To the Editor: Amyloid light chain (AL) amyloidosis is a rare disease caused by the tissue deposition of misfolded immunoglobulin light chains (LCs), produced by clonal plasma cells, that potentially cause organ dysfunction and death 1. Plasma cell‐directed (PCD) therapies inhibit abnormal LC production but do not address existing amyloid deposits. Organ responses that may accompany hematologic response (HR; reduction or complete removal of involved free LCs) are often variable and incomplete, creating a significant need for amyloid‐targeted therapies to halt and potentially reverse organ dysfunction.
In preclinical studies, an anti‐LC antibody, 2A4, was shown to specifically bind soluble and insoluble aggregated LCs and mediate antibody‐dependent phagocytosis 2. We have reported encouraging organ responses in a first‐in‐human, phase 1/2 clinical trial (NCT01707264) with NEOD001 3, a monoclonal antibody derived from 2A4. Herein, we report detailed organ responses of two patients treated with NEOD001 after months to years of persistent organ dysfunction despite previous HR.
The phase 1/2 study design assessed NEOD001 safety and has been described 3. Each patient had a diagnosis of AL amyloidosis and previously experienced partial or better HR to systemic therapy. Each was enrolled in a dose‐escalating cohort for intravenous NEOD001 infusion every 28 days (q28d) and ultimately escalated to the maximum tolerated dose (MTD; 24 mg/kg). Each patient provided informed consent, and the trial was conducted in accordance with International Committee on Harmonisation Good Clinical Practice guidelines and the tenets of the Declaration of Helsinki.
In November 2009, a 60‐year‐old man received a diagnosis of AL amyloidosis with only renal involvement. He was previously treated with three different lines of PCD therapy. Although the patient achieved complete HR to combined bortezomib, lenalidomide, and steroid treatment, his proteinuria persisted >3 years later.
At enrollment in January 2014, the patient's urinary protein was 5,129 mg/day (normal, <150 mg/day). We note that persistently elevated proteinuria, especially >5,000 mg/day, will eventually lead to end‐stage renal disease and necessitate dialysis 4. This patient also had slightly elevated serum creatinine (1.4 mg/dL; normal range, 0.8–1.3 mg/dL), below normal creatinine clearance (62 mL/min; normal range, 77–160 mL/min), and an eGFR not significantly impacted at baseline.
The patient began NEOD001 treatment 40.3 months after his last exposure to PCD therapy. He was treated for 9 months with 16 mg/kg NEOD001 q28d, then was escalated to 24 mg/kg (MTD) for the remainder of this trial. At the 5‐month follow‐up visit, after 4 infusions of NEOD001, proteinuria was reduced by 36%, which constitutes consensus‐defined renal response 4. As of the trial cutoff date (May 9, 2016), he had received 29 infusions (20 at the MTD; Fig. 1A). His best response, recorded after 23 months of treatment, revealed an 88% reduction in proteinuria from baseline (602 mg/day). The patient's serum creatinine levels had stabilized at 1.1 mg/dL, and his creatinine clearance had risen to fluctuate within the normal range (74–92 mL/min). The patient's estimated glomerular filtration rate did not change from screening (52–60 mL/min/1.73 m2). He also experienced progressive functional improvement, complete resolution of edema, and no more fatigue.
Figure 1.
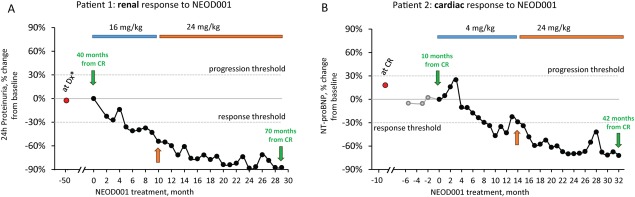
Renal and cardiac responses of patients after NEOD001 administration. A: The urinary protein level of patient 1 (60‐year‐old man) was 5,129 mg/day at screening 40 months after he achieved CR from previous plasma cell‐directed therapy (lenalidomide and steroid, then bortezomib and lenalidomide and steroid, then high‐dose melphalan followed by autologous stem cell transplantation). After infusion 4 (16 mg/kg NEOD001), reductions in his 24‐hr proteinuria met renal response consensus criteria 4 (−36%; proteinuria 3,285 mg/day). The patient's dose was escalated to the MTD at infusion 10 (orange arrow). Best renal response was measured after 23 infusions (−88% of baseline; proteinuria 602 mg/day), and he experienced no SAEs (grade ≥3) or dose interruptions. Clinically, he experienced progressive functional improvement; edema was completely resolved, and he was no longer fatigued. B: Patient 2 (46‐year‐old man) had elevated NT‐proBNP levels (>3,000 pg/mL) both after he achieved CR from previous chemotherapy (combined cyclophosphamide, bortezomib, and dexamethasone) and at screening 9.6 months later. After infusion 8 (4 mg/kg NEOD001), his NT‐proBNP level dropped to 2196 pg/mL (−33.7% of baseline) and met consensus criteria for cardiac response 5. At infusion 14, his dose was escalated to the MTD (orange arrow), and his best response to date was recorded after 31 infusions (−72% of baseline; 929 pg/mL NT‐proBNP). The patient did experience one grade 3 SAE (chest pain), but it was deemed unrelated to NEOD001, and he has not had any dose interruptions. Clinically, he experienced progressive functional improvement and significantly improved edema with a reduction in diuretic needs. *At diagnosis, the patient had renal involvement >0.5 g/day urinary protein excretion. He achieved CR 10 months after diagnosis. CR, complete hematologic response; MTD, maximum tolerated dose; NT‐proBNP, N‐terminal fragment of probrain natriuretic peptide; SAEs, serious adverse events.
During the trial, the patient did not experience any serious (grade ≥3) adverse events (AEs) but did experience mild to moderate AEs possibly related to the study drug (fatigue, muscle spasms, infusion site reaction, and thrombocytopenia).
A second patient (46‐year‐old man) received a diagnosis of AL amyloidosis in October 2012 and presented with only cardiac involvement (Mayo cardiac stage II, New York Heart Association [NYHA] functional classification II). He was treated with PCD therapy and experienced complete HR. However, his N‐terminal fragment of probrain natriuretic peptide (NT‐proBNP) level remained elevated and unimproved (3,914 pg/mL; >332 pg/mL predicts poor prognosis 5), indicating persistent cardiac dysfunction.
The patient began treatment with 4 mg/kg NEOD001 q28d in October 2013, 9.6 months after he experienced complete HR, at which point his baseline NT‐proBNP level was 3,312 pg/mL. His cardiac troponin level was <0.06 ng/mL, and his NYHA classification was II. After eight infusions, he achieved a cardiac response by consensus criteria 5 (NT‐proBNP level was 2,196 pg/mL; 34% reduction from baseline) and normal cardiac troponin (<0.01 ng/mL). The patient was escalated to 24 mg/kg at his 14‐month infusion, and his NT‐proBNP continued to decrease (Fig. 1B). His lowest NT‐proBNP measure (929 pg/mL; 72% reduction from baseline) was recorded after 31 months of treatment. His cardiac troponin levels remained normal throughout the study, and, after 14 months of treatment, his NYHA classification stabilized at class I. In addition to these biomarker improvements, the patient experienced progressive functional improvement and significantly improved edema with a reduction in diuretic needs.
He did experience one serious AE (noncardiac chest pain necessitating hospitalization [grade 3]), but it was deemed unrelated to the study drug, and he experienced no mild or moderate AEs related to the study drug.
Since the study cutoff date reported here, these patients' conditions have continued to improve. In August 2016, the renal patient's proteinuria was 378 mg/day and the cardiac patient's NT‐proBNP level was 421 pg/mL.
Previous HR should be considered when assessing organ response after NEOD001 treatment. However, organ responses in both these patients might have been specifically related to NEOD001 treatment because organ improvement was not evident in the months to years after PCD treatment, suggesting that toxic amyloid did not resolve with complete HR. Additionally, in the overall study population, neither the depth of best or last HR nor the time since best or last HR predicted response to NEOD001.
These cases highlight the potential of an emerging class of AL amyloidosis drugs that directly target deposited amyloid 6, administered as combined treatment with PCD therapies or to patients who experience HR to previous systemic therapy but have persistent organ dysfunction. These cases strongly suggest that administration of NEOD001 can result in organ responses not solely attributable to HR. NEOD001 is being evaluated further in two randomized, placebo‐controlled, global trials assessing NEOD001 treatment in patients with newly diagnosed AL amyloidosis receiving standard of care (VITAL phase 3/NCT02312206) and in patients with cardiac dysfunction refractory to past systemic therapy (PRONTO phase 2b/NCT02632786).
Authors Contribution
MAG treated patient 1. HL and BW treated patient 2. All authors wrote the manuscript and approved the final version to be submitted for publication.
Morie A. Gertz,1* Heather J. Landau,2 and Brendan M. Weiss3 1Division of Hematology, Mayo Clinic, Rochester, Minnesota; 2Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York; 3Abramson Cancer Center, Division of Hematology‐Oncology, University of Pennsylvania, Philadelphia, Pennsylvania
Acknowledgments
The authors thank all the patients who participated in this study and the investigators not highlighted here (Raymond L. Comenzo, David Seldin, Jeffrey A. Zonder, Giampaolo Merlini, Stefan Schönland, and Michaela Liedtke) who treated the patients. Medical editorial assistance was provided by ApotheCom (San Francisco, CA).
Contract grant sponsor: Prothena Biosciences Inc (South San Francisco, CA).
Conflict of interest: M.A.G. received honoraria from Celgene, Novartis, Millennium, Med Learning Group, Research to Practice, Onyx, Isis Pharmaceuticals, Sanofi, and Prothena; served in a consulting/advisory role for Prothena; and received travel reimbursements from Prothena, Celgene, and Novartis. H.L. received honoraria from Takeda; served in a consulting/advisory role for Onyx, Spectrum, Takeda, and Prothena; and performed research for Onyx. B.M.W. served in a consulting/advisory role for Janssen R&D, Millennium, and GlaxoSmithKline; performed research for Janssen R&D and Prothena; and received travel reimbursements from Janssen R&D, Millennium, and Prothena.
References
- 1. Merlini G, Comenzo RL, Seldin DC, et al. Immunoglobulin light chain amyloidosis. Exp Rev Gastroenterol Hepatol 2014;7:143–156. [DOI] [PubMed] [Google Scholar]
- 2. Zago W, Renz M, Torres R, et al. NEOD001 specifically binds aggregated light chain infiltrates in multiple organs from patients with AL amyloidosis and promotes phagocytic clearance of AL aggregtes in vitro. Poster presented at: 57th Annual Meeting of the American Society of Hematology; December 5–8, 2015; Orlando, Florida.
- 3. Gertz MA, Landau H, Comenzo RL, et al. First‐in‐human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J Clin Oncol 2016;34:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 2014;124:2325–2332. [DOI] [PubMed] [Google Scholar]
- 5. Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light‐chain amyloidosis. Leukemia 2012;26:2317–2325. [DOI] [PubMed] [Google Scholar]
- 6. Weiss BM, Wong SW, Comenzo RL. Beyond the plasma cell: Emerging therapies for immunoglobulin light chain amyloidosis. Blood 2016;127:2275–2280. [DOI] [PubMed] [Google Scholar]


