Abstract
Objective
To locate the organic cation transporter 2 (OCT2) in the cochlea of three different species and to modulate the ototoxicity of cisplatin in the guinea pig by pretreatment with phenformin, having a known affinity for OCT2.
Study Design
Immunohistochemical and in vivo study.
Methods
Sections from the auditory end organs were subjected to immunohistochemical staining in order to identify OCT2 in cochlea from untreated rats, guinea pigs, and a pig. In the in vivo study, guinea pigs were given phenformin intravenously 30 minutes before cisplatin administration. Electrophysiological hearing thresholds were determined, and hair cells loss was assessed 96 hours later. The total amount of platinum in cochlear tissue was determined using mass spectrometry.
Results
Organic cation transporter 2 was found in the supporting cells and in type I spiral ganglion cells in the cochlea of all species studied. Pretreatment with phenformin did not reduce the ototoxic side effect of cisplatin. Furthermore, the concentration of platinum in the cochlea was not affected by phenformin.
Conclusions
The localization of OCT2 in the supporting cells and type I spiral ganglion cells suggests that this transport protein is not primarily involved in cisplatin uptake from the systemic circulation. We hypothesize that OCT2 transport intensifies cisplatin ototoxicity via transport mechanisms in alternate compartments of the cochlea.
Level of Evidence
N/A. Laryngoscope, 125:E320–E325, 2015
Keywords: Cisplatin, ototoxicity, OCT2, phenformin
INTRODUCTION
Cisplatin is a platinum‐based anticancer drug causing nephro‐ and ototoxic side effects.1 It has been shown in a number of studies that cisplatin is toxic primarily to the outer hair cells (OHCs) in the organ of Corti. Stria vascularis and the spiral ganglion cells are also reportedly affected by the drug.2, 3 After cisplatin administration, OHCs are initially damaged in the base of the cochlea, leading to a high‐frequency hearing loss.4 There is an individual susceptibility to cisplatin ototoxicity and no otoprotective treatments are clinically proven.1
Nephrotoxic side effects resulting from damage to the proximal tubule cells can be alleviated by prehydration and forced diuresis.5 It is not known how cisplatin is transported from the systemic circulation to the target cells in the inner ear, but active transport mechanisms are suspected.6, 7 Organic cation transporters (OCTs) located in kidney and liver are known to be involved in the excretion of drugs from the systemic circulation.8, 9 Various isoforms of OCTs have specific species‐ and tissue‐distribution patterns.10, 11 Organic cations, such as the antidiabetic drugs metformin12, 13 and phenformin14, 15 and the histamine receptor antagonists cimetidine and ranitidine,16 are transported by OCTs in the renal endothelial cells.
In previous studies, organic transport protein 2 (OCT2) was found to be involved in cisplatin‐induced nephro‐17, 18 and ototoxicity.6 Organic transport protein 2 has been shown to transport cisplatin to the renal proximal tubule cells,17, 19, 20, 21, 22 and immunostaining has localized OCT2 to the cochlea of the murine inner ear.6, 23 Depleted ototoxic and nephrotoxic side effects of cisplatin were demonstrated in OCT2‐deficient mice and mice treated with cimetidine.6, 17
We have studied OCT2 because of its suggested causative involvement in the ototoxic side effect of cisplatin. Organic transport protein 2 is not reported to be a transport protein for cisplatin into cancer cells.24 Therefore, a protective therapy utilizing transport pathways would not counteract the antitumour effect of cisplatin. In this study, phenformin was chosen as an otoprotector because previous studies have shown a competitive inhibitory effect of OCT2.14, 25 Hence, the aim of the study was two‐fold, namely to locate OCT2 by immunohistochemical means in the rat, guinea pig, and pig cochlea and to establish if pretreatment with phenformin reduces cisplatin‐induced ototoxicity in the guinea pig in vivo.
MATERIALS AND METHODS
Animals
Guinea pigs (Duncun‐Hartley, Lidköpings kaninfarm AB, Sweden) and rats (Sprague‐Dawley, Scanbur AB, Sollenuna, Sweden) were housed at the animal facility at Karolinska University Hospital in Uppsala, Sweden, and kept in enrichment cages at a room temperature of 21°C, with a 12‐hour light–dark cycle and free access to food and tap water. A pig cochlea (Sus Scrofa) was harvested at the Department of Anesthesia, Uppsala University Hospital. Care and use of the animals reported in this study were approved in accordance with ethical standards (Ethical permits N135/11 and C315/5). For immunohistochemical staining, tissues from four untreated female albino rats, four untreated female albino guinea pigs, and an untreated pig were used. In the in vivo study, 15 female albino guinea pigs (range 261–337 g) were used, anesthetized with an intramuscular injection of ketamine (40 mg/kg) and xylazine (10 mg/kg). The weight of the animals was monitored daily, and they were given a daily subcutaneous injection of 5 ml saline. The end of the experiment was set to 96 hours after cisplatin was given; thereafter, the animals were sacrificed with an overdose of pentobarbiturate and cochlea were harvested.
Immunohistochemistry of the Cochleae of Three Different Species
Parvalbumin is known to exert both a cytoplasmatic and a nuclear immunoreactivity in type I spiral ganglion cells and in hair cells. Costaining with parvalbumin and OCT2 was used to identify inner hair cells (IHCs) and OHCs.26, 27 Kidney served as positive control for OCT2, as shown in previous studies.20, 28 The perilymphatic space of rat, guinea pig, and pig cochlea was perfused with 4% paraformaldehyde after decapitation, followed by decalcification. One‐half of the kidney was fixed with 4% paraformaldehyde. Rat and guinea pig cochlea and kidney were embedded in paraffin, sectioned 5‐µm thick, and mounted. The tissue sections were deparaffinized and boiled for 10 minutes in an antigen unmasking solution (Vector Laboratories, Burlingame, CA). A pig cochlea was embedded in Tissue‐Tek (OCT Polysciences, Warrington, PA), rapidly frozen, and sectioned at 8 to 10 µm with a cryostat.29 The frozen sections were collected into gelatin/chrome alum‐coated slides and stored below −70°C prior to immunohistochemistry. All sections were incubated with blocking serum (5% goat serum) for 30 minutes at room temperature. Rabbit antirat organic cation transporter 2 (OCT2) polyclonal antibodies, diluted 1:100 (Alpha Diagnostic International, San Antonio, TX), and mouse antiparvalbumin, diluted 1:250 (Chemicon International, Hants, United Kingdom) were used as primary antibodies. All antibodies were diluted in phosphate‐buffered saline (PBS). For negative controls, the primary antibodies were excluded and only PBS was used. One separate slide and one section on each slide were used as a negative control. All sections were incubated overnight with primary antibodies at 4°C. Organic transport protein 2 antibodies were visualized by means of indocarbocyanine Cy3 (red dye) conjugated goat antirabbit antibody, diluted 1:200 (Jackson Immuno Research Laboratories Inc., West Grove, PA). Parvalbumin antibodies were visualized by means of Alexa fluor 488 (green dye) conjugated goat antimouse antibody, diluted 1:400 (Invitrogen, Carlsbad, Cal., USA). The secondary antibodies were applied for 60 minutes at room temperature. All slides were mounted with Vecta Shield mounting medium with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Vector Laboratories, Burlingame, CA), and nuclei of all sections were then counterstained with DAPI (blue color). Fluorescent signals were analyzed with a light microscope (Zeiss Axio Scope; Carl Zeiss, Jena, Germany) or with a laser confocal microscope (Nikon TE2000; Nikon Co, Tokyo, Japan).
Modulation of Ototoxicity by OCT Inhibition: An In Vivo Study
Fifteen guinea pigs were used, randomized into two groups. The right internal jugular vein was catheterized for drug administration along the venous flow, that is, against the heart. The study group (n = 8) were given phenformin 20 mg/kg intravenously (phenformin hydrochloride 7.5 mg/ml; Sigma‐Aldrich, Saint Louis, MO) 30 minutes prior to intravenous administration of cisplatin 8 mg/kg (Platinol 1 mg/ml; Bristol‐Myers Squibb Pharmaceuticals, New York, NY). Control animals (n = 7) were given only cisplatin 8 mg/kg intravenously. The drugs were administered at a flow rate of 1 ml/minute. The ototoxic side effects of cisplatin were assessed and compared between the two groups. All calculated results were “blind.” Ototoxicity was evaluated by measuring the electrophysiological hearing thresholds with auditory brainstem response (ABR) and assessing the morphological loss of IHCs and OHCs. The levels of platinum in cochlear tissue were assessed and compared between the two groups. Tucker‐Davis Technologies (Gainesville, FL) equipment was used to measure the electrophysiological hearing thresholds of anesthetized animals. Auditory threshold shifts were determined in the left ear at 6.3 kHz, 12.5 kHz, and 20 kHz and calculated as the difference between thresholds obtained before and 96 hours after drug treatment, expressed as dB change.30 The temporal bones were immediately removed after the animals were euthanized. For morphological calculation of the loss of IHCs and OHCs, the left cochlea was perfused and fixed in 4% paraformaldehyde. After the bone was removed, the hair cells were labeled with phalloidin (diluted 1:200). Hair cells (scar formations) were counted along the cochlea in a Zeiss fluorescence microscope (Carl Zeiss). The percentage of missing hair cells per mm was calculated.31 Before cisplatin administration, one 0.25‐ml blood sample was aspirated from the left jugular vein of control group animals. After 96 hours, 0.25 ml blood was aspirated from the heart from all animals before euthanization. The right cochlea was used to determine the total platinum content in the tissue. From all cochlea, entire basilar membranes, including the organ of Corti and stria vascularis, were pooled together to form one sample. Inductively coupled plasma mass spectrometry (Analytica AB, Luleå, Sweden) was used for the analytical procedure.32
Statistical Evaluation
For descriptive data, median (maximum–minimum) values were used. The Mann‐Whitney nonparametric test was used to compare the two independent groups. Differences for which P values were 0.05 or less were deemed statistically significant.
RESULTS
Immunohistochemistry
Positive immunolabeling for OCT2 (red) was seen in endothelial tubular cells from renal tissue (Fig. 1A). This was found in previous studies and served as a positive control.20, 28 Intense cytoplasmic immunoreactivity for OCT2 (red) was observed in the supporting cells of organ of Corti and in type I spiral ganglion cells. Similar immunolabeling for OCT2 was identified in the rat, guinea pig, and pig (Fig. 1B and Fig. 2A–F). Positive staining for OCT2 was seen in DeitersÇ cells, HensenÇs cells, outer sulcus (ClaudiusÇ cells) and inner sulcus cells, outer and inner pillar cells, and in the tympanic covering layer localized under the basilar membrane (Fig. 2A, C, E). Type I spiral ganglion cells showed intense staining for OCT2 (Fig. 2B, D, F). Guinea pig and pig cochlea showed positive cytoplasmic and nuclear immunoreactivity to parvalbumin (green) in the IHCs with the connecting axons. Weak staining was also seen in the three rows of OHCs (Fig 2C and E). Double staining with parvalbumin showed no detectable immunoreactivity to OCT2 in the hair cells. Type I spiral ganglion cells showed both nuclear and cytoplasmic immunoreactivity to parvalbumin (Fig. 2D and F).
Figure 1.
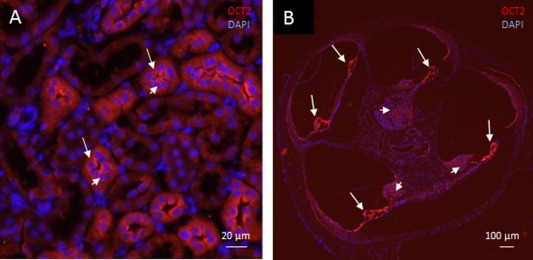
Light microscopy. Organic cation transporter 2 (red) and nuclear 4Ç,6‐diamidino‐2‐phenylindole (DAPI) staining (blue). (A) Rat kidney. Positive immunoreactivity to organic cation transporter 2 (OCT2) (long arrows) is evident in the endothelial tubular cells. Nuclear staining with DAPI (short arrows) confirms that OCT2 is localized in the cell cytoplasm. Similar immunolabeling of OCT2 is seen in guinea pig kidney. (B) Guinea pig cochlea (overview). Intense immunoreactivity to OCT2 is evident in the organ of Corti (long arrows) and in type I spiral ganglion cells (short arrows).
Figure 2.
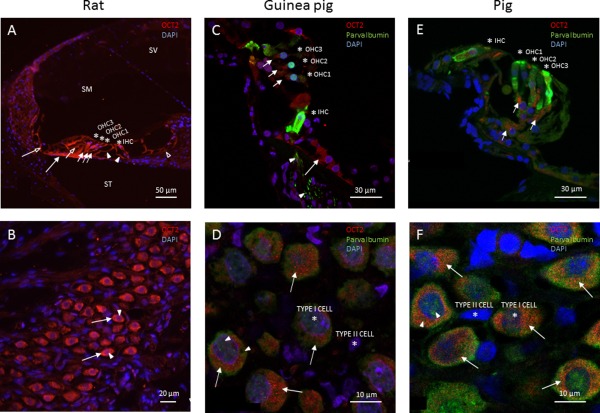
Cochlear sections. (A and B) Light microscopy. (C–F) Laser confocal microscopy. Organic cation transporter 2 (red), parvalbumin (green), and nuclear 4Ç,6‐diamidino‐2‐phenylindole (DAPI) staining (blue). Scala media (SM), scala tympani (ST), and scala vestibuli (SV). Asterisks identify the inner hair cells (IHC), first row of outer hair cells (OHC1), second row of outer hair cells (OHC2), and third row of outer hair cells (OHC3). (A) Rat organ of Corti. Organic cation transporter 2 immunoreactivity is visualized in DeitersÇ cells (short arrows), inner sulcus cells (opened arrowhead), outer sulcus cells, cells of ClaudiusÇ (opened long arrow), HensenÇs cells (short opened arrow), inner and outer pillar cells (arrowheads), and the tympanic covering layer (long arrow). (B) Rat spiral ganglion cells. Cytoplasm staining of OCT2 in type I spiral ganglion cells (long arrows). Cell nuclei are stained with DAPI (arrowheads). (C) Guinea pig organ of Corti. Positive immunoreactivity for parvalbumin is seen in the IHCs and all rows of OHCs. Neurites beneath the IHCs display parvalbumin (arrowheads). Organic cation transporter 2 immunoreactivity is evident in DeitersÇ cells (short arrows) and inner sulcus cells (long arrow). (D) Guinea pig spiral ganglion cells. DAPI‐positive nuclei of type I and II spiral ganglion cells (asterisks). Positive immunoreactivity to parvalbumin is seen both in cytoplasm and in nuclei of type I spiral ganglion cells (arrowheads). Cytoplasmic staining of OCT2 is evident in type I spiral ganglion cells (long arrows). (E) Pig organ of Corti. Asterisks identify the inner hair cells (IHC), first row of outer hair cells (OHC1), second row of outer hair cells (OHC2), and third row of outer hair cells (OHC3). Positive immunoreactivity to parvalbumin is seen in the IHCs and in OHCs. Organic cation transporter 2 immunoreactivity is shown in the DeitersÇ cells (short arrows). (F) Pig spiral ganglion cells. DAPI identifies the nuclei of type I and II spiral ganglion cells (asterisks). Positive immunoreactivity to parvalbumin is identified both in the cytoplasm and in the nuclei of type I spiral ganglion cells (arrowheads). Cytoplasmic staining of OCT2 is identified in type I spiral ganglion cells (long arrows).
In Vivo Study
The guinea pigs had electrophysiological hearing thresholds in the normal range before treatment. After the intravenous injections of drugs, two animals in the study group died the day after treatment and were excluded. No weight differences were noted between the two groups over the study period (P > 0.05). Generally, there was considerable individual variability of ototoxicity in both groups following cisplatin treatment (Table 1). No differences in auditory threshold shifts were seen between the two groups. Morphological evaluation revealed a pronounced loss of OHCs, whereas the IHCs were preserved in both groups. No difference in OHCs loss was seen between the two groups (P > 0.05). The platinum content in cochlear tissues did not differ between the groups (P > 0.05) (Table 1).
Table 1.
Ototoxicity and Platina in Cochlear Tissue.
| Animal | 1 | 2 | 3 | 4 | 5 | 6 | Median | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Study group (cisplatin and phenformin) | ABR threshold shifts | 6.3 kHz | 15 | 40 | 70 | 10 | 20 | 10 | 18 | |
| 12.5 kHz | 10 | 60 | 70 | 5 | 20 | 30 | 25 | |||
| 20 kHz | 45 | 70 | 85 | 15 | 45 | 60 | 53 | |||
| Hair cells loss % | OHC 1 | 28 | 51 | 61 | 19 | 16 | 29 | 33 | ||
| OHC 2 | 12 | 27 | 39 | 8 | 8 | 16 | 14 | |||
| OHC 3 | 9 | 19 | 30 | 9 | 8 | 13 | 11 | |||
| Platina µg/g | 0.24 | 0.96 | 0.39 | 0.59 | 1.02 | 0.99 | 0.77 |
| Animal | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Median | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Control group (cisplatin) | ABR threshold shifts | 6.3 kHz | 55 | 35 | 10 | 0 | 35 | 60 | 65 | 35 |
| 12.5 kHz | 35 | 65 | 25 | 5 | 55 | 55 | 70 | 55 | ||
| 20 kHz | 45 | 55 | 30 | 10 | 60 | 55 | 90 | 55 | ||
| Hair cells loss % | OHC 1 | 26 | 100 | 27 | 15 | 27 | 57 | 76 | 27 | |
| OHC 2 | 15 | 98 | 14 | 8 | 13 | 41 | 66 | 15 | ||
| OHC 3 | 14 | 99 | 10 | 7 | 8 | 30 | 55 | 15 | ||
| Plaina µg/g | 1.59 | 0.89 | 0.74 | 0.91 | 0.88 | 0.97 | 1.21 | 0.91 |
ABR = auditory brainstem response; OHC 1 = first row of outer hair cells; OHC 2 = second row of outer hair cells; OHC3 = third row of outer hair cells; platina = concentration in cochlea tissue 96 hours after drug treatment.
DISCUSSION
Organic cation transporters may play an important part in the influx of cisplatin, a highly cytotoxic drug, to cochlear target cells. Organic cation transporter 2 is one transport protein that has been identified to facilitate cisplatin uptake and thereby intensifies to its ototoxic side effect. Inner ears from three different species were analyzed by immunohistochemistry using confocal microscopy, particularly regarding the cellular distribution of OCT2. Positive immunoreactivity to OCT2 was localized in the supporting cells and in the type I spiral ganglion cells from rat, guinea pig, and pig cochlea. These findings may partly corroborate previous studies and indicate a possible involvement of OCT2 in cisplatin ototoxicity.6 However, administration of phenformin could not demonstrate any reduction of cisplatin ototoxicity or drug uptake, although earlier experimental studies have shown that cisplatin is transported actively by OCT2.6, 17, 33
The concept of cisplatin ototoxicity as a consequence of active transport is based mainly on immunohistochemistry and polymerase chain reaction in the murine cochlea.6 In previous studies, the immunoreactivity to OCT2 was identified in stria vascularis, IHCs, OHCs, and in spiral ganglion cells.6 Our results localize positive immunostaining of OCT2 in the supporting cells without any expression in the hair cells previously described by More et al.23 Species differences are less likely to explain the inconsistent findings of the cochlear expression of OCT2. A possible explanation for the discrepancy appears more to be related to differences in immunochemical staining and microscopy techniques. In an effort to improve the resolution, confocal immunofluorescence technique was used in the present study. Furthermore, costaining with parvalbumin was carried out, which made it possible to separate the positive immunoreactivity in the organ of Corti between the hair cells and supporting cells, thereby excluding the presence of OCT2 both in the IHCs and OHCs. No immunoreactivity to OCT2 could be visualized in the vicinity of the cochlear vascular networks of the spiral ligament and stria vascularis.34, 35 These findings casts doubt as to whether OCT2 is involved in cisplatin transport across the blood–perilymph or intrastrial fluid–blood barriers. Because earlier studies using the mouse model have identified OCT2 expression in the stria vascularis,6, 23 further studies are needed to clarify the role of OCT2 for the uptake from the systemic circulation.
Nuclear immunolabeling with DAPI confirmed a cytoplasmic immunostaining of OCT2,6, 23 which is known to have 12 transmembrane domains with intra‐ and extracellular loops.36 The cytoplasmic staining seen in the present study might be due to the antibody binding to amino acids in the endoplasmic reticulum and Golgi apparatus. The uptake of cisplatin in the cochlea is obligatory for the ototoxic side effects. Our previous findings based on pharmacokinetic studies suggested that the ototoxic effect of cisplatin is related to the drug concentration in the perilymphatic compartments.32, 37 The findings in this study give no support that OCT2 is primarily involved in the uptake of cisplatin from the systemic circulation but does not exclude an OCT2‐mediated transport from deeper compartments of the cochlea to the target cells for cisplatin. The complex processes that take place in cisplatin‐induced ototoxicity probably involve several series of events, of which influx of the drug to target cells is the crucial first step. The question arises concerning whether OCT2, by acting in the supporting cells, implies an active uptake of cisplatin at these cellular sites and thus if that could be of major importance for the distribution of the drug to the OHCs. Alternatively, the uptake of cisplatin by the supporting cells could lead to apoptosis in these cells as an early event in the ototoxic process preceding injury of the OHCs. Cisplatin is reported to induce toxic lesions on the supporting cells in the organ of Corti, mainly on the DeitersÇ cells that connect the OHCs to the basal membrane.38, 39 The supporting cells are important for the bolster and survival of the OHCs. Several ion channels40 and gap junction proteins such as connexins are located in the supporting cells.27 They are probably involved in the transport and recycling of the potassium that is released from the hair cells and recycled to the endolymphatic compartment.41 In this study, distinct positivity for OCT2 was identified in DeitersÇ cells, and immunoreactivity of OCT2 was also seen in the pillar cells that form the tunnel of Corti, as well in HensenÇs and ClaudiusÇ cells situated more laterally in the organ of Corti.42
Organic cation transporter 2 immunoreactivity in the type I spiral ganglion cells also confirms a direct uptake of cisplatin by these cells. The spiral ganglion cells connect the hair cells to the central nervous system, and the relatively large type I cells comprise 95% of the spiral ganglion cells. Their peripheral processes are connected to the primary sensory cells, the IHCs.43 No positive immunoreactivity could be identified in the type II spiral ganglion cells.
Recent studies indicate that several protein transporters are involved in the transport mechanisms of cisplatin. The copper transporter Ctr1 has been studied both in the kidney and in the inner ear in relation to the side effects of cisplatin. It has been identified immunohistochemically in the inner ear.23 An intratympanic administration of copper sulphate prior to cisplatin injection has been found to prevent hearing loss.23 Because cancer cells reportedly contain Ctrl,44, 45 local administration of a competitive binder to Ctr1 is preferred, with the intention of reducing the ototoxic effect while preserving antineoplastic activity.
In this study, no reduction of the ototoxic side effects induced by cisplatin could be established in guinea pigs pretreated the potential OCT2 antagonist phenformin. This finding was corroborated by the fact that the concentration of total platinum in cochlear tissue did not differ between the two groups. There are several possible explanations for this finding in various combinations. First, phenformin in the guinea pig may not reach the deeper compartments of the inner ear where OCT2 was found to be localized; thus, the passage of phenformin over the blood‐perilymph and intrastrial fluid‐blood barrier may be restricted.46, 47 Second, due to an anticipated risk of metabolic acidosis, only a limited dose of phenformin was given in vivo48; hence, the concentration of phenformin that reached OCT2 in the cochlea might have been too weak for a competitive antagonistic effect. And finally, OCT2 in the guinea pig might not primarily be involved in cisplatin transport to the inner ear structures. One limitation of this study was that we did not evaluate the pharmacokinetics of phenformin in blood and scala tympani perilymph.
CONCLUSION
More knowledge about cisplatin transport into the inner ear compartments is necessary in order to understand the evolution of the toxic process. In this study, OCT2 was identified in the cochlea of rat, guinea pig, and pig. One possible problem in our interpretation, in the light of earlier observation, concerns the localization of OCT2 and possible active transport of cisplatin to the target cells via the intrastrial fluid–blood barrier. In contrast to earlier findings, no OCT2 expression was localized to the stria vascularis. Organic cation transporter 2 was located in the supporting cells and type I spiral ganglion cells. The localization of OCT2 in this study gives no support that this transport protein is involved in the uptake of cisplatin from the vascular networks in the cochlea. Systemically administered phenformin did not alleviate cisplatin‐induced ototoxicity, possibly due to restricted passage across the intrastrial fluid blood barrier. This present findings afford new insight into the possible mechanism of active cisplatin uptake by target cells. More studies are necessary, however, to obtain a complete picture of these processes as related to various transcellular transport mechanisms in the ear.
Acknowledgments
The authors thank Anette Fransson, PhD, for tissue preparations and Professor Staffan Eksborg for statistical support.
Supported by AFA Insurance, Foundation Tysta Skolan, and Foundation Acta Oto‐Laryngologica. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 2007;33:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cardinaal RM, de Groot JC, Huizing EH, Veldman JE, Smoorenburg GF. Dose‐dependent effect of 8‐day cisplatin administration upon the morphology of the albino guinea pig cochlea. Hear Res 2000;144:135–146. [DOI] [PubMed] [Google Scholar]
- 3. van Ruijven MW, de Groot JC, Klis SF, Smoorenburg GF. The cochlear targets of cisplatin: an electrophysiological and morphological time‐sequence study. Hear Res 2005;205:241–248. [DOI] [PubMed] [Google Scholar]
- 4. Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg 2007;15:364–369. [DOI] [PubMed] [Google Scholar]
- 5. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 2008;73:994–1007. [DOI] [PubMed] [Google Scholar]
- 6. Ciarimboli G, Deuster D, Knief A, et al. Organic cation transporter 2 mediates cisplatin‐induced oto‐ and nephrotoxicity and is a target for protective interventions. Am J Pathol 2010;176:1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Ding D, Jiang H, Fu Y, Salvi R. Co‐administration of cisplatin and furosemide causes rapid and massive loss of cochlear hair cells in mice. Neurotox Res 2011;20:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Arch 2004;447:666–676. [DOI] [PubMed] [Google Scholar]
- 9. Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch 2004;447:465–468. [DOI] [PubMed] [Google Scholar]
- 10. Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 2007;24:1227–1251. [DOI] [PubMed] [Google Scholar]
- 11. Ciarimboli G. Role of organic cation transporters in drug‐induced toxicity. Expert Opin Drug Metab Toxicol 2011;7:159–174. [DOI] [PubMed] [Google Scholar]
- 12. Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1–3). J Pharmacol Exp Ther 2004;308:2–9. [DOI] [PubMed] [Google Scholar]
- 13. Kimura N, Masuda S, Tanihara Y, et al. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet 2005;20:379–386. [DOI] [PubMed] [Google Scholar]
- 14. Umehara KI, Iwatsubo T, Noguchi K, Kamimura H. Comparison of the kinetic characteristics of inhibitory effects exerted by biguanides and H2‐blockers on human and rat organic cation transporter‐mediated transport: Insight into the development of drug candidates. Xenobiotica 2007;37:618–634. [DOI] [PubMed] [Google Scholar]
- 15. Shitara Y, Nakamichi N, Norioka M, Shima H, Kato Y, Horie T. Role of organic cation/carnitine transporter 1 in uptake of phenformin and inhibitory effect on complex I respiration in mitochondria. Toxicol Sci 2013;132:32–42. [DOI] [PubMed] [Google Scholar]
- 16. Bourdet DL, Pritchard JB, Thakker DR. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J Pharmacol Exp Ther 2005;315:1288–1297. [DOI] [PubMed] [Google Scholar]
- 17. Ciarimboli G, Ludwig T, Lang D, et al. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 2005;167:1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol 2009;296:F505–F511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biermann J, Lang D, Gorboulev V, et al. Characterization of regulatory mechanisms and states of human organic cation transporter 2. Am J Physiol Cell Physiol 2006;290:C1521–C1531. [DOI] [PubMed] [Google Scholar]
- 20. Karbach U, Kricke J, Meyer‐Wentrup F, et al. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am J Physiol Renal Physiol 2000;279:F679–F687. [DOI] [PubMed] [Google Scholar]
- 21. Ludwig T, Riethmuller C, Gekle M, Schwerdt G, Oberleithner H. Nephrotoxicity of platinum complexes is related to basolateral organic cation transport. Kidney Int 2004;66:196–202. [DOI] [PubMed] [Google Scholar]
- 22. Motohashi H, Inui KI. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J 2013;15:581–588. doi: 10.1208/s12248‐013‐9465‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum‐induced ototoxicity. J Neurosci 2010;30:9500–9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sprowl JA, van Doorn L, Hu S, et al. Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: influence on antitumor efficacy and systemic clearance. Clin Pharmacol Ther 2013;94:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sogame Y, Kitamura A, Yabuki M, Komuro S, Takano M. Transport of biguanides by human organic cation transporter OCT2. Biomed Pharmacother 2013;67:425–430. [DOI] [PubMed] [Google Scholar]
- 26. Gomide VC, de Francisco AC, Chadi G. Localization of neurotensin immunoreactivity in neurons and organ of Corti of rat cochlea. Hear Res 2005;205:1–6. [DOI] [PubMed] [Google Scholar]
- 27. Liu W, Bostrom M, Kinnefors A, Rask‐Andersen H. Unique expression of connexins in the human cochlea. Hear Res 2009;250:55–62. [DOI] [PubMed] [Google Scholar]
- 28. Motohashi H, Sakurai Y, Saito H, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol 2002;13:866–874. [DOI] [PubMed] [Google Scholar]
- 29. Liu W, Bostrom M, Rask‐Andersen H. Expression of peripherin in the pig spiral ganglion—aspects of nerve injury and regeneration. Acta Otolaryngol 2009;129:608–614. [DOI] [PubMed] [Google Scholar]
- 30. Ekborn A, Laurell G, Andersson A, Wallin I, Eksborg S, Ehrsson H. Cisplatin‐induced hearing loss: influence of the mode of drug administration in the guinea pig. Hear Res 2000;140:38–44. [DOI] [PubMed] [Google Scholar]
- 31. Berglin CE, Pierre PV, Bramer T, et al. Prevention of cisplatin‐induced hearing loss by administration of a thiosulfate‐containing gel to the middle ear in a guinea pig model. Cancer Chemother Pharmacol 2011;68:1547–1556. [DOI] [PubMed] [Google Scholar]
- 32. Hellberg V, Wallin I, Eriksson S, et al. Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity. J Natl Cancer Inst 2009;101:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yonezawa A, Inui K. Organic cation transporter OCT/SLC22A and H(+)/organic cation antiporter MATE/SLC47A are key molecules for nephrotoxicity of platinum agents. Biochem Pharmacol 2011;81:563–568. [DOI] [PubMed] [Google Scholar]
- 34. Cohen‐Salmon M, Regnault B, Cayet N, et al. Connexin30 deficiency causes instrastrial fluid‐blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A 2007;104:6229–6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang W, Dai M, Fridberger A, et al. Perivascular‐resident macrophage‐like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid‐blood barrier. Proc Natl Acad Sci USA 2012;109:10388–10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koepsell H. Organic cation transporters in intestine, kidney, liver, and brain. Annu Rev Physiol 1998;60:243–266. [DOI] [PubMed] [Google Scholar]
- 37. Dammeyer P, Hellberg V, Wallin I, et al. Cisplatin and oxaliplatin are toxic to cochlear outer hair cells and both target thioredoxin reductase in organ of Corti cultures. Acta Otolaryngol 2014;134:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laurell G, Bagger‐Sjoback D. Degeneration of the organ of Corti following intravenous administration of cisplatin. Acta Otolaryngol 1991;111:891–898. [DOI] [PubMed] [Google Scholar]
- 39. Ramirez‐Camacho R, Garcia‐Berrocal JR, Bujan J, Martin‐Marero A, Trinidad A. Supporting cells as a target of cisplatin‐induced inner ear damage: therapeutic implications. Laryngoscope 2004;114:533–537. [DOI] [PubMed] [Google Scholar]
- 40. Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K‐Cl co‐transporter Kcc4. Nature 2002;416:874–878. [DOI] [PubMed] [Google Scholar]
- 41. Mistrik P, Ashmore J. The role of potassium recirculation in cochlear amplification. Curr Opin Otolaryngol Head Neck Surg 2009;17:394–399. [DOI] [PubMed] [Google Scholar]
- 42. Glueckert R, Pfaller K, Kinnefors A, Rask‐Andersen H, Schrott‐Fischer A. The human spiral ganglion: new insights into ultrastructure, survival rate and implications for cochlear implants. Audiol Neurootol 2005;10:258–273. [DOI] [PubMed] [Google Scholar]
- 43. Rask‐Andersen H, Tylstedt S, Kinnefors A, Illing R. Synapses on human spiral ganglion cells: a transmission electron microscopy and immunohistochemical study. Hear Res 2000;141:1–11. [DOI] [PubMed] [Google Scholar]
- 44. Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A 2002;99:14298–14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zisowsky J, Koegel S, Leyers S, et al. Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem Pharmacol 2007;73:298–307. [DOI] [PubMed] [Google Scholar]
- 46. Zhang F, Dai M, Neng L, et al. Perivascular macrophage‐like melanocyte responsiveness to acoustic trauma—a salient feature of strial barrier associated hearing loss. FASEB J 2013;27:3730–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neng L, Zhang F, Kachelmeier A, Shi X. Endothelial cell, pericyte, and perivascular resident macrophage‐type melanocyte interactions regulate cochlear intrastrial fluid‐blood barrier permeability. J Assoc Res Otolaryngol 2013;14:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bando K, Ochiai S, Kunimatsu T, et al. Comparison of potential risks of lactic acidosis induction by biguanides in rats. Regul Toxicol Pharmacol 2010;58:155–160. [DOI] [PubMed] [Google Scholar]


