Abstract
Members of several families of cell surface and secreted proteins bind glycosaminoglycans (GAGs), the structurally heterogeneous polysaccharides found on proteoglycans. To understand the physiological significance of the interactions of proteins with GAGs, it is critical that relationships between GAG structure and binding be analyzed. It is particularly important that interactions depending on common structural features of GAGs (e.g., size, charge density, and disaccharide repeat unit) be distinguished from those mediated by specific sequences of carbohydrate modification. Gathering the information needed to make such distinctions has so far been difficult, however, partly because structurally homogeneous samples of GAGs are lacking but also because of technical difficulties associated with performing and interpreting assays of protein-GAG binding. We describe an electrophoretic method useful for both measuring affinity and evaluating structural selectivity in protein-GAG binding. Data are presented on the binding of the GAG heparin to the protease inhibitor antithrombin III, the acidic and basic fibroblast growth factors, and the extracellular matrix protein fibronectin. Results obtained with fibronectin are consistent with a model in which high-affinity binding (Kd approximately 34 nM) is mediated through the recognition of specific carbohydrate sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeler D., Rosenberg R., Jordan R. Fractionation of low molecular weight heparin species and their interaction with antithrombin. J Biol Chem. 1979 Apr 25;254(8):2902–2913. [PubMed] [Google Scholar]
- Björk I., Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982 Oct 29;48(3):161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- Danielsson A., Björk I. Binding to antithrombin of heparin fractions with different molecular weights. Biochem J. 1981 Feb 1;193(2):427–433. doi: 10.1042/bj1930427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosso M., Cappelletti R., Viti M., Vannucchi S., Chiarugi V. Binding of the basement-membrane glycoprotein laminin to glycosaminoglycans. An affinity-chromatography study. Biochem J. 1981 Dec 1;199(3):699–704. doi: 10.1042/bj1990699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P. J., Silbert C. K., Silbert J. E. Effects of heparan sulfate removal on attachment and reattachment of fibroblasts and endothelial cells. Biochemistry. 1986 Jan 28;25(2):405–410. doi: 10.1021/bi00350a020. [DOI] [PubMed] [Google Scholar]
- Glabe C. G., Harty P. K., Rosen S. D. Preparation and properties of fluorescent polysaccharides. Anal Biochem. 1983 Apr 15;130(2):287–294. doi: 10.1016/0003-2697(83)90590-0. [DOI] [PubMed] [Google Scholar]
- Homandberg G. A. Characterization of the interactions of an amino-terminal fibronectin fragment with the native molecule: implications for polymerization of fibronectin. Biopolymers. 1987 Dec;26(12):2087–2098. doi: 10.1002/bip.360261208. [DOI] [PubMed] [Google Scholar]
- Horejsí V. Review: Affinity electrophoresis. Anal Biochem. 1981 Mar 15;112(1):1–8. doi: 10.1016/0003-2697(81)90252-9. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Busch S. J., Cardin A. D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991 Apr;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Johansson S., Hök M. Heparin enhances the rate of binding of fibronectin to collagen. Biochem J. 1980 May 1;187(2):521–524. doi: 10.1042/bj1870521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R. E., Oosta G. M., Gardner W. T., Rosenberg R. D. The binding of low molecular weight heparin to hemostatic enzymes. J Biol Chem. 1980 Nov 10;255(21):10073–10080. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Laterra J., Ansbacher R., Culp L. A. Glycosaminoglycans that bind cold-insoluble globulin in cell-substratum adhesion sites of murine fibroblasts. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6662–6666. doi: 10.1073/pnas.77.11.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C., Tengblad A., Thunberg L., Hök M., Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978 Nov 1;175(2):691–701. doi: 10.1042/bj1750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Thunberg L., Bäckström G., Riesenfeld J., Nordling K., Björk I. Extension and structural variability of the antithrombin-binding sequence in heparin. J Biol Chem. 1984 Oct 25;259(20):12368–12376. [PubMed] [Google Scholar]
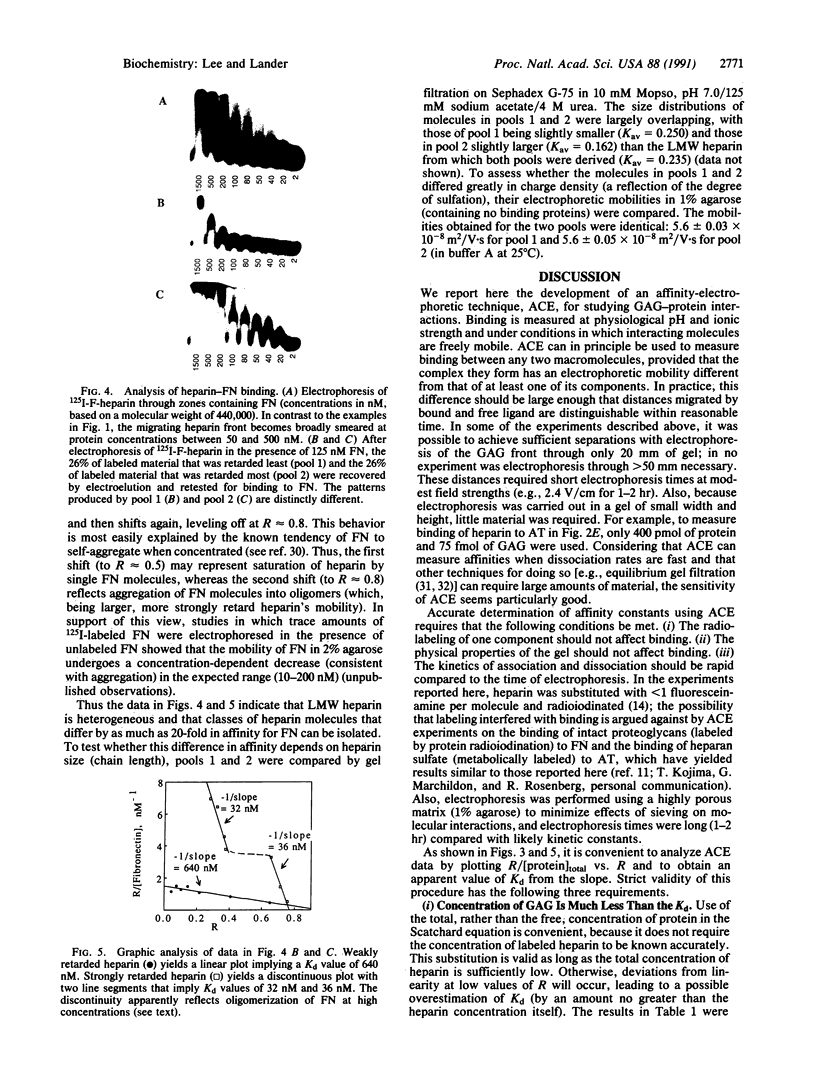
- Lobb R. R., Fett J. W. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 1984 Dec 18;23(26):6295–6299. doi: 10.1021/bi00321a001. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Harper J. W., Fett J. W. Purification of heparin-binding growth factors. Anal Biochem. 1986 Apr;154(1):1–14. doi: 10.1016/0003-2697(86)90487-2. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987 Apr;131(1):123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Osterlund E., Eronen I., Osterlund K., Vuento M. Secondary structure of human plasma fibronectin: conformational change induced by calf alveolar heparan sulfates. Biochemistry. 1985 May 21;24(11):2661–2667. doi: 10.1021/bi00332a011. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Jordan R. E., Favreau L. V., Lam L. H. Highly active heparin species with multiple binding sites for antithrombin. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1319–1324. doi: 10.1016/0006-291x(79)90260-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Oosta G. M., Jordan R. E., Gardner W. T. The interaction of heparin with thrombin and antithrombin. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1200–1208. doi: 10.1016/0006-291x(80)90079-0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Complexing of fibronectin glycosaminoglycans and collagen. Biochim Biophys Acta. 1980 Aug 13;631(2):350–358. doi: 10.1016/0304-4165(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Saunders S., Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol. 1988 Feb;106(2):423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz A. P., McCarthy J. B., Charonis A. S., Furcht L. T. Localization of three distinct heparin-binding domains of laminin by monoclonal antibodies. J Biol Chem. 1988 Apr 5;263(10):4861–4868. [PubMed] [Google Scholar]
- Smith J. W., Knauer D. J. Ligand blotting with 125I-fluoresceinamine-heparin. Anal Biochem. 1987 Jan;160(1):105–114. doi: 10.1016/0003-2697(87)90619-1. [DOI] [PubMed] [Google Scholar]
- Stamatoglou S. C., Keller J. M. Interactions of cellular glycosaminoglycans with plasma fibronectin and collagen. Biochim Biophys Acta. 1982 Oct 28;719(1):90–97. doi: 10.1016/0304-4165(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Walicke P. A., Feige J. J., Baird A. Characterization of the neuronal receptor for basic fibroblast growth factor and comparison to receptors on mesenchymal cells. J Biol Chem. 1989 Mar 5;264(7):4120–4126. [PubMed] [Google Scholar]
- Welsh E. J., Frangou S. A., Morris E. R., Rees D. A., Chavin S. I. Tyrosine optical activity as a probe of the conformation and interactions of fibronectin. Biopolymers. 1983 Mar;22(3):821–831. doi: 10.1002/bip.360220305. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Kimata K., Pratt R. M. Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem. 1980 Jul 10;255(13):6055–6063. [PubMed] [Google Scholar]