Abstract
Objective
To evaluate the effect of secukinumab (interleukin‐17A inhibitor) on patient‐reported outcomes in patients with active ankylosing spondylitis (AS).
Methods
In this phase III study, 371 patients were randomized (1:1:1) to receive intravenous (IV) secukinumab 10 mg/kg at baseline and weeks 2 and 4 followed by subcutaneous (SC) secukinumab 150 mg every 4 weeks (IV→150 mg group), or SC secukinumab 75 mg every 4 weeks (IV→75 mg group), or placebo. Patient‐reported outcomes included the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), BASDAI criteria for 50% improvement (BASDAI 50), Short Form 36 (SF‐36) physical component summary (PCS) score and mental component summary (MCS) score, Ankylosing Spondylitis Quality of Life (ASQoL) questionnaire, Bath Ankylosing Spondylitis Functional Index (BASFI), EuroQol 5‐domain (EQ‐5D) questionnaire, Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F), and Work Productivity and Activity Impairment–General Health questionnaire (WPAI‐GH).
Results
At week 16, secukinumab IV→150 mg or IV→75 mg was associated with statistically and clinically significant improvements from baseline versus placebo in the BASDAI (−2.3 for both regimens versus −0.6; P < 0.0001 and P < 0.001, respectively), SF‐36 PCS (5.6 for both regimens versus 1.0; P < 0.0001 and P < 0.001, respectively), and ASQoL (−3.6 for both regimens versus −1.0; P < 0.0001 and P < 0.001, respectively). Clinically significant improvements in the SF‐36 MCS, BASFI, EQ‐5D, and BASDAI 50 were observed with both secukinumab groups versus placebo at week 16; improvements were also observed in the FACIT‐F and WPAI‐GH. All improvements were sustained through week 52.
Conclusion
Our findings indicate that secukinumab provides significant and sustained improvements in patient‐reported disease activity and health‐related quality of life, and reduces functional impairment, fatigue, and impact of disease on work productivity in patients with active AS.
Ankylosing spondylitis (AS), part of the larger disease group of axial spondyloarthritis, is a chronic immune‐mediated inflammatory disease 1, 2. With an estimated worldwide prevalence of 0.2–1.4% 2, 3, 4, 5, 6, 7, AS represents a significant personal, societal, and economic health‐related burden. The progressive nature of AS can lead to structural damage of the spine, worsening of joint function, physical disability, and significant functional impairment, culminating in reduced health‐related quality of life (HRQoL) 8. Indeed, individuals with AS not only have pain and physical function limitations, but also experience diminished social functioning and work disability.
Traditional treatment options for patients with AS include nonsteroidal antiinflammatory drugs (NSAIDs) and physical therapy. However, over the long term, NSAID use is associated with gastrointestinal and cardiovascular adverse events, while disease‐modifying antirheumatic drugs have been shown to have limited efficacy 9 in peripheral arthritis only and not in axial disease. Consequently, anti–tumor necrosis factor (anti‐TNF) therapy is recommended in patients in whom NSAIDs fail to achieve adequate disease control or those patients with high disease activity, and these therapies have been shown to improve outcomes in patients with AS, reducing pain and improving mobility and HRQoL 9. However, it has been reported that 25–40% of AS patients with moderate to severe disease do not respond to or are intolerant of anti‐TNF agents and, therefore, are left with no alternative treatment 10, 11, 12, 13, 14. Hence, there is an unmet need for novel therapies that offer long‐term disease control in AS.
Therapeutic strategies targeting various inflammatory pathways, including interleukin‐6 receptor (IL‐6R) blockade, T cell costimulation inhibition, and IL‐1R antagonism, have largely failed to show significant clinical efficacy in AS 12, 15, 16. IL‐17A has been implicated in the pathogenesis of AS, with elevated levels of IL‐17–producing cells found in the circulation and target tissues of patients with this disease 17, 18, 19.
Secukinumab (AIN457) is a high‐affinity, fully human IgG1κ monoclonal antibody that selectively binds to and neutralizes IL‐17A. In a phase II proof‐of‐concept trial, secukinumab was well tolerated and rapidly reduced clinical and biologic signs of active AS 20. MEASURE 1 is an ongoing, 2‐year, phase III, randomized trial, followed by a 3‐year extension period, designed to assess the long‐term efficacy and safety of secukinumab in patients with active AS. Secukinumab was shown to improve the signs and symptoms of AS through the first 52 weeks of therapy 21. Here, we report the effects of secukinumab treatment over 52 weeks on patient‐reported outcomes.
PATIENTS AND METHODS
Study design and patients
The detailed study design and methods for MEASURE 1 have been described previously 21. Briefly, eligible patients were ages ≥18 years and were diagnosed as having AS with prior documented radiologic evidence fulfilling the modified New York criteria 22 and active disease defined as a score of ≥4 on the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 23 and spinal pain ≥4 cm on a 10‐cm visual analog scale at baseline, despite treatment with maximum tolerated doses of NSAIDs. Key exclusion criteria included total spinal ankylosis, evidence of infection or malignancy on chest radiograph, and previous treatment with cell‐depleting therapies or biologic agents other than anti‐TNF therapy 21. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Institutional review board or ethics committee approval and written informed consent from patients were obtained prior to study procedures being initiated. This study is registered with ClinicalTrials.gov (identifier NCT01358175).
This phase III double‐blind, placebo‐controlled study was conducted at 65 centers across Belgium, Bulgaria, Canada, France, Germany, Italy, Mexico, The Netherlands, Peru, Russia, Taiwan, Turkey, the UK, and the US. Patients were randomized (1:1:1) to receive intravenous (IV) secukinumab 10 mg/kg (at baseline and weeks 2 and 4) followed by subcutaneous (SC) secukinumab 150 mg, or 75 mg every 4 weeks (IV→150 mg group and IV→75 mg group, respectively), or placebo on the same IV and SC dosing schedule. Responders were defined as patients in whom Assessment of SpondyloArthritis international Society criteria for 20% improvement in disease activity (ASAS20) was achieved. In those patients who were originally assigned to receive placebo at baseline, nonresponders and responders were re‐randomized (1:1) to receive secukinumab 150 mg SC or 75 mg SC at weeks 16 and 24, respectively.
Patient‐reported outcome assessments
A brief overview of the patient‐reported outcomes assessed in this study is presented in Table 1. The patient‐reported outcomes were assessed as prespecified end points and included mean change from baseline to weeks 16 and 52 in the BASDAI 21, 23, Short Form 36 (SF‐36) health survey physical component summary (PCS) score 21, 24, SF‐36 mental component summary (MCS) score 24, Ankylosing Spondylitis Quality of Life (ASQoL) measure 21, 25, Bath Ankylosing Spondylitis Functional Index (BASFI) 26, EuroQol 5‐domain (EQ‐5D) questionnaire 27, Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F) 28, 29, and Work Productivity and Activity Impairment–General Health (WPAI‐GH) 30 (Table 1). Additional assessments included the proportion of patients in whom BASDAI 50 was achieved (defined as at least a 50% improvement [decrease] from baseline in the total BASDAI score) and the proportion of patients with improvements from baseline in the SF‐36 PCS and MCS that exceeded the minimum clinically important difference (MCID; defined as an improvement of ≥2.5 points) at week 16 and other time points. Patients in whom such an improvement was achieved are referred to here as SF‐36 PCS or MCS responders.
Table 1.
Overview of patient‐reported outcome measuresa
| Instrument (ref.) | Description | Assessment | MCID |
|---|---|---|---|
| SF‐36 PCS and MCS 24 | Summary of SF‐36 domain scores separately as physical components and mental components | Range 0–50 points for each component (a score of 50 [±10 SD] indicates normal function) |
Improvement: ≥2.5 points Deterioration: −0.8 points |
| SF‐36 version 2 (acute form) 24 | Assesses HRQoL using 8 subscales that can be scored individually: physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional, and mental health | Range 0–100 points (worst to best) |
Improvement: 5.0 points Deterioration: −2.5 points |
| ASQoL 25, 39 | Self‐administered questionnaire designed to assess HRQoL in adult patients with AS | Dichotomous yes or no (1 or 0) scale for 18 items, with a total score range of 0–18; high scores indicate worse QoL | Improvement: ≥1.8 |
| EQ‐5D 27, 40 | Assesses health status; the first section of the questionnaire has 5 questions (regarding mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression), and the second section has a health state assessment using a VAS | Each dimension has 3 levels (no problems, some problems, and major problems) | Improvement: 10.0 points |
| FACIT‐F 28, 29 | Assesses self‐reported fatigue and its impact on daily activities and function; consists of a 13‐item questionnaire evaluated on a 5‐point scale | Range 0–4 points, where 0 = not at all and 4 = very much | Improvement: ≥4 points |
| WPAI‐GH 30 | Six questions are evaluated; each has unique response options, and 4 outcome scores can be derived (percent work time missed due to health, percent impairment while working due to health, percent overall work impairment due to health, and percent activity impairment due to health) | Expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity | Not available |
| BASFI 26, 39 | Measures self‐reported functional status using a set of 10 questions designed to determine the degree of functional limitation in patients with AS | The mean of 10 scales gives the BASFI score, a value between 0 and 10, where 0 = no restriction of function and 10 = maximum restriction of function | ≥7 mm or 17.5% |
| BASDAI 23, 39 | Measures self‐reported disease activity, using 2 VAS to measure the effect of AS on the respondent's well‐being, the first estimated over the last week, the second over the last 6 months | Range 0–10 points, where 0 = no problem and 10 = worst problem | ≥10 mm or 22.5% |
MCID = minimum clinically important difference; SF‐36 = Short Form 36 health survey; PCS = physical component summary; MCS = mental component summary; HRQoL = health‐related quality of life; ASQoL = Ankylosing Spondylitis Quality of Life; AS = ankylosing spondylitis; EQ‐5D = EuroQol 5‐domain; VAS = visual analog scale; FACIT‐F = Functional Assessment of Chronic Illness Therapy–Fatigue; WPAI‐GH = Work Productivity and Activity Impairment–General Health; BASFI = Bath Ankylosing Spondylitis Functional Index; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index.
BASDAI and BASFI were assessed at baseline and at weeks 1, 2, and 4, then every 4 weeks to week 32, and then at weeks 40 and 52. All other patient‐reported outcomes were assessed at baseline and weeks 4, 8, 12, 16, 24, and 52, except for the WPAI‐GH, which was assessed at baseline and weeks 16, 24, and 52.
Statistical analysis
Sample size calculation and detailed statistical analyses for primary and secondary end points have been reported previously 21. All of the patient‐reported outcomes were analyzed in the full analysis set that comprised all patients from the randomized set who had been assigned to receive study treatment. The difference between secukinumab and placebo treatment for continuous variables at weeks 16 and 52 in patient‐reported outcomes (except WPAI‐GH, which was analyzed using observed data) were analyzed using mixed‐effects model repeated measures (MMRM), with treatment groups, visit, and anti‐TNF status as factors, and respective baseline score and weight as covariates. Treatment‐by‐visit and respective baseline score–by‐visit were included as interaction terms. An unstructured covariance structure was assumed for the model. The significance of the treatment effects for secukinumab regimens at different analysis visits was determined from the pairwise comparisons performed between secukinumab regimens and placebo.
A subgroup analysis assessed BASDAI, SF‐36 PCS, and ASQoL, which were part of the predefined testing strategy, according to previous anti‐TNF status (patients who were naive for anti‐TNF therapy or those with a history of inadequate response to or intolerance of these agents). The week 16 results were analyzed using MMRM. The week 52 results are provided as observed data. Additionally, at weeks 16 and 52, the least squares mean (LSM) change from baseline in the total BASDAI score was assessed as a function of baseline high‐sensitivity C‐reactive protein (hsCRP) level (≤10 mg/liter and >10 mg/liter) using MMRM, with treatment groups, study visit, and anti‐TNF status as factors, and baseline BASDAI score and weight as covariates. Treatment‐by‐visit and baseline BASDAI score–by‐visit were included as interaction terms.
For the BASDAI 50, SF‐36 PCS, and SF‐36 MCS responder analyses, treatment groups were compared with respect to response to treatment using a logistic regression model, with treatment and anti‐TNF status as factors and baseline scores (for SF‐36 scores [PCS or MCS]) and weight as covariates. In addition, odds ratios (ORs) with corresponding 95% confidence intervals were estimated for ASAS20, ASAS40, BASDAI 50, SF‐36 PCS, and SF‐36 MCS.
RESULTS
Characteristics of the patients
Between November 9, 2011 and January 21, 2013, a total of 371 patients with AS were randomized to 1 of the 3 treatment groups: secukinumab IV→150 mg (n = 125), secukinumab IV→75 mg (n = 124), or placebo (n = 122). A country‐specific breakdown of enrolled patients is available upon request from the corresponding author. Of the 371 patients randomized, 351 (94.6%) remained in the study at week 16 and 319 (86.0%) remained in the study at week 52.
Baseline demographics, disease characteristics, and prior or concomitant medication use were similar across study groups (Table 2) and have been reported in detail elsewhere 21. Mean SF‐36 PCS scores at baseline ranged from 36.3 to 37.6 across the treatment groups, and ASQoL scores ranged from 10.8 to 11.7, indicating impaired physical function and HRQoL (Table 3).
Table 2.
Demographic and baseline characteristics of the patients with AS (full analysis set)*
| Secukinumab IV→150 mg (n = 125) | Secukinumab IV→75 mg (n = 124) | Placebo (n = 122) | |
|---|---|---|---|
| Age, mean ± SD years | 40.1 ± 11.6 | 42.3 ± 13.2 | 43.1 ± 12.4 |
| Sex, no. (%) male | 84 (67.2) | 88 (71.0) | 85 (69.7) |
| Weight, mean ± SD kg | 74.7 ± 16.2 | 77.7 ± 19.6 | 76.7 ± 14.4 |
| Race, no. (%) | |||
| White | 69 (55.2) | 76 (61.3) | 81 (66.4) |
| Asian | 21 (16.8) | 23 (18.5) | 19 (15.6) |
| American Indian or Alaska Native | 8 (6.4) | 3 (2.4) | 3 (2.5) |
| Other | 27 (21.6) | 22 (17.7) | 19 (15.6) |
| Time since AS diagnosis, mean ± SD years | 6.5 ± 6.9 | 7.9 ± 9.7 | 8.3 ± 8.9 |
| HLA–B27 positive, no. (%) | 86 (68.8) | 99 (79.8) | 90 (73.8) |
| Anti‐TNF naive, no. (%) | 92 (73.6) | 90 (72.6) | 89 (73.0) |
AS = ankylosing spondylitis; IV = intravenous; anti‐TNF = anti–tumor necrosis factor.
Table 3.
Change in patient‐reported outcomes from baseline to week 16 and week 52 in patients with ankylosing spondylitis, according to treatment groupa
| Secukinumab IV→150 mg (n = 125) | Secukinumab IV→75 mg (n = 124) | Placebo (n = 122) | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient‐reported outcome | Baseline | Change from baseline to week 16 | Change from baseline to week 52 | Baseline | Change from baseline to week 16 | Change from baseline to week 52 | Baseline | Change from baseline to week 16 |
| BASDAI | 6.3 ± 1.6 | −2.3 ± 0.2b | −2.8 ± 0.2 | 6.0 ± 1.4 | −2.3 ± 0.2c | −2.7 ± 0.2 | 6.5 ± 1.5 | −0.6 ± 0.2 |
| SF‐36 PCS | 36.8 ± 6.8 | 5.6 ± 0.6b | 6.7 ± 0.6 | 37.6 ± 6.4 | 5.6 ± 0.6c | 6.6 ± 0.6 | 36.3 ± 6.4 | 1.0 ± 0.6 |
| SF‐36 MCS | 40.0 ± 10.5 | 3.4 ± 0.8d | 4.5 ± 0.8 | 41.5 ± 10.2 | 3.3 ± 0.8d | 5.5 ± 0.8 | 39.2 ± 10.2 | 0.6 ± 0.9 |
| ASQoL | 10.9 ± 4.7 | −3.6 ± 0.4b | −4.4 ± 0.4 | 10.8 ± 4.9 | −3.6 ± 0.4c | −4.2 ± 0.4 | 11.7 ± 4.2 | −1.0 ± 0.4 |
| BASFI | 5.6 ± 2.2 | −1.8 ± 0.2¶ | −2.2 ± 0.2 | 5.4 ± 2.2 | −1.7 ± 0.2¶ | −1.9 ± 0.2 | 5.8 ± 2.0 | −0.4 ± 0.2 |
| EQ‐5D health state assessment | 45.2 ± 19.9 | 13.3 ± 1.9¶ | 16.4 ± 1.9 | 47.1 ± 18.6 | 15.2 ± 1.9¶ | 19.4 ± 1.9 | 46.5 ± 20.5 | 2.0 ± 2.0 |
| FACIT‐F | 25.6 ± 10.7 | 6.8 ± 0.8e | 9.1 ± 0.8 | 27.5 ± 9.6 | 6.6 ± 0.9e | 7.5 ± 0.8 | 24.5 ± 9.4 | 2.5 ± 0.9 |
| WPAI‐GH | ||||||||
| % work time missed due to health | 11.6 ± 21.6 | −1.0 ± 21.5 | −2.1 ± 22.9 | 7.2 ± 16.0 | −3.9 ± 12.0 | −2.8 ± 11.7 | 15.3 ± 25.7 | 1.9 ± 22.4 |
| % impairment while working due to health | 45.3 ± 24.1 | −20.1 ± 24.8 | −20.2 ± 23.1 | 42.0 ± 23.9 | −15.1 ± 24.7 | −20.5 ± 21.6 | 51.7 ± 18.7 | −12.8 ± 26.0 |
| % overall work impairment due to health | 49.7 ± 26.2 | −20.8 ± 26.1 | −21.2 ± 24.5 | 44.1 ± 25.4 | −16.1 ± 25.8 | −20.1 ± 23.8 | 56.7 ± 19.8 | −10.2 ± 27.0 |
| % activity impairment due to health | 56.7 ± 23.9 | −18.7 ± 25.9 | −25.4 ± 25.7 | 55.6 ± 22.2 | −20.2 ± 25.9 | −24.8 ± 24.1 | 58.9 ± 21.3 | −7.0 ± 27.2 |
For the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Short Form 36 (SF‐36) physical component summary (PCS) score, SF‐36 mental component summary (MCS) score, Ankylosing Spondylitis Quality of Life (ASQoL) questionnaire, Bath Ankylosing Spondylitis Functional Index (BASFI), EuroQol 5‐domain questionnaire (EQ‐5D), and Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F), the baseline values are the mean ± SD from observed data, and the week 16 and week 52 values are the least squares mean ± SEM from mixed‐effects model repeated measures 21. For Work Productivity and Activity Impairment–General Health (WPAI‐GH), the baseline, week 16, and week 52 values are the mean ± SD from observed data.
P < 0.0001 versus placebo, adjusted for multiple testing.
P < 0.001 versus placebo, adjusted for multiple testing.
P < 0.05 versus placebo, adjusted for multiple testing.
P < 0.0001 versus placebo.
P < 0.001 versus placebo.
Patient‐reported outcomes
At week 16, improvements in the BASDAI score were significantly greater in patients receiving either regimen of secukinumab than in those receiving placebo 21. The LSM changes in both secukinumab regimens also exceeded MCID values (Table 1). Additionally, the OR (>1) favored a higher BASDAI 50 response with both secukinumab regimens versus placebo (Figure 1).
Figure 1.
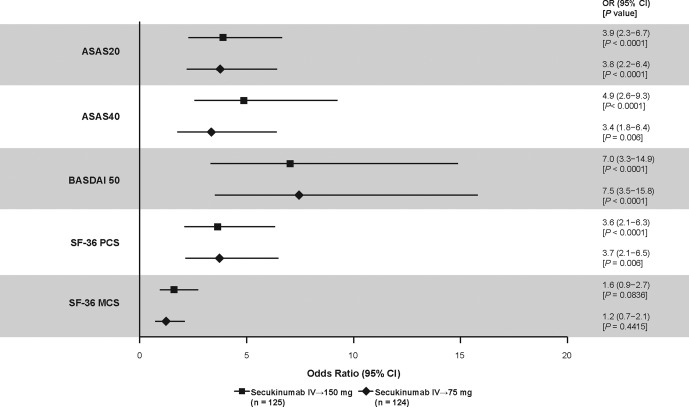
Odds ratios (ORs) for Assessment of SpondyloArthritis international Society criteria for 20% improvement in disease activity (ASAS20), ASAS40, Bath Ankylosing Spondylitis Disease Activity Index criteria for 50% improvement (BASDAI 50), and Short Form 36 (SF‐36) physical component summary (PCS) and mental component summary (MCS) responses at week 16 in AS patients treated with secukinumab versus those treated with placebo. ASAS20/40 and SF‐36 PCS were analyzed as part of a predefined hierarchical testing strategy, with P values adjusted for multiple testing; P values for BASDAI 50 and SF‐36 MCS are unadjusted. Missing data were imputed as nonresponse. 95% CI = 95% confidence interval; IV = intravenous.
Improvements in the total BASDAI score were sustained through week 52 (Figure 2A and Table 3) 21. Furthermore, at week 16, LSM change from baseline in the BASDAI score was greater in patients treated with secukinumab than in those treated with placebo regardless of hsCRP level at baseline. In patients with hsCRP levels ≤10 mg/liter, LSM ± SEM changes from baseline to week 16 were −1.9 ± 0.2 in those treated with secukinumab IV→150 mg and −2.2 ± 0.2 in those treated with secukinumab IV→75 mg versus 0.1 ± 0.4 in those treated with placebo. In patients with hsCRP levels >10 mg/liter, LSM ± SEM changes from baseline to week 16 were −2.9 ± 0.2 in those treated with secukinumab IV→150 mg and −2.5 ± 0.2 in those treated with secukinumab IV→75 mg versus 0.5 ± 0.4 in those treated with placebo. These improvements from baseline in BASDAI score were mostly sustained at week 52 in patients treated with secukinumab who had hsCRP levels ≤10 mg/liter (LSM ± SEM changes of −2.5 ± 0.2 with secukinumab IV→150 mg and −2.7 ± 0.2 with secukinumab IV→75 mg) and those who had hsCRP levels >10 mg/liter (LSM ± SEM changes of −3.3 ± 0.3 with secukinumab IV→150 mg and −2.8 ± 0.3 with secukinumab IV→75 mg).
Figure 2.
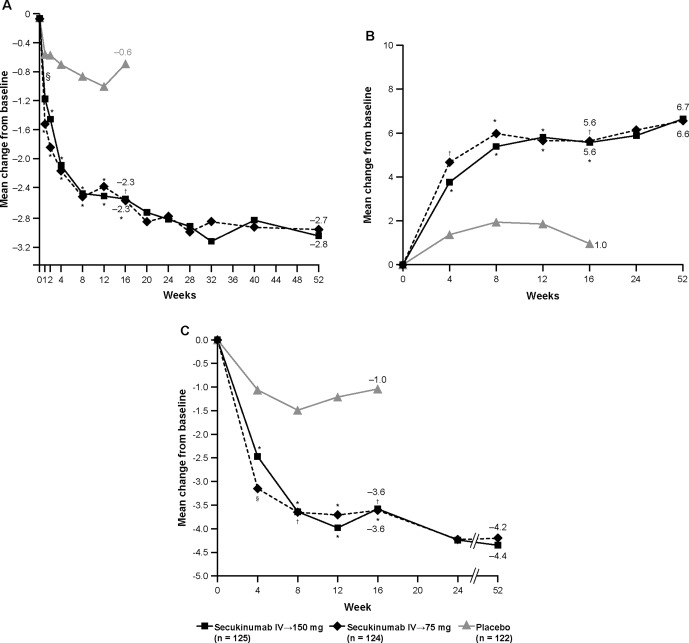
Mean change from baseline through week 52 in the Bath Ankylosing Spondylitis Disease Activity Index (A), Short Form 36 physical component summary score (B), and Ankylosing Spondylitis Quality of Life questionnaire (C). Least squares mean data are from mixed‐effects model repeated measures through week 52. P values at week 16 were adjusted for multiple testing. ∗ = P < 0.0001; † = P < 0.001; § = P < 0.01, versus placebo. IV = intravenous.
Patients treated with secukinumab also showed improvements in the total BASDAI score at weeks 16 and 52 (21) irrespective of anti‐TNF status (naive versus inadequate response). At week 16, LSM ± SEM changes from baseline in anti‐TNF–naive patients were −2.7 ± 0.2 in patients treated with secukinumab IV→150 mg and −2.6 ± 0.2 in patients treated with secukinumab IV→75 mg versus −0.7 ± 0.2 in patients treated with placebo (both P < 0.0001). For patients with an inadequate response to anti‐TNF agents, changes from baseline to week 16 were −1.7 ± 0.3 in those treated with secukinumab IV→150 mg and −2.2 ± 0.3 in those treated with secukinumab IV→75 mg versus −0.7 ± 0.3 in those treated with placebo (P < 0.05 for secukinumab IV→150 mg versus placebo and P < 0.01 for secukinumab IV→75 mg versus placebo). At week 52, further improvements in the BASDAI score were observed in patients treated with secukinumab who were anti‐TNF naive (mean ± SD change from baseline −3.3 ± 2.3 with secukinumab IV→150 mg and −2.9 ± 1.9 with secukinumab IV→75 mg) and those who had an inadequate response to anti‐TNF agents (mean ± SD change 2.8 ± 1.9 with secukinumab IV→150 mg and −2.7 ± 1.9 with secukinumab IV→75 mg).
At week 16, improvements in SF‐36 PCS and ASQoL were also significantly greater in patients treated with either secukinumab regimen compared with those treated with placebo 21. Improvements in SF‐36 PCS and ASQoL exceeded MCID values and were sustained through 52 weeks with both secukinumab regimens (Figures 2B and C and Table 1). The OR favored higher SF‐36 PCS and MCS responses in patients treated with either secukinumab regimen versus those treated with placebo, although the P values for SF‐36 MCS were >0.05 for both secukinumab regimens (Figure 1). Greater ASAS20 and ASAS40 response rates with secukinumab versus placebo 21 were also indicated by the ORs (>1 for both parameters), which are also shown for comparison (Figure 1).
Both anti‐TNF–naive patients and those with an inadequate response to anti‐TNF showed improvements in SF‐36 PCS and ASQoL. For anti‐TNF–naive patients, LSM ± SEM changes in SF‐36 PCS from baseline to week 16 were 6.9 ± 0.6 in those treated with secukinumab IV→150 mg and 6.1 ± 0.7 in those treated with secukinumab IV→75 mg versus 1.3 ± 0.7 in those treated with placebo (both P < 0.0001). For patients with an inadequate response to anti‐TNF agents, LSM ± SEM changes in SF‐36 PCS from baseline to week 16 were 3.6 ± 1.2 in those treated with secukinumab IV→150 mg and 6.5 ± 1.2 in those treated with secukinumab IV→75 mg versus 2.0 ± 1.3 in those treated with placebo (P = 0.35 for secukinumab IV→150 mg versus placebo and P < 0.05 for secukinumab IV→75 mg versus placebo). At week 52, further improvement in SF‐36 PCS was observed with secukinumab IV→150 mg in patients in both subgroups and with secukinumab IV→75 mg in anti‐TNF–naive patients. The mean ± SD change from baseline to week 52 was 8.3 ± 7.4 in anti‐TNF–naive patients treated with secukinumab IV→150 mg, 7.1 ± 6.2 in anti‐TNF–naive patients treated with secukinumab IV→75 mg, 4.9 ± 6.2 in patients with an inadequate response to anti‐TNF agents treated with secukinumab IV→150 mg, and 6.8 ± 7.8 in patients with an inadequate response to anti‐TNF agents treated with secukinumab IV→75 mg.
The LSM ± SEM changes from baseline to week 16 in ASQoL in the anti‐TNF–naive subgroup were −4.4 ± 0.5 in patients treated with secukinumab IV→ 150 mg and −3.7 ± 0.5 in patients treated with secukinumab IV→75 mg versus −1.3 ± 0.5 in patients treated with placebo (P < 0.0001 for secukinumab IV→150 mg versus placebo and P < 0.001 for secukinumab IV→75 mg versus placebo). In the subgroup of patients with an inadequate response to anti‐TNF, the LSM ± SEM changes from baseline to week 16 were −1.9 ± 0.9 in patients treated with secukinumab IV→150 mg and −4.4 ± 0.9 in patients treated with secukinumab IV→75 mg versus −1.0 ± 0.9 in patients treated with placebo (P = 0.47 for secukinumab IV→150 mg versus placebo and P < 0.01 for secukinumab IV→75 versus placebo). These scores were similar or improved with both secukinumab regimens at week 52. The mean ± SD change from baseline to week 52 was −5.0 ± 5.3 in anti‐TNF–naive patients treated with secukinumab IV→150 mg, −4.1 ± 4.3 in anti‐TNF–naive patients treated with secukinumab IV→75 mg, −3.4 ± 3.9 in patients with an inadequate response to anti‐TNF agents treated with secukinumab IV→150 mg, and −5.7 ± 5.3 in patients with an inadequate response to anti‐TNF agents treated with secukinumab IV→75 mg.
Mean changes from baseline to week 16 for the BASFI (Figure 3), EQ‐5D, and FACIT‐F were greater in patients treated with either secukinumab regimen than in those treated with placebo (Table 3). Improvements in BASFI and EQ‐5D also exceeded MCID values in patients treated with secukinumab (Table 1).
Figure 3.
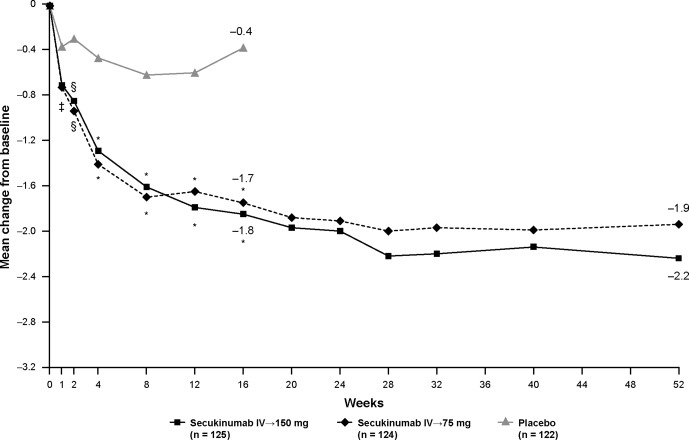
Mean change from baseline through week 52 in the Bath Ankylosing Spondylitis Functional Index. Least squares mean data are from mixed‐effects model repeated measures through week 52. ∗ = P < 0.0001; § = P < 0.01; ‡ = P < 0.05 versus placebo. IV = intravenous.
The percent of work time missed due to health decreased from baseline to week 16 in patients treated with secukinumab and increased in patients treated with placebo (−1.0% in patients treated with secukinumab IV→150 mg and −3.9% in patients treated with secukinumab IV→75 mg versus 1.9% in patients treated with placebo). Similarly, percentage improvements from baseline to week 16 in all other WPAI‐GH outcomes (impairment while working due to health, overall work impairment due to health, and activity impairment due to health) were also greater in patients treated with secukinumab than in those treated with placebo. All of these outcomes were sustained or further improved through week 52 in both secukinumab groups.
DISCUSSION
The 52‐week results from the MEASURE 1 study showed significant and sustained improvements in the signs and symptoms of AS with secukinumab 21. The patient‐reported outcomes assessed in MEASURE 1 showed that in addition to significant and sustained improvement in the signs and symptoms of AS 21, patients treated with secukinumab showed statistically and clinically significant improvements in multiple facets of physical functioning and HRQoL at week 16 compared with those treated with placebo. The improvements were sustained in the secukinumab regimens over the long term, i.e., through week 52.
These results are clinically meaningful, since patients with active AS experience poor HRQoL due to back pain, discomfort, and fatigue, which ultimately restricts their physical function and work productivity 31. The significant improvements observed with both secukinumab regimens versus placebo at week 16 in BASDAI, SF‐36 PCS, and ASQoL 21 were maintained irrespective of the baseline anti‐TNF status of the patients. Improvements in BASDAI scores were also better with secukinumab versus placebo at week 16 regardless of baseline hsCRP level and were sustained up to week 52. All improvements observed at week 16 were sustained through week 52, and the OR favored better responses with the 2 secukinumab regimens versus placebo.
Both secukinumab regimens provided improvements in BASFI scores and all 5 domains of health status on the EQ‐5D in comparison to placebo, suggesting improvements in the physical function and health status of the patients receiving secukinumab.
Patients with AS frequently experience fatigue due to pain, stiffness, and poor sleep 32, 33. Although the impact of fatigue on patients with AS has not been a prominent focus of clinical research in the past, recent research has established the impact of treatment on this important patient‐reported outcome 34, 35. However, only a few AS studies have directly assessed fatigue using a focused tool, such as the FACIT‐F scale, and considered fatigue as a major symptom in the majority of patients with AS 32, 36, 37. In the current trial, secukinumab treatment resulted in a greater reduction in fatigue and impact of AS on daily activities and function at week 16 than placebo, as measured by the FACIT‐F scale. Moreover, the higher maintenance dose used in the secukinumab IV→150 mg arm resulted in further reductions in FACIT‐F score as well as improvements in daily activities and function at week 52.
The disabling nature of AS may also lead to premature withdrawal from active employment and a decrease in work productivity 38. In our study, greater reductions in work or activity impairment at week 16, as assessed by WPAI‐GH, were observed with secukinumab than with placebo, and sustained or further improvements were noted at week 52.
A limitation of this study concerns the methodology used to assess the statistical significance of differences in mean patient‐reported outcome score changes across groups. Although the use of all patient‐reported outcome assessments was prespecified in the study, only the changes from baseline in the BASDAI, SF‐36 PCS, and ASQoL were included in the predefined hierarchical testing strategy that accounted for increases in Type I error due to multiple testing. However, consistent trends in improvements across multiple patient‐reported outcome measures assessing several disease dimensions reflect the clinically meaningful impact of secukinumab treatment on AS.
Secukinumab is the first biologic agent other than TNF inhibitors to demonstrate significant improvements in the signs and symptoms of AS in a phase III trial. The additional results from MEASURE 1 presented here build on these findings to show that secukinumab provided significant and sustained improvements in patient‐reported disease activity, HRQoL, functional impairment, physical and mental health status, fatigue levels, and work productivity in patients with active AS.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Deodhar had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Deodhar, Dougados, Baeten, Wei, Geusens, Readie, Richards, Martin, Porter.
Acquisition of data. Deodhar, Dougados, Baeten, Wei, Geusens, Readie, Richards, Martin, Porter.
Analysis and interpretation of data. Deodhar, Dougados, Baeten, Wei, Geusens, Readie, Richards, Martin, Porter.
ROLE OF THE STUDY SPONSOR
The study was designed by the scientific steering committee and Novartis personnel. All authors had access to the data, contributed to its interpretation, and collaborated in the development of the manuscript. The initial draft of the manuscript was written by a medical writer employed by the study sponsor (Vasundhara Pathak, Novartis Healthcare, Hyderabad, India). All authors critically reviewed and provided feedback on subsequent versions. All authors made the decision to submit the manuscript for publication and vouch for the accuracy and completeness of the data and fidelity of this report to the study protocol. Statistical analyses were performed by statisticians employed by the study sponsor. Publication of this article was not contingent upon approval by Novartis.
ACKNOWLEDGMENTS
The authors would like to thank the patients and the investigators who participated in the study. The authors also thank John Gallagher, medical consultant for Novartis Pharma AG, Basel, Switzerland.
ClinicalTrials.gov identifier: NCT01358175.
Supported by Novartis Pharma AG (Basel, Switzerland).
Dr. Deodhar has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, Boehringer Ingelheim, Janssen, Novartis, Pfizer, Sun Pharma, and UCB (less than $10,000 each) and has testified as an expert witness on behalf of Novartis.
Dr. Dougados has received consulting fees, speaking fees, and/or honoraria from AbbVie, Bristol‐Myers Squibb, Eli Lilly, Merck, Pfizer, UCB, Roche, and Novartis (less than $10,000 each) and has received research grants from AbbVie, Bristol‐Myers Squibb, Eli Lilly, Merck, Pfizer, and Novartis.
Dr. Baeten has received research grants and consulting fees, speaking fees, and/or honoraria from Boehringer Ingelheim, Janssen, MSD, Novartis, Pfizer, AbbVie, Bristol‐Myers Squibb, Eli Lilly, Roche, and UCB (less than $10,000 each).
Dr. Wei has received research grants from AbbVie, Bristol‐Myers Squibb, Celgene, Chugai, Eisai, Janssen, Novartis, Pfizer, Sanofi‐Aventis, TSH Taiwan, and UCB Pharma.
Dr. Geusens has received speaking fees from Pfizer, Abbott, Eli Lilly, Amgen, MSD, Will‐Pharma, Bio Minerals, Roche, and Novartis (less than $10,000 each). Ms Readie and Drs. Richards and Porter own stock or stock options in Novartis.
REFERENCES
- 1. Deodhar A. Axial spondyloarthritis criteria and modified NY criteria: issues and controversies. Clin Rheumatol 2014;33:741–7. [DOI] [PubMed] [Google Scholar]
- 2. Garg N, van den Bosch F, Deodhar A. The concept of spondyloarthritis: where are we now? Best Pract Res Clin Rheumatol 2014;28:663–72. [DOI] [PubMed] [Google Scholar]
- 3. Gran J, Husby G, Hordvik M. Prevalence of ankylosing spondylitis in males and females in a young middle‐aged population of Tromsø, northern Norway. Ann Rheum Dis 1985;44:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaipiainen‐Seppanen O, Aho K, Heliovaara M. Incidence and prevalence of ankylosing spondylitis in Finland. J Rheumatol 1997;24:496–9. [PubMed] [Google Scholar]
- 5. Alamanos Y, Papadopoulos NG, Voulgari PV, Karakatsanis A, Siozos C, Drosos AA. Epidemiology of ankylosing spondylitis in Northwest Greece, 1983–2002. Rheumatology 2004;43:615–8. [DOI] [PubMed] [Google Scholar]
- 6. Dai SM, Han XH, Zhao DB, Shi YQ, Liu Y, Meng JM. Prevalence of rheumatic symptoms, rheumatoid arthritis, ankylosing spondylitis, and gout in Shanghai, China: a COPCORD study. J Rheumatol 2003;30:2245–51. [PubMed] [Google Scholar]
- 7. Hukuda S, Minami M, Saito T, Mitsui H, Matsui N, Komatsubara Y, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol 2001;28:554–9. [PubMed] [Google Scholar]
- 8. Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369:1379–90. [DOI] [PubMed] [Google Scholar]
- 9. Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos‐Vargas R, Collantes‐Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glintborg B, Ostergaard M, Krogh NS, Dreyer L, Kristensen HL, Hetland ML. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti‐tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis 2010;69:2002–8. [DOI] [PubMed] [Google Scholar]
- 11. Toussirot É. Current therapeutics for spondyloarthritis. Expert Opin Pharmacother 2011;12:2469–77. [DOI] [PubMed] [Google Scholar]
- 12. Song IH, Poddubnyy D. New treatment targets in ankylosing spondylitis and other spondyloarthritides. Curr Opin Rheumatol 2011;23:346–51. [DOI] [PubMed] [Google Scholar]
- 13. Zochling J, van der Heijde D, Burgos‐Vargas R, Collantes E, Davis JC Jr, Dijkmans B, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2006;65:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heiberg MS, Koldingsnes W, Mikkelsen K, Rødevand E, Kaufmann C, Mowinckel P, et al. The comparative one‐year performance of anti‐tumor necrosis factor α drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum 2008;59:234–40. [DOI] [PubMed] [Google Scholar]
- 15. Haibel H, Rudwaleit M, Listing J, Sieper J. Open label trial of anakinra in active ankylosing spondylitis over 24 weeks. Ann Rheum Dis 2005;64:296–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sieper J, Braun J, Kay J, Badalamenti S, Radin AR, Jiao L, et al. Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double‐blind, placebo‐controlled study (ALIGN). Ann Rheum Dis 2015;74:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowness P, Ridley A, Shaw J, Chan AT, Wong‐Baeza I, Fleming M, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA‐B27 homodimers are increased in ankylosing spondylitis. J Immunol 2011;186:2672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Caňete JD, et al. Interleukin‐17–positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum 2012;64:99–109. [DOI] [PubMed] [Google Scholar]
- 19. Mei Y, Pan F, Gao J, Ge R, Duan Z, Zeng Z, et al. Increased serum IL‐17 and IL‐23 in the patient with ankylosing spondylitis. Clin Rheumatol 2011;30:269–73. [DOI] [PubMed] [Google Scholar]
- 20. Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti‐interleukin‐17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double‐blind, placebo‐controlled trial. Lancet 2013;382:1705–13. [DOI] [PubMed] [Google Scholar]
- 21. Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin‐17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. [DOI] [PubMed] [Google Scholar]
- 22. Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 23. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 24. Ware JE Jr, Snow KK, Kosinski M, Gandek B. SF‐36 health survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 25. Doward LC, Spoorenberg A, Cook SA, Whalley D, Helliwell PS, Kay LJ, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 2003;62:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 27. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–43. [DOI] [PubMed] [Google Scholar]
- 28. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997;13:63–74. [DOI] [PubMed] [Google Scholar]
- 29. Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811–9. [PubMed] [Google Scholar]
- 30. Loeppke R, Hymel PA, Lofland JH, Pizzi LT, Konicki DL, Anstadt GW, et al. Health‐related workplace productivity measurement: general and migraine‐specific recommendations from the ACOEM Expert Panel. J Occup Environ Med 2003;45:349–59. [DOI] [PubMed] [Google Scholar]
- 31. Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl 2006;78:4–11. [PubMed] [Google Scholar]
- 32. Aissaoui N, Rostom S, Hakkou J, Berrada Ghziouel K, Bahiri R, Abouqal R, et al. Fatigue in patients with ankylosing spondylitis: prevalence and relationships with disease‐specific variables, psychological status, and sleep disturbance. Rheumatol Int 2012;32:2117–24. [DOI] [PubMed] [Google Scholar]
- 33. Deodhar A, Braun J, Inman RD, Mack M, Parasuraman S, Buchanan J, et al. Golimumab reduces sleep disturbance in patients with active ankylosing spondylitis: results from a randomized, placebo‐controlled trial. Arthritis Care Res (Hoboken) 2010;62:1266–71. [DOI] [PubMed] [Google Scholar]
- 34. Brophy S, Davies H, Dennis MS, Cooksey R, Husain MJ, Irvine E, et al. Fatigue in ankylosing spondylitis: treatment should focus on pain management. Semin Arthritis Rheum 2013;42:361–7. [DOI] [PubMed] [Google Scholar]
- 35. Hammoudeh M, Zack DJ, Li W, Stewart VM, Koenig AS. Associations between inflammation, nocturnal back pain and fatigue in ankylosing spondylitis and improvements with etanercept therapy. J Int Med Res 2013;41:1150–9. [DOI] [PubMed] [Google Scholar]
- 36. Revicki DA, Rentz AM, Luo MP, Wong RL. Psychometric characteristics of the short form 36 health survey and functional assessment of chronic illness Therapy‐Fatigue subscale for patients with ankylosing spondylitis. Health Qual Life Outcomes 2011;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pathan E, Abraham S, van Rossen E, Withrington R, Keat A, Charles PJ, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in ankylosing spondylitis. Ann Rheum Dis 2013;72:1475–80. [DOI] [PubMed] [Google Scholar]
- 38. Boonen A, Chorus A, Miedema H, van der Heijde D, Landewe R, Schouten H, et al. Withdrawal from labour force due to work disability in patients with ankylosing spondylitis. Ann Rheum Dis 2001;60:1033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS‐G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ‐S). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S47–58. [DOI] [PubMed] [Google Scholar]
- 40. EuroQol Group. EuroQol: a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]


