Abstract
Background & Objectives
Red blood cells (RBCs) may be stored up to 42 days before transfusion, per US and EU standards. Although there is ample evidence that RBCs undergo deleterious changes during storage, studies assessing outcomes relative to storage time report conflicting findings. This study investigated RBC storage duration perspectives and practices among blood banking and transfusion professionals.
Materials & Methods
A survey was administered at the American Association of Blood Banking annual meeting in October 2014 (N = 69).
Results
On average, participants believed RBC storage should not exceed 34 days (median: 35; range: 1–52), and estimated that RBCs are typically stored 21 days before transfusion at their institutions (median: 20; range: 10–40). There was 97% agreement that minimizing/reversing changes during RBC storage may produce clinical benefits; however, 80% believed the research does not consistently demonstrate worse outcomes using older blood. Two‐thirds agreed that RBC storage duration is a major concern, but 81% agreed most institutions are not pursuing measures to shorten storage.
Conclusions
This study found that many transfusion professionals believe RBCs should be stored for fewer than the 42 days currently allowed and that further efforts are warranted to abrogate changes in stored RBCs. These findings suggest a need for increased awareness of potential consequences of extended RBC storage and for strategies to maximize transfusion benefits.
Keywords: Blood storage, RBC storage lesion, transfusion
Introduction
Globally, approximately 85 million units of red blood cells (RBCs) is transfused each year 1. Blood transfusion is one of the most common procedures performed during hospitalization in the United States. In 2010, 11% of US hospital stays involving a procedure required transfusion – a rate that has been rising steadily, primarily due to the ageing population 2, 3, 4. Overall, nearly 13.8 million units of blood was transfused, mostly to hospitalized patients 5. Blood use in the United States is generally reported as being higher than in Europe; for example, per capita US blood utilization is estimated to be at least 10% higher than that in the United Kingdom 5, 6.
According to standards set by the US Food and Drug Administration (FDA), RBCs may be stored with standard preservative solutions for up to 42 days before transfusion 7. In Europe, RBCs may be stored for as many as 49 days, depending on the additive solution used and other validated conditions (e.g. mean 24‐h post‐transfusion red cell survival ≥75% and <0·8% haemolysis at end of storage) 8, 9. However, it is well established that RBCs undergo a series of changes during storage that impair membrane health and oxygen delivery and that may have an adverse impact on clinical outcomes 10, 11, 12, 13. These changes – collectively called ‘storage lesion’ – include metabolic alterations (e.g. reduced adenosine triphosphate [ATP] and 2,3‐diphosphoglycerate [2,3‐DPG]), enzymatic changes (e.g. plasma membrane breakdown, protein damage), oxidative effects (e.g. changes in cell shape, membrane loss) and physiologic changes (e.g. protein restructuring, eryptosis signalling) 10, 11, 12, 13.
The age of RBCs may also increase the potential for inappropriately low perfusion and under‐oxygenation of the microvasculature 10, 14. In as few as 10–14 days of storage, the ability of RBCs to move through capillaries and deliver oxygen is diminished 11, 14, 15. Moreover, when stored blood is transfused, RBCs compromised by in vitro ageing are eliminated from circulation within 24 hours, resulting in a loss of up to 30% of the transfused unit 16. Despite these storage‐acquired deficits, transfusion of RBCs stored for extended durations remains common; the average age of blood used for transfusion in the United States was estimated at 18 days, according to the 2011 National Blood Collection and Utilization Survey (NBCUS) conducted by the Department of Health and Human Services with the American Association of Blood Banks (AABB) 5.
Thus, because stored RBCs have a limited life span during which they offer clinical benefit, the appropriate storage time warrants careful consideration. The large number of studies and reviews on the effects of blood storage speaks to the importance of this issue 17, 18, but the results have not been conclusive. Twenty studies, reviews and meta‐analyses published from 1997 to 2013 reported harmful effects of prolonged RBC storage, including increased risk of morbidity and mortality 17, 19, increased risk of multi‐organ failure and renal failure 17, 20, increased risk of infection 17 and longer hospital stays 17. In contrast, from 1989 to 2014, 15 studies of varying design reported no independent effect of blood storage time on patient survival or length of stay in the intensive care unit 17, 21, 22, nor any clinically harmful effect associated with older transfused blood 17, 22. The large randomized, controlled RECESS and ABLE trials more recently reported no negative impact of older stored blood for transfusion 22, 23. However, some experts feel that studies conducted to date were not adequately designed to measure differences in outcomes by blood storage time. In part, this is because these studies actually compared fresh with ‘middle‐aged’ blood and included few participants who received blood following prolonged storage (35–42 days) 17, 18, 24.
Meanwhile, there is no consensus on whether the potential risks of older stored blood warrant shorter storage time or other approaches to retaining the benefits of fresher blood. There is also a gap in our understanding of experts’ impressions of the mixed evidence base and the clinical practices relating to RBC storage time prior to transfusion; until now, no published study has assessed these particular viewpoints. It is in this environment of uncertainty and debate that this cross‐sectional survey study aimed to investigate current perspectives and practices regarding RBC storage duration among members of the blood banking and transfusion medicine community.
Materials and methods
A cross‐sectional survey was administered to attendees of the AABB annual meeting in October 2014. Data were collected over 4 days during the meeting and analysed from November 2014 to January 2015.
Survey instrument
The survey questionnaire used in this study was developed based on a comprehensive review of the literature on the storage of blood used for transfusion, using the PubMed search engine from the US National Library of Medicine National Institutes of Health. Survey development included careful review and revision of initial draft survey items to ensure the clarity, relevance, and validity of each item relative to the constructs being assessed.
Two screening questions were used to assess eligibility: participants were required to be currently involved in clinical, scientific or administrative aspects of blood transfusion and/or currently involved in the care of patients requiring transfusion. The survey comprised 30 questions designed to gather information about the respondent's primary role and function related to transfusion, years of experience and work setting (four multiple‐choice questions); to capture opinions on the appropriate maximum duration of RBC storage and the estimated average age of RBCs transfused at each respondent's institution (two open‐ended questions allowing numerical response, in days); and to measure level of agreement with 24 statements related to RBC storage and its impact on blood quality and transfusion outcomes (four‐point Likert scale: strongly agree, agree, disagree and strongly disagree). The full survey is available online with this study's supporting information (Fig. S1).
Data collection
Data were collected from attendees of the AABB Annual Meeting, held 25–28 October 2014, in Philadelphia, Pennsylvania. The congress was attended by approximately 6500 professionals, including over 5000 registrants from North America and more than 500 from Europe; attendees represented a variety of roles, including physicians, nurses, technicians and administrators working in various aspects of blood banking, transfusion medicine and cellular therapy. Congress attendees were recruited at two venues using two survey modalities: (i) electronic surveys on iPad tablets (using surveymonkey ® software, Palo Alto, CA, USA) in the AABB meeting exhibit hall and (ii) pen‐and‐paper surveys prior to an industry‐sponsored symposium held on 27 October 2014. This symposium on the topic of blood storage and the quality of blood used for transfusion was sponsored by Biomet Biologics, LLC, the manufacturer of an FDA‐approved solution for rejuvenation of liquid stored RBCs. Subjects were informed that the survey was intended to assess perspectives on storage of blood used for transfusion, but they were not told that survey data would form the basis of a study submitted for peer‐reviewed publication.
Data analysis
All survey data were compiled and verified using Microsoft Excel, and analyses were conducted using sas version 9.3 (SAS Institute, Inc. Atlanta, GA, USA). Descriptive statistics, including univariate summaries, were calculated for each survey item (frequency counts for categorical variables: means, standard deviations, medians, lower/upper quartiles and ranges for continuous variables). Incomplete surveys were allowed to be included in the study, which is reflected in the variable sample sizes across different analyses.
Inferential analyses were employed to further assess relationships between variables, using a significance level of P < 0·05. Fisher's exact test was used to assess relationships between categorical variables, including differences in level of agreement to the Likert‐type perspective questions according to institution type and discrete measures of blood storage (i.e. days divided into ranges). Spearman correlation coefficients (r s) were calculated to assess relationships between categorical and continuous variables, including differences in level of agreement to the Likert‐type perspective questions according to measures of RBC storage duration (respondent opinion on the appropriate maximum number of RBC storage days and estimated average age of RBCs used for transfusion, in days). An exploratory factor analysis was performed using a principal component methodology on all surveys with complete data; all factors with eigenvalues ≥1 were considered for the model.
Results
A total of 44 electronic surveys were collected at the AABB exhibit hall (representing <1% of the approximate 6500 meeting attendees), plus 26 paper‐and‐pen surveys at the industry‐sponsored symposium (46·4% response rate at the symposium); 1 electronic survey was later found to be ineligible and excluded from the analysis, resulting in a final study sample of 69 respondents.
Participants represented a range of roles and settings in blood banking and transfusion medicine (Table 1). The most commonly reported primary roles associated with blood transfusion were ‘supplying blood’ (31·9%) and ‘providing care to patients requiring transfusions’ (17·4%); fewer were involved in collecting blood (11·6%), research (11·6%), conducting transfusions (8·7%), ordering blood (7·2%) and patient blood management (2·9%). As a whole, participants reported working in transfusion medicine for an average of 20·4 years (median: 19 years; range: 1–47 years). More than one‐third of subjects (36·2%) reported that their primary function within transfusion medicine was as a clinician, transfusion medicine physician or laboratory director, while 24·6% were administrators, 17·4% were technicians, and 11·6% were researchers (Table 1). More than half of study participants worked in hospitals, nearly one‐third worked at non–hospital‐based blood centres, and the remaining 13·3% worked in university/research centres or other settings. In all, 89·1% of participants reported being aware of the published AABB guidelines that aim to reduce the number and volume of blood transfusions as part of patient blood management strategies.
Table 1.
Participant characteristics
| Primary role associated with blood transfusion, percentage of respondents (n = 69) | |
| Supplying blood | 31·9% |
| Providing care to patients requiring transfusions | 17·4% |
| Collecting blood | 11·6% |
| Research | 11·6% |
| Conducting transfusions | 8·7% |
| Ordering blood | 7·2% |
| Patient blood management | 2·9% |
| Othera | 8·7% |
| Type of institution where employed, percentage of respondents (n = 68) | |
| Hospital | 57·4% |
| Blood centre (non–hospital‐based) | 29·4% |
| University/research centre/otherb | 13·3% |
| Number of years working in transfusion medicine, median (range) (n = 69) | 19 (1–47) |
| Primary function in relation to transfusion medicine, percentage of respondents (n = 69) | |
| Clinician/transfusion medicine physician/laboratory director | 36·2% |
| Administrator | 24·6% |
| Technician | 17·4% |
| Research | 11·6% |
| Otherc | 10·1% |
Other responses: quality (n = 1); genotyping (n = 1); research & development (n = 2); industry (n = 1); blood management (n = 1).
Other responses: government (n = 1); rARC (n = 1); manufacturing (n = 1); blood centre and hospital (n = 1).
Other responses: industry (n = 1); transfusion safety officer (n = 2); quality (n = 2); teaching (n = 2).
Blood storage duration: appropriate versus actual RBC storage time
Participants’ opinions on the appropriate time limit for RBC storage prior to transfusion averaged 33·7 days (median: 35 days; range: 1–52 days; n = 67; Fig. S2a). A substantial number of respondents (43·3%) viewed 42 days as appropriate, corresponding exactly with the maximum RBC storage duration allowed by the FDA 7. Overall, participants estimated that RBC units are on average 21·2 days old at the time of transfusion at their respective institutions (median: 20 days; range: 10–40 days; n = 49; Fig. S2b). The majority of participants who provided both responses (81·6%) reported that the average age of RBCs transfused at their institutions was less than what they considered to be the appropriate maximum storage duration (Fig. 1a). Far fewer participants (14·3%) indicated that the average age of RBCs transfused at their institutions was greater than what they considered to be the appropriate storage limit (Fig. 1b).
Figure 1.
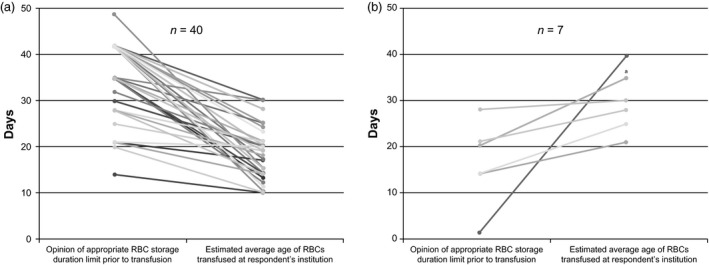
Comparison of participants’ opinion of the appropriate maximum RBC storage duration vs. the estimated average age of RBCs used for transfusion at participants’ institutions, in days. Each line represents a single respondent, and all respondents shown provided responses to both questions. (a) Participants who indicated that the average age of RBCs transfused at their institutions was less than what they considered to be the appropriate storage limit (n = 40). (b) Participants who indicated that the average age of RBCs transfused at their institutions was more than what they considered to be the appropriate storage limit (n = 7). aLine represents two subjects who provided identical responses.
Perspectives on the storage of blood used for transfusion
Participants indicated their level of agreement with 24 statements related to RBC storage. Due to sparse responses at the extreme ends of the original 4‐point Likert‐type scale, results were dichotomized into agree (strongly agree and agree combined) and disagree (strongly disagree and disagree combined). Responses to selected statements are presented in Fig. 2 (Fig. S3 includes responses to all 24 statements). Nearly all participants (96·9%) agreed that minimizing or reversing the changes that occur over time in stored RBCs may impact the clinical benefits of blood transfusions; however, 79·7% also concurred that the research does not consistently demonstrate that blood transfusions lead to worse outcomes with older stored RBCs when compared with fresher blood. Although 65·1% of respondents agreed that duration of blood storage prior to use is a major concern among transfusion medicine professionals, 81·3% agreed that most institutions are not pursuing measures to reduce the age of stored blood at time of transfusion.
Figure 2.
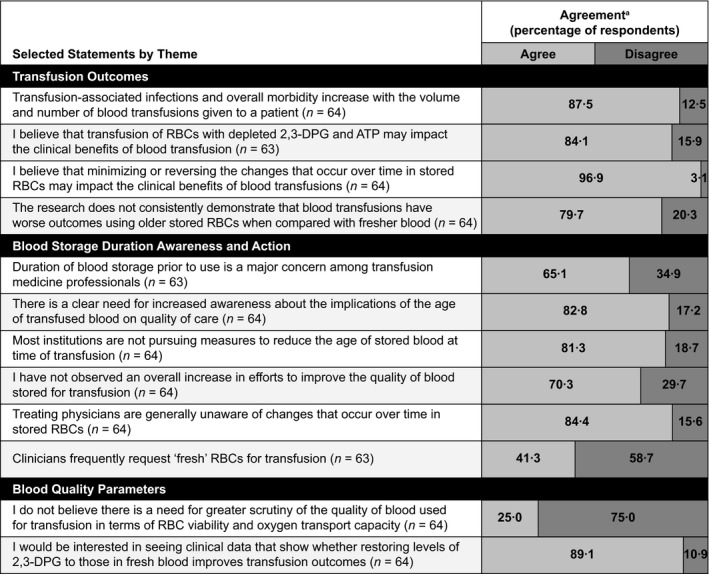
Rates of participant agreement with selected statements related to RBC storage and its impact on blood quality and transfusion outcomes. aResponses have been dichotomized into agree and disagree from original 4‐point scale (strongly agree, agree, disagree and strongly disagree).
Correlations
Several significant relationships were identified between variables assessed in the survey. The greater the number of days that participants indicated as the appropriate storage duration limit for RBCs, the more likely they were to disagree (i) that use of fewer units to treat each patient requiring transfusion would be cost saving (r s = –0·42995; P = 0·0005) and (ii) that the parameters defining the acceptable duration of RBC usability do not take into consideration the capacity of stored RBCs to effectively deliver oxygen to tissues (r s = –0·33968; P = 0·0079). On the other hand, these participants, who designated longer appropriate RBC storage limits, were more likely to agree that there is no need for greater scrutiny of the quality of blood for transfusion with respect to RBC viability and oxygen transport (r s = 0·30395; P = 0·0163). When appropriate RBC storage limit responses were stratified into discreet categories (≤21, 22–41 and ≥42 days), respondents who indicated that RBCs should be stored for a maximum of 21 days were significantly more likely to disagree that there is a need for well‐designed, adequately powered, controlled, randomized trials to answer questions about the impact of blood storage on patient outcomes (P = 0·0345); those who indicated that RBC storage for 42 days or longer is appropriate were significantly more likely to disagree that the parameters currently defining the acceptable duration of RBC usability do not take into consideration the capacity of stored RBCs to effectively deliver oxygen to tissues (P = 0·0032).
In relation to actual practices at participants’ institutions, the greater the number of days that a participant estimated as the average age of RBCs used for transfusion at their institution, the more likely they were to agree (i) that they have not seen an overall increase in efforts to improve the quality of blood stored for transfusion (r s = 0·34699; P = 0·0169), (ii) that a restrictive transfusion threshold should be used in the vast majority of hospitalized stable patients (r s = 0·33808; P = 0·0201) and (iii) that treating physicians are generally unaware of changes that occur over time in stored RBCs (r s = 0·31989; P = 0·0284).
Factor analysis
Exploratory factor analysis was performed on all surveys with complete data (n = 56) using principal component methodology to identify potential factors accounting for variance in the data as well as any possible underlying causal influence of these latent factors. These results are available online as supporting information (Fig. S4 and Table S1).
Discussion
This survey assessed perspectives and current practices related to RBC storage among 69 blood banking and transfusion medicine professionals. Although this study does not represent the full spectrum of possible perspectives from all professionals involved in blood storage and transfusion medicine, it may serve as a useful representation of the larger situation.
Our finding of 21 days as the average estimated age of RBCs used for transfusion at participants’ institutions is consistent with the average of 18 days reported by the 2011 NBCUS report and suggests that extended blood storage is not uncommon 5. Our results reveal some conflicting perspectives with respect to the significance of extended RBC storage time. Two‐thirds of respondents agreed that duration of blood storage prior to use is a major concern among transfusion medicine professionals, although respondents from blood centres were significantly less likely to agree with this statement compared with those working in other settings. The survey data imply that the vast majority of all respondents recognize the impact of storage on blood quality and transfusion outcomes, at least from a pathophysiologic perspective: about 84% agreed that transfusion of RBCs with depleted 2,3‐DPG and ATP may impact the clinical benefits of blood transfusion, and 97% agreed that minimizing or reversing the changes that occur over time in stored RBCs may improve the clinical benefits of blood transfusions. However, when asked for their opinion on the ‘appropriate’ storage time limit for RBCs prior to transfusion, participants indicated an overall average of 34 days – a point at which 2,3‐DPG is almost fully degraded and ATP has declined significantly in stored blood 14. Average reported appropriate RBC storage limit did not differ according to practice setting (e.g. for blood centres vs. other settings). It appears that regulatory guidance exerts a major influence on perspectives, as 29 of 67 respondents (43%) indicated 42 days as the appropriate storage duration limit for RBCs prior to transfusion (matching the maximum storage duration allowed by the FDA). The average 34‐day appropriate RBC storage limit may also be influenced by existing practices at respondents’ institutions, as well as practical considerations/limitations that would make a shorter storage limit unrealistic, as participants were asked for their opinion of the ‘appropriate’ storage limit – not the ‘ideal’ limit.
These findings are likely also a reflection of the lack of definitive evidence on the impact of stored blood age on clinical outcomes; 80% of respondents agreed that the link between longer storage and poorer outcomes has not been consistently demonstrated and more than 90% agreed that there is a need for well‐designed, adequately powered, randomized, controlled trials to answer questions about the impact of blood storage on patient outcomes. This viewpoint may have been shaped by anticipation for the RECESS and ABLE trials 22, 23, whose results were not yet published at the time this survey was conducted. Many participants (89%) also expressed interest in seeing data on the clinical impact of restoring 2,3‐DPG in stored RBCs to the level found in fresh blood, which was very likely influenced by the fact that approximately one‐third of the survey sample was recruited at a symposium on transfusion blood quality and reversing the impact of storage lesion.
The vast majority of respondents also agreed that there is a need for increased awareness about the implications of the age of transfused blood on quality of care (83%), as well as for greater scrutiny of the quality of blood used for transfusion, including RBC viability and oxygen transport capacity (75%). Given the clear need for increased awareness and clinical strategies to address issues related to extended blood storage, these findings support the scientific priorities recently recommended by The Working Group on Strategies to Optimize RBC Products, convened in 2012 by the National Heart, Lung, and Blood Institute, including 25:
Improvement or development of new methods to measure oxygen delivery to tissues;
Identification and development of surrogate markers related to oxygen delivery;
Identification of markers that are correlated with good or poor RBC survival;
Clinical studies that link RBC in vitro characteristics with recipient outcome;
Development of improved RBC storage or rejuvenation; and
Studies to improve storage bags.
Current RBC storage practices appear to be affected by an elevated threshold for action at individual and institutional levels. In this study, over 80% of study participants, only a portion of whom are physicians themselves, believed that treating physicians are generally unaware of changes that occur over time in stored RBCs, and only 40% perceived that clinicians frequently request ‘fresh’ RBCs. About 70% of participants had not observed an overall increase in efforts by their institution to improve the quality of blood stored for transfusion, and over 80% believed that most institutions are not pursuing measures to reduce the age of stored blood used for transfusion. Based on the survey results, the potential risks of extended RBC storage duration do not appear to be an institutional priority nor a major concern for the respondents, who have likely weighed the available data along with their own clinical experiences. These perspectives represent a barrier for stakeholders who continue to pursue strategies to reduce RBC storage duration and maximize RBC quality.
Limitations
Our study has several limitations that may affect the strength and generalizability of the findings. Of course, there are many possible drawbacks inherent to questionnaire‐based research, such as the potential misinterpretation of questions and responses based on the relative subjectivity of the survey instrument and biases of the subjects and researchers. Accordingly, every effort was made to develop clear, valid survey items rooted in an understanding of current practices and literature related to blood storage and quality. Additionally, this study's sample size was relatively small and sampling was conducted via a non‐probability, convenience methodology. Interpretation of the practices and perspectives reported here may not be representative of all blood banking and transfusion professionals, as survey participants were limited to attendees of the AABB congress willing to complete the survey; additionally, participants recruited from the industry‐sponsored symposium on this topic may have had greater knowledge, concerns and/or involvement in issues surrounding the quality of blood used for transfusion compared with non‐attendees. Analysis of the study data was further limited by the fairly high level of variability in the data relative to the small sample size (e.g. participants reported a broad range of roles and functions within blood banking and transfusion medicine).
Conclusions
This survey study demonstrates conflicting perspectives on the clinical impact and institutional prioritization of RBC storage prior to blood transfusion. The vast majority of participants recognize a potential negative impact of storage on blood quality and transfusion outcomes; however, extended blood storage remains commonly accepted. Professionals across a range of roles related to transfusion medicine maintain the perspective that RBCs may be stored for 42 days per US regulatory standards despite recognition of changes in structure and function that may compromise RBC health and impair oxygen delivery. This analysis suggests that these perspectives may be related to a lack of consensus on the link between RBC age and transfusion outcomes, the influence of existing regulatory guidance on RBC storage limits, a lack of awareness on the part of treating clinicians and institutional inaction. These results highlight the need for further research and increased awareness of the potential relationship between extended RBC storage duration and adverse events, as well as the potential to implement strategies that maximize transfusion benefits.
Conflict of interests
The author acknowledges research contract support from Biomet, as well as grant support from the National Institutes of Health and the National Blood Foundation, to study the effects of blood storage age on recipient outcomes. The author is a consultant to Zimmer Biomet.
Supporting information
Figure S1. Full survey questionnaire.
Figure S2. Appropriate RBC storage limit prior to transfusion, in days, according to participant opinion.
Figure S3. Full listing of participant agreement with 24 statements related to RBC storage and its impact on blood quality and transfusion outcomes.
Figure S4. Exploratory factor analysis was performed on all surveys with complete data (n = 56) using principal component methodology.
Table S1. Survey items contributing to each of the 3 main factors identified by principal component analysis.
Acknowledgements
The author was involved in the conception, writing and editing of this manuscript. The author acknowledges The Lockwood Group (Andrea Burdett, MPH, and Jane Radix, EdD) for editorial assistance and Michael Schwiers for data analysis and statistical assistance. Funding for the study and for editorial and statistical assistance was provided by Biomet Biologics, LLC, a Zimmer Biomet company.
References
- 1. Carson JL, Grossman BJ, Kleinman S, et al: Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2012; 157:49–58 [DOI] [PubMed] [Google Scholar]
- 2. Pfuntner A, Wier LM, Stocks C: Statistical brief #149: most frequent procedures performed in U.S. Hospitals, 2010. February 2013. Agency for Healthcare Research and Quality (AHRQ). http://www.hcup-us.ahrq.gov/reports/statbriefs/sb149.pdf. Last accessed 18 May 2015. [PubMed]
- 3. Hofmann A, Farmer S, Shander A: Five drivers shifting the paradigm from product‐focused transfusion practice to patient blood management. Oncologist 2011; 16(suppl 3):3–11 [DOI] [PubMed] [Google Scholar]
- 4. Blood, tissues and cells from human origin: the European blood alliance perspective. In: Folléa G, de Wit J, eds. 2015. http://europeanbloodalliance.eu/eba-book; accessed 20 July 2016
- 5. United States Department of Health and Human Services : The 2011 national blood collection and utilization survey report. Washington. 2011; http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Last accessed 18 May 2015; 9 March 2015.
- 6. NHS . Blood transfusion – risks. 2013. www.nhs.uk/Conditions/Blood-transfusion/Pages/Risks.aspx. Last accessed 9 March 2015.
- 7. United States General Accounting Office : Public health: blood supply generally adequate despite new donor restrictions. 2002; http://www.gao.gov/assets/240/235234.pdf. Last accessed 18 May 2015.
- 8. Joint United Kingdom Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee : Shelf life of red cells in additive solution (SAG‐M). www.transfusionguidelines.org/document-library/documents/shelf-life-of-red-cells-in-additive-solution-sag-m. Published May 2005. Updated September 2014. Last accessed 5 May 2016.
- 9. European Directorate for the Quality of Medicines & Healthcare : Guide to the Preparation, Use and Quality Assurance of Blood Components, 18th edn Strasbourg, France, Council of Europe, 2015. [Google Scholar]
- 10. Hess JR: Red cell changes during storage. Transfus Apher Sci 2010; 43:51–59 [DOI] [PubMed] [Google Scholar]
- 11. Doctor A, Spinella P: Effect of processing and storage on red blood cell function in vivo. Semin Perinatol 2012; 36:248–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim‐Shapiro DB, Lee J, Gladwin MT: Storage lesion: role of red blood cell breakdown. Transfusion 2011; 51:844–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kor DJ, Van Buskirk CM, Gajic O: Red blood cell storage lesion. Bosn J Basic Med Sci 2009; 9(suppl 1):21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valeri CR, Ragno G: The effects of preserved red blood cells on the severe adverse events observed in patients infused with hemoglobin based oxygen carriers. Artif Cells Blood Substit Immobil Biotechnol 2008; 36:3–18 [DOI] [PubMed] [Google Scholar]
- 15. Zimrin AB, Hess JR: Current issues relating to the transfusion of stored red blood cells. Vox Sang 2009; 96:93–103 [DOI] [PubMed] [Google Scholar]
- 16. Bosman GJ, Werre JM, Willekens FL, et al: Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med 2008; 18:335–347 [DOI] [PubMed] [Google Scholar]
- 17. Aubron C, Nichol A, Cooper DJ, et al: Age of red blood cells and transfusion in critically ill patients. Ann Intensive Care 2013; 3:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang D, Sun J, Solomon SB, et al: Transfusion of older stored blood and risk of death: a meta‐analysis. Transfusion 2012; 52:1184–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janz DR, Zhao Z, Koyama T, et al: Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann Intensive Care 2013; 3:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schimmer C, Hamouda K, Ozkur M, et al: Influence of storage time and amount of red blood cell transfusion on postoperative renal function: an observational cohort study. Heart Lung Vessel 2013; 5:148–157 [PMC free article] [PubMed] [Google Scholar]
- 21. van de Watering L, Lorinser J, Versteegh M, et al: Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion 2006; 46:1712–1718 [DOI] [PubMed] [Google Scholar]
- 22. Steiner ME, Triulzi D, Assmann SF, et al Randomized trial results: red cell storage age is not associated with a significant difference in multiple‐organ dysfunction score or mortality in transfused cardiac surgery patients [Pleanry P2‐030A]. Transfusion 2014; 54(suppl S2):15A. [Google Scholar]
- 23. Lacroix J, Hébert PC, Fergusson DA, et al: Age of transfused blood in critically ill adults. N Engl J Med 2015; 372:1410–1418 [DOI] [PubMed] [Google Scholar]
- 24. Stowell CP: Effects of storage on the biology and clinical efficacy of the banked red blood cell. Transfus Apher Sci 2010; 43:45–47 [DOI] [PubMed] [Google Scholar]
- 25. Wagner SJ, Glynn SA, Welniak LA: Research opportunities in optimizing storage of red blood cell products. Transfusion. 2014; 54:483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Full survey questionnaire.
Figure S2. Appropriate RBC storage limit prior to transfusion, in days, according to participant opinion.
Figure S3. Full listing of participant agreement with 24 statements related to RBC storage and its impact on blood quality and transfusion outcomes.
Figure S4. Exploratory factor analysis was performed on all surveys with complete data (n = 56) using principal component methodology.
Table S1. Survey items contributing to each of the 3 main factors identified by principal component analysis.


