Abstract
Information regarding Helicobacter pylori antibiotic resistance in Indonesia was previously inadequate. We assessed antibiotic susceptibility for H. pylori in Indonesia, and determined the association between virulence genes or genetic mutations and antibiotic resistance. We recruited 849 dyspeptic patients who underwent endoscopy in 11 cities in Indonesia. E-test was used to determine the minimum inhibitory concentration of five antibiotics. PCR-based sequencing assessed mutations in 23S rRNA, rdxA, gyrA, gyrB, and virulence genes. Next generation sequencing was used to obtain full-length sequences of 23S rRNA, infB, and rpl22. We cultured 77 strains and identified 9.1% with clarithromycin resistance. Low prevalence was also found for amoxicillin and tetracycline resistance (5.2% and 2.6%, respectively). In contrast, high resistance rates to metronidazole (46.7%) and levofloxacin (31.2%) were demonstrated. Strains isolated from Sumatera Island had significantly higher metronidazole resistance than those from other locations. Metronidazole resistant strains had highly distributed rdxA amino acid substitutions and the 23S rRNA A2143G mutation was associated with clarithromycin resistance (42.9%). However, one strain with the highest MIC value had a novel mutation in rpl22 without an A2143G mutation. Mutation at Asn-87 and/or Asp-91 of gyrA was associated with levofloxacin-resistance and was related to gyrB mutations. In conclusions, although this is a pilot study for a larger survey, our current data show that Indonesian strains had the high prevalence of metronidazole and levofloxacin resistance with low prevalence of clarithromycin, amoxicillin, and tetracycline resistance. Nevertheless, clarithromycin- or metronidazole-based triple therapy should be administered with caution in some regions of Indonesia.
Introduction
Asia is a very important continent for Helicobacter pylori infection, a common chronic bacterial infection in humans that is associated with peptic ulcer disease, gastric cancer, and primary gastric B-cell lymphoma [1]. Asia is the continent with the largest populace (4.4 billion people) and the highest frequency of gastric cancer in the world [2]. With some exceptions that some population with low incidence of gastric cancer even with a high prevalence of H. pylori known as the “Asian enigma” [3], the incidence of gastric cancer in several regions tends to mirror the prevalence of H. pylori infection [4]. Recent guidelines have proposed indications and regimens for the Asia-Pacific region and three countries in East Asia [5–8]. Nevertheless, the development of drug resistance in H. pylori is a major issue and the viability of some regimens has been truly challenged as they are becoming unsuccessful [9]. Creating an updated suitable first-line regimen is fundamental to counteract revised treatment courses that result in perpetuation of secondary antibiotic resistance [2].
Indonesia is a multi-ethnic nation in Southeast Asia that consists of more than 13,600 islands, with Sumatra, Papua, Kalimantan, Sulawesi, and Java being the five main islands. Currently, hospitals that provide GI endoscopy services in Indonesia are limited, and most are located on Java Island [10]. The prevalence of H. pylori infection in Javanese, the predominant ethnicity, is low (2.4%), even when using a combination of five different diagnostic methods [11,12]. However, several ethnic groups have much higher prevalence of H. pylori infection (42.9%, 40.0%, and 36.7% for Papuan, Batak, and Buginese individuals, respectively) [12]. Additionally, they harbor more virulent genotypes such as cagA positive, oipA ‘on’, and iceA1 positive [13]. The Kyoto Global Consensus Conference on H. pylori Gastritis in 2014 expressed that H. pylori gastritis should be characterized as an infectious disease, notwithstanding asymptomatic patients and irrespective of complications such as peptic ulcers and gastric cancer [14]. It has been proposed that H. pylori should be eradicated, even in Indonesia. To our knowledge, only a single study has reported the rates of H. pylori antibiotic resistance in Indonesia, identifying 72 strains in the low prevalence H. pylori region, Jakarta, in 2006 [15]. In this study, the rates clarithromycin (CAM), amoxicillin (AMX), metronidazole (MNZ), and levofloxacin (LVX) resistance were 27.8%, 19.4%, 100.0%, and 1.4%, respectively [15]. In addition, there was no report regarding tetracycline (TCN) resistance in Indonesian in H. pylori strains. TCN is a basic antibiotic, used in quadruple regimens, for H. pylori eradication. Since antibiotic resistance is expanding globally [16,17], it is necessary to examine the drug resistance rates in Indonesia.
The understanding of H. pylori antibiotic resistance mechanisms, which primarily occur because of mutations in chromosomal genes, is important as a premise for the establishment of rational antibiotic combinations. Critically, although numerous point mutations have appeared, the positions of such mutations were not uniform for every topographical area [18,19]. For example, although two nucleotide substitutions, specifically, A2142G or A2142C and A2143G in the peptidyl transferase loop of 23S rRNA, cause primary CAM resistance in H. pylori strains isolated in Western countries [20], they account for only 23% of resistant strains in Asia [20]. Our previous report demonstrated the synergic effect of mutated sequences in hp1048 (infB), hp1314 (rpl22), and A2143G, which resulted in higher MICs [21]. Unlike the mutational patterns of 23S rRNA, the mechanisms of MNZ resistance are complex and largely associated with inactivating mutations in rdxA, through frameshift mutations, insertions, and deletions [22]. Moreover mutations in the sequences of gyrase subunit A (gyrA) and gyrB, encoding translational proteins, greatly reduce the antimicrobial ability of fluoroquinolones [23].
In this study, we aimed to determine the antibiotic susceptibility of H. pylori in 11 cities, covering the five largest islands, of Indonesia. We also analyzed the association between virulence genes and antibiotic resistance rates. Furthermore, we determined the presence of genetic mutations that are associated with antibiotic resistance.
Materials and Methods
Patients and H. pylori
We conducted a prospective study from August 2012 to November 2015. This study included 849 adult dyspeptic patients who underwent endoscopy examinations in 11 cities including cities on the five largest islands of Indonesia. Patients with bleeding related to esophageal varices, a history of partial gastric resection and previous H. pylori eradication therapy were excluded from this study. Included were patients from Surabaya (296 patients), Jakarta (31), and Malang (97) on Java Island, Aceh (38) and Medan (93) on Sumatera Island, Pontianak (90) on Kalimantan Island, Makassar (30) and Manado (57) on Sulawesi Island, Jayapura (21) on Papua Island, Bangli (61) on Bali Island, and Kupang (35) on Timor Island. Peptic ulcer diseases were diagnosed by endoscopic observation, whereas chronic gastritis was determined by histologic examination. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. There were no minors or children enrolled in our study, therefore not needed informed consent from the next of kin, caretakers, or guardians on behalf of them. Written informed consent was obtained from all participants, and the study protocol was approved by the Institutional Review Board or the Ethics Committee of Dr. Cipto Mangunkusumo Teaching Hospital (Jakarta, Indonesia), Dr. Soetomo Teaching Hospital (Surabaya, Indonesia), Dr. Wahidin Sudirohusodo Teaching Hospital (Makassar, Indonesia), and Oita University Faculty of Medicine (Yufu, Japan).
For H. pylori culture, antral biopsy specimens were homogenized and inoculated onto antibiotics selection plate, subsequently subcultured onto Mueller Hinton II Agar medium (Becton Dickinson, NJ, USA) supplemented with 10% horse blood without antibiotics. The plates were incubated for up to 10 days at 37°C under microaerophilic conditions (10% O2, 5% CO2, and 85% N2). H. pylori isolates were identified based on colony morphology, Gram staining results, and positive reactions for oxidase, catalase, and urease. Isolated strains were stored at -80°C in Brucella Broth (Difco, NJ, USA) containing 10% dimethyl sulfoxide and 10% horse serum.
Antibiotic susceptibility testing
The E-test method (Biomerieux, France) was used to determine the minimum inhibitory concentration (MIC) of AMX, MNZ, TCN, CAM, and LVX. Mueller-Hinton II Agar medium (Becton Dickinson) supplemented with 10% defibrinated horse blood was used as culture media. The bacterial suspension, adjusted to be equivalent to a McFarland opacity standard of 3.0, was inoculated onto the plates. After 72 h of incubation, the MIC of each antibiotic was determined. Quality control was performed using H. pylori ATCC 43504. The resistance breakpoints were determined as described by the European Committee on Antimicrobial Susceptibility Testing (EUCAST; available at http://www.eucast.org/). Strains were considered to be resistant for MICs of >0.125 mg/L for AMX, 0.25 mg/L for CAM, 8 mg/L for MNZ, and 1 mg/L for TCN and LVX.
Virulence factors and resistant strains molecular detection
H. pylori DNA was extracted from H. pylori, cultured to confluence on plates, using a commercially available kit (Qiagen, Hilden, Germany). The DNA was stored at -20°C until used for gene amplification. Mutations in gyrA, gyrB, rdxA, and 23S rRNA were assessed in antibiotic-resistant strains by PCR-based sequencing using the primers as described previously [24–26]. As a control, we sequenced five randomly selected strains sensitive to MNZ and LVX and one CAM-sensitive strain from the collection of Indonesian strains. The sequences were then compared with the published sequence of H. pylori strain 26695 (GenBank accession number AE000511.1 GI: 6626253) using MAFFT version 7 (available at http://mafft.cbrc.jp/alignment/server/) and confirmed by visual inspection. Direct sequencing of the cagA EPIYA repeat region and determination of oipA status (“on” or “off”) were determined by PCR-based sequencing as described previously [27–29]. The presence of vacA (s1 or s2, m1 or m2 and i1, i2 or i3), iceA (iceA1 or iceA2), dupA, jhp0562, and β-(1,3)galT genotypes were determined based on PCR product size as described previously [30–35].
To find other genetic mutations, associated with high MIC values, but not involving typical 23S rRNA mutations, we also obtained full-length 23S rRNA, hp1048 (infB), and hp1314 (rpl22) [21] from next-generation sequencing data (MiSeq next-generation sequencer; Illumina, Inc., San Diego, CA). MiSeq output was integrated into contig sequences by CLC Genomics Workbench 7.0.4. Genomics Workbench was also used for gene prediction and translation to protein sequences.
Statistical analysis
Discrete variables were tested using the chi-square test, whereas continuous variables were tested using the Mann-Whitney U and t-tests. A multivariate logistic regression model was used to calculate the odds ratios (OR) of clinical outcomes including age, sex, H. pylori antibiotic resistance, ethnicity, and location. All determinants with P values < 0.10 were entered together into the full logistic regression model. The OR and 95% confidence interval (CI) were used to estimate risk. A P value of < 0.05 was accepted as statistically significant. The SPSS statistical software package version 18.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses.
Results
Prevalence of antibiotic resistance
Despite obtaining a large amount of samples, only 77 strains could be isolated. The low number of isolated strains was related to the low prevalence of H. pylori infection in Indonesia, and was confirmed by histology and immunohistochemistry (88/849, 10.4%, Table 1). Half of the samples were obtained from Java Island (424 samples) and had a H. pylori infection prevalence of only 4.0% (17/424; Table 1). In contrast, the most recent survey of Timor Island found the prevalence of H. pylori infection to be 40.0% (14/35). The host individuals consisted of 39 males (age range, 24 to 80 years; mean age, 49.6 ± 10.8 years) and 38 female patients (age range, 28 to 68 years; mean age 48.9 ± 15.4 years). Strains were isolated from Surabaya (12 strains), Jakarta (1), Malang (1), Medan (18), Pontianak (5), Makassar (6), Manado (7), Jayapura (7), Bali (6), and Kupang (14 strains). Among the patients, 70 had chronic gastritis and seven had peptic ulcer diseases.
Table 1. Prevalence of H. pylori infection in Indonesia based on multiple tests.
| Island (city) | Year | N | Diagnostic Method (%) | ||
|---|---|---|---|---|---|
| Culture | Histology confirmed by IHC | At least one method | |||
| Bali (Bangli)* | 2015 | 61 | 6 (9.8) | 7 (11.5) | 7 (11.5) |
| Java | 424 | 14 (3.3) | 15 (3.5)*** | 17 (4.0) | |
| (Surabaya) | 2012–2015 | 296 | 12 (4.1) | 14 (4.7) | 15 (5.1) |
| (Jakarta) | 2013 | 31 | 1 (0.1) | 1 (0.1) | 1 (0.1) |
| (Malang)* | 2014 | 97 | 1 (1.0) | ** | 1 (1.0) |
| Kalimantan (Pontianak) | 2014 | 90 | 5 (5.6) | 4 (4.4) | 6 (6.7) |
| Papua (Jayapura) | 2013 | 21 | 9 (42.9) | 9 (42.9) | 9 (42.9) |
| Sumatera | 131 | 19 (14.5) | 20 (15.3) | 26 (19.8) | |
| (Medan) | 2014 | 93 | 19 (20.4) | 20 (21.5) | 26 (27.9) |
| (Aceh)* | 2014 | 38 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sulawesi | 87 | 13 (14.9) | 13 (14.9) | 13(14.9) | |
| (Manado)* | 2015 | 57 | 7 (12.3) | 7 (12.3) | 7 (12.3) |
| (Makassar) | 2014 | 30 | 6 (20.0) | 6 (20.0) | 6 (20.0) |
| Timor (Kupang)* | 2015 | 35 | 14 (40.0) | 12 (34.3) | 14 (40.0) |
| Total | 849 | 80 (9.4) | 80 (9.4)*** | 88 (10.4) | |
IHC: Immunohistochemistry
* The most recent surveys that are not including in the previous publication (Syam AF, et al., 2015)
** Sample obtained only from culture, there was no sample for histology examination
*** The total number does not include the Malang survey
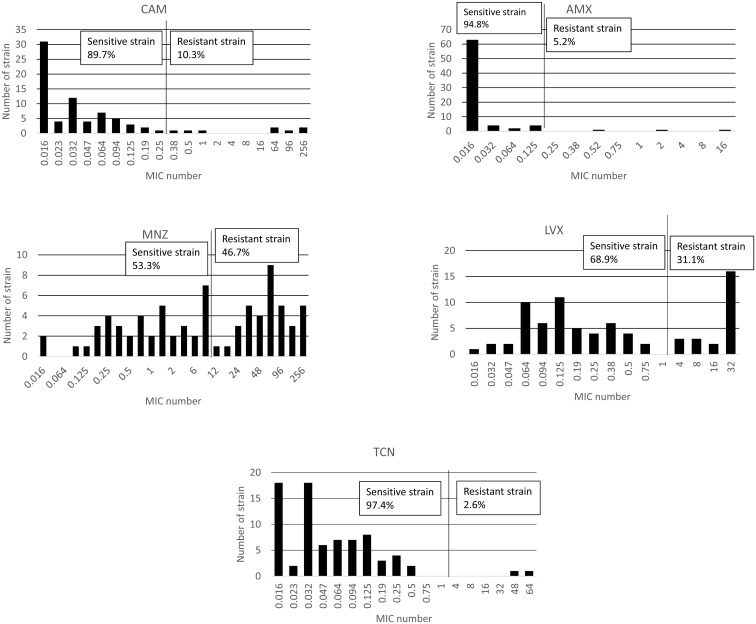
Overall, there were 28 strains that were sensitive to all antibiotics (36.4%). In contrast with the previous study in Indonesia [15], we found a low prevalence of CAM resistance (7/77, 9.1%, compared to 27.8% in the previous study). Low prevalence was also observed for AMX resistance (4/77, 5.2%) and TCN resistance (2/77, 2.6%, Table 2). In contrast, and in accordance with the trend of increasing resistance Asia [36], there was high rate of resistance to MNZ (36/77, 46.8%). Moreover, most strains were associated with MIC values ≥ 48 mg/L (26/36, 72.2%, Fig 1). In addition, we detected a high prevalence of LVX resistance (24/77, 31.2%) with a high distribution of MIC values that were predominantly ≥ 16 mg/L. The distribution of patient age and antimicrobial resistance of the isolates is shown in Table 2. Antibiotic resistance rate did not differ among different age groups (P = 0.37, 0.42, 0.07, 0.56, and 0.45 for AMX, CAM, MNZ, LVX, and TCN, respectively). There were no associations between antibiotic resistance and gender or clinical outcomes (P >0.05).
Table 2. Prevalence of antibiotic resistance of H. pylori isolates in Indonesia.
| Characteristic | N | Antibiotics (%) | ||||
|---|---|---|---|---|---|---|
| CAM | AMX | MNZ | LVX | TCN | ||
| Total | 77 | 7 (9.1) | 4 (5.2) | 36 (46.7) | 24 (31.2) | 2 (2.6) |
| Gender | ||||||
| Male | 38 | 3 (7.9) | 2 (5.2) | 21 (55.2) | 11 (28.9) | 1 (2.7) |
| Female | 39 | 4 (10.2) | 2 (5.1) | 15 (38.4) | 13 (33.3) | 1 (2.6) |
| Age Groups | ||||||
| 17–30 | 9 | 0 (0.0) | 0 (0.0) | 6 (66.6) | 3 (33.3) | 0 (0.0) |
| 31–40 | 10 | 2 (20.0) | 0 (0.0) | 6 (60.0) | 3 (30.0) | 1 (10.0) |
| 41–50 | 21 | 2 (9.1) | 1 (4.7) | 5 (22.7) | 4 (18.1) | 1 (4.5) |
| 51–60 | 25 | 1 (4.1) | 1 (4.0) | 14 (58.3) | 10 (41.6) | 0 (0.0) |
| >61 | 12 | 2 (16.6) | 2 (16.6) | 5 (41.6) | 4 (33.3) | 0 (0.0) |
| Clinical Outcome | ||||||
| Gastritis | 70 | 6 (8.9) | 4 (5.9) | 30 (44.7) | 21 (31.3) | 0 (0.0) |
| PUD | 7 | 0 (0.0) | 0 (0.0) | 3 (42.8) | 0 (0.0) | 0 (0.0) |
Abbreviations: AMX, amoxicillin; CAM, clarithromycin; MNZ, metronidazole; TCN, tetracycline; LVX, levofloxacin; PUD, peptic ulcer disease
Fig 1. Distribution of antibiotic MIC values in Indonesia.
The resistance rates to metronidazole and levofloxacin were high; in contrast, we revealed a low prevalence of clarithromycin, amoxicillin, and tetracycline resistance.
Antibiotic resistance rates according to location
The prevalence of antibiotic resistance based on location is shown in Table 3. Strains from Kalimantan were resistant to only two antibiotics. Strains obtained from Java and Bali Islands had CAM resistance rates greater than 15%, which is the permitted limit of CAM resistance recommended to be used for eradication therapy without checking the resistant rate [37,38]. AMX resistance was not detected in strains obtained from three islands (Bali, Java, and Kalimantan). Only Java Island showed signs of increased resistance to TCN (2/14, 14.3%). The highest LVX resistance was in strains isolated from Java and Sumatera Islands (50.0% and 44.4%, respectively). Strains isolated from Sumatera Island showed significantly higher MNZ resistance (88.9%) than those of other locations (50.0%, 42.9%, 33.3%, 30.8%, 21.4% and 20.0% for Java, Papua, Bali, Sulawesi, Timor, and Kalimantan, respectively, P = 0.003). Even after adjustment by age and sex in multivariate analysis, strains from Sumatera Island had significantly higher MNZ resistance rates than strains from other islands (OR = 7.9, OR = 10.5, OR = 15.3, OR = 28.3, OR = 17.5, and OR = 30.4 for Java, Papua, Bali, Sulawesi, Timor, and Kalimantan Island, respectively, P <0.05).
Table 3. Prevalence of H. pylori antibiotic resistance in Indonesia by location.
| Island | N | Resistance (%) | ||||
|---|---|---|---|---|---|---|
| CAM | AMX | MNZ* | LVX | TCN | ||
| Bali | 6 | 1 (16.7) | 0 (0.0) | 2 (33.3) | 1 (16.6) | 0 (0.0) |
| Java | 14 | 3 (21.4) | 0 (0.0) | 7 (50.0) | 7 (50.0) | 2 (14.3) |
| Kalimantan | 5 | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (20.0) | 0 (0.0) |
| Papua | 7 | 1 (14.3) | 1 (14.3) | 3 (42.9) | 2 (28.6) | 0 (0.0) |
| Sulawesi | 13 | 1 (7.7) | 1 (7.7) | 4 (30.8) | 2 (15.4) | 0 (0.0) |
| Sumatera | 18 | 1 (5.6) | 1 (5.6) | 16 (88.9) | 8 (44.4) | 0 (0.0) |
| Timor | 14 | 0 (0.0) | 1 (7.1) | 3 (21.4) | 3 (21.4) | 0 (0.0) |
Abbreviations: AMX, amoxicillin; CAM, clarithromycin; MNZ, metronidazole; TCN, tetracycline; LVX, levofloxacin.
* Strains isolated from Sumatera Island had significantly higher MNZ resistance rates than strains isolated from other islands even after adjusting for age and sex (P <0.05).
Antibiotic resistance rate according to ethnic group
The predominant ethnic groups from different islands and their association with antibiotic resistance rates are shown in Table 4. Strains isolated from Ambonese, Chinese, and Balinese individuals displayed a high prevalence of CAM resistance (50.0%, 20.0%, and 16.6%, respectively, Table 4).
Table 4. Prevalence of antibiotic resistance in H. pylori in Indonesia by ethnicity.
| Ethnicity | Island | N | Resistance (%) | ||||
|---|---|---|---|---|---|---|---|
| CAM | AMX | MNZ | LVX | TCN | |||
| Javanese | Java | 3 | 0 (0.0) | 0 (0.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) |
| Chinese** | Java and Kalimantan | 10 | 2 (20.0) | 0 (0.0) | 5 (50.0) | 5 (50.0) | 1 (10.0)* |
| Batak | Sumatera | 19 | 1 (5.2) | 1 (5.2) | 16 (84.2)* | 8 (42.1) | 0 (0.0) |
| Papuan | Papua | 7 | 1 (14.3) | 1 (14.3) | 3 (42.9) | 2 (28.6) | 0 (0.0) |
| Dayak | Kalimantan | 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Buginese | Sulawesi | 6 | 0 (0.0) | 1 (16.6) | 2 (33.3) | 0 (0.0) | 0 (0.0) |
| Balinese | Bali | 6 | 1 (16.6) | 0 (0.0) | 2 (33.3) | 1 (16.6) | 0 (0.0) |
| Timor | Timor | 15 | 0 (0.0) | 1 (6.6) | 3 (20.0) | 4 (26.6) | 0 (0.0) |
| Minahasanese | Sulawesi | 7 | 1 (14.3) | 0 (0.0) | 2 (28.6) | 2 (28.6) | 0 (0.0) |
| Ambonese*** | Java | 2 | 1 (50.0) | 0 (0.0) | 2 (100.0)* | 1 (50.0) | 1 (50.0)* |
Abbreviations: AMX, amoxicillin; CAM, clarithromycin; MNZ, metronidazole; TCN, tetracycline; LVX, levofloxacin.
* P < 0.05
** Chinese-Indonesians are dispersing throughout the archipelago. In this study, the strains were obtained from Chinese individuals who lived in Surabaya, Java Island, Pontianak, and Kalimantan Island.
*** Ambonese are the predominant group of Ambon Island in Maluku, an island group east of Sulawesi. In this study, the strains were obtained from Ambonese who lived in Surabaya, Java Island.
Only those from Buginese individuals showed AMX resistance >15%. Among three ethnicities with the highest prevalence of H. pylori in Indonesia [12], Papuan and Batak individuals were associated with a large number of antibiotic resistance types, including LVX, which is part of a second-line regimen, in contrast to strains isolated from Buginese individuals. Ambonese individuals were associated with higher rates of MNZ and TCN resistance than other ethnicities (both P = 0.01). Only strains from Dayak individuals were sensitive to all five antibiotics.
Multidrug resistance
No strain was resistant to all tested antibiotics (Table 5). Fifteen strains showed dual-drug resistance (11 strains for MNZ-LVX, two for CAM-LVX and one each for MNZ-AMX and LVX-AMX). Resistance to three antibiotics was observed in two (2.6%) strains that were isolated from Java Island; one was resistant to a combination of CAM, MNZ, and LVX. In addition, two strains (obtained from Sumatera and Java Island) were recognized as resistant to four drugs including LVX. Overall, Java (six strains) and Sumatera Island (seven strains) showed a higher prevalence of multidrug resistance than other locations. No differences were observed for clinical outcomes between single-drug and multidrug resistant infections (P = 0.53).
Table 5. Prevalence of multidrug resistance among Indonesian strains.
| Antibiotics | Total | Number of Patients (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Bali N = 6 | Kalimantan N = 5 | Java N = 14 | Papua N = 7 | Sulawesi N = 13 | Sumatera N = 18 | Timor N = 14 | ||
| Double drugs | ||||||||
| MNZ + AMX | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.7) | 0 (0.0) | 0 (0.0) |
| MNZ + LVX | 11 | 1 (16.7) | 0 (0.0) | 2 (14.3) | 1 (14.3) | 0 (0.0) | 6 (33.3) | 1 (7.1) |
| CAM + LVX | 2 | 0 (0.0) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 1 (7.7) | 0 (0.0) | 0 (0.0) |
| LVX + AMX | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Triple drugs | ||||||||
| CAM + MNZ + LVX | 1 | 0 (0.0) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| TCN + MNZ + LVX | 1 | 0 (0.0) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Quadruple drugs | ||||||||
| CAM + MNZ + AMX + LVX | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) |
| CAM + MNZ + TCN + LVX | 1 | 0 (0.0) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: AMX, amoxicillin; CAM, clarithromycin; MNZ, metronidazole; TCN, tetracycline; LVX, levofloxacin.
Virulence genes of Indonesian strains and antibiotic resistance types
Table 6 shows the association between virulence genes and the pattern of antibiotic resistance. Although there was a significant association between TCN resistance and cagA positivity (P = 0.004), it was only 2 resistant strains which were insufficient to make conclusion. jhp0562-positive/β-(1,3)galT-negative H. pylori types tended to have higher MNZ resistance than jhp0562-negative/β-(1,3)galT-positive types (P = 0.06). There was no association between other virulence factors and antibiotics resistance types (P >0.05).
Table 6. Association between virulence genes and antibiotic resistance pattern (%).
| Virulence Genes | CAM (%) | MNZ (%) | LVX (%) | |||
|---|---|---|---|---|---|---|
| S | R | S | R | S | R | |
| Strain (number) | 70 | 7 | 41 | 36 | 53 | 24 |
| cagA positive | 94.3 | 100 | 95.1 | 94.4 | 96.2 | 91.7 |
| cagA type | ||||||
| • East-Asian-type | 60.0 | 85.7 | 53.7 | 72.2 | 58.5 | 70.8 |
| • Western-type | 25.7 | 0.0 | 29.3 | 16.7 | 28.3 | 12.5 |
| • ABB-type | 8.6 | 14.3 | 12.1 | 5.6 | 9.4 | 8.3 |
| vacA s1/m1 | 65.7 | 85.7 | 75.6 | 58.3 | 67.9 | 66.7 |
| iceA1 genotype | 64.3 | 71.4 | 61.0 | 69.4 | 67.9 | 58.3 |
| jhp0562 genotype | 20.0 | 0.0 | 24.4 | 11.1 | 22.6 | 8.3* |
| oipA “on” | 91.4 | 100.0 | 90.2 | 94.4 | 92.5 | 91.7 |
| dupA negative | 82.9 | 100 | 80.5 | 88.9 | 88.7 | 75.0 |
* P = 0.06The data about the tetracycline and amoxicillin were excluded from the table, for which the resistant strains were 5 or less.
Abbreviations: AMX, amoxicillin; CAM, clarithromycin; MNZ, metronidazole; TCN, tetracycline; LVX, levofloxacin; iceA1 genotype, iceA1 positive/ iceA2 negative; jhp0562 genotype, jhp0562 positive/ β-(1,3)galT negative.
Detection of H. pylori gene mutations and association with antimicrobial resistance
Two MNZ-resistant strains did not show identifiable specific bands for rdxA after targeted PCR; therefore, 34 MNZ-resistant and 5-sensitive control Indonesian strains were analyzed in this study. DNA sequence analysis of rdxA from all MNZ-sensitive strains revealed intact reading frames (lacking nonsense mutations). Pairwise alignment identified that the MNZ-sensitive strains shared 94.9–97.0% identity with the reference strain, 26695. In contrast, most MNZ-resistant strains contained missense mutations (16/34, 47.1%), resulting in amino acid substitutions (Table 7). In addition, five strains had nonsense mutations that resulted in the introduction of a premature stop codon. Moreover, the rdxA alleles of nine strains (26.5%) contained nucleotide deletions and/or insertions that resulted in a translational frameshift.
Table 7. Mutation type of rdxA related to metronidazole resistance.
| No | Strain | MIC | Sites of Mutation | Type of mutation |
|---|---|---|---|---|
| 1 | Jayapura16 | 32 | R16C, A183V | Missense mutation |
| 2 | Kupang23 | >256 | E15Q, R16H, E32D, E34stop | Premature stop codon |
| 3 | Kupang 30 | 96 | R90K, P106S, V111A | Missense mutation |
| 4 | Kupang 41 | 64 | Q6H, R16C, R90K, E175Q, N178D, A183V | Missense mutation |
| 5 | Kupang 5 | 12 | A68V | Missense mutation |
| 6 | Kupang 73 | 48 | K64N, H97T, P106S, H127Y, Q197stop | Premature stop codon |
| 7 | Malang1 | 48 | 16frameshift | Frameshift mutation |
| 8 | Manado26 | 64 | None | Non-specific mutation |
| 9 | Manado 31 | 48 | 65frameshift | Frameshift mutation |
| 10 | Medan3 | 16 | 65frameshift | Frameshift mutation |
| 11 | Medan10 | >256 | H97Y, P106T, G122S | Missense mutation |
| 12 | Medan15 | 64 | P51S, A68T, E175Q, R176H | Missense mutation |
| 13 | Medan17 | 128 | R16C, A68T, G122S, C159Y | Missense mutation |
| 14 | Medan18 | 64 | R90K, 195frameshift | Frameshift mutation |
| 15 | Medan19 | 128 | Q6H, R16C, R90K, H97T, A118S, E175Q, A183V, V204I | Missense mutation |
| 16 | Medan20 | 64 | K64N, H97Y, P106T, G122S, 137frameshift | Frameshift mutation |
| 17 | Medan22 | 32 | R16H, K64N, H97Y, P106M, G122S, E175Q | Missense mutation |
| 18 | Medan23 | 24 | V55M, K64N, G122S, E175Q, A183V, | Missense mutation |
| 19 | Medan25 | 64 | M1V, R16C, K64N, A80T, H97Y, P106T, G122S, E175Q | Missense mutation |
| 20 | Medan27 | >256 | 4frameshift | Frameshift mutation |
| 21 | Medan28 | 48 | P51S, K64N, M102stop, P106L, 173frameshift | Frameshift mutation |
| 22 | Medan30 | 96 | None | Non-specific mutation |
| 23 | Medan31 | 96 | K64N, H97I, P106T, G122S, E175stop | Premature stop codon |
| 24 | Medan32 | 32 | R10I, A68V, G145R | Missense mutation |
| 25 | Medan33 | 96 | R10S, H97Y, P106T, G122S, 169frameshift | Frameshift mutation |
| 26 | Makassar52 | 64 | None | Non-specific mutation |
| 27 | Makassar55 | 96 | Q6H, R16H, M56I, R90K, H97Y, P166S, | Missense mutation |
| 28 | Pontianak20 | >256 | G163D, E173stop | Premature stop codon |
| 29 | Surabaya68 | 24 | R16H, P106T, V204I | Missense mutation |
| 30 | Surabaya69 | 32 | None | Non-specific mutation |
| 31 | Surabaya79 | 128 | Q6H, R16C, M56I, A80T, R90K, H97Y | Missense mutation |
| 32 | Surabaya137 | >256 | 23frameshift | Frameshift mutation |
| 33 | Surabaya283 | 32 | Q146stop | Premature stop codon |
| 34 | Surabaya304 | 64 | I182V | Missense mutation |
R16C means Cysteine replaced Arginine amino acid in the position 16; E34stop means stop codon replaced Glutamate amino acid in the position 34; 16frameshift means frameshift mutation in the position 16.
Among the five LVX-sensitive control Indonesian strains, no mutations in both gyrA and gyrB subunits were identified. In contrast, among 24 LVX-resistant strains, 22 had amino acid variants of the gyrA subunit (Table 8). Eleven LVX-resistant strains (45.8%) had an amino acid substitution at Asp-91, whereas six strains had an amino acid substitution at Asn-87. Both of these mutations (13/15, 86.7%) were predominantly associated with the highest MIC values observed (32 mg/L). In addition, two strains exhibited amino acid substitutions at Arg-484 and at Ser-479 in the gyrB subunit. When analyzing the association between these two genes and LVX-resistance, it was demonstrated the four strains (16.7%) had mutations in both gyrA and gyrB; 18/24 (75.0%) in gyrA only, and no strains had a mutation in gyrB only. We also found two of 24 (8.3%) LVX-resistant strains with no gyrA and gyrB mutations, which were associated with MIC values of 8 and 25 mg/L. There was no correlation between the degree of LVX resistance and the types or number of mutations in both genes.
Table 8. Mutation type of gyrA and gyrB related to levofloxacin resistance.
| No | Strain | MIC (mg/L) | gyrA mutation | gyrB mutation |
|---|---|---|---|---|
| 1 | Jayapura1 | >32 | N87K | None |
| 2 | Jayapura21 | >32 | N87K | None |
| 3 | Kupang2 | 4 | D91N, A129T | S479G |
| 4 | Kupang11 | >32 | D91Y | None |
| 5 | Kupang23 | >32 | A129T | S479G |
| 6 | Kupang41 | 8 | D91N | R484K |
| 7 | Malang1 | 16 | D91N | None |
| 8 | Manado18 | 8 | None | None |
| 9 | Manado20 | 8 | D91Y | None |
| 10 | Medan3 | >32 | N87I | None |
| 11 | Medan 10 | 25 | None | None |
| 12 | Medan15 | >32 | R140K, D192N | None |
| 13 | Medan 17 | 16 | D34N | None |
| 14 | Medan18 | 4 | D91G, D161N | None |
| 15 | Medan22 | >32 | D91N | None |
| 16 | Medan 23 | 4 | D34Y, R140K | None |
| 17 | Medan30 | >32 | D91N | None |
| 18 | Pontianak50 | >32 | D91G | None |
| 19 | Surabaya71 | >32 | D91N | None |
| 20 | Surabaya79 | >32 | N87Y | R484K |
| 21 | Surabaya137 | >32 | N87K | None |
| 22 | Surabaya151 | >32 | N87K | None |
| 23 | Surabaya283 | >32 | D91Y | None |
| 24 | Surabaya304 | >32 | D91G | None |
N87K means Lysine replaced Asparagine amino acid in the position 87.
Based on 23Sr RNA sequencing in the seven CAM-resistant strains, three (42.9%) exhibited an interesting point mutation, specifically A2143G (Table 9), and two of these strains were associated with high MIC values (64 and 96 mg/L). In contrast, we found minimal nucleotide variation on the one control Indonesian CAM-sensitive strain. Interestingly, there was no specific mutation in the strain associated with the highest MIC values (Surabaya137). Based on the previous report [21], we performed next generation sequencing of the Surabaya137 strain (average sequencing depth was 46.9× and overall %GC was 38.4). Using strain 26695 and the control CAM-sensitive strain Medan27, we could not identify any mutations in full-length 23S rRNA. Although we found point mutations, (T189C and T198C) in hp1048 (infB), these were silent mutations. In contrast, compared to strains 29965 and Medan27, we confirmed the involvement of novel mutated sequences in hp1314 (rpl22) including 19 bp deletions at position 535 (Table 9).
Table 9. Gene mutations related to clarithromycin resistance.
| No | Strain | MIC (mg/L) | 23SrRNA | rpl22 (hp1314) | infB (hp1048) |
|---|---|---|---|---|---|
| 1 | Jayapura6 | 96 | A2143G | - | - |
| 2 | Kupang64 | 32 | None | - | - |
| 3 | Manado20 | 64 | None | - | - |
| 4 | Medan15 | 0.5 | None | - | - |
| 5 | Surabaya71 | 64 | A2143G | - | - |
| 6 | Surabaya137 | >256 | None | None | A352G, C361T, G374A, A391G, C406A, T437C, G529A, C634D, G635T, G671A, T672C, A673T, 535del, T946C, G995T, G2245A |
| 7 | Surabaya304 | 1 | A2143G, G2172T | - | - |
A2143G means Guanine replaced Adenosine in the position 2143.
Nucleotide sequencing
Nucleotide sequence data reported are available under the DDBJ accession numbers LC174777-LC174814 and LC175851 (rdxA), LC174815-LC174843 (gyrB), LC174844-LC174852 and LC174855-LC174874 (gyrA), LC175228-LC175232 and LC175234-LC175236 (23srRNA), LC175237-LC175238 (infB), LC175239-LC175240 (rpl22), LC174875-LC174908 (cagA) and LC175814-LC175850 (oipA).
Discussion
Southeast Asia is a region with low CAM resistance rates [2]. The previous studies reported that CAM resistance rates among H. pylori isolates of Thailand and Malaysia, neighboring countries, were very low (3.7% and 5.2%, respectively) [18,39]. In agreement with this data, we revealed a low prevalence of CAM resistance. It is suggested that CAM-based triple therapy might still be useful as an initial treatment of H. pylori infection in Indonesia. However, further analysis based on the ethnicity of the host revealed that Ambonese, Chinese, and Balinese individuals were associated with strains with CAM resistance rates, exceeding those of the recommendations from the Maastricht guidelines (>15–20%) [37,38]. Furthermore, breakdown based on location showed that strains isolated from Bali and Java Island demonstrated high CAM resistance. Our report was consistent with a previous study in Indonesia, in which isolates from Java Island had a CAM resistance rate of 27.8% [15]. A recent report from Singapore, a neighboring country, also demonstrated a changing CAM-resistance profile over 15 years (7.9–17.1%) [40]. Therefore, triple therapy with CAM should be utilized with caution or should be culture-based in some areas, and involving particular ethnicities, in Indonesia.
We identified a mononucleotide substitution from A to G at site 2143 in CAM-resistant strains. Moreover, this was associated with high CAM MIC values (strains Jayapura6 and Surabaya304). A previous study reported the A2143G mutation has a much stronger impact on CAM resistance than the A2142G and A2142C mutations [41]. However, we failed to identify T2183C and A2223G mutations, which are frequently found to be the cause of CAM resistance in Asian, rather than in Western, countries [19]. Our current study also confirmed that hp1314 (rpl22) mutations are associated with CAM resistance [21], although we identified different mutation types compared to those of Vietnamese strains (3 bp deletion and 9 bp insertion in rpl22). In contrast to the current study, we previously demonstrated that a single mutation in infB or rpl22 resulted in low MIC values and showed the involvement of infB, rpl22, and 23S rRNA in higher MIC values. Therefore, we suggested that rpl22 mutations might not only result in synergistic effects, but also could be independent causes of CAM resistance.
Aside from isolates from Buginese individuals, for which the AMX resistance rate was greater than 15%, in general, our study revealed that Indonesian isolates had low resistance to AMX. We found only one resistant strain each from four islands. This is in contrast to the previous report in Indonesia [15], but similar to other typical Southeast Asian antibiotic resistance patterns [2]. This distinction might be based on differences in research facility reproducibility, caused by a lack of standardized testing protocols or different regional practices. Thus, AMX might be useful as a secondary antibiotic, for use in cases exhibiting poor responses to CAM-based triple therapy in Indonesia. This drug has been the first antibiotic utilized for H. pylori treatment because of the assumed absence of resistance [42]. Nevertheless, increasing primary AMX-resistance rates have been reported in South Korea (7.1–18.5%) [43], and in India and Pakistan (72.5% and 37.0%, respectively) [44,45]. Additionally, AMX can be obtained without prescription and has been one of the most commonly used antibiotics in Indonesia in recent years. A strict policy for antibiotic use is necessary to counteract the failure of primary antibiotic treatment for H. pylori in Indonesia.
TCN is an anti-microbial, to which resistance is occasionally experienced. The reason for this is that to achieve resistance, three point changes are required [9]. In agreement with most countries [46], we detected low TCN resistance in Indonesian strains. Although Ambonese individuals were associated with significantly higher resistance than strains isolated from other locations, the observation of only two resistant strains is of some concern. This is the first study to examine the TCN resistance rate in Indonesia. Due to the important role of TCN as a salvage quadruple therapy, our data are vital for guiding second line regimens for eliminating H. pylori infections.
The resistance rate for MNZ was high in Indonesia. Moreover, two H. pylori groups, specifically, Batak and Papua, had rates higher than the preferential number outlined by the Maastricht III Consensus Report (>40%) [37]. However, in Papua, the number of strains was small. In Asia, only Japan, Thailand, and Malaysia have populations associated with <40% MNZ resistance [2]. Therefore, regimens including MNZ are not suitable and should not be chosen as first-line treatments in Indonesia. We distinguished diverse mutations involving rdxA in most MNZ-resistant strains. Complex genetic events (insertions, deletions, and missense and frameshift mutations) were simultaneously present in the strains. Previously it was shown that only 11.8% of MNZ-resistant strains did not harbor any mutation in rdxA, and this was probably related to frxA [22], rpsU [47], dppA or dapF [48] alterations. Conversely, MNZ-sensitive strains had high resemblance against reference strains. Because of different mutations in rdxA, molecular antibiotic susceptibility testing is not applicable for metronidazole.
LVX was proposed a decade ago based on its role in salvage treatment regimens after the failure of clarithromycin-based treatments [49]. Nevertheless, the frequency of LVX resistance is by all accounts expanding around the world, which might diminish the efficacy LVX-based treatment regimens [50,51]. Our findings showed a high prevalence of primary resistance to LVX (33.1%). Similarly, LVX is not sufficiently effective for inclusion in treatment regimens in Indonesia. Point mutations in the quinolones resistance-determining region (QRDR) of gyrA abrogate binding between the antibiotic and the enzyme, resulting in bacterial antibiotic resistance [52]. In the present study we found the predominant mutations at amino acid 87 (Asn to Lys, Tyr, or Ile) and 91 (Asp to Asn, Gly, or Tyr), which have previously been described [18,53,54]. Additionally, these point mutations were present at a high frequency (86.7%) and were associated with high MIC values. Although we found amino acid substitutions at Arg-484 and at Ser-479 in gyrB subunits, they had were associated with the gyrA mutations in amino acid 87/91, which most likely minimizes the influence of these gyrB mutations in Indonesian LVX-resistant strains. Therefore, in Indonesia, screening for gyrA mutations could be adequate for recognizing LVX-resistant strains.
The number of H. pylori strains demonstrating triple or quadruple resistance in this study appeared to be a serious challenge in the fight against infections and a hindrance to the success of eradication regimens. Java and Sumatera Island could be two locations with a higher risk for H. pylori treatment failure in Indonesia due to the associated high antibiotic resistance type area. Several regions of Indonesia are associated with a high prevalence of H. pylori infections; increased resistance to the antibiotics used to treat this bacterium might result in increased recurrence rates. It is therefore important to perform susceptibility-guided re-treatment using a case-by-case approach, if available, in patients demonstrating initial treatment failure. Recently, a high accuracy DNA strip genotyping test was developed combining PCR and hybridization that permits the molecular identification of mutations in gyrA and 23S rRNA within 6 h [55]. Our genotypic resistance results are vital to guide follow-up treatment protocols after first-line regimens fail.
The number of samples in this study was relatively low, which certainly suggests the major limitations of this study. We considered that a larger sample size among region is necessary to elucidate the prevalence of H. pylori antibiotic resistance in Indonesia. The current study is a pilot study for a larger survey and we are now continuing the similar surveys to that performed in this study, to increase sample numbers and expand geographically to other islands. In addition, we only determined the presence of well-known genetic mutations associated with antibiotic resistance. H. pylori contains approximately 1,600 genes, and it is likely that only a fraction of genomic changes that are related to drug resistance have been identified. Next-generation sequencing technology is beneficial in that it can yield enormous numbers of DNA sequences in less time and at lower cost, which could be used to clarify the evolution and pathogenicity of H. pylori. To guide antibiotic regimens in Indonesia, the locations were perhaps more important than the ethnicities of the patients. Most antibiotic resistance is related to local antibiotic consumption [56]. Moreover, such resistance is primarily due to the H. pylori genotype, rather than the human genotype.
Conclusions
The rates of resistance to MNZ and LVX were high in Indonesia, which implies that MNZ-, and LVX-based triple therapies are not valuable for first-line treatment of H. pylori in Indonesia. In general, we revealed a low prevalence of CAM, AMX, and TCN resistance. Nevertheless, individuals of several ethnicities were shown to be associated with a high prevalence of CAM, TC, and MNZ resistance. In this manner, CAM- or MNZ-based triple therapy should be used with caution or should be demographic-based in some regions of Indonesia. National epidemiological surveillance of resistance rates is required to further determine optimal treatment strategies in Indonesia.
Data Availability
Nucleotide sequence data reported are available under the DDBJ accession numbers LC174777-LC174814 and LC175851 (rdxA), LC174815-LC174843 (gyrB), LC174844-LC174852 and LC174855-LC174874 (gyrA), LC175228-LC175232 and LC175234-LC175236 (23srRNA), LC175237-LC175238 (infB), LC175239-LC175240 (rpl22), LC174875-LC174908 (cagA) and LC175814-LC175850 (oipA).
Funding Statement
This study was funded by grants from the National Institutes of Health (DK62813) and the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (24406015, 24659200, 25293104, 26640114,15H02657 and 221S0002) (YY). It was also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Young Researcher Overseas Visits (YY) and the Strategic Funds for the Promotion of Science and Technology from Japan Science and Technology Agency (JST) (YY).
References
- 1.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002; 2: 28–37. 10.1038/nrc703 [DOI] [PubMed] [Google Scholar]
- 2.Miftahussurur M, Yamaoka Y. Appropriate first-line regimens to combat Helicobacter pylori antibiotic resistance: an Asian perspective. Molecules. 2015; 20: 6068–6092. 10.3390/molecules20046068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002; 97: 1106–1112. 10.1111/j.1572-0241.2002.05663.x [DOI] [PubMed] [Google Scholar]
- 4.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010; 25: 479–486. 10.1111/j.1440-1746.2009.06188.x [DOI] [PubMed] [Google Scholar]
- 5.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24: 1587–1600. 10.1111/j.1440-1746.2009.05982.x [DOI] [PubMed] [Google Scholar]
- 6.Kim SG, Jung HK, Lee HL, Jang JY, Lee H, Kim CG, et al. [Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition]. Korean J Gastroenterol. 2013; 62: 3–26. [DOI] [PubMed] [Google Scholar]
- 7.Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013; 132: 1272–1276. 10.1002/ijc.27965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinese Society of Gastroenterology CSGoHp, Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013; 14: 211–221. 10.1111/1751-2980.12034 [DOI] [PubMed] [Google Scholar]
- 9.Megraud F. The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therap Adv Gastroenterol. 2012; 5: 103–109. 10.1177/1756283X11432492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdullah AA, Abdullah M, Fauzi A, Syam AF, Simadibrata M, Makmun D. The effectiveness of endoscopic retrograde cholangiopancreatography in the management of patients with jaundice at Cipto Mangunkusumo Hospital, Jakarta. Acta Med Indones. 2012; 44: 298–303. [PubMed] [Google Scholar]
- 11.Miftahussurur M, Shiota S, Suzuki R, Matsuda M, Uchida T, Kido Y, et al. Identification of Helicobacter pylori infection in symptomatic patients in Surabaya, Indonesia, using five diagnostic tests. Epidemiol Infect. 2015; 143: 986–996. 10.1017/S095026881400154X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syam AF, Miftahussurur M, Makmun D, Nusi IA, Zain LH, Zulkhairi, et al. Risk Factors and Prevalence of Helicobacter pylori in Five Largest Islands of Indonesia: A Preliminary Study. PLoS One. 2015; 10: e0140186 10.1371/journal.pone.0140186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miftahussurur M, Syam AF, Makmun D, Nusi IA, Zein LH, Zulkhairi, et al. Helicobacter pylori virulence genes in the five largest islands of Indonesia. Gut Pathog. 2015; 7: 26 10.1186/s13099-015-0072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015; 64: 1353–1367. 10.1136/gutjnl-2015-309252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumala W, Rani A. Patterns of Helicobacter pylori isolate resistance to fluoroquinolones, amoxicillin, clarithromycin and metronidazoles. Southeast Asian J Trop Med Public Health. 2006; 37: 970–974. [PubMed] [Google Scholar]
- 16.Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, Takahashi S, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007; 45: 4006–4010. 10.1128/JCM.00740-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuadrado-Lavin A, Salcines-Caviedes JR, Carrascosa MF, Mellado P, Monteagudo I, Llorca J, et al. Antimicrobial susceptibility of Helicobacter pylori to six antibiotics currently used in Spain. J Antimicrob Chemother. 2012; 67: 170–173. [DOI] [PubMed] [Google Scholar]
- 18.Teh X, Khosravi Y, Lee WC, Leow AH, Loke MF, Vadivelu J, et al. Functional and molecular surveillance of Helicobacter pylori antibiotic resistance in Kuala Lumpur. PLoS One. 2014; 9: e101481 10.1371/journal.pone.0101481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ierardi E, Giorgio F, Losurdo G, Di Leo A, Principi M. How antibiotic resistances could change Helicobacter pylori treatment: A matter of geography? World J Gastroenterol. 2013; 19: 8168–8180. 10.3748/wjg.v19.i45.8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oleastro M, Ménard A, Santos A, Lamouliatte H, Monteiro L, Barthélémy P, et al. Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori. J Clin Microbiol. 2003; 41: 397–402. 10.1128/JCM.41.1.397-402.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binh TT, Shiota S, Suzuki R, Matsuda M, Trang TT, Kwon DH, et al. Discovery of novel mutations for clarithromycin resistance in Helicobacter pylori by using next-generation sequencing. J Antimicrob Chemother. 2014; 69: 1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerrits MM, van der Wouden EJ, Bax DA, van Zwet AA, van Vliet AH, de Jong A, et al. Role of the rdxA and frxA genes in oxygen-dependent metronidazole resistance of Helicobacter pylori. J Med Microbiol. 2004; 53: 1123–1128. 10.1099/jmm.0.45701-0 [DOI] [PubMed] [Google Scholar]
- 23.Rimbara E, Noguchi N, Kawai T, Sasatsu M. Fluoroquinolone resistance in Helicobacter pylori: role of mutations at position 87 and 91 of GyrA on the level of resistance and identification of a resistance conferring mutation in GyrB. Helicobacter. 2012; 17: 36–42. [DOI] [PubMed] [Google Scholar]
- 24.Kwon DH, Pena JA, Osato MS, Fox JG, Graham DY, Versalovic J. Frameshift mutations in rdxA and metronidazole resistance in North American Helicobacter pylori isolates. J Antimicrob Chemother. 2000; 46: 793–796. [DOI] [PubMed] [Google Scholar]
- 25.Wang LH, Cheng H, Hu FL, Li J. Distribution of gyrA mutations in fluoroquinolone-resistant Helicobacter pylori strains. World J Gastroenterol. 2010; 16: 2272–2277. 10.3748/wjg.v16.i18.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho SL, Tan EL, Sam CK, Goh KL. Clarithromycin resistance and point mutations in the 23S rRNA gene in Helicobacter pylori isolates from Malaysia. J Dig Dis. 2010; 11: 101–105. 10.1111/j.1751-2980.2010.00423.x [DOI] [PubMed] [Google Scholar]
- 27.Matsunari O, Shiota S, Suzuki R, Watada M, Kinjo N, Murakami K, et al. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol. 2012; 50: 876–883. 10.1128/JCM.05562-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida T, Nguyen LT, Takayama A, Okimoto T, Kodama M, Murakami K, et al. Analysis of virulence factors of Helicobacter pylori isolated from a Vietnamese population. BMC Microbiol. 2009; 9: 175 10.1186/1471-2180-9-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaoka Y, Kikuchi S, el-Zimaity H, Gutierrez O, Osato M, Graham D. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002; 123: 414–424. [DOI] [PubMed] [Google Scholar]
- 30.Oleastro M, Monteiro L, Lehours P, Mégraud F, Ménard A. Identification of markers for Helicobacter pylori strains isolated from children with peptic ulcer disease by suppressive subtractive hybridization. Infect Immun. 2006; 74: 4064–4074. 10.1128/IAI.00123-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atherton JC, Cao P, Peek RM, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995; 270: 17771–17777. [DOI] [PubMed] [Google Scholar]
- 32.Yamaoka Y, El-Zimaity H, Gutierrez O, Figura N, Kim J, Kodama T, et al. Relationship between the cagA 3' repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology. 1999; 117: 342–349. [DOI] [PubMed] [Google Scholar]
- 33.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999; 37: 2274–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi A, Shiota S, Matsunari O, Watada M, Suzuki R, Nakachi S, et al. Intact Long-Type dupA as a Marker for Gastroduodenal Diseases in Okinawan Subpopulation, Japan. Helicobacter. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, et al. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007; 133: 926–936. 10.1053/j.gastro.2007.06.056 [DOI] [PubMed] [Google Scholar]
- 36.De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010; 19: 409–414. [PubMed] [Google Scholar]
- 37.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007; 56: 772–781. 10.1136/gut.2006.101634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut. 2012; 61: 646–664. 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- 39.Vilaichone RK, Gumnarai P, Ratanachu-Ek T, Mahachai V. Nationwide survey of Helicobacter pylori antibiotic resistance in Thailand. Diagn Microbiol Infect Dis. 2013; 77: 346–349. 10.1016/j.diagmicrobio.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 40.Ang TL, Fock KM, Ang D, Kwek AB, Teo EK, Dhamodaran S. The Changing Profile of Helicobacter pylori Antibiotic Resistance in Singapore: A 15-Year Study. Helicobacter. 2016. [DOI] [PubMed] [Google Scholar]
- 41.De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani L, Burattini O, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006; 144: 94–100. [DOI] [PubMed] [Google Scholar]
- 42.Francesco VD, Zullo A, Hassan C, Giorgio F, Rosania R, Ierardi E. Mechanisms of Helicobacter pylori antibiotic resistance: An updated appraisal. World J Gastrointest Pathophysiol. 2011; 2: 35–41. 10.4291/wjgp.v2.i3.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JW, Kim N, Kim JM, Nam RH, Chang H, Kim JY, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18: 206–214. 10.1111/hel.12031 [DOI] [PubMed] [Google Scholar]
- 44.Pandya HB, Agravat HH, Patel JS, Sodagar N. Emerging antimicrobial resistance pattern of Helicobacter pylori in central Gujarat. Indian J Med Microbiol. 2014; 32: 408–413. 10.4103/0255-0857.142256 [DOI] [PubMed] [Google Scholar]
- 45.Khan A, Farooqui A, Manzoor H, Akhtar SS, Quraishy MS, Kazmi SU. Antibiotic resistance and cagA gene correlation: a looming crisis of Helicobacter pylori. World J Gastroenterol. 2012; 18: 2245–2252. 10.3748/wjg.v18.i18.2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Megraud F. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology. 1998; 115: 1278–1282. [DOI] [PubMed] [Google Scholar]
- 47.Binh TT, Suzuki R, Trang TT, Kwon DH, Yamaoka Y. Search for Novel Candidate Mutations for Metronidazole Resistance in Helicobacter pylori Using Next-Generation Sequencing. Antimicrob Agents Chemother. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li AQ, Dai N, Yan J, Zhu YL. Screening for metronidazole-resistance associated gene fragments of H pylori by suppression subtractive hybridization. World J Gastroenterol. 2007; 13: 1847–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cammarota G, Cianci R, Cannizzaro O, Cuoco L, Pirozzi G, Gasbarrini A, et al. Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000; 14: 1339–1343. [DOI] [PubMed] [Google Scholar]
- 50.Glocker E, Stueger HP, Kist M. Quinolone resistance in Helicobacter pylori isolates in Germany. Antimicrob Agents Chemother. 2007; 51: 346–349. 10.1128/AAC.00614-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto Y, Miki I, Aoyama N, Shirasaka D, Watanabe Y, Morita Y, et al. Levofloxacin- versus metronidazole-based rescue therapy for H. pylori infection in Japan. Dig Liver Dis. 2005; 37: 821–825. 10.1016/j.dld.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 52.Nishizawa T, Suzuki H, Hibi T. Quinolone-Based Third-Line Therapy for Helicobacter pylori Eradication. J Clin Biochem Nutr. 2009; 44: 119–124. 10.3164/jcbn.08-220R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishizawa T, Suzuki H, Kurabayashi K, Masaoka T, Muraoka H, Mori M, et al. Gatifloxacin resistance and mutations in gyra after unsuccessful Helicobacter pylori eradication in Japan. Antimicrob Agents Chemother. 2006; 50: 1538–1540. 10.1128/AAC.50.4.1538-1540.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia M, Raymond J, Garnier M, Cremniter J, Burucoa C. Distribution of spontaneous gyrA mutations in 97 fluoroquinolone-resistant Helicobacter pylori isolates collected in France. Antimicrob Agents Chemother. 2012; 56: 550–551. 10.1128/AAC.05243-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cambau E, Allerheiligen V, Coulon C, Corbel C, Lascols C, Deforges L, et al. Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori. J Clin Microbiol. 2009; 47: 3600–3607. 10.1128/JCM.00744-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013; 62: 34–42. 10.1136/gutjnl-2012-302254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nucleotide sequence data reported are available under the DDBJ accession numbers LC174777-LC174814 and LC175851 (rdxA), LC174815-LC174843 (gyrB), LC174844-LC174852 and LC174855-LC174874 (gyrA), LC175228-LC175232 and LC175234-LC175236 (23srRNA), LC175237-LC175238 (infB), LC175239-LC175240 (rpl22), LC174875-LC174908 (cagA) and LC175814-LC175850 (oipA).