Abstract
Objective
To assess whether the time between the last rituximab infusion and initiation of a different biologic agent influenced infection risk in patients with rheumatoid arthritis (RA).
Methods
Patients with RA who newly initiated rituximab within the Consortium of Rheumatology Researchers of North America registry were included if they switched to a nonrituximab biologic agent and had ≥1 followup visit within 12 months of switching. Patients were categorized by duration of time between their last rituximab infusion and initiation of a subsequent biologic agent (≤5 months, 6–11 months, and ≥12 months). The primary outcome was time to first infectious event. Adjusted Cox regression models estimated the association between time to starting a subsequent biologic agent and infection.
Results
A total of 44 overall infections (7 serious, 37 nonserious) were reported during the 12‐month followup in the 215 patients included in this analysis (104 switched at ≤5 months, 67 at 6–11 months, and 44 at ≥12 months). Median (interquartile range) time to infection was 4 (2–5) months. Infection rates per patient‐year in the ≤5‐month, 6–11‐month, and ≥12‐month groups were 0.34 (95% confidence interval [95% CI] 0.22–0.52), 0.30 (95% CI 0.17–0.52), and 0.41 (95% CI 0.22–0.77), respectively. After adjustment, time to switch to a subsequent biologic agent was not associated with infection, which remained unchanged when number and rate of rituximab retreatments were included in the models.
Conclusion
In this real‐world cohort of patients with RA, infection rates ranged from 0.30 to 0.41 per patient‐year, with no significant difference in the rate between patients who initiated a subsequent biologic agent earlier versus later after rituximab treatment.
Introduction
Patients with rheumatoid arthritis (RA) have an increased risk of infection compared with the general population due to extended use of disease‐modifying antirheumatic drugs (DMARDs) and biologic agent therapy, as well as the disease itself 1. The safety of switching between biologic agent therapies is an important consideration, particularly regarding the risk of infection. Although such risk is well documented when switching within a class (e.g., cycling between different tumor necrosis factor inhibitors [TNFi]) 2, 3, 4, limited data exist on infection rates when switching between biologic agent classes, particularly in patients who switched to a biologic agent with a different mechanism of action after rituximab.
Box 1. Significance & Innovations.
In a real‐world setting in patients with rheumatoid arthritis, duration of time between the last rituximab infusion and the switch to a biologic agent with a different mechanism of action was not associated with an increased risk of infection.
The rate and number of rituximab treatments did not influence the risk of infection associated with subsequent biologic agent use.
For patients not responding to rituximab, switching to a different biologic agent may represent an appropriate therapeutic option, rather than delaying treatment due to perceived concerns regarding residual effects of rituximab and risk of infection.
These results may inform clinical decisions regarding safety concerns when considering a switch between classes of biologic agents.
Rituximab is an anti‐CD20 monoclonal antibody with a unique mechanism of action that targets and depletes CD20+ B cells. Rituximab (2 × 1,000 mg, 2 weeks apart, every 24 weeks or based on clinical evaluation) is approved in combination with methotrexate for the treatment of patients with RA who have had an inadequate response to ≥1 TNFi. Immunosuppression due to prolonged B cell depletion from repeated doses of rituximab may be associated with an increased risk of infection, both with persistent rituximab treatment and due to residual effects of rituximab when switching to a subsequent biologic agent 5.
Currently, little is known about safety outcomes when switching between classes of biologic agents. Of particular interest is the time from last rituximab dose to the first dose of the subsequent nonrituximab biologic agent. Additionally, in clinical trial settings, patients typically receive repeat doses of rituximab every 4–6 months, as needed; however, the rate and number of rituximab retreatments vary in real‐world clinical practice. Understanding how both the duration of time between rituximab and a subsequent biologic agent and the frequency of rituximab use may impact safety outcomes can assist treatment decisions in clinical practice.
The objective of this study was to assess whether time between the last rituximab infusion and the switch to a biologic agent with a different mechanism of action, as well as the intensity of rituximab use (rate and number of retreatments), influenced risk of infection in a real‐world clinical setting in patients with RA.
Patients and methods
Study design and population
The Consortium of Rheumatology Researchers of North America (CORRONA) registry is an independent, prospective, observational cohort of patients with RA recruited at more than 160 private and academic practice sites in the US, with more than 600 participating rheumatologists. As of May 30, 2015, data on more than 40,989 patients with RA have been collected.
Inclusion criteria for this analysis were diagnosis of RA at any time between March 1, 2006 and March 3, 2014; first‐time initiation of rituximab within CORRONA; subsequent initiation of a nonrituximab biologic agent after rituximab use, with no other biologic agents used during the interim; ≥1 followup appointment within 12 months after subsequent biologic agent initiation; and available data on rituximab infusion dates to calculate the duration of time between the switch from rituximab to a subsequent biologic agent.
Analytic design and cohort
The analytic design is illustrated in Supplementary Figure 1 (available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22912/abstract). First‐time initiators of rituximab in CORRONA who initiated a subsequent biologic agent after rituximab discontinuation were included. Patients were categorized by the duration of time between their last rituximab infusion and the switch to a subsequent biologic agent (≤5 months, 6–11 months, and ≥12 months). Patients were followed up from the time they initiated the subsequent biologic agent (index date) to the time of the first infectious event (infectious events occurring within 90 days after the discontinuation or switch were counted as an event). Followup ended with the first infectious event or with the earliest of the following: 12‐month elapse, discontinuation or switch of the subsequent biologic agent, switch back to rituximab, or exit from CORRONA.
Assessments and outcomes
The primary outcomes were time to infection and infection rates by patient category. The effect of the number and rate of rituximab retreatment prior to the index date (the time of initiation of a subsequent biologic agent) on risk of infection was also evaluated.
Data on infection were collected by rheumatologists at routine visits in CORRONA, at both enrollment and followup. Serious infections were defined as those requiring intravenous antibiotics or hospitalization. For time to all infections, the time to first infection was used and for serious infections, the time to first serious infection was used. Physicians reported whether the patient ever had the infection and the year (month and year at followup) of its occurrence. If the patient had the infection more than once in the past, the year (month and year at followup) of the most recent occurrence was collected. For each infection type, rheumatologists reported pathogen types and whether the patient was hospitalized or received parenteral antibiotics.
Statistical analysis
Patient demographic and clinical characteristics at baseline (the time of initiation of a subsequent biologic agent) were compared across patient groups. Kaplan‐Meier analyses estimated the time to infection and cumulative infection rates for each patient group. A Cox regression model estimated the unadjusted association between the time to the switch to a subsequent biologic agent and infection. A multivariable model estimated the association between the time to the switch to a subsequent biologic agent and infection, adjusted for potential confounders. Potential confounders (measured at baseline and defined as characteristics that differed by time to initiation and were associated with infection in the unadjusted models) were included in the model, and the adjusted hazard ratio (HR) for time to initiation was estimated. Potential confounders included disease activity assessments, age, sex, duration of RA, smoking status, medical history variates, and number of prior nonbiologic DMARDs and nonrituximab biologic agents. The number and rate of rituximab retreatments per year were added to the model regardless of the unadjusted association with infections.
Results
Analytic population and patient characteristics
Within CORRONA, 215 patients switched to a subsequent biologic agent after rituximab use and were included in the analysis (see Supplementary Figure 2, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22912/abstract). Of these patients, 104, 67, and 44 switched to a subsequent biologic agent at ≤5 months (median 3.5 [interquartile range (IQR) 2–5]), 6–11 months (median 7 [IQR 6–9]), and ≥12 months (median 17 [IQR 15–24]), respectively, after their last rituximab infusion. A total of 130 patient‐years were included in this analysis (≤5 months [patient‐years 62.1], 6–11 months [patient‐years 43.4], and ≥12 months [patient‐years 24.3]). A total of 103 patients switched from rituximab to a TNFi, and 112 patients switched from rituximab to a non‐TNFi biologic agent.
The average age of the analytic cohort was 56.8 years, and the mean duration of RA was 14.8 years. Baseline demographic and clinical characteristics were mostly similar between the groups; however, patients who switched to a subsequent biologic agent earliest (≤5 months) had higher tender joint counts, and patients who switched later (>6 months) were more likely to have Medicare coverage (Table 1). Patients in the ≤5‐month group also tended to be younger (although not significantly), which may account for the less frequent Medicare coverage. A history of serious infections was observed in 6.5%, 11.5%, and 9.8% of patients in the ≤5‐month, 6–11‐month, and ≥12‐month groups, respectively. There was a significantly lower proportion of patients who were current smokers in the 6–11‐month group compared with the ≤5‐month and ≥12‐month groups (P = 0.03).
Table 1.
Baseline characteristics, comorbidities, and medication historya
| Time to switch to a subsequent biologic agent | ||||
|---|---|---|---|---|
| Characteristic | ≤5 months (n = 104) | 6–11 months (n = 67) | ≥12 months (n = 44) | P |
| Age, mean ± SD years | 55.6 ± 11.1 | 57.9 ± 11.7 | 57.8 ± 12.3 | 0.36 |
| Women | 75.7 | 83.6 | 68.2 | 0.17 |
| Time to switch, median (IQR) months | 3.5 (2–5) | 7 (6–9) | 17 (15–24) | NA |
| White | 92.3 | 94.0 | 93.2 | 0.94 |
| Duration of RA, mean ± SD years | 13.3 ± 9.6 | 16.3 ± 9.9 | 15.8 ± 9.6 | 0.12 |
| BMI, mean ± SD kg/m2 | 30.9 ± 7.8 | 29.6 ± 8.0 | 31.3 ± 8.0 | 0.46 |
| Insuranceb | ||||
| Private | 80.8 | 77.6 | 72.7 | 0.53 |
| Medicare | 26.0 | 46.3 | 45.5 | < 0.01 |
| Medicaid | 6.7 | 6.0 | 9.1 | 0.83 |
| No insurance | 0.0 | 1.5 | 0.0 | 0.52 |
| Medical history | ||||
| Serious infections | 6.5 | 11.5 | 9.8 | 0.51 |
| Diabetes mellitus | 12.5 | 11.9 | 11.4 | 1.00 |
| Lung disease/pulmonary fibrosis | 3.8 | 6.0 | 4.5 | 0.91 |
| Liver disorder/hepatic events | 5.8 | 7.5 | 4.5 | 0.87 |
| Malignancy | 13.5 | 10.5 | 9.1 | 0.75 |
| Cardiovascular disease | 6.7 | 14.9 | 11.4 | 0.21 |
| Current smoker | 23.3 | 9.0 | 25.0 | 0.03 |
| RF seropositivec | 63.6 | 67.6 | 77.8 | 0.44 |
| ACPA seropositivec | 57.6 | 59.1 | 75.0 | 0.60 |
| CDAI, median (IQR) | 24.0 (14.0–34.5) | 21.0 (12.8–28.5) | 21.3 (13.5–29.9) | 0.13 |
| TJC (0–28), median (IQR) | 9.0 (3.0–15.0) | 6.0 (1.0–10.0) | 5.0 (1.5–10.0) | 0.03 |
| SJC (0–28), median (IQR) | 5.0 (2.0–10.0) | 6.0 (2.0–8.0) | 4.0 (1.0–12.0) | 0.76 |
| Patient pain (0–100), mean ± SD | 55.2 ± 25.9 | 55.8 ± 24.1 | 52.8 ± 28.3 | 0.83 |
| DAS28‐CRP, mean ± SDc | 4.7 ± 1.4 | 4.5 ± 1.7 | 4.1 ± 1.5 | 0.27 |
| M‐HAQ score, mean ± SD | 0.7 ± 0.5 | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.58 |
| Medication history | ||||
| Currently receiving prednisone, no. (%) | 47 (45.2) | 34 (50.7) | 21 (47.7) | 0.77 |
| No. of prior DMARDs, including current, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (2–3.5) | 0.73 |
| No. of prior biologic agents, excluding rituximab | ||||
| 0 | 1.9 | 1.5 | 11.4 | 0.07 |
| 1 | 21.2 | 22.4 | 27.3 | 0.07 |
| ≥2 | 76.9 | 76.1 | 61.4 | 0.07 |
| Rituximab retreatments, mean ± SD | 1.5 ± 2.2 | 1.5 ± 1.9 | 1.1 ± 1.7 | 0.54 |
| No. of rituximab retreatments | ||||
| 0 | 52.9 | 40.3 | 50.0 | 0.44 |
| 1 | 16.4 | 19.4 | 22.7 | 0.44 |
| ≥2 | 30.8 | 40.3 | 27.3 | 0.44 |
| Rate of rituximab treatment per year, mean ± SD | 0.95 ± 1.2 | 0.72 ± 0.7 | 0.37 ± 0.4 | < 0.01 |
Values are the percentage, unless indicated otherwise. Baseline was defined as the time of initiation of the subsequent biologic agent. IQR = interquartile range; NA = not applicable; RA = rheumatoid arthritis; BMI = body mass index; RF = rheumatoid factor; ACPA = anti–cyclic citrullinated peptide antibody; CDAI = Clinical Disease Activity Index; TJC = tender joint count; SJC = swollen joint count; DAS28‐CRP = Disease Activity Score in 28 joints using the C‐reactive protein level; M‐HAQ = modified Health Assessment Questionnaire; DMARDs = disease‐modifying antirheumatic drugs.
Insurance categories were not mutually exclusive, and patients may have had dual coverage.
Laboratory measures were not mandated by the registry protocol and were not obtained in routine clinical practice. In the ≤5‐month, 6–11‐month, and ≥12‐month categories, respectively, n = 66, n = 37, and n = 27 for RF; n = 33, n = 22, and n = 12 for ACPA; and n = 45, n = 33, and n = 21 for DAS28‐CRP.
Regarding medication history, patients who switched to a subsequent biologic agent earliest (≤5 months) had a higher rate of rituximab retreatment per year compared with the 6–11‐month and ≥12‐month groups; however, the mean total number of rituximab retreatments was similar across the groups (Table 1). The proportion of patients receiving prednisone and the number of prior DMARDs was similar across groups.
Infection rates and types
Overall, there were 44 infections reported in the 215 patients during the 12‐month followup (Table 2). There were 7 serious infections (defined as requiring intravenous antibiotics or hospitalization) at a rate of 0.054 events per patient‐year. The overall all‐infection rates (events per patient‐year) were 0.34, 0.30, and 0.41 for the ≤5‐month, 6–11‐month, and ≥12‐month groups, respectively. The most common types of nonserious infections observed across all patient groups were upper respiratory infection (n = 15), urinary tract infection (n = 7), and >1 organ system infection (n = 3; sinusitis/urinary tract infection [n = 1] and urinary tract/upper respiratory tract infection [n = 2]). No opportunistic infections were reported.
Table 2.
Infection rates and types by time to the switch to a subsequent biologic agent
| Time to switch | ||||
|---|---|---|---|---|
| ≤5 months (n = 104) | 6–11 months (n = 67) | ≥12 months (n = 44) | Total | |
| Total duration of followup, patient‐years | 62.08 | 43.38 | 24.25 | 129.71 |
| No. of infections | 21 | 13 | 10 | 44 |
| Infection rate per patient‐year (95% confidence interval) | 0.34 (0.22–0.52) | 0.30 (0.17–0.52) | 0.41 (0.22–0.77) | 0.34 (0.25–0.46) |
| Infection type, no. | ||||
| Serious infectionsa | 1 | 4 | 2 | 7 |
| Nonserious infections | 20 | 9 | 8 | 37 |
| Upper respiratoryb | 9 | 3 | 3 | 15 |
| Urinary tract | 5 | 0 | 2 | 7 |
| Multiorgan systemc | 0 | 2 | 1 | 3 |
| Skin (cellulitis) | 1 | 1 | 0 | 2 |
| Musculoskeletal (joint bursa) | 0 | 0 | 1 | 1 |
| Other | 5 | 3 | 1 | 9 |
Serious infections were defined as infections requiring intravenous antibiotics or hospitalization.
Includes bronchitis, bronchitis/other, pneumonia, sinusitis, sinusitis/bronchitis, sinusitis/upper respiratory infection, and upper respiratory infection.
Includes sinusitis/urinary tract infection and urinary tract infection/upper respiratory tract infection.
Time to infection
Of the patients who had an infection during the 12‐month followup, the median (IQR) time to infection was 4 2, 3, 4, 5 months after initiation of the subsequent biologic agent. The time to the first infectious event after starting the subsequent biologic agent was similar between patient groups (Figure 1A). The probability of infection at 3, 6, 9, and 12 months after initiation of the subsequent biologic agent was also unaffected by the duration of time between the last rituximab infusion and the initiation of a subsequent biologic agent (Figure 1B). When patients were stratified by type of subsequent biologic agent used (TNFi versus non‐TNFi), the probability of infection over time was not significantly different between the patient groups (data not shown).
Figure 1.
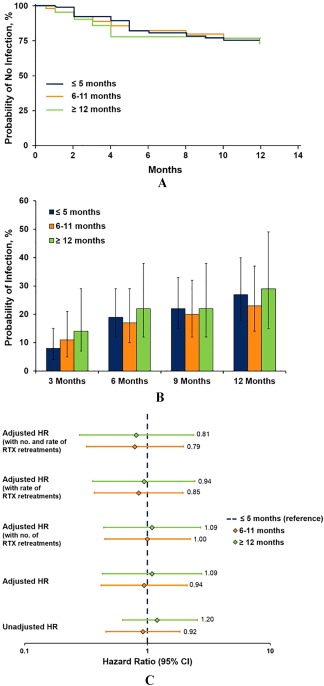
Summary of infections. A, survival analysis of the probability of not experiencing an infection over time by time to the switch. Estimates of time to infection and probability of infection were similar when analyzing tumor necrosis factor inhibitor (TNFi) initiators and non‐TNFi initiators separately. B, probability of infection at 3, 6, 9, and 12 months by time to the switch. C, unadjusted and adjusted hazard ratios (HRs) for infection rate by time to the switch. Cox regression models were adjusted for age, sex, duration of rheumatoid arthritis, smoking status, patient pain, modified Health Assessment Questionnaire, Clinical Disease Activity Index, presence of subcutaneous nodules, history of cardiovascular disease, history of liver disease, history of serious infection, number of prior disease‐modifying antirheumatic drugs, and number of prior biologic agents (excluding rituximab [RTX]). 95% CI = 95% confidence interval.
Association between time to the start of a subsequent biologic agent and infection
Based on Cox regression models, the unadjusted HRs (95% confidence interval [95% CI]) for infection were 0.92 (0.46–1.84) and 1.20 (0.57–2.55) in the 6–11‐month and ≥12‐month groups, respectively, relative to the ≤5‐month group (Figure 1C). After adjusting for potential confounders, the HRs (95% CIs) for infection were 0.94 (0.42–2.10) and 1.09 (0.43–2.76) in the 6–11‐month and ≥12‐month groups, respectively, relative to the ≤5‐month group, demonstrating that there was no association between time to the switch and infection. Addition of the number (0, 1, or <2) and/or rate of rituximab retreatments prior to initiation of the subsequent biologic agent to the models did not impact these results (Figure 1C).
Discussion
This analysis, conducted in a large, real‐world setting of patients with established RA and moderate to high disease activity, assessed the risk of infection based on duration of time between switching from rituximab to another biologic agent. Of the 215 patients who switched from rituximab to a subsequent biologic agent, 44 infections (7 serious) were reported during the 12‐month followup. The rate of any infection while patients were exposed to a nonrituximab biologic agent in this study was 0.34 per patient‐year, and the rate of serious infection was 0.054 per patient‐year, similar to previous reports of 0.23 to 0.58 per patient‐year and 0.055 to 0.064 per patient‐year, respectively 6, 7, 8. No opportunistic infections were reported, and the distribution of nonserious infections by organ system was as expected based on the patient population 1.
The rate of infection was compared between patients categorized by time (≤5 months, 6–11 months, and ≥12 months) from last rituximab infusion to initiation of a subsequent biologic agent. The rate of infection after initiation of the subsequent biologic agent was not significantly different across these groups, suggesting that there was no increase in risk of infection when switching early (within 5 months of the last rituximab infusion) compared with switching later (≥1 year after the last rituximab infusion). Additionally, the risk of infection was adjusted for the number and rate of rituximab retreatments and compared across patients in the ≤5‐month, 6–11‐month, and ≥12‐month groups. After adjusting for the number and rate of rituximab retreatments, the HRs for risk of infection after initiation of the subsequent biologic agent were not significantly different across patients in the ≤5‐month, 6–11‐month, and ≥12‐month groups. This lack of difference suggests that the intensity of rituximab use prior to the switch did not influence the risk of infection associated with the subsequent biologic agent, regardless of the duration of time between the last rituximab infusion and initiation of a subsequent biologic agent.
Previous studies have examined the risk of infection when switching between biologic agents of the same class and between classes. Switching from one TNFi to another TNFi was not associated with increased infection risk in adjusted models 2, 4. Two studies have examined the safety of biologic agents after rituximab treatment 7, 9. One retrospective study examined 22 patients with RA who initiated a subsequent biologic agent at a mean of 4 months (range 1–12 months); after a mean followup time of 14 months, there were no serious infections, and the duration of time between stopping rituximab and initiating a subsequent biologic agent was unrelated to the occurrence of nonserious infections 9. In another study, data on serious infections were collected from a cohort of 185 patients with RA from an international rituximab clinical trial program 7. The majority of patients were peripherally B cell depleted when a subsequent biologic agent was initiated at a median of 7 months after the last rituximab infusion. The overall rate of serious infections was 0.055 per patient‐year after exposure to the subsequent biologic agent, similar to the observed rate of 0.054 in this study.
Additionally, as in our study, the nature of the infections reported (upper respiratory tract and urinary tract) were consistent with typical infections seen in patients with RA treated with DMARDs or other biologic agents.
Although the current study was based on a small cohort of patients from an observational study, it provides a unique opportunity to examine the impact of the timing of biologic agent therapy on safety in patients who are likely B cell depleted from rituximab. These results provide additional confidence that the risk of infections is not substantially higher in patients with a short time interval between 2 different biologic agents. For patients needing to switch from rituximab, these data suggest that switching to another biologic agent with the potential for suppression of disease activity is a valid approach. Providers should therefore be encouraged to optimize therapy according to disease activity levels after an inadequate response to rituximab, rather than delay initiation of another biologic agent due to concerns regarding residual immunosuppressive effects of rituximab and the risk of infection.
The strengths of this analysis stem from the large, population‐based nature of the CORRONA registry, representing routine care from more than 160 rheumatology clinical practices in the US, allowing for generalizability of findings. The limitations of this study include the small sample size for analysis, limited followup, and wide 95% CIs. Additionally, recall bias of the actual dates of the qualifying infection may influence the results. Further, the status of the patients' B cell depletion was not measured in this analysis. Future studies will be necessary to determine whether the peripheral B cell level after rituximab treatment affects the safety of subsequent biologic agents.
In this sample of patients with RA, rates of infection were consistent with previously published estimates. The types of infections acquired were consistent with observations in patients with RA 1. Duration of time between the last rituximab infusion and the switch to subsequent biologic agents with different mechanisms of action did not influence the risk of infection in this small cohort. For patients with an inadequate response to rituximab, switching to another class of drug 4 months after the last infusion may be an appropriate therapeutic approach rather than delaying treatment. Taken together, these results may inform clinical decisions regarding safety outcomes when switching between classes of biologic agents.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Harrold had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Harrold, Reed, Karki, Magner, Shewade, John, Kremer, Greenberg.
Acquisition of data
Harrold, Reed, Karki, Magner, Shewade, John, Kremer, Greenberg.
Analysis and interpretation of data
Harrold, Reed, Karki, Magner, Shewade, John, Kremer, Greenberg.
ADDITIONAL DISCLOSURES
Authors Shewade and John are employees of Genentech. Support for third‐party writing assistance for this manuscript, furnished by Ellen Mercado, PhD, of Health Interactions, was provided by F. Hoffmann‐La Roche, Ltd.
Supporting information
Supplementary Figure 1. Analysis design. The primary exposure of interest (blue) was the time (in months) from the last rituximab infusion to the start of a subsequent (non‐rituximab) biologic. Patients were categorized by the duration of time between their last rituximab infusion and the switch to a subsequent biologic (≤ 5 months, 6‐11 months, and ≥ 12 months). For patients who discontinued or switched their subsequent biologic before infection, infectious events occurring within 90 days following the discontinuation or switch were counted as an event as the medication may still have an immunosuppressant effect.
Supplementary Figure 2. Patient attrition. A total of 215 patients with rheumatoid arthritis from the Corrona registry were selected for this analysis based on the inclusion and exclusion criteria. Baseline visit was at the time of subsequent (non‐rituximab) biologic initiation. If this initiation occurred between visits, then baseline information was obtained at the visit within 4 months prior to initiation. There were 104 patients who were excluded because the actual visit date for initiation of the subsequent drug was unclear (“no baseline visit date”). Because patients are classified based on the time interval between medications, this information was critical for inclusion.
ACKNOWLEDGMENT
The authors thank Tmirah Haselkorn (Genentech, Inc.) for her insightful and critical review of the manuscript.
Supported by the Consortium of Rheumatology Researchers of North America (CORRONA). Over the past 2 years, the CORRONA registry has been supported by AbbVie, Amgen, AstraZeneca, Bristol‐Myers Squibb, Crescendo, Genentech, Horizon Pharma, Janssen, Eli Lilly, Novartis, Pfizer, and UCB through contracted subscriptions.
Dr. Harrold is an employee of and owns stock options in Corrona, has received grant funding from Pfizer, and has received consultancy fees from Roche (less than $10,000 each).
Dr. Kremer is a shareholder and an employee of Corrona, has received research support from Genentech, and has received consulting fees from AbbVie, Amgen, Bristol‐Myers Squibb, Genentech, GlaxoSmithKline, Eli Lilly, MedImmune, Pfizer, and Sanofi (less than $10,000 each).
Dr. Greenberg is an employee of and shareholder in Corrona, and has received consulting fees from Novartis, Genentech, Janssen, Eli Lilly, and Pfizer (less than $10,000 each).
REFERENCES
- 1. Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population‐based study. Arthritis Rheum 2002;46:2287–93. [DOI] [PubMed] [Google Scholar]
- 2. Curtis JR, Xie F, Chen L, Baddlet JW, Beukelman T, Saag KG, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis 2011;70:1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnston SS, Turpcu A, Shi N, Fowler R, Chu BC, Alexander K. Risk of infections in rheumatoid arthritis patients switching from anti‐TNF agents to rituximab, abatacept, or another anti‐TNF agent, a retrospective administrative claims analysis. Semin Arthritis Rheum 2013;43:39–47. [DOI] [PubMed] [Google Scholar]
- 4. Nguyen‐Khoa BA, Goehring EL Jr, Alexander KA, Dong W, Napalkov P, Jones JK. Risk of significant infection in rheumatoid arthritis patients switching anti‐tumor necrosis factor‐α drugs. Semin Arthritis Rheum 2012;42:119–26. [DOI] [PubMed] [Google Scholar]
- 5. Brinkman IH, van de Laar MA, Jansen TL, van Roon EN. The potential risk of infections during (prolonged) rituximab therapy in rheumatoid arthritis. Expert Opin Drug Saf 2011;10:715–26. [DOI] [PubMed] [Google Scholar]
- 6. Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova‐Scholz M, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 2005;52:3403–12. [DOI] [PubMed] [Google Scholar]
- 7. Genovese MC, Breedveld FC, Emery P, Cohen S, Keystone E, Matteson EL, et al. Safety of biological therapies following rituximab treatment in rheumatoid arthritis patients. Ann Rheum Dis 2009;68:1894–7. [DOI] [PubMed] [Google Scholar]
- 8. Lang VR, Englbrecht M, Rech J, Nüsslein H, Manger K, Schuch F, et al. Risk of infections in rheumatoid arthritis patients treated with tocilizumab. Rheumatology (Oxford) 2012;51:852–7. [DOI] [PubMed] [Google Scholar]
- 9. Mishra R, Singh V, Pritchard CH. Safety of biologic agents after rituximab therapy in patients with rheumatoid arthritis. Rheumatol Int 2011;31:481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Analysis design. The primary exposure of interest (blue) was the time (in months) from the last rituximab infusion to the start of a subsequent (non‐rituximab) biologic. Patients were categorized by the duration of time between their last rituximab infusion and the switch to a subsequent biologic (≤ 5 months, 6‐11 months, and ≥ 12 months). For patients who discontinued or switched their subsequent biologic before infection, infectious events occurring within 90 days following the discontinuation or switch were counted as an event as the medication may still have an immunosuppressant effect.
Supplementary Figure 2. Patient attrition. A total of 215 patients with rheumatoid arthritis from the Corrona registry were selected for this analysis based on the inclusion and exclusion criteria. Baseline visit was at the time of subsequent (non‐rituximab) biologic initiation. If this initiation occurred between visits, then baseline information was obtained at the visit within 4 months prior to initiation. There were 104 patients who were excluded because the actual visit date for initiation of the subsequent drug was unclear (“no baseline visit date”). Because patients are classified based on the time interval between medications, this information was critical for inclusion.


