Abstract
With increasing demand for donor organs for transplantation, machine perfusion (MP) promises to be a beneficial alternative preservation method for donor livers, particularly those considered to be of suboptimal quality, also known as extended criteria donor livers. Over the last decade, numerous studies researching MP of donor livers have been published and incredible advances have been made in both experimental and clinical research in this area. With numerous research groups working on MP, various techniques are being explored, often applying different nomenclature. The objective of this review is to catalog the differences observed in the nomenclature used in the current literature to denote various MP techniques and the manner in which methodology is reported. From this analysis, we propose a standardization of nomenclature on liver MP to maximize consistency and to enable reliable comparison and meta‐analyses of studies. In addition, we propose a standardized set of guidelines for reporting the methodology of future studies on liver MP that will facilitate comparison as well as clinical implementation of liver MP procedures.
Keywords: clinical research/practice, liver transplantation/hepatology, donors and donation: extended criteria, guidelines, organ perfusion and preservation
Short abstract
The authors report on the current nomenclature and methodology of machine perfusion of donor livers and propose the establishment of standardized nomenclature and data reporting for reliable and valid comparison of future studies.
Abbreviations
- MP
machine perfusion
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- SCS
static cold storage
Introduction
In an effort to meet the demand for donor organs needed for transplantation, livers considered to be of suboptimal quality and function are increasingly being transplanted. Given the increased vulnerability of these organs and the potential injury incurred during procurement and storage/transportation, machine perfusion (MP) is a promising alternative to static cold storage (SCS), the current standard of care in donor liver preservation. Following the first successful series of extra‐corporeally perfused canine liver grafts performed by Brettschneider and Starzl et al. in 1967 1, MP has been explored as a method to achieve the preservation of donor livers under conditions simulating normal in vivo physiology in an attempt to minimize ischemia‐related injury associated with SCS. Research into MP has established three major benefits: the capability to preserve donor organs while providing them with oxygen and nutrients at various temperatures (optimal and prolonged preservation); the ability to recondition and optimize the function of donor organs, particularly extended criteria organs, with, for instance, oxygen perfsufflation, de‐fatting techniques for steatotic livers and pharmaceutical intervention (organ resuscitation and function recovery); and lastly, to provide the possibility of testing the function and viability of the organ prior to transplantation (ex situ viability testing) by MP at 37°C.
With the number of publications on liver MP to date exceeding 500, the last 10 years has seen an incredible advancement in both experimental and clinical research into donor liver MP. Several groups have been exploring different methods of MP with the major technique differences relating to the temperatures used and the provision of oxygen and whether the technique is flow or pressure controlled. Given that MP is a nascent technology with many technical aspects continuing to be explored, adapted and improved, the publications on MP have exhibited great discrepancies. These include the nomenclature used to describe the different MP techniques (abbreviations included), the temperatures considered to be hypo‐, subnormo, or normothermic and the manner in which certain details of the methodology are reported. The absence of standardized nomenclature and guidelines for reporting technical details pertaining to MP gives rise to the relatively large variation that exists among studies. This makes it difficult to compare different studies, perform meta‐analyses and, in some cases, attempt to reexecute the methodology used.
With the number of clinical studies on MP of donor livers rapidly increasing, it is important that a consensus is reached on the nomenclature applied and which necessary aspects of the methodology should be included in a paper. The objective of this review is to catalog the differences observed in the nomenclature used in the current literature to denote various techniques of liver MP and the manner in which the methodology is described. From our analysis, we aim to address these discrepancies, propose recommendations for nomenclature and develop a standardized set of guidelines for the reporting methodology for future studies on MP of donor livers.
Methods
Literature search strategy
A comprehensive literature search for all published articles regarding MP of donor livers was performed using the PubMed, EMBASE, MEDLINE, Web of Science, and Cochrane Library databases. The final date of the search was February 17, 2015. To ensure all potentially relevant articles were included in the search, no specific date limits were set. The search was conducted using the medical subject heading (MeSH) terms and Emtree keywords “machine perfusion, machine preservation, liver transplantation, and hepatic transplantation” combined with free text terms regarding machine perfusion of donor livers such as “hypothermic,” “normothermic,” and “subnormothermic.”
Selection criteria and data collection
Study selection was performed independently by two authors (S.A.K and R.J.P.) in a standardized fashion using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) method 2 . Study inclusion was carried out in three phases. An initial title search was carried out whereby relevant titles were screened and studies whose titles were unrelated to the aims of this review were excluded. The abstracts of the remaining studies were then acquired and independently assessed for eligibility. Full papers of the abstracts regarded as potentially eligible were retrieved and underwent complete review and assessment until a final compilation of articles was made. For articles in which an inconsistency between the two authors occurred, a discussion about these articles was held to reach to a consensus. Figure 1 illustrates the study selection procedure and the inclusion and exclusion criteria.
Figure 1.
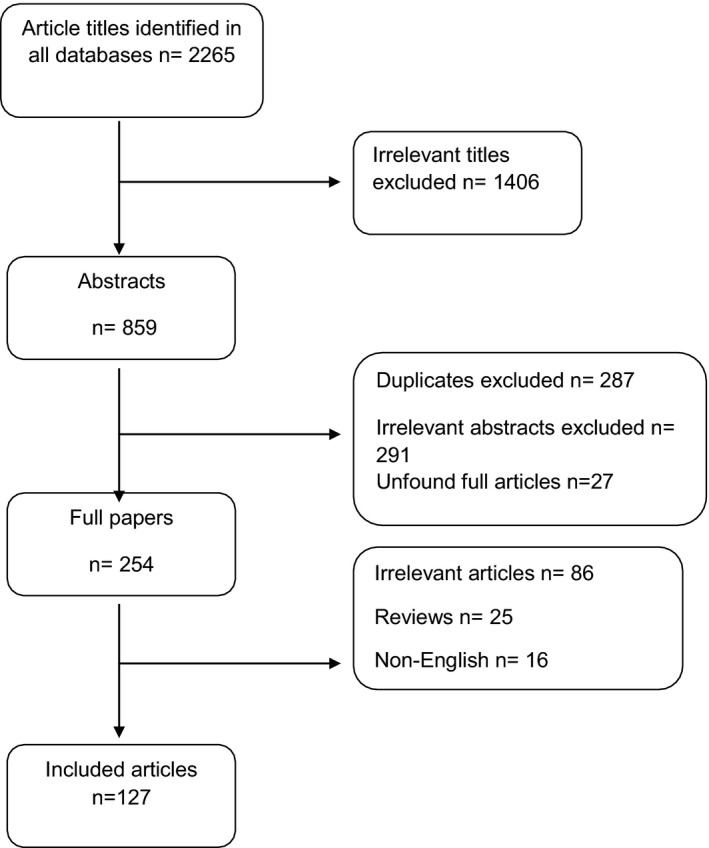
Flow chart illustrating study selection and inclusion procedure. Irrelevant titles included studies mainly involving in vivo perfusion (and not machine perfusion), in vitro cell studies, follow‐up studies on MP or studies involving analysis of data from studies on MP of donor livers without including the MP procedure description in the methodology.
Inclusion criteria
All articles on machine perfusion of donor livers
Fully accessible articles written in English and published in scientific journals
Human and animal studies
Exclusion criteria
Irrelevant to title and objective of review
Non‐English
Articles about MP of other organs
Full version inaccessible
Data extraction and analysis
The data from the included studies was assessed, with the main focus of these articles being the Materials and Methods section. The primary aim of this study was to investigate the manner in which the methodology of these studies was reported and to determine how certain aspects concerning the MP procedure were mentioned. The recommendations and guidelines that this review provides were extensively discussed and agreed on by all authors of this paper.
Results
Of the 2265 articles identified from the initial literature search, 127 of these ultimately met all inclusion criteria (Figure 1). These papers were published between 1997 and 2015 and constituted both animal and human clinical studies. From our analyses, we observed several differences in the manner in which the same type of MP techniques was referred to as well as marked variation in the temperatures used. In the following paragraphs and tables, we highlight and assess these differences as well as provide recommendations to establish uniformity in the manner in which data are reported.
Timing of machine perfusion
In all of the studies reviewed in this paper, MP was conducted either for (almost) the entire duration of the preservation phase of the transplantation process or before or after a period of traditional SCS. A significant majority of the research groups that conducted animal studies 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 and studies in which the donor and recipient center was the same location 35 nearly eliminated the SCS phase, thus perfusing donor organs immediately after procurement until the point of implantation. The remaining studies, particularly most human studies, performed MP for various time periods after a few hours of SCS (during transport of the organs from the donor to recipient centers) or as a result of prolonged cold ischemia times due to various logistical or unforeseen circumstances 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50. Interesting to note, as opposed to performing MP for the entire preservation phase, the first clinical studies conducted by Guarrera et al. and Dutkowski et al. chose to focus on exclusively conducting MP after a period of traditional SCS and immediately prior to implantation (<2 h prior) 35, 40, 45, 46, 51.
Nomenclature and abbreviations used to identify the type of machine perfusion
As the pioneering technique of MP, a number of different terms, as shown in Table 1, have been used to describe hypothermic MP in the past two decades. Even though the majority of the studies mention the term hypothermic within the title and/or article itself, a number of papers simply use the term machine perfusion, without specific indication of the temperature used. Other types of MP include subnormothermic and normothermic perfusion. For all three major types of MP, despite referring to the same procedure, numerous abbreviations are used to describe the type of MP performed (Table 1).
Table 1.
Nomenclature and abbreviations currently used for the different types of liver machine perfusion
| Type of machine perfusion | References |
|---|---|
| Hypothermic (oxygenated) machine perfusion (HMP) | 1, 2, 3, 4, 5, 6, 7, 8, 15, 22, 24, 26, 30, 31, 35, 41, 42, 43, 44, 45, 46, 48, 49, 50, 56, 71, 72, 80, 82, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 |
| Hypothermic oxygenated perfusion (HOPE) | 8, 9, 13, 34, 51, 61, 100, 101, 102, 103, 104, 105, 106, 107 |
| Continuous hypothermic oxygenated machine perfusion (CHOP) | 108 |
| Machine perfusion (MP) or Machine perfusion preservation (MPP) | 53, 57, 63, 64, 73, 74, 75, 109, 110, 111 |
| Cold perfusion | 23 |
| Subnormothermic machine perfusion (SMP) | 11, 112 |
| Subnormothermic machine perfusion (SNMP) | 3, 10, 18, 39, 76, 113, 114, 115 |
| Subnormothermic ex vivo liver perfusion (SNEVLP) | 17 |
| Subnormothermic machine perfusion (MP20) | 60, 65, 66, 67, 116, 117, 118 |
| Normothermic machine perfusion (NMP) | 16, 19, 21, 25, 29, 36, 37, 38, 40, 62, 68, 81, 119, 120, 121, 122, 123 |
| Normothermic extracorporeal perfusion (NELP) | 12, 14, 77, 124, 125 |
| Normothermic extracorporeal perfusion (NECMO) | 83 |
| Normothermic ex vivo liver perfusion (NEVLP) | 5 |
| Warm perfusion | 33, 54, 55, 62 |
Additionally, for subnormothermic and normothermic MP, a major difference lay in the additional emphasis of whether these perfusions were performed extra corporeally or not.
Temperatures used during machine perfusion
Although, in general, three types of MP can be recognized (i.e. hypothermic, subnormothermic and normothermic), we noted marked inconsistency in the actual temperatures denoted by these terms (see Table 2). Despite including a description of the technique of MP, a number of papers 7, 15, 52, 53 failed to specify what particular temperatures were used in their respective studies while some descriptions used arbitrary and unspecific terms such as “warm,” “cold,” or “room temperature” 23, 33, 39, 54, 55 to denote the temperatures used during MP. Of the 58 studies on hypothermic MP, 26 (45%) reported perfusing the livers at 4°C, whereas the rest performed MP at different temperatures within the 0°C–10°C range. All studies on subnormothermic MP were generally conducted at temperatures between 20°C and 30°C. However, the majority of these studies reported using 20°C or 21°C, which in all cases the authors referred to as being room temperature. Normothermic MP was primarily carried out at the physiological body temperature of humans or the animal of study, although small discrepancies were seen in the temperatures stated as being the physiological body temperature of the different animals.
Table 2.
Various temperatures currently used for the different types of liver machine perfusion
| References | |
|---|---|
| Hypothermic temperatures | |
| 0°C–4°C | 126 |
| 1°C–3°C | 127 |
| 2°C ± 1°C | 119 |
| 3°C–5°C | 50 |
| 3°C–6°C | 47, 48 |
| 4°C | 4, 8, 50, 53, 75, 78, 100, 110, 111 |
| 4°C–6°C | 27, 29, 30, 41, 63 |
| 4°C–8°C | 12, 21, 63 |
| 5°C | 121 |
| 5°C–8°C | 122, 123, 124, 125 |
| 8°C | 60, 61 |
| 8°C–10°C | 77 |
| 10°C | 24, 49, 53, 54, 55, 56, 57, 83, 126 |
| Subnormothermic temperatures | |
| 20°C | 68, 80, 81, 93, 95, 96, 121, 127 |
| 21°C | 11, 69, 70, 78, 82, 88, 89, 128 |
| 25°C | 71 |
| 33°C | 90 |
| 20°C–30°C | 94 |
| Normothermic temperatures | |
| Porcine 38°C | 100, 101, 106, 116 |
| Human 35.5°C–37.5°C | 76 |
| Rat 36.5°C–37°C | 129 |
| Human/rabbit/rat 37°C | 9, 52, 74, 75, 102, 103, 104, 105, 110, 114, 130 |
| Rat 37.5°C | 14, 121, 125 |
| (Porcine) 39°C | 97 |
| “Warm” | 33, 52 |
Other aspects of methodology
In addition to the discrepancies in temperature, analysis of the literature exhibited variation in the description of certain technical aspects of the machine perfusion procedure. For instance, seven studies lack a clear description of whether the liver underwent single (via the hepatic artery or portal vein) or dual perfusion 16, 23, 24, 56, 57, 58, 59. As opposed to the vast majority (92%) of the studies that stipulated the use of a pressure or flow controlled system and provided specifications of the settings used, a number of studies failed to specify this 7, 12, 16, 24, 56, 59, 60, 61, 62, 63, 64, 65. All studies that provided oxygenation during MP explicitly stated this in the methodology; however, a number of studies went further to specifically outline the details such as the O2/CO2 mixture or the oxygen tension 2, 3, 5, 6, 44, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 as opposed to simply mentioning the presence of an oxygenator within the MP system 6, 11, 18, 20, 22, 24, 59, 60, 62, 64, 65, 66, 67, 68, 79, 80, 81. Lastly, a significant number of the studies also clearly mentioned the type of pump used during MP 2, 3, 5, 6, 26, 64, 65, 66, 67, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 82, 83, 84, which gives an indication of the flow pattern through the liver.
Discussion
In an effort to initiate and facilitate a standardization of nomenclature as well as to establish guidelines on the experimental and clinical reporting of MP of donor livers, this systematic literature review assessed the differences in the nomenclature, temperatures and techniques currently used and reported in published articles.
The timing of machine perfusion
Given that the timing and duration of MP during the entire preservation and transportation period is essentially correlated to the specific benefits MP is intended to provide to the organ, it is important that the period at which MP is performed is specified, for instance, organ reconditioning and optimization can be applied either prior to or after static cold storage, whereas viability testing is generally performed shortly before implantation.
It is evident from the reviewed literature that MP can be performed mainly at three particular time points; (1) immediately after organ procurement, before the organ is stored on ice for transportation (prestatic cold storage); (2) (shortly) before organ implantation, especially in instances with longer cold ischemia times (poststatic cold storage); and (3) for the entire preservation period between procurement and implantation, thus (nearly) eliminating the need for SCS. In the case of the latter method, we propose the term preservation MP. When applying preservation MP, a short period of SCS is still required during and immediately after organ procurement, when the organ is prepared for connection to the perfusion device, and shortly before implantation to avoid warm ischemia during the anastomosis time. We thus propose to use the term preservation MP when the time period of SCS either before or after MP is less than a maximum of 3 h (Figure 2). This 3‐h time frame is based on the experience of the authors of this paper with various techniques of machine perfusion. It was generally agreed that in reality it normally takes approximately 1.5–2 h from the point of in situ cold flush, donor hepatectomy, back table procedure to connection of the organ onto the perfusion device. However, there are a number of cases in which this may be delayed, for example, in livers with aberrant arterial vasculature that require vascular reconstructions, and thus extra back table time is needed before the liver can be connected to the device. This 3‐h time frame is therefore the recommended maximum time period that allows for unavoidable circumstances that may cause a delay before machine perfusion can be started. Similarly, it generally takes 40–60 min to make the vascular anastomoses in the recipient until reperfusion can be initiated. When this is added to the time needed to take a donor liver off the machine, flush out machine perfusion fluid, remove the cannulas and perform the last back table work (i.e. trimming of vessels and preparation of the venacava in the donor for piggy back anastomosis), one may expect a total time period of 1–3 h before graft reperfusion in the recipient occurs. Therefore, this 3 h of SCS reflects a maximum time period. If the duration of SCS is longer than 3 h and MP is applied either prior (immediately after procurement) or after SCS (shortly before implantation), we propose to call this pre‐SCS MP and post‐SCS MP, respectively.
Figure 2.
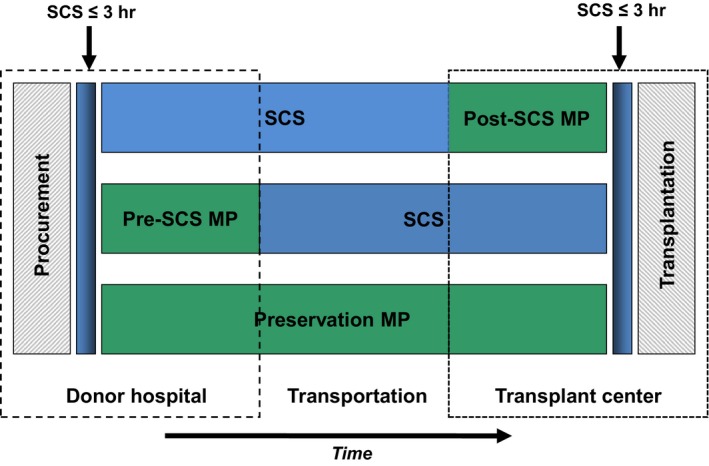
Charts illustrating classification of the timing of machine perfusion. MP conducted within 3 h of organ procurement and followed by a period of SCS is considered as pre‐SCS MP, whereas that performed after a period of at least 3 h of SCS preservation prior to implantation is considered as post‐SCS MP. Additionally, MP can be performed between periods of SCS. Duration of SCS and preservation MP conducted within the 3 h windows on either end of the procedure remains unspecified and can be widely varied. Lastly, MP can also be performed for the entire preservation period (immediately after organ procurement until just before implantation).
Nomenclature and abbreviations
A number of different terms and abbreviations have been used in the observed studies describing generally similar MP methods. In some of these cases, a few aspects such as oxygenation or single/dual perfusion may have differed and were incorporated. To minimize confusion and tackle the heterogeneity in the nomenclature, we believe that authors of future publications should avoid adapting other aspects of perfusion into the nomenclature and retain simplicity. Given the importance of specifying certain aspects of MP performed, the choice to use certain terms in the title and throughout the publication remains within the discretion of the author, although it is advised that the use of the standardized abbreviations for the respective types of MP—HMP (hypothermic machine perfusion), MMP (mid‐thermic machine perfusion), SMP (subnormothermic machine perfusion) and NMP (normothermic machine perfusion)—be maintained.
Temperature ranges
As described in the Results section, experiments conducted on MP of donor livers have generally been performed at three temperature ranges: hypothermically at 0°C–10°C,subnormothermically at 20°C–33°C and normothermically at 35°C–38°C (depending on the species used in study). Based on common practice of the various research groups working on liver MP and following a discussion with the authors involved in this review, the following classification of the standardized temperature ranges is proposed.
Hypothermic MP (0°C–12°C)
All studies involving HMP so far have been conducted at temperatures of 10°C and below with the major reason being that the rate of metabolism and enzymatic reactions in mammalian cells decreases to rates as low as 20% or even less 85, 86 (Figure 3). The benefit of HMP is that it minimizes preservation injury while improving organ viability and, for oxygenated livers, replenishes adenosine tri‐phosphate (ATP) stores. Because the rates of numerous energy dependent reactions of liver mitochondrial enzymes exhibit a significant change at 12.5°C 87, the proposed cut‐off point for HMP is 12°C.
Figure 3.
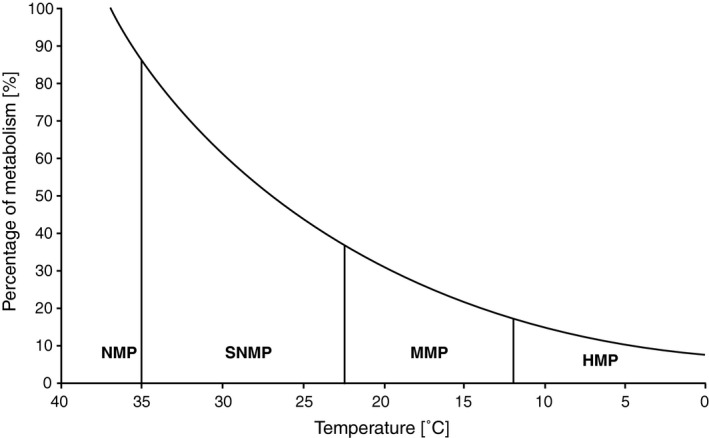
Graphic presentation of the change in the rate of metabolism with decreasing temperature. Based on Van't Hoff's principle (expressed as ), this graph demonstrates the significantly reduced metabolism at hypothermic temperatures (0°C–12°C). The vertical lines in the graphs indicate the lower endpoint of temperature ranges of the different types of MP proposed. NMP; normothermic machine perfusion (35°C–38°C); SMP, subnormothermic machine perfusion (25°C–34°C); MMP, mid‐thermic machine perfusion (13°C–24°C); HMP, hypothermic machine perfusion (0°C–12°C).
Midthermic MP (13°C–24°C) and subnormothermic MP (25°C–34°C)
The term subnormothermic has been considered for temperature ranges varying between 12°C and 35°C even though MP was performed at 20°C–22°C in the majority of studies in which the temperature was referred to as subnormothermic. This broad temperature range shows a great difference in the rate of metabolism at, for example, 12°C as compared to 33°C (Figure 2). Furthermore, it can be argued that temperatures as low as 15°C, 18°C or 20°C are too low to be considered as subnormothermic as not only does this term suggest being slightly below normal body temperature but also at such low temperatures a living person would be defined as (extremely) hypothermic. Whereas at higher temperatures such as 30°C–33°C, the rate of metabolism increases close to 70% of the normal rate at body temperature (Figure 3). Based on this, we propose to use the term mid‐thermic (13°C–24°C) to distinguish the lower temperatures (0°C–12°C) from the less physiologically abnormal subnormothermic temperature range (25°C–34°C).
Normothermic MP (35°C–38°C)
The term normothermic should refer to the normal core body temperature of the species used in the study, i.e. 37°C for human and rodent studies and 38°C in studies with porcine models.
Ex vivo or ex situ MP
An additional aspect of MP that demonstrated particular variation in the literature was the referral of MP as being performed ex vivo or ex situ. Given that MP involves perfusion of donor livers outside the body of a deceased donor, the term ex vivo, which refers to “outside of the living body,” does not seem appropriate. Therefore, the term ex situ, which refers to “outside original location/position,” is proposed as a more representative description of what occurs during MP.
Other technical aspects and reporting guidelines
Along with discrepancies in the nomenclature and temperature ranges, reporting of other aspects, particularly technical aspects belonging to methodology, were observed. Given the ongoing advancement in the field of MP, it is important that certain methodological aspects are explicitly stated to ensure that studies can be reproduced as well as objectively compared with each other. Moreover, with additional clinical trials currently being performed, this will facilitate future meta‐analyses with maximum validity and reliability. The authors of this review reached a consensus on various aspects of the MP procedure that were considered fundamental and developed a checklist that can be utilized and referred to when preparing a report on liver MP (Table 3). Important aspects in this checklist include clear descriptions of the flushing technique, all of the technical aspects of the MP procedure, type of perfusion fluid used and clarification of the time point, duration and temperatures at which MP is conducted. Furthermore, to make valid comparisons of experimental outcomes, the manner in which data is presented and described, particularly in the Results section of publications, is important. The selection of (clinically) relevant endpoints during MP was not the objective of this paper, but the reader is referred to other recent reviews that have summarized the various types of biomarkers that can be used during MP for graft viability assessment 69, 70. Naturally, in clinical trials traditional outcome parameters such as graft and patient survival rates, as well as hepatic and systemic postoperative complications, will be relevant endpoints. In case of donation after cardiac death (DCD) liver transplantation, a major clinical endpoint should be the incidence of postoperative biliary complications.
Table 3.
Checklist with recommended guidelines for the reporting of relevant aspects of the methodology used in liver machine perfusion
| 1. Phase of preservation |
|
| 2. Environment and temperature |
|
| 3. Technical aspects |
|
| 4. Perfusion fluid composition and oxygenation |
|
| 5. Pre‐ and Post‐MP phase |
|
Both at baseline as well as compounds that are continuously or intermittently administered during perfusion.
Conclusion
As experimental and clinical research into MP of donor livers advances, a standardization of nomenclature and reporting of technical aspects of MP is required to minimize heterogeneity and to facilitate more reliable and valid comparison analyses of studies. We hope this paper provides a useful overview on current nomenclature and will be helpful in the reporting of future research studies on liver MP.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Karangwa SA, Dutkowski P, Fontes P, Friend PJ, Guarrera JV, Markmann JF, Mergental H, Minor T, Quintini C, Selzner M, Uygun K, Watson CJ & Porte RJ. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am J Transplant 2016; 16: 2932–2942
[The copyright line for this article was changed after original online publication on 13 June 2016].
References
- 1. Yanaga K, Makowka L, Lebeau G, et al. A new liver perfusion and preservation system for transplantation research in large animals. J Invest Surg 1990; 3: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berendsen TA, Bruinsma BG, Lee J, et al. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res 2012; 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berendsen TA, Bruinsma BG, Puts CF, et al. Supercooling enables long‐term transplantation survival following 4 days of liver preservation. Nat Med 2014; 20: 790–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boehnert MU, Yeung JC, Bazerbachi F, et al. Normothermic acellular ex vivo liver perfusion reduces liver and bile duct injury of pig livers retrieved after cardiac death. Am J Transplant 2013; 13: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 6. Carnevale ME, Balaban CL, Guibert EE, Bottai H, Rodriguez JV. Hypothermic machine perfusion versus cold storage in the rescuing of livers from non‐heart‐beating donor rats. Artif Organs 2013; 37: 985–991. [DOI] [PubMed] [Google Scholar]
- 7. Compagnon P, Clement B, Campion JP, Boudjema K. Effects of hypothermic machine perfusion on rat liver function depending on the route of perfusion. Transplantation 2001; 72: 606–614. [DOI] [PubMed] [Google Scholar]
- 8. Dutkowski P, Furrer K, Tian Y, Graf R, Clavien P. Novel short‐term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non‐heart beating donor. Ann Surg 2006; 244: 968–976; discussion 976‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dutkowski P, Schonfeld S, Odermatt B, Heinrich T, Junginger T. Rat liver preservation by hypothermic oscillating liver perfusion compared to simple cold storage. Cryobiology 1998; 36: 61–70. [DOI] [PubMed] [Google Scholar]
- 10. Fujiyoshi M, Taketomi A. Sub‐normothermic machine perfusion preservation for graft selection and therapy in a mouse liver transplantation model. Hepatology 2014; 60: 241A–241A. [Google Scholar]
- 11. Gringeri E, Bonsignore P, Bassi D, et al. Subnormothermic machine perfusion for non‐heart‐beating donor liver grafts preservation in a swine model: A new strategy to increase the donor pool? Transplant Proc 2012; 44: 2026–2028. [DOI] [PubMed] [Google Scholar]
- 12. Habib MM, Hafez TS, Parkes HG, Seifalian AM, Fuller BJ, Davidson BR. A comparison of bile composition from heart‐beating and non‐heart‐beating rabbit organ donors during normothermic extracorporeal liver perfusion: Experimental evaluation using proton magnetic resonance spectroscopy. Transplant Proc 2004; 36: 2914–2916. [DOI] [PubMed] [Google Scholar]
- 13. Hessheimer AJ, Fondevila C, Maathuis MHJ, et al. Hypothermic oxygenated perfusion improves hepatocellular but causes kupffer and endothelial cell injury in porcine DCD liver transplant. Am J Transplant 2012; 12: 131. [Google Scholar]
- 14. Tolboom H, Pouw RE, Izamis M, et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation 2009; 87: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jain S, Lee CY, Baicu S, et al. Hepatic function in hypothermically stored porcine livers: Comparison of hypothermic machine perfusion vs cold storage. Transplant Proc 2005; 37: 340–341. [DOI] [PubMed] [Google Scholar]
- 16. Jamieson RW, Zilvetti M, Roy D, et al. Hepatic steatosis and normothermic perfusion‐preliminary experiments in a porcine model. Transplantation 2011; 92: 289–295. [DOI] [PubMed] [Google Scholar]
- 17. Knaak JM, Spetzler VN, Goldaracena N, et al. Subnormothermic ex vivo liver perfusion reduces endothelial cell and bile duct injury after donation after cardiac death pig liver transplantation. Liver Transpl 2014; 20: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 18. Liu Q, Berendsen T, Izamis ML, Uygun B, Yarmush ML, Uygun K. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant Proc 2013; 45: 3209–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Q, Nassar A, Farias K, et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl 2014; 20: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuno N, Obara H, Watanabe R, et al. Rewarming preservation by organ perfusion system for donation after cardiac death liver grafts in pigs. Transplant Proc 2014; 46: 1095–1098. [DOI] [PubMed] [Google Scholar]
- 21. Nassar A, Liu Q, Farias K, et al. Ex vivo normothermic machine perfusion is safe, simple, and reliable: Results from a large animal model. Surg Innov 2015; 22: 61–69. [DOI] [PubMed] [Google Scholar]
- 22. Obara H, Matsuno N, Enosawa S, et al. Pretransplant screening and evaluation of liver graft viability using machine perfusion preservation in porcine transplantation. Transplant Proc 2012; 44: 959–961. [DOI] [PubMed] [Google Scholar]
- 23. Lauschke H, Olschewski P, Tolba R, Schulz S, Minor T. Oxygenated machine perfusion mitigates surface antigen expression and improves preservation of predamaged donor livers. Cryobiology 2003; 46: 53–60. [DOI] [PubMed] [Google Scholar]
- 24. Olschewski P, Tolba R, Akbar S, Minor T. Use of HTK solution for hypothermic machine perfusion: An alternative for the preservation of less than optimal donor livers? An experimental study in rats. Transplant Proc 2003; 35: 767. [DOI] [PubMed] [Google Scholar]
- 25. op den Dries S, Karimian N, Weeder PD, Porte RJ. Normothermic acellular machine perfusion and bile duct injury in pig livers retrieved after cardiac death. Am J Transplant 2013; 13: 3289. [DOI] [PubMed] [Google Scholar]
- 26. Op Den Dries S, Wiersma‐Buist J, Leuvenink HGD, De Boer MT, Lisman T, Porte RJ. Hypothermic oxygenated machine preservation of livers from donation after cardiac death: Does it reduce bile duct Injury? Liver Transpl 2012; 18: S184. [Google Scholar]
- 27. ‘t Hart NA, der van Plaats A, Leuvenink HGD, et al. Determination of an adequate perfusion pressure for continuous dual vessel hypothermic machine perfusion of the rat liver. Transplant Int 2007; 20: 343–352. [DOI] [PubMed] [Google Scholar]
- 28. Vekemans K, Liu Q, Brassil J, Komuta M, Pirenne J, Monbaliu D. Influence of flow and addition of oxygen during porcine liver hypothermic machine perfusion. Transplant Proc 2007; 39: 2647–2651. [DOI] [PubMed] [Google Scholar]
- 29. Xu H, Berendsen T, Kim K, et al. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia. J Surg Res 2012; 173: E83–E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luer B, Koetting M, Efferz P, Minor T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl Int 2010; 23: 944–950. [DOI] [PubMed] [Google Scholar]
- 31. Fondevila C, Hessheimer AJ, Maathuis MJ, et al. Hypothermic oxygenated machine perfusion in porcine donation after circulatory determination of death liver transplant. Transplantation 2012; 94: 22–29. [DOI] [PubMed] [Google Scholar]
- 32. Reddy S, Greenwood J, Maniakin N, et al. Non‐heart‐beating donor porcine livers: The adverse effect of cooling. Liver Transpl 2005; 11: 35–38. [DOI] [PubMed] [Google Scholar]
- 33. Brockmann J, Reddy S, Coussios C, et al. Normothermic perfusion: A new paradigm for organ preservation. Ann Surg 2009; 250: 1–6. [DOI] [PubMed] [Google Scholar]
- 34. Schlegel A, Graf R, Brockmann J, Clavien P, Dutkowski P. Rescue of liver grafts after cardiac arrest: First study comparing warm versus cold machine perfusion strategies in rodent models of liver transplantation. Transplant Int 2013; 26: 52–52. [Google Scholar]
- 35. Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am J Transplant 2010; 10: 372–381. [DOI] [PubMed] [Google Scholar]
- 36. Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS ONE 2014; 9: e110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant 2013; 13: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 38. op den Dries S, Karimian N, Porte RJ. Normothermic machine perfusion of discarded liver grafts. Am J Transplant 2013; 13: 2504–2504. [DOI] [PubMed] [Google Scholar]
- 39. Martins P, Bruinsma B, Farmer A, et al. Subnormothermic machine perfusion for recovery and viability testing of the discarded human liver. Transplant Int 2013; 26: 316. [Google Scholar]
- 40. Graham JA, Guarrera JV. “Resuscitation” of marginal liver allografts for transplantation with machine perfusion technology. J Hepatol 2014; 61: 418–431. [DOI] [PubMed] [Google Scholar]
- 41. Henry SD, Arrington BO, Nachbar E, Samstein B, Emond JC, Guarrera JV. Hypothermic machine perfusion attenuates molecular markers of preservation injury in human liver transplantation. Am J Transplant 2010; 10: 107–108. [Google Scholar]
- 42. Henry S, Arrington B, Chen SW, Lee HT, Emond JC, Guarrera JV. Hypothermic machine perfusion reduces molecular damage cascades in human liver transplantation. Hepatology 2009; 50: 637A–637A. [Google Scholar]
- 43. Guarrera J, Estevez J, Boykin J, et al. Hypothermic machine perfusion of liver grafts for transplantation: Technical development in human discard and miniature swine models. Transplant Proc 2005; 37: 323–325. [DOI] [PubMed] [Google Scholar]
- 44. Guarrera JV, Henry SD, Chen SWC, et al. Hypothermic machine preservation attenuates ischemia/reperfusion markers after liver transplantation: Preliminary results. J Surg Res 2011; 167: E365–E373. [DOI] [PubMed] [Google Scholar]
- 45. Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant 2015; 15: 161–169. [DOI] [PubMed] [Google Scholar]
- 46. Henry SD, Nachber E, Tulipan J, et al. Hypothermic machine preservation reduces molecular markers of ischemia/reperfusion injury in human liver transplantation. Am J Transplant 2012; 12: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 47. Bae C, Henry SD, Guarrera JV. Is extracorporeal hypothermic machine perfusion of the liver better than the ‘good old icebox'? Curr Opin Organ Transplant 2012; 17: 137–142. [DOI] [PubMed] [Google Scholar]
- 48. Tulipan JE, Stone J, Samstein B, et al. Molecular expression of acute phase mediators is attenuated by machine preservation in human liver transplantation: Preliminary analysis of effluent, serum, and liver biopsies. Surgery 2011; 150: 352–360. [DOI] [PubMed] [Google Scholar]
- 49. Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev 2012; 26: 163–175. [DOI] [PubMed] [Google Scholar]
- 50. Bae C, Pichardo EM, Huang H, Henry SD, Guarrera JV. The benefits of hypothermic machine perfusion are enhanced with vasosol and alpha‐tocopherol in rodent donation after cardiac death livers. Transplant Proc 2014; 46: 1560–1566. [DOI] [PubMed] [Google Scholar]
- 51. Dutkowski P, Schlegel A, de Oliveira M, Muellhaupt B, Neff F, Clavien P. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol 2014; 60: 765–772. [DOI] [PubMed] [Google Scholar]
- 52. Butler AJ, Rees MA, Wight DG, et al. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation 2002; 73: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 53. Bessems M, Doorschodt BM, Kolkert JLP, et al. Preservation of steatotic livers: A comparison between cold storage and machine perfusion preservation. Liver Transpl 2007; 13: 497–504. [DOI] [PubMed] [Google Scholar]
- 54. Reddy SP, Bhattacharjya S, Maniakin N, et al. Preservation of porcine non‐heart‐beating donor livers by sequential cold storage and warm perfusion. Transplantation 2004; 77: 1328–1332. [DOI] [PubMed] [Google Scholar]
- 55. Hara Y, Akamatsu Y, Maida K, et al. A new liver graft preparation method for uncontrolled non‐heart‐beating donors, combining short oxygenated warm perfusion and prostaglandin E1. J Surg Res 2013; 184: 1134–1142. [DOI] [PubMed] [Google Scholar]
- 56. Jain S, Xu H, Duncan H, et al. Ex‐vivo study of flow dynamics and endothelial cell structure during extended hypothermic machine perfusion preservation of livers. Cryobiology 2004; 48: 322–332. [DOI] [PubMed] [Google Scholar]
- 57. Lee CY, Jain S, Duncan HM, et al. Survival transplantation of preserved non‐heart‐beating donor rat livers: Preservation by hypothermic machine perfusion. Transplantation 2003; 76: 1432–1436. [DOI] [PubMed] [Google Scholar]
- 58. Nagrath D, Xu H, Tanimura Y, et al. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion ex vivo . Metab Eng 2009; 11: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu H, Zhang JX, Jones JW, Southard JH, Clemens MG, Lee CY. Hypothermic machine perfusion of rat livers preserves endothelial cell function. Transplant Proc 2005; 37: 335–337. [DOI] [PubMed] [Google Scholar]
- 60. Boncompagni E, Gini E, Ferrigno A, et al. Decreased apoptosis in fatty livers submitted to subnormothermic machine‐perfusion respect to cold storage. Eur J Histochem 2011; 55: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dutkowski R, Graf R, Clavien P. Rescue of the cold preserved rat liver by hypothermic oxygenated machine perfusion. Am J Transplant 2006; 6: 903–912. [DOI] [PubMed] [Google Scholar]
- 62. St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non‐heart‐beating donor livers with normothermic machine perfusion. Br J Surg 2002; 89: 609–616. [DOI] [PubMed] [Google Scholar]
- 63. Uchiyama M, Kozaki K, Nemoto T, et al. Liver transplantation from non‐heart‐beating donors: Effect of machine perfusion preservation and pentoxifylline. Transplant Proc 1998; 30: 3798–3800. [DOI] [PubMed] [Google Scholar]
- 64. Uchiyama M, Matsuno N, Hama K, et al. Comparison between nonpulsatile and pulsatile machine perfusion preservation in liver transplantation from non‐heart‐beating donors. Transplant Proc 2001; 33: 936–938. [DOI] [PubMed] [Google Scholar]
- 65. Vairetti M, Ferrigno A, Rizzo V, et al. Subnormothermic machine perfusion protects against rat liver preservation injury: A comparative evaluation with conventional cold storage. Transplant Proc 2007; 39: 1765–1767. [DOI] [PubMed] [Google Scholar]
- 66. Tarantola E, Bertone V, Milanesi G, et al. Preservation of obese rat livers by subnormothermic machine perfusion protects dipeptidylpeptidase‐IV activity and expression in the biliary tree. Dig Liver Dis 2012; 44: S24. [Google Scholar]
- 67. Tarantola E, Bertone V, Milanesi G, et al. Dipeptidylpeptidase‐IV activity and expression reveal decreased damage to the intrahepatic biliary tree in fatty livers submitted to subnormothermic machine‐perfusion respect to conventional cold storage. Eur J Histochem 2014; 58: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. op den Dries S, Karimian N, Sutton ME, et al. Successful ex‐vivo normothermic machine perfusion and viability testing of discarded human donor livers. Transplant Int 2013; 26: 337. [DOI] [PubMed] [Google Scholar]
- 69. Verhoeven CJ, Farid WR, de Jonge J, Metselaar HJ, Kazemier G, van der Laan LJ. Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation. J Hepatol 2014; 61: 672–684. [DOI] [PubMed] [Google Scholar]
- 70. Schlegel A, Kron P, Dutkowski P. Hypothermic oxygenated liver perfusion: Basic mechanisms and clinical application. Curr Transplant Rep 2015; 2: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Monbaliu D, Heedfeld V, Liu Q, et al. Hypothermic machine perfusion of the liver: Is it more complex than for the kidney? Transplant Proc 2011; 43: 3445–3450. [DOI] [PubMed] [Google Scholar]
- 72. Henry SD, Arrington B, Samstein B, et al. Preservation/reperfusion injury is attenuated by hypothermic machine perfusion in human liver transplantation. Am J Transplant 2009; 9: 234–234. [Google Scholar]
- 73. Lee CY, Zhang JX, deSilva H, Coger RN, Clemens MG. Heterogeneous flow patterns during hypothermic machine perfusion preservation of livers. Transplantation 2000; 70: 1797–1802. [DOI] [PubMed] [Google Scholar]
- 74. Bessems M, Doorschodt B, Dinant S, de Graaf W, van Gulik T. Machine perfusion preservation of the pig liver using a new preservation solution, polysol. Transplant Proc 2006; 38: 1238–1242. [DOI] [PubMed] [Google Scholar]
- 75. Bessems M, Doorschodt B, Hooijschuur O, van Vliet A, van Gulik T. Optimization of a new preservation solution for machine perfusion of the liver: Which is the preferred colloid? Transplant Proc 2005; 37: 329–331. [DOI] [PubMed] [Google Scholar]
- 76. Bruinsma BG, Berendsen TA, Izamis M, Yarmush ML, Uygun K. Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int J Artif Organs 2013; 36: 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hafez TS, Habib MM, Seifalian AM, Fuller BJ, Davidson BR. Near‐infrared spectroscopic assessment of mitochondrial oxygenation status–comparison during normothermic extracorporeal liver perfusion by buffer only or buffer fortified with washed red blood cells: An experimental study. Transplant Proc 2004; 36: 1265–1267. [DOI] [PubMed] [Google Scholar]
- 78. Dirkes MC, Post ICJH, Heger M, van Gulik TM. A novel oxygenated machine perfusion system for preservation of the liver. Artif Organs 2013; 37: 719–724. [DOI] [PubMed] [Google Scholar]
- 79. Shigeta T, Matsuno N, Huai‐Che H, et al. A basic consideration for porcine liver preservation using a novel continuous machine perfusion device. Transplant Proc 2012; 44: 942–945. [DOI] [PubMed] [Google Scholar]
- 80. Obara H, Matsuno N, Shigeta T, Hirano T, Enosawa S, Mizunuma H. Temperature controlled machine perfusion system for liver. Transplant Proc 2013; 45: 1690–1692. [DOI] [PubMed] [Google Scholar]
- 81. Imber CJ, St Peter SD, Lopez de Cenarruzabeitia I, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation 2002; 73: 701–709. [DOI] [PubMed] [Google Scholar]
- 82. Kim JS, Boudjema K, D'Alessandro A, Southard JH. Machine perfusion of the liver: Maintenance of mitochondrial function after 48‐hour preservation. Transplant Proc 1997; 29: 3452–3454. [DOI] [PubMed] [Google Scholar]
- 83. Fondevila C, Hessheimer AJ, Maathuis MJ, et al. Superior preservation of DCD livers with continuous normothermic perfusion. Ann Surg 2011; 254: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 84. Lee CY, Zhang JX, deSilva H, Coger RN, Clemens MG. Heterogeneous flow patterns during hypothermic machine perfusion preservation of livers. Transplantation 2000; 70: 1797–1802. [DOI] [PubMed] [Google Scholar]
- 85. Bruinsma BG, Berendsen TA, Izamis M, Yarmush ML, Uygun K. Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int J Artif Organs 2013; 36: 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dirkes MC, Post ICJH, Heger M, van Gulik TM. A novel oxygenated machine perfusion system for preservation of the liver. Artif Organs 2013; 37: 719–724. [DOI] [PubMed] [Google Scholar]
- 87. Lee MP, Gear AR. The effect of temperature on mitochondrial membrane‐linked reactions. J Biol Chem 1974; 249: 7541–7549. [PubMed] [Google Scholar]
- 88. Manekeller S, Schuppius A, Stegemann J, Hirner A, Minor T. Role of perfusion medium, oxygen and rheology for endoplasmic reticulum stress‐induced cell death after hypothermic machine preservation of the liver. Transplant Int 2008; 21: 169–177. [DOI] [PubMed] [Google Scholar]
- 89. Monbaliu D, Liu Q, Libbrecht L, et al. Preserving the morphology and evaluating the quality of liver grafts by hypothermic machine perfusion: A proof‐of‐concept study using discarded human livers. Liver Transpl 2012; 18: 1495–1507. [DOI] [PubMed] [Google Scholar]
- 90. Monbaliu D, Liu Q, Libbrecht L, et al. Preservation of normal morphology of human livers after 24 hours of hypothermic machine perfusion. A first‐in‐man study. Transplant Int 2011; 24: 151. [Google Scholar]
- 91. Monbaliu D, Vekemans K, De Vos R, et al. Hemodynamic, biochemical, and morphological characteristics during preservation of normal porcine livers by hypothermic machine perfusion. Transplant Proc 2007; 39: 2652–2658. [DOI] [PubMed] [Google Scholar]
- 92. Monbaliu DR, Debbaut C, Hillewaert WJ, et al. Flow competition between hepatic arterial and portal venous flow during hypothermic machine perfusion preservation of porcine livers. Int J Artif Organs 2012; 35: 119–131. [DOI] [PubMed] [Google Scholar]
- 93. op den Dries S, Sutton ME, Karimian N, et al. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS ONE 2014; 9: e88521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. op den Dries S, Leuvenink H, De Boer MT, Lisman T, Porte RJ. Hypothermic oxygenated machine preservation of donor livers after prolonged ischemia in a porcine model of donation after cardiac death. HPB 2012; 14: 482. [Google Scholar]
- 95. Henry SD, Tulipan JE, Stone J, et al. Quantification of inflammatory biomarkers in perfusion effluent collected during the first liver machine perfusion clinical trial. Am J Transplant 2011; 11: 454–455. [Google Scholar]
- 96. Jain S, Lee SH, Korneszczuk K, et al. Improved preservation of warm ischemic livers by hypothermic machine perfusion with supplemented University of Wisconsin solution. J Invest Surg 2008; 21: 83–91. [DOI] [PubMed] [Google Scholar]
- 97. Jomaa A, Gurusamy K, Siriwardana PN, et al. Does hypothermic machine perfusion of human donor livers affect risks of sinusoidal endothelial injury and microbial infection? A feasibility study assessing flow parameters, sterility, and sinusoidal endothelial ultrastructure. Transplant Proc 2013; 45: 1677–1683. [DOI] [PubMed] [Google Scholar]
- 98. Liu Q, Vekemans K, van Pelt J, et al. Discriminate liver warm ischemic injury during hypothermic machine perfusion by proton magnetic resonance spectroscopy: A study in a porcine model. Transplant Proc 2009; 41: 3383–3386. [DOI] [PubMed] [Google Scholar]
- 99. Liu Q, Vekemans K, Iania L, et al. Assessing warm ischemic injury of pig livers at hypothermic machine perfusion. J Surg Res 2014; 186: 379–389. [DOI] [PubMed] [Google Scholar]
- 100. de Rougemont O, Breitenstein S, Leskosek B, et al. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg 2009; 250: 674–683. [DOI] [PubMed] [Google Scholar]
- 101. Dutkowski P, Odermatt B, Heinrich T, et al. Hypothermic oscillating liver perfusion stimulates ATP synthesis prior to transplantation. J Surg Res 1998; 80: 365–372. [DOI] [PubMed] [Google Scholar]
- 102. Schlegel A, Graf R, Clavien P, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol 2013; 59: 984–991. [DOI] [PubMed] [Google Scholar]
- 103. Schlegel A, Graf R, Kron P, Clavien P, Dutkowski P. Warm or cold machine perfusion to rescue DCD liver grafts prior to transplantation. Br J Surg 2014; 101: 18–19. [Google Scholar]
- 104. Schlegel AA, Graf R, Clavien P, Dutkowski P. Hypothermic oxygenated machine perfusion (HOPE) prevents biliary injury after transplantation of DCD liver grafts. Liver Transpl 2013; 19: S86. [DOI] [PubMed] [Google Scholar]
- 105. Schlegel A, de Rougemont O, Graf R, Clavien P, Dutkowski P. Protective mechanisms of end‐ischemic cold machine perfusion in DCD liver grafts. J Hepatol 2013; 58: 278–286. [DOI] [PubMed] [Google Scholar]
- 106. Schlegel A, Dutkowski P. Role of hypothermic machine perfusion in liver transplantation. Transpl Int 2015; 28: 677–689. [DOI] [PubMed] [Google Scholar]
- 107. Schlegel A, Kron P, Graf R, Clavien P, Dutkowski P. Hypothermic oxygenated machine perfusion (HOPE) down‐regulates the immune response in a rat model of liver transplantation. Liver Transpl 2014; 20: S137–S138. [DOI] [PubMed] [Google Scholar]
- 108. Lu L, Rao J, Zhou H, Wang X. Effect of continuous hypothermic oxygenated machine perfusion (CHOP) on liver graft from donors after cardiac death (DCD) following liver transplantation. Hepatology 2014; 60: 829A–829A. [Google Scholar]
- 109. Uchiyama M, Matsuno N, Nakamura Y, et al. Usefulness of preservation by machine perfusion of liver grafts from non‐heart‐beating donors‐a porcine model. Transplant Proc 2003; 35: 105–106. [DOI] [PubMed] [Google Scholar]
- 110. Bessems M, Doorschodt B, van Vliet A, van Gulik T. Improved rat liver preservation by hypothermic continuous machine perfusion using Polysol, a new, enriched preservation solution. Liver Transpl 2005; 11: 539–546. [DOI] [PubMed] [Google Scholar]
- 111. Bessems M, Doorschodt B, van Vliet A, van Gulik T. Machine perfusion preservation of the non‐heart‐beating donor rat livers using polysol, a new preservation solution. Transplant Proc 2005; 37: 326–328. [DOI] [PubMed] [Google Scholar]
- 112. Gringeri E, Polacco M, D'Amico FE, et al. A new liver autotransplantation technique using subnormothermic machine perfusion for organ preservation in a porcine model. Transplant Proc 2011; 43: 997–1000. [DOI] [PubMed] [Google Scholar]
- 113. Bruinsma BG, Sridharan GV, Weeder PD, et al. Dynamic characterization of human livers during ex vivo machine perfusion. Hepatology 2014; 60: 200A. [Google Scholar]
- 114. Bruinsma B, Sridharan G, Weeder P, et al. Indicators of liver viability in the ex vivo perfused human liver. Transplantation 2014; 98: 373–374. [Google Scholar]
- 115. Bruinsma BG, Yeh H, Ozer S, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant 2014; 14: 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vairetti M, Ferrigno A, Carlucci F, et al. Subnormothermic machine perfusion protects steatotic livers against preservation injury: A potential for donor pool increase? Liver Transpl 2009; 15: 20–29. [DOI] [PubMed] [Google Scholar]
- 117. Vairetti M, Ferrigno A, Carlucci F, et al. Subnormothermic machine perfusion protects steatotic liver graft: Implications for organ transplantation. Transplant Int 2007; 20: 280–280. [Google Scholar]
- 118. Tolboom H, Izamis ML, Sharma N, et al. Subnormothermic machine perfusion at both 20 degrees C and 30 degrees C recovers ischemic rat livers for successful transplantation. J Surg Res 2012; 175: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. St Peter SD, Imber CJ, Kay J, James T, Friend PJ. Hepatic control of perfusate homeostasis during normothermic extrocorporeal preservation. Transplant Proc 2003; 35: 1587–1590. [DOI] [PubMed] [Google Scholar]
- 120. op den Dries S, Karimian N, Sutton M, et al. Successful ex‐vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant 2013; 13: 519. [DOI] [PubMed] [Google Scholar]
- 121. Izamis ML, Tolboom H, Uygun B, Berthiaume F, Yarmush ML, Uygun K. Resuscitation of ischemic donor livers with normothermic machine perfusion: A metabolic flux analysis of treatment in rats. PLoS ONE 2013; 8: e69758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. op den Dries S, Karimian N, Sutton M, et al. Normothermic oxygenated machine preservation reduces reperfusion injury of DCD livers but seems less useful in DBD livers: A comparative study in a rat model. Liver Transpl 2013; 19: S202. [Google Scholar]
- 123. op den Dries S, Karimian N, Sutton ME, et al. Normothermic machine preservation reduces bile duct injury in DCD livers: A comparative study in a rat model. Transplant Int 2013; 26: 57. [Google Scholar]
- 124. Tolboom H, Milwid JM, Izamis ML, Uygun K, Berthiaume F, Yarmush ML. Sequential cold storage and normothermic perfusion of the ischemic rat liver. Transplant Proc 2008; 40: 1306–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tolboom H, Pouw R, Uygun K, et al. A model for normothermic preservation of the rat liver. Tissue Eng 2007; 13: 2143–2151. [DOI] [PubMed] [Google Scholar]
- 126. van der Plaats A, Maathuis MH, ‘T Hart NA, et al. The Groningen hypothermic liver perfusion pump: Functional evaluation of a new machine perfusion system. Ann Biomed Eng 2006; 34: 1924–1934. [DOI] [PubMed] [Google Scholar]
- 127. ‘t Hart N, van der Plaats A, Leuvenink H, et al. Hypothermic machine perfusion of the liver and the critical balance between perfusion pressures and endothelial injury. Transplant Proc 2005; 37: 332–334. [DOI] [PubMed] [Google Scholar]
- 128. Belzer FO, Southard JH. Principles of solid organ preservation by cold storage. Transplantation 1988; 45: 673–676. [DOI] [PubMed] [Google Scholar]
- 129. Nishino H, Nakaya J, Nishi S, Kurosawa T, Ishibashi T. Temperature‐induced differential kinetic properties between an initial burst and the following steady state in membrane‐bound enzymes: Studies on lathosterol 5‐desaturase. Arch Biochem Biophys 1997; 339: 298–304. [DOI] [PubMed] [Google Scholar]
- 130. Lee MP, Gear ARL. The effect of temperature on mitochondrial membrane‐linked reactions. J Biol Chem 1974; 249: 7541–7549. [PubMed] [Google Scholar]


