTs display nuclease activity in vitro, probably killing their target bacteria [27, 33, 46]. The CdiA-CT of E. coli EC869 has DNase activity, but its amino-acid sequence is very different from that of CdiA-CTFRM16 (30% identity) and its nuclease activity is Zn2+-dependent [47, 48]. This finding may reflect highly convergent evolution for the nuclease activity of CdiA-CT fragments.
We found that cdiBFRM16, cdiCFRM16, cdiAFRM16 and cdiIFRM16 were cotranscribed as an operon in both the exponential and the stationary phases of growth in LB culture. Moreover, we performed phylogenetic analysis, which displays that cdiBFRM16, cdiCFRM16 and cdiAFRM16 genes co-evolved. We therefore hypothesize that the product of the cdiC gene is involved in the functional CDI system. Due to the similarity of the CdiC protein with HlyC [36], CdiCFRM16 might play a role in activation of CDI through lysine acetylation. In our growth inhibition assay in E. coli, we did not observe any effect of the cdiCFRM16 expression on CdiA-CTFRM16 activity. Some authors suggest that CdiC activity may promote CdiA or CdiB association with membrane [49]. Indeed, CdiCFRM16 may play an important role in membrane-associated CDI functions, such as CdiBA biogenesis, CdiA target cell binding or toxin translocation through the cell envelope.
We identified and characterized the function of CdiIFRM16 in protecting the bacteria producing it from the toxic activity of CdiA-CTFRM16. Our phylogenetic analysis suggested that cdiBCA and cdiI were different evolutionary units. Ruhe and coworkers previously observed that, in many CDI toxin-immunity protein pairs, the CdiI proteins display much greater sequence diversity than the CdiA-CT toxins [26]. They suggest that cdiI evolution is rapid as long as CdiI maintains sufficient affinity for CdiA-CT to provide immunity [26]. Investigation of the genetic context of cdiIFRM16 orthologs in available complete genomes showed that they are either orphan genes or participate in orphan cdiA-CT-cdiI pairs (J-C. Ogier, unpublished data). These orphan cdiI genes or cdiA-CT-cdiI pairs may be horizontally exchanged between bacteria, potentially enabling bacteria to protect themselves against neighbouring bacteria that are producing and delivering CdiA-CT toxins. Interestingly, no cdiIFRM16 orthologs were found in Xenorhabdus genomes, and the closest cdiIFRM16orthologs, associated or unassociated with cdiA-CT, were mostly found in Photorhabdus strains, a genus having a life cycle similar to that of Xenorhabdus, including a major stage in the insect cadaver [22].
Several attempts for assessing role of the in vivo activity of the cdiBCAI locus were conducted between X. doucetiae FRM16 and strains of Xenorhabdus that naturally do not harbor any cdi loci. However, no clear effect could be detected (data not shown). This is likely due to the highly redundant arsenal of Xenorhabdus factors involved in antagonism between bacteria [4]. For example, a locus encoding a tail-phage bacteriocin is responsible for eliminating phylogenetically close bacteria from the insect cadaver [15].
In conclusion, Xenorhabdus genus is a pertinent resource for identifying genomic loci potentially involved in interbacterial competition systems, such as cdi loci. In order to determine the specificities of the cdiBCAI-type locus, further studies should be conducted.
Supporting Information
(DOCX)
(XLSX)
(XLSX)
(XLSX)
Comparison of the topologies of the phylogenetic trees of CdiBFRM16 (panel A), CdiCFRM16 (panel B) and CdiAFRM16 (panel C) in a limited set of bacterial species, as identified by BlastP (i.e. selection of 16 strains with complete cdiBCAI loci, covering species diversity). The phylogenetic trees were built by the maximum likelihood (ML) method, and branch support values (estimated by the aLRT(SH-like) method) are indicated at the nodes. The branch length scale bar below the phylogenetic tree reflects the number of amino-acid substitutions per site. The Xd_FRM16 sequences are highlighted in blue. For some taxa, orthologs are highlighted in red when the topology is not congruent with the species tree. The sequences from “E. coli-type” and “Burkholderia-type” cdi loci are used as outgroups, and are highlighted in bold. Accession numbers of the sequences are indicated in S2 Table.
(PPTX)
Acknowledgments
We thank William Ourliac for assistance with growth inhibition assays and Nadège Ginibre for bacterial mating experiments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by 1) Institut National de la Recherche Agronomique: grant n°010-1133-01 from the department "Santé des Plantes et Environnement"; 2) Université Montpellier: grant n°2011.
References
- 1.Forst S, Dowds B, Boemare N, Stackebrandt E. Xenorhabdus and Photorhabdus spp.: Bugs that kill bugs. Annu Rev Microbiol. 1997;51:47–72. [DOI] [PubMed] [Google Scholar]
- 2.Goodrich-Blair H, Clarke DJ. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol Microbiol. 2007;64(2):260–8. [DOI] [PubMed] [Google Scholar]
- 3.Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS. Insect pathogens as biological control agents: Back to the future. J Invertebr Pathol. 2015;132:1–41. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen-LeRoux C, Gaudriault S, Ramarao N, Lereclus D, Givaudan A. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr Opin Microbiol. 2012; 15(3):220–31. 10.1016/j.mib.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 5.Bode HB. Entomopathogenic bacteria as a source of secondary metabolites. Curr Opin Chem Biol. 2009;13(2):224–30. 10.1016/j.cbpa.2009.02.037 [DOI] [PubMed] [Google Scholar]
- 6.Park D, Ciezki K, van der Hoeven R, Singh S, Reimer D, Bode HB, et al. Genetic analysis of xenocoumacin antibiotic production in the mutualistic bacterium Xenorhabdus nematophila. Mol Microbiol. 2009;73(5):938–49. 10.1111/j.1365-2958.2009.06817.x [DOI] [PubMed] [Google Scholar]
- 7.Reimer D, Luxenburger E, Brachmann AO, Bode HB. A New Type of Pyrrolidine Biosynthesis Is Involved in the Late Steps of Xenocoumacin Production in Xenorhabdus nematophila. Chembiochem. 2009;10(12):1997–2001. 10.1002/cbic.200900187 [DOI] [PubMed] [Google Scholar]
- 8.Reimer D, Pos KM, Thines M, Grun P, Bode HB. A natural prodrug activation mechanism in nonribosomal peptide synthesis. Nat Chem Biol. 2011;7(12):888–90. [DOI] [PubMed] [Google Scholar]
- 9.Reimer D, Nollmann FI, Schultz K, Kaiser M, Bode HB. Xenortide Biosynthesis by Entomopathogenic Xenorhabdus nematophila. J Nat Prod. 2014;77(8):1976–80. 10.1021/np500390b [DOI] [PubMed] [Google Scholar]
- 10.Crawford JM, Portmann C, Kontnik R, Walsh CT, Clardy J. NRPS Substrate Promiscuity Diversifies the Xenematides. Org Lett. 2011;13(19):5144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs SW, Proschak A, Jaskolla TW, Karas M, Bode HB. Structure elucidation and biosynthesis of lysine-rich cyclic peptides in Xenorhabdus nematophila. Org Biomol Chem. 2011;9(9):3130–2. 10.1039/c1ob05097d [DOI] [PubMed] [Google Scholar]
- 12.Gualtieri M, Aumelas A, Thaler JO. Identification of a new antimicrobial lysine-rich cyclolipopeptide family from Xenorhabdus nematophila. J Antibiot. 2009;62(6):295–302. [DOI] [PubMed] [Google Scholar]
- 13.Riley MA. Molecular mechanisms of bacteriocin evolution. Annu Rev Genet. 1998;32:255–78. [DOI] [PubMed] [Google Scholar]
- 14.Riley MA, Wertz JE. Bacteriocin diversity: ecological and evolutionary perspectives. Biochimie. 2002;84(5–6):357–64. [DOI] [PubMed] [Google Scholar]
- 15.Morales-Soto N, Forst SA. The xnp1 P2-Like Tail Synthesis Gene Cluster Encodes Xenorhabdicin and Is Required for Interspecies Competition. J Bacteriol. 2011;193(14):3624–32. 10.1128/JB.00092-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaler JO, Baghdiguian S, Boemare N. Purification and Characterization of Xenorhabdicin, a Phage Tail-Like Bacteriocin, from the Lysogenic Strain F1 of Xenorhabdus-Nematophilus. Appl Environ Microbiol. 1995;61(5):2049–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh J, Banerjee N. Transcriptional analysis and functional characterization of a gene pair encoding iron-regulated xenocin and immunity proteins of Xenorhabdus nematophila. J Bacteriol. 2008;190(11):3877–85. 10.1128/JB.00209-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz S, Hood RD, Mougous JD. What is type VI secretion doing in all those bugs? Trends Microbiol. 2010;18(12):531–7. 10.1016/j.tim.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hood RD, Singh P, Hsu FS, Guvener T, Carl MA, Trinidad RRS, et al. A Type VI Secretion System of Pseudomonas aeruginosa Targets, a Toxin to Bacteria. Cell Host Microbe. 2010;7(1):25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A. 2010;107(45):19520–4. 10.1073/pnas.1012931107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng J, Ho B, Mekalanos JJ. Genetic Analysis of Anti-Amoebae and Anti-Bacterial Activities of the Type VI Secretion System in Vibrio cholerae. PLoS One. 2011;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaston JM, Suen G, Tucker SL, Andersen AW, Bhasin A, Bode E, et al. The entomopathogenic bacterial endosymbionts Xenorhabdus and Photorhabdus: convergent lifestyles from divergent genomes. PLoS One. 2011;6(11):e27909 10.1371/journal.pone.0027909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogier JC, Pages S, Bisch G, Chiapello H, Medigue C, Rouy Z, et al. Attenuated Virulence and Genomic Reductive Evolution in the Entomopathogenic Bacterial Symbiont Species, Xenorhabdus poinarii. Genome Biol Evol. 2014;6(6):1495–513. 10.1093/gbe/evu119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki SK, Poole SJ, Hayes CS, Low DA. Toxin on a stick Modular CDI toxin delivery systems play roles in bacterial competition. Virulence. 2011;2(4):356–9. 10.4161/viru.2.4.16463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes CS, Koskiniemi S, Ruhe ZC, Poole SJ, Low DA. Mechanisms and Biological Roles of Contact-Dependent Growth Inhibition Systems. Cold Spring Harb Perspect Med. 2014;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruhe ZC, Low DA, Hayes CS. Bacterial contact-dependent growth inhibition. Trends Microbiol. 2013;21(5):230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki SK, Diner EJ, de Roodenbeke CT, Burgess BR, Poole SJ, Braaten BA, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468(7322):439–42. 10.1038/nature09490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruhe ZC, Nguyen JY, Chen AJ, Leung NY, Hayes CS, Low DA. CDI Systems Are Stably Maintained by a Cell-Contact Mediated Surveillance Mechanism. PLoS Genet. 2016;12(6):e1006145 10.1371/journal.pgen.1006145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruhe ZC, Wallace AB, Low DA, Hayes CS. Receptor Polymorphism Restricts Contact-Dependent Growth Inhibition to Members of the Same Species. Mbio. 2013;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309(5738):1245–8. 10.1126/science.1115109 [DOI] [PubMed] [Google Scholar]
- 31.Mercy C, Ize B, Salcedo SP, de Bentzmann S, Bigot S. Functional Characterization of Pseudomonas Contact Dependent Growth Inhibition (CDI) Systems. PLoS One. 2016;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson MS, Garcia EC, Cotter PA. The Burkholderia bcpAIOB Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems. PLoS Genet. 2012;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolakakis K, Amber S, Wilbur JS, Diner EJ, Aoki SK, Poole SJ, et al. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol Microbiol. 2012;84(3):516–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Givaudan A, Lanois A. flhDC, the flagellar master operon of Xenorhabdus nematophilus: Requirement for motility, lipolysis, extracellular hemolysis, and full virulence in insects. J Bacteriol. 2000;182(1):107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapelle M, Girard PA, Cousserans F, Volkoff NA, Duvic B. Lysozymes and lysozyme-like proteins from the fall armyworm, Spodoptera frugiperda. Mol Immunol. 2009;47(2–3):261–9. 10.1016/j.molimm.2009.09.028 [DOI] [PubMed] [Google Scholar]
- 36.Trent MS, Worsham LMS, Ernst-Fonberg ML. The biochemistry of hemolysin toxin activation: Characterization of HlyC, an internal protein acyltransferase. Biochemistry. 1998;37(13):4644–52. 10.1021/bi971588y [DOI] [PubMed] [Google Scholar]
- 37.Daborn PJ, Waterfield N, Silva CP, Au CPY, Sharma S, Ffrench-Constant RH. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc Natl Acad Sci U S A. 2002;99(16):10742–7. 10.1073/pnas.102068099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law RJ, Hamlin JNR, Sivro A, McCorrister SJ, Cardama GA, Cardona ST. A Functional Phenylacetic Acid Catabolic Pathway Is Required for Full Pathogenicity of Burkholderia cenocepacia in the Caenorhabditis elegans Host Model. J Bacteriol. 2008;190(21):7209–18. 10.1128/JB.00481-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luberto C, Toffaletti DL, Wills EA, Tucker SC, Casadevall A, Perfect JR, et al. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C-neoformans. Genes Dev. 2001;15(2):201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seth-Smith HMB, Fookes MC, Okoro CK, Baker S, Harris SR, Scott P, et al. Structure, diversity, and mobility of the Salmonella Pathogenicity Island 7 family of Integrative and Conjugative Elements within Enterobacteriaceae. J Bacteriol. 2012;194(6):1494–504. 10.1128/JB.06403-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jubelin G, Pagès S, Lanois A, Boyer MH, Gaudriault S, Ferdy JB, et al. Studies of the dynamic expression of the Xenorhabdus FliAZ regulon reveal atypical iron-dependent regulation of the flagellin and haemolysin genes during insect infection. Environ Microbiol. 2011;13(5):1271–84. [DOI] [PubMed] [Google Scholar]
- 42.Bisch G, Pagès S, McMullen JG, Stock SP, Duvic B, Givaudan A, et al. Xenorhabdus bovienii CS03, the bacterial symbiont of the entomopathogenic nematode Steinernema weiseri, is a non-virulent strain against lepidopteran insects. J Invertebr Pathol. 2015;124:15–22. [DOI] [PubMed] [Google Scholar]
- 43.Willett JL, Ruhe ZC, Goulding CW, Low DA, Hayes CS. Contact-Dependent Growth Inhibition (CDI) and CdiB/CdiA Two-Partner Secretion Proteins. J Mol Biol. 427(23):3754–65. 10.1016/j.jmb.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burrus V, Waldor MK. Shaping bacterial genomes with integrative and conjugative elements. Res Microbiol. 2004;155(5):376–86. 10.1016/j.resmic.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 45.Guglielmini J, Quintais L, Garcillan-Barcia MP, de la Cruz F, Rocha EPC. The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet. 2011;7(8):e1002222 10.1371/journal.pgen.1002222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole SJ, Diner EJ, Aoki SK, Braaten BA, de Roodenbeke CT, Low DA, et al. Identification of Functional Toxin/Immunity Genes Linked to Contact-Dependent Growth Inhibition (CDI) and Rearrangement Hotspot (Rhs) Systems. PLoS Genet. 2011;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morse RP, Nikolakakis KC, Willett JLE, Gerrick E, Low DA, Hayes CS, et al. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc Natl Acad Sci U S A. 2012;109(52):21480–5. 10.1073/pnas.1216238110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webb JS, Nikolakakis KC, Willett JLE, Aoki SK, Hayes CS, Low DA. Delivery of CdiA Nuclease Toxins into Target Cells during Contact-Dependent Growth Inhibition. PLoS One. 2013;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willett JLE, Gucinski GC, Fatherree JP, Low DA, Hayes CS. Contact-dependent growth inhibition toxins exploit multiple independent cell-entry pathways. Proc Natl Acad Sci U S A. 2015;112(36):11341–6. 10.1073/pnas.1512124112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
(XLSX)
(XLSX)
Comparison of the topologies of the phylogenetic trees of CdiBFRM16 (panel A), CdiCFRM16 (panel B) and CdiAFRM16 (panel C) in a limited set of bacterial species, as identified by BlastP (i.e. selection of 16 strains with complete cdiBCAI loci, covering species diversity). The phylogenetic trees were built by the maximum likelihood (ML) method, and branch support values (estimated by the aLRT(SH-like) method) are indicated at the nodes. The branch length scale bar below the phylogenetic tree reflects the number of amino-acid substitutions per site. The Xd_FRM16 sequences are highlighted in blue. For some taxa, orthologs are highlighted in red when the topology is not congruent with the species tree. The sequences from “E. coli-type” and “Burkholderia-type” cdi loci are used as outgroups, and are highlighted in bold. Accession numbers of the sequences are indicated in S2 Table.
(PPTX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.