ABSTRACT
Although some reports on neurostimulation are positive, no effective treatment method for camptocormia in Parkinson's disease (PD) is known to date. We aim to identify prognostic factors for a beneficial DBS effect on camptocormia. In an observational cohort study, we investigated 25 idiopathic PD patients, who suffered additionally from camptocormia, and underwent bilateral neurostimulation of the subthalamic nucleus (STN) to improve classical PD symptoms. Using an established questionnaire, we examined deep brain stimulation (DBS) effects on camptocormia in addition to general neurostimulation effects. A beneficial neurostimulation effect on camptocormia was defined as an improvement in the bending angle of a least 50%. In 13 patients, the bending angle of camptocormia improved, in 12 patients it did not. A multifactorial analysis revealed a short duration between onset of camptocormia and start of neurostimulation to be the relevant factor for outcome. All patients with duration of camptocormia up to 1.5 years showed a beneficial effect; patients between 1.5 and ∼3 years showed mixed results, but none with a duration of more than 40 months improved except for 1 patient whose camptocormia was levodopa responsive. The bending angle was not a prognostic factor. Our data indicate that the main prognostic factor for a beneficial DBS effect on camptocormia is its short duration. As an explanation, we suggest that neurostimulation may improve camptocormia only as long as muscle pathology is limited. Our findings may help to elucidate the mode of action of neurostimulation. A prospective study is necessary. © 2015 International Parkinson and Movement Disorder Society
Keywords: Parkinson's disease, camptocormia, deep brain stimulation, nucleus subthalamicus, proprioception
Camptocormia (from the Greek “kamptein”=to bend and “kormos”=trunk) is an anterior flexion of the thoracolumbar spine with or without laterodeviation, which is a most disabling symptom for patients and has negative effects, particularly for their social interactions.1 Camptocormia occurs in nearly 10% of Parkinson's disease (PD) patients.2 It is important to distinguish it from stooped posture, which is a common manifestation of PD that can be aggravated in OFF phases, and from OFF dystonia as well. The deep brain stimulation (DBS) effect on stooped posture might not to be separated from pharmacological effects on the bending ankle. In some patients, a levodopa‐responsive camptocormia was observed.3
We recently reported that camptocormia in PD is associated with a myopathy that can be defined by pathological core features. Camptocormia in PD showed a consistent lesion pattern composed of myopathic changes with type 1 fiber hypertrophy, loss of type 2 fibers, loss of oxidative enzyme activity, and acid phosphatase reactivity of lesions.4 Ultrastructurally, myofibrillar disorganization and Z‐band streaming up to electron‐dense patches/plaques were observed in the lesions. The myopathological changes may explain the loss of muscle strength given that they show a disorganization of myofibers and, later on, an intrafascicular fibrosis and fatty degeneration.4 It is unknown whether this myopathy is primary or secondary.
Attempts to treat camptocormia in PD have mostly been unsuccessful. Camptocormia in PD is usually not responsive to l‐dopa, dopamine agonists, or anticholinergics.5 Injections of botulinum toxin have been reported to be successful,5 but have not yet been confirmed by other studies. Physiotherapy may improve camptocormia at the beginning of symptoms for some time, but is without long‐lasting effects.6 Backpack therapy,7 orthoses, or surgical stabilization were not able to revert or even stop the syndrome.6
Neurostimulation of the subthalamic nucleus (STN) does improve the core symptoms of PD.8 However, the results of neurostimulation on camptocormia were inconsistent. Here, we aim to identify prognostic factors for the DBS effect on camptocormia.
Patients and Methods
Participants
The patients in this study suffered from idiopathic PD and camptocormia and were retrospectively assessed in 2011‐2013. The diagnosis of PD was made according to the UK Parkinson's Disease Society Brain Bank criteria.9 Camptocormia is defined here as a marked anterior flexion of the thoracolumbar spine of at least 30 degrees with or without laterodeviation,1 appearing in the standing or walking position under optimal anti‐PD medication, and disappearing in the recumbent position, but without any signs of a fixed kyphosis as in osteoporotic kyphosis. Absence of clinically detectable flexor dystonia was required. In our experience, an anterior flexion of at least 30 degrees served as the best parameter to separate camptocormia patients from those suffering from stooped posture. No improvement of the camptocormia was observed by optimizing the anti‐PD medication. Informed written consent for participation was given by all patients, and the study was approved by the ethical committee of the Medical Faculty Kiel.
Instruments and Design
Patients and their caregivers were interviewed using a standardized questionnaire.1 Onset of PD was defined by prescribing the first anti‐PD medication and onset of camptocormia by the diagnosis of a neurologist, photodocumentation, or reports from the patients' family. Severity of clinical symptoms was scored as previously described,1 in particular by visual analog scales (VAS; 0‐10, 10 most severe complaints) for back pain and handicaps resulting from camptocormia. Questions pertaining to the effects of neurostimulation on PD‐ and camptocormia‐related symptoms were included, and the response to symptoms of camptocormia in relation to general DBS response was noted. Severity of motor symptoms was rated with the UPDRS‐III (baseline compared to conditions 6‐12 months after implantation). The bending angle was taken from clinical examinations (medical records) or measured from photos or video freeze frames. A positive DBS effect on camptocormia was defined by an improvement in the bending angle of at least 50%. Individual camptocormia‐related complaints and the DBS effect on these complaints were documented.
Multivariate analysis was used to detect factors that influence the DBS effect on camptocormia. Factors that were correlated with the improvement of the bending angle by DBS were: age and sex of the patients; duration of PD before DBS; duration of camptocormia before DBS; UPDRS‐III before DBS; bending angle of camptocormia before DBS; l‐dopa equivalent dose before DBS; VAS of pain before DBS; VAS of handicaps resulting from camptocormia before and after DBS; and the effect of l‐dopa (using regular l‐dopa doses) on the UPDRS‐III item 28 (posture, score: 0‐4) during the preoperative test.
Statistical Analysis
Multivariate analysis was done using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA).
Results
Patient Characteristics
We investigated 4 women and 21 men with an average age of 67 years (range: 54‐83). The duration of PD was 3 to 27 years with a mean of 16 years at the time of investigation (Table 1). Our patients developed camptocormia 10.2 years (mean, range: 1‐26 years) after the diagnosis of PD. The bending angle was between 30 and 90 degrees with an average of 53 degrees. Twenty‐three of the twenty‐five patients also showed a laterodeviation (19 to the right, 4 to the left), but no patient suffered from a laterodeviation without marked forward bending.
Table 1.
Characterization of Patients
| Variables | All patients (n = 25) | Responders (n = 13) | Nonresponders (n = 12) |
|---|---|---|---|
| Age at assessment | 67.1 (54‐83) | 65.8 (54‐72) | 68.6 (59‐83) |
| Age at onset of PD | 50.3 (35‐62) | 49.8 (35‐62) | 50.8 (44‐62) |
| Sex (male/female) | 21/4 | 11/2 | 10/2 |
| Total duration of PD, years | 15.4 (3‐27) | 14.7 (3‐27) | 17 (12‐25) |
| Period of PD without CC, years | 10.2 (1‐26) | 10.7 (1‐26) | 9.6 (6‐17) |
| Duration of CC until surgery, months | 35 (8‐90) | 19.8 (8‐61) | 51.4 (21‐90) |
| Interval between DBS and last assessment, months | 30.9 (6‐66) | 30.0 (7‐66) | 31.9 (6‐64) |
| Bending angle before DBS | 53.2 (30‐90) | 52.7 (30‐90) | 53.8 (30‐90) |
| Bending angle at last assessment | 34.8 (0‐90) | 9.6 (0‐30) | 62.1 (40‐90) |
| UPDRS‐III before DBS (ON) | 22.5 (11‐37) | 21.4 (11‐34) | 24.1 (13‐37) |
| UPDRS‐III 6‐12 months after DBS (ON + stimulation) | 15.2 (6.0‐23.5) | 12.9 (6‐19) | 18.2 (15.0‐23.5) |
| LEDD before DBS | 1,044 (525‐2,250) | 926 (525‐1,600) | 1,172 (575‐2,250) |
| LEDD 6‐12 months after DBS | 561 (150‐1,365) | 544 (225‐1,250) | 580 (150‐1,365) |
Variables are expressed as mean (range). UPDRS‐III = motor examination of the UPDRS.
CC, camptocormia; LEDD = l‐dopa equivalent daily dose.
Nineteen patients reported retrospectively to have suffered from back pain of the lumbal region before neurostimulation. The pain was characterized as stabbing, burning, spasmodic, or obtuse. Its intensity, when present, was estimated, on average, as 6.9 on the VAS.
Effect of Neurostimulation on Camptocormia
All patients underwent bilateral DBS of the STN between 8 and 90 months after onset of the camptocormia, and 1 patient additionally underwent pallidal stimulation without effect on camptocormia. Twenty‐three of twenty‐five patients reported that their PD‐related symptoms improved after DBS. The mean UPDRS‐III in the medication ON condition improved from 22.5 preoperatively to 15.2 postoperatively (medication ON/stimulation ON). The l‐dopa equivalent daily dose (LEDD)10 could be reduced, on average, by 46% within 6 to 12 months after DBS.
At investigation, the bending angle had improved between 30 and 70 degrees (67%–100%) in 11 patients and by 15 and 20 degrees in 2 additional patients who showed a mild camptocormia of 30 degrees. The laterodeviation improved in parallel to the bending angle. In 11 of the 13 responders, no worsening of the bending angle was reported during an observation period of 7 to 66 months (Table 1), but 2 patients with a residual bending angle of 15 degrees stated that they had been completely upright in the first years after DBS. In 12 patients, no improvement was observed, and 6 of these even demonstrated a worsening of their camptocormia at 6 to 66 months' follow up. No worsening of the camptocormia directly related to DBS was reported by the patients or noticed in the medical records.
The VAS for handicaps resulting from camptocormia improved from 7.1 to 3.7 (48%), and the VAS of pain was reduced from 6.9 to 4.2 (39.9%). To further evaluate the DBS effect, we asked the patients for their major individual complaint related to the camptocormia and its change after DBS. Typical complaints were: inability to drive a car (because of the inability to look backward); inability to look people in the eyes; inability to carry something in front of the body; inability to pick up things that lay higher than the table; and problems with drinking or breathing. Individual camptocormia‐related complaints in 12 of 13 camptocormia‐responsive patients improved (information in 1 patient was missing). In the 12 patients without improvement of the bending angle, their individual complaints did not improve at all, or even worsened (Supporting Table 2).
Multifactorial Analysis of the DBS Effect on Camptocormia
There was a strong negative correlation between the duration of the camptocormia before neurostimulation and the improvement of the bending angle after DBS (r2 = 0.58; P < 0.0001; Fig. 1A). All patients with duration of camptocormia of less than 1.5 years improved, but only 1 patient with longer camptocormia duration (over 40 months) improved substantially. Conversely, the subjective impact of camptocormia was more severe when neurostimulation was applied later after onset of camptocormia (Fig. 1B). A strong correlation between the objective improvement of the bending angle and the subjective VAS of handicap after neurostimulation could be observed (r2 = 0.72; P < 0.0001). There was no correlation between the improvement of the bending angle and duration of PD, UPDRS‐III, bending angle before DBS, sex, age of patient, or the LEDD at baseline. An improvement by a maximum of 1 point of UPDRS‐III item 28 during the preoperative l‐dopa challenge, as it was rated for 9 of 21 patients, was not a predictive factor of improvement in the bending angle after DBS.
Figure 1.
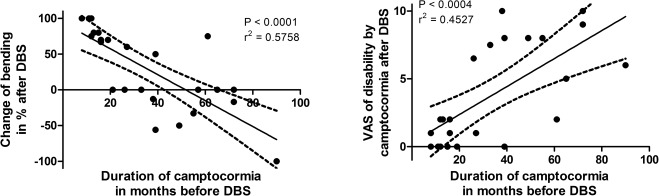
An improvement of camptocormia correlates negatively with the duration of the symptom. The shorter the duration of camptocormia before neurostimulation, the better is the DBS effect (A). The same correlation is reflected by the restrictions in daily activities resulting from the camptocormia. For long‐lasting camptocormia before neurostimulation, the camptocormia‐related disabilities are more severe than in short symptom duration before neurostimulation (B). The observation period of the DBS effect is 31 months in the mean (see Table 1). R2 = coefficient of determination, which describes the quality of the correlation r.
Discussion
In our case series, we found a remarkably beneficial effect of DBS on camptocormia in all patients with a duration of the symptom up to 18 months, in 3 of 8 patients with a camptocormia between 18 and 39 months, but (with one exception) not in camptocormia of longer duration. The effect of DBS on camptocormia in PD has been reported for 42 patients thus far5, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 (Table 2). In 25 STN‐stimulated and 3 globus pallidus interna (GPi)‐stimulated patients, a benefit was observed, but not in the remaining 14. In 11 of the 42 patients, the duration of camptocormia before DBS was documented at between 1 and 11 years.11, 12, 16, 17, 20, 21 The 3 patients with camptocormia duration of up to 2 years before DBS all improved. In the remaining 8 patients, 3 showed mild improvement (i.e., less than 20 degrees) and 1 a cessation of the camptocormia even after 5‐year camptocormia before surgery. In this patient, the camptocormia was l‐dopa responsive, as in our patient who improved after camptocormia duration of 61 months.12 The reported cases thus far seem to fit the pattern of response of camptocormia to neurostimulation found in our cases.
Table 2.
PD Patients With Camptocormia, Who Underwent Neurostimulation
| Authors | Patients (n) | Age | DBS Target | Outcome | |
|---|---|---|---|---|---|
| Effective | Not Effective | ||||
| Schäbitz et al., 200311 | 2 | 61, 65 | STN bilateral | 0 | 2 |
| Azher and Jankovic, 20055 | 1 | STN bilateral | 0 | 1 | |
| Yamada et al., 200612 | 1 | 71 | STN bilateral | 1 | 0 |
| Hellmann et al., 200613 | 1 | 53 | STN bilateral | 1 | 0 |
| Sako et al., 200914 | 6 | 44‐60 | STN bilateral | 6 | 0 |
| Umemura et al., 201015 | 18 | 56‐79 | STN bilateral | 12 | 6 |
| Asahi et al., 201116 | 4 | 60‐69 | STN bilateral | 3 | 1 |
| Lyons et al., 201217 | 1 | 63 | STN bilateral | 1 | 0 |
| Capelle et al., 201018 | 2 | 65, 73 | STN bilateral | [1] | 1 |
| 1 | 64 | GPi bilateral | 1 | 0 | |
| Upadhyaya et al. 201019 | 1 | 59 | STN bilateral | 0 | 1 |
| 1 | 59 | GPi bilateral | 0 | 1 | |
| Micheli et al., 200520 | 1 | 62 | GPi bilateral | 1 | 0 |
| O'Riordan et al., 200921 | 2 | 62, 63 | GPi bilateral | [1] | 1 |
| Schulz‐Schaeffer et al. | 24 | 54‐83 | STN bilateral | 13 | 11 |
| 1 | 59 | STN+GPi bilateral | 0 | 1 | |
| Summary | 67 | 53‐83 | 41 | 26 | |
Brackets indicate outcome not unequivocally clear.
DBS Effect on Camptocormia
The cause of camptocormia is unknown; central and peripheral causes have been proposed.22 The myopathic changes we have reported for paraspinal muscles in PD‐camptocormia (i.e., the degree of intrafascicular fibrosis) correlate with the severity of camptocormia symptoms.4 The myopathy seems to be progressive, and in the later course leads to an intrafascicular fibrosis and fatty degeneration of the muscle.1 The dynamic of muscle pathology might explain why camptocormia can only be treated with DBS within a certain time window. Additionally, skeletal degenerative changes may develop in long‐lasting camptocormia and may contribute to the lack of improvement of camptocormia by DBS in single patients.
A challenging but unresolved question is why STN‐DBS even shows an effect on camptocormia. As reported previously, the morphological changes in the paraspinal muscles in PD‐camptocormia show remarkable similarities to those of experimental tenotomy, but these similarities were observed only when the mono‐ and polysynaptic reflex circuits of tenotomized muscles were intact. This led to the assumption that camptocormia in PD may be a symptom of proprioceptive dysregulation.4 An impairment of the proprioception in PD is known.23 DBS of the STN can improve, but obviously not fully restore the proprioceptive function.24 This may be an explanation of the DBS effect on camptocormia.
Limitations of the Study
This study has obvious limitations. It is a retrospective analysis based on a personal assessment of the patients at different time points after surgery. However, the core data obtained are reliable and the extent of the effect is well documented. The bending angle and the VAS of subjective complaints resulting from camptocormia sufficiently measure the therapy effect. Another limitation of our study is that we did not have data to correlate histopathological parameters and magnetic resonance imaging of the paraspinal muscle with the DBS outcome.
Conclusion
We aimed to identify prognostic factors for a positive DBS effect on camptocormia. The data of our observational study show that a short time period between onset of camptocormia and neurostimulation is the most important factor related to a beneficial effect of DBS on camptocormia in PD. Our hypothesis is that camptocormia leads to an impaired axial tone by proprioceptive dysregulation. In consequence, a myopathy, characterized by myofibrillar disarrangement, occurs that becomes aggravated with time by secondary changes, such as intrafascicular fibrosis and fatty degeneration. STN‐DBS may partially restore the proprioception and thus may be an efficacious treatment for camptocormia, as long as secondary myopathic changes are not yet advanced. A controlled study is needed and should include analyses of muscle morphology. The long‐term effect of DBS on camptocormia needs to be examined prospectively.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft; B. Review and Critique.
W.J.S.S.: 1A, 1C, 2A, 3A
N.G.M.: 1B, 1C, 2A, 3B
S.M.: 1B, 1C, 2C, 3B
A.W.: 1B, 2A, 2B, 3B
C.B.: 1C, 2C, 3B
G.D.: 1A, 2A, 3A
C.O.: 1A, 1B, 2C, 3C
Financial Disclosures
W.J.S.‐S. served as consultant for Bayer Healthcare and Piramal Imaging and received institutional funding from the German Research Council, the German Ministry of Health, the Alberta Prion Research Institute, Bayer Healthcare, and Piramal Imaging. N.G.M. received a travel grant from Grifols and a lecture fee from Merz Pharmazeuticals. C.B. received lecture fees from GlaxoSmithKline (GSK), Medtronic, Orion Pharma, and UCB; received institutional funding from the Georg & Jürgen Rickertsen Stiftung Hamburg; and served on scientific advisory boards for GSK and UCB Pharma. G.D. has received lecture fees from UCB, Medtronic, and Desitin; has been serving as a consultant for Medtronic, Sapiens, Boston Scientific, and Britannica; received royalties from Thieme; and is a government employee and receives through his institution funding for his research from the German Research Council, the German Ministry of Education, and Health and Medtronic. C.O. received lecture fees from UCB, Medtronic, St. Jude, and Desitin; served as consultant for Medtronic and St. Jude; and received institutional funding from MSD, UCB, Lundbeck, Meda‐Pharma, Lilly, Desitin, Teva, and Novartis.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web‐site.
Supplementary Information Table 1.
Acknowledgments
The authors thank Marita Oehlwein for her help in data acquisition and acknowledge the excellent neurosurgical skills of the universities in Kiel (Prof. M.H. Mehdorn), Hamburg (Drs. W. Hamel and J.A. Köppen), Freiburg (Prof. G. Nikkhah and Dr. M. Pinsker), and Leipzig (Dr. D. Winkler). The authors thank the patients for their participation in this study.
Relevant conflicts of interest/financial disclosures: Nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
The copyright line for this article was changed on 21 November 2016 after original online publication.
References
- 1. Margraf NG, Wrede A, Rohr A, et al. Camptocormia in idiopathic Parkinson's disease: a focal myopathy of the paravertebral muscles. Mov Disord 2010;25:542–551. [DOI] [PubMed] [Google Scholar]
- 2. Yoritaka A, Shimo Y, Takanashi M, et al. Motor and non‐motor symptoms of 1453 patients with Parkinson's disease: prevalence and risks. Parkinsonism Relat Disord 2013;19:725–731. [DOI] [PubMed] [Google Scholar]
- 3. Ho B, Prakash R, Morgan JC, Sethi KD. A case of levodopa‐responsive camptocormia associated with advanced Parkinson's disease. Nat Clin Pract Neurol 2007;3:526–530. [DOI] [PubMed] [Google Scholar]
- 4. Wrede A, Margraf NG, Goebel HH, Deuschl G, Schulz‐Schaeffer WJ. Myofibrillar disorganization characterizes myopathy of camptocormia in Parkinson's disease. Acta Neuropathol 2012;123:419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azher SN, Jankovic J. Camptocormia: pathogenesis, classification, and response to therapy. Neurology 2005;65:355–359. [DOI] [PubMed] [Google Scholar]
- 6. Finsterer J, Strobl W. Presentation, etiology, diagnosis, and management of camptocormia. Eur Neurol 2010;64:1–8. [DOI] [PubMed] [Google Scholar]
- 7. Gerton BK, Theeler B, Samii A. Backpack treatment for camptocormia. Mov Disord 2010;25:247–248. [DOI] [PubMed] [Google Scholar]
- 8. Deuschl G, Schade‐Brittinger C, Krack P, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 9. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deuschl G, Schupbach M, Knudsen K, et al. Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson's disease: concept and standards of the EARLYSTIM‐study. Parkinsonism Relat Disord 2013;19:56–61. [DOI] [PubMed] [Google Scholar]
- 11. Schäbitz WR, Glatz K, Schuhan C, et al. Severe forward flexion of the trunk in Parkinson's disease: focal myopathy of the paraspinal muscles mimicking camptocormia. Mov Disord 2003;18:408–414. [DOI] [PubMed] [Google Scholar]
- 12. Yamada K, Goto S, Matsuzaki K, et al. Alleviation of camptocormia by bilateral subthalamic nucleus stimulation in a patient with Parkinson's disease. Parkinsonism Relat Disord 2006;12:372–375. [DOI] [PubMed] [Google Scholar]
- 13. Hellmann MA, Djaldetti R, Israel Z, Melamed E. Effect of deep brain subthalamic stimulation on camptocormia and postural abnormalities in idiopathic Parkinson's disease. Mov Disord 2006;21:2008–2010. [DOI] [PubMed] [Google Scholar]
- 14. Sako W, Nishio M, Maruo T, et al. Subthalamic nucleus deep brain stimulation for camptocormia associated with Parkinson's disease. Mov Disord 2009;24:1076–1079. [DOI] [PubMed] [Google Scholar]
- 15. Umemura A, Oka Y, Ohkita K, Yamawaki T, Yamada K. Effect of subthalamic deep brain stimulation on postural abnormality in Parkinson disease. J Neurosurg 2010;112:1283–1288. [DOI] [PubMed] [Google Scholar]
- 16. Asahi T, Taguchi Y, Hayashi N, et al. Bilateral subthalamic deep brain stimulation for camptocormia associated with Parkinson's disease. Stereotact Funct Neurosurg 2011;89:173–177. [DOI] [PubMed] [Google Scholar]
- 17. Lyons M, Boucher O, Patel N, Birch B, Evidente V. Long‐term benefit of bilateral subthalamic deep brain stimulation on camptocormia in Parkinson's disease. Turk Neurosurg 2012;22:489–492. [DOI] [PubMed] [Google Scholar]
- 18. Capelle HH, Schrader C, Blahak C, et al. Deep brain stimulation for camptocormia in dystonia and Parkinson's disease. J Neurol 2011;258:96–103. [DOI] [PubMed] [Google Scholar]
- 19. Upadhyaya CD, Starr PA, Mummaneni PV. Spinal deformity and Parkinson disease: a treatment algorithm. Neurosurg Focus 2010;28:E5. [DOI] [PubMed] [Google Scholar]
- 20. Micheli F, Cersosimo MG, Piedimonte F. Camptocormia in a patient with Parkinson disease: beneficial effects of pallidal deep brain stimulation. Case report. J Neurosurg 2005;103:1081–1083. [DOI] [PubMed] [Google Scholar]
- 21. O'Riordan S, Paluzzi A, Liu X, Aziz TZ, Nandi D, Bain PG. Camptocormia—response to bilateral globus pallidus interna stimulation in three patients. Mov Disord 2009;24(Suppl 1):489. [Google Scholar]
- 22. Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson's disease. Lancet Neurol 2011;10:538–549. [DOI] [PubMed] [Google Scholar]
- 23. Zia S, Cody F, O'Boyle D. Joint position sense is impaired by Parkinson's disease. Ann Neurol 2000;47:218–228. [PubMed] [Google Scholar]
- 24. Maschke M, Tuite PJ, Pickett K, Wachter T, Konczak J. The effect of subthalamic nucleus stimulation on kinaesthesia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2005;76:569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's web‐site.
Supplementary Information Table 1.


