Abstract
The dosing regimen of prasugrel adjusted for Japanese patients was compared with that of clopidogrel by analyzing the pharmacokinetics and pharmacodynamics in 40 healthy Japanese subjects in a randomized, single‐blind crossover study. In period 1, the subjects received either 300 mg clopidogrel or 20 mg prasugrel; after a >2‐week interval (period 2), the drug was switched. Blood samples of 36 of the 40 subjects were collected for analysis of pharmacokinetics, pharmacodynamics, and CYP2C19 genotypes. The plasma concentration of the active metabolite of prasugrel increased rapidly and reached its peak 30 minutes postadministration, whereas that of the active metabolite of clopidogrel reached its peak 1 hour postadministration. The mean AUC and Cmax of the active metabolite of clopidogrel, but not those of prasugrel, were CYP2C19 genotype dependent. Prasugrel rapidly inhibited platelet aggregation, reaching its maximum effect 1 hour postadministration. Clopidogrel, on the other hand, showed maximum inhibition 2 hours postadministration. Platelet aggregation inhibition by clopidogrel was significantly lower in the poor‐metabolizer subjects than in the extensive‐metabolizer subjects. Overall, prasugrel inhibited platelet aggregation more rapidly and more effectively in healthy Japanese subjects than was observed for clopidogrel.
Keywords: prasugrel, clopidogrel, cytochrome P450 2C19, polymorphism, platelet
Prasugrel and clopidogrel are thienopyridine derivatives with adenosine diphosphate (ADP)–antagonistic activity and are widely used in clinics for the prevention of ischemic events in patients with ST‐segment‐elevated and non‐ST‐segment‐elevated myocardial infarction (STEMI and NSTEMI, respectively) who are undergoing percutaneous coronary intervention (PCI).1, 2, 3 Rapid, potent, and consistent inhibition of platelet aggregation is important in patients with acute STEMI undergoing PCI.4, 5 Compared with the effect of clopidogrel, prasugrel rapidly and more potently inhibits platelet aggregation.6 In STEMI patients undergoing PCI, prasugrel has been shown to be more effective than clopidogrel in reducing the risk of cardiovascular mortality, myocardial infarction, and stroke, as well as stent thrombosis.1
Prasugrel and clopidogrel are inactive prodrugs that are converted to active metabolites that bind irreversibly to the platelet ADP receptor P2Y12. The conversion of clopidogrel to the active metabolite has been reported to be a 2‐step, CYP‐dependent process (mainly CYP2C19).7, 8 On the other hand, prasugrel is metabolized by esterases in the intestines and is subsequently metabolized by the CYP450 3A, 2B6, 2C9, and 2C19 isoforms.9, 10 In previous clinical studies, 20% to 30% of the patients treated with clopidogrel showed low or no inhibition of ADP‐induced platelet aggregation by CYP2C19 polymorphisms, as measured by ex vivo aggregometry.11, 12
To our knowledge, the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel at a dose adjusted for Japanese patients have not been compared in a clinical study. Therefore, we investigated the clinically relevant pharmacological parameters of prasugrel at a loading dose of 20 mg in healthy Japanese subjects and compared them with those of clopidogrel at a loading dose of 300 mg. Furthermore, we studied the potential effect of CYP2C19 polymorphisms on these parameters.
Methods
Subjects
The present study was conducted in accordance with the Declaration of Helsinki and practice and ethical guidelines for clinical studies. The study protocol was approved by the Institutional Review Board for Human Studies of Hamamatsu University School of Medicine, Hamamatsu, Japan (approval number RG14‐024). Written informed consent was obtained from each subject before participation in the study. Forty healthy Japanese subjects were enrolled. None of them had taken any drug or supplement for at least 1 week before the start of the study.
Study Design
This study was a randomized, single‐blind crossover study. In period 1, the subjects received either 300 mg clopidogrel (Sanofi‐Aventis) or 20 mg prasugrel (Daiichi Sankyo) as a single dose during the fasting state in the morning as determined by randomization via computer‐generated random sequences in a 1:1 ratio. The subjects’ blood samples were collected for pharmacokinetic and pharmacodynamic analysis. After a more than 2‐week interval (period 2), the drug was switched, and the subjects’ blood samples were collected again for pharmacokinetic and pharmacodynamic analysis according to the same collection schedule as used during period 1.
Determination of the Active Drug Metabolites in Plasma
Venous blood samples for determining the plasma concentrations of the active metabolites of prasugrel and clopidogrel were collected in 1/10th volume of 3.2% trisodium citrate before and 0.25, 0.5, 1, 2, and 4 hours after the respective drug administration. The samples were centrifuged at 1710g immediately after collection and were stored at –80°C until analysis. The inhibition of platelet aggregation by prasugrel and clopidogrel was measured before and 1, 2, 4, 6, and 8 hours and 10 days after the respective drug administration. The plasma concentrations of the active metabolites were determined using validated liquid chromatography–tandem mass spectrometry methods.13, 14 The lower limit of detection of both active metabolites is 0.50 ng/mL.
Pharmacokinetic Analysis
The area under the plasma concentration–time curves (AUC) from 0 to 4 hours and the peak concentration (Cmax) of the active metabolites were derived from the concentration–time data using noncompartmental methods. The AUC values were calculated by the linear trapezoidal method.
Measurement of Platelet Aggregation by Light Transmission Aggregometry
Light transmission aggregometry (LTA) was performed on an MCM hematracer 313‐M (SSR Engineering Co., Ltd., Tokyo, Japan).15 Platelet‐rich plasma (PRP) and platelet‐poor plasma (PPP) were prepared from the trisodium citrate–treated venous blood samples by differential centrifugation at room temperature.
PRP and PPP were used for the baseline reading and as a reference corresponding to 100% aggregation, respectively. Aggregation was performed using ADP (20 μM; Chrono‐Log Corp., Havertown, Pennsylvania). The optical density was recorded for 10 minutes at the start of platelet aggregation. The maximal aggregation response (MPA) was recorded and was used for the subsequent analyses. The inhibition of platelet aggregation (IPA) was calculated from the observed MPA at each scheduled point of each treatment using the following formula: IPA (%) = ([MPAbaseline – MPApostdose]/MPAbaseline) × 100.
VerifyNow Assay
The VerifyNow system (Accumetrics, San Diego, California) is a turbidimetric‐based optical detection system that measures platelet aggregation as an increase in light transmittance in whole blood. The assay uses reagents based on microbead agglutination technology, in particular, a lyophilized preparation of human fibrinogen‐coated beads, platelet agonists, a preservative, and a buffer solution.16
Citrate‐anticoagulated whole blood was automatically dispensed from the blood collection tube into the assay device by the instrument. ADP was incorporated into the assay channel to induce platelet activation. Light transmittance increased as activated platelets bound and aggregated the fibrinogen‐coated beads. The instrument measured this change in the optical signal, and reported the results in P2Y12 (PRU). A higher PRU reflected greater ADP‐mediated platelet reactivity.
Determination of the Vasodilator‐Stimulated Phosphoprotein Phosphorylation Ratio
The vasodilator‐stimulated phosphoprotein (VASP) phosphorylation state was determined using an enzyme‐linked immunosorbent assay (CY‐QUANT VASP/P2Y12; France) per the manufacturer's instructions.17 The platelet reactivity index (PRI) was calculated in the presence of PGE1 alone or PGE1 and ADP.
CYP2C19 Genotyping
Five milliliters of blood was collected from each subject for CYP2C19 genotyping. The crude DNA was extracted from the leukocytes of each subject using a commercially bioavailable kit (Third Wave Technologies, Madison, Wisconsin). The genotyping of the mutated genes CYP2C19 *2 and *3 was performed using a polymerase chain reaction–restriction fragment length polymorphism method.18
Data Analysis
The data are presented as the mean ± standard deviation (SD). All data were evaluated using Welch's t test or a paired t test. For the IPA, PRU, and PRI time course determinations, the data were analyzed by repeated‐measures analysis of variance (ANOVA) and the Bonferroni test using SAS software (version 9.2; SAS Institute, Cary, North Carolina). For the AUC, the data were analyzed by ANOVA and Dunnett's test, using SPSS software (version 22; Japan IBM, Tokyo, Japan). A P ≤ .05 was considered statistically significant.
Results
Subjects’ Demographics and Adverse Events
Thirty‐six of the 40 subjects (14 female and 22 male) completed the study according to the protocol. Two subjects were withdrawn from the study in period 1 because of adverse events (hypotension and pain in fingers), which were, however, not related to prasugrel and clopidogrel. Two subjects withdrew their consent after period 1. Slight prasugrel‐related subcutaneous bleeding occurred in 1 subject; however, the subject completed the study according to the protocol. The CYP2C19 genotyping revealed that there were 8 extensive metabolizers (*1/*1), 21 intermediate metabolizers (*1/*2 and *1/*3), and 7 poor metabolizers (*2/*2, *2/*3, and *3/*3).
Pharmacokinetics of the Active Metabolite of Prasugrel and Clopidogrel
The mean plasma concentration–time curves of the active metabolites of prasugrel and clopidogrel after a single oral dose of 20 mg prasugrel or 300 mg clopidogrel are shown in Figure 1a. The plasma concentration of the active metabolite of prasugrel increased rapidly and reached its peak 30 minutes after administration. In contrast, the plasma concentration of the active metabolite of clopidogrel reached its peak 1 hpir after administration. The plasma concentration of prasugrel is shown in Figure 1b and that of clopidogrel in Figure 1c according to the respective CYP2C19 polymorphism. The mean AUC of the active metabolite of clopidogrel differed significantly between the extensive and intermediate metabolizers, as well as between the extensive and poor metabolizers (Table 1, Figure 1c). The Cmax in the poor metabolizers was significantly lower than that in the extensive metabolizers, but it was quite small (Table 1, Figure 1c); however, the mean AUC and Cmax of the active metabolite of prasugrel were not affected by the CYP2C19 polymorphism (Table 1, Figure 1b).
Figure 1.
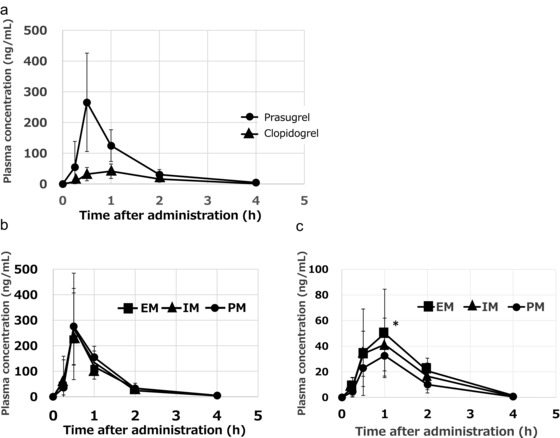
The plasma concentration of the active metabolites of prasugrel and clopidogrel in 36 subjects after a single oral administration of 20 mg of prasugrel or 300 mg of clopidogrel (a). The plasma concentration of prasugrel (b) and clopidogrel (c) according to the respective CYP2C19 polymorphism. The data represent the mean ± SD; *P ≤ .05 versus the poor metabolizers.
Table 1.
Pharmacokinetic Parameters of the Active Metabolites of Prasugrel and Clopidogrel
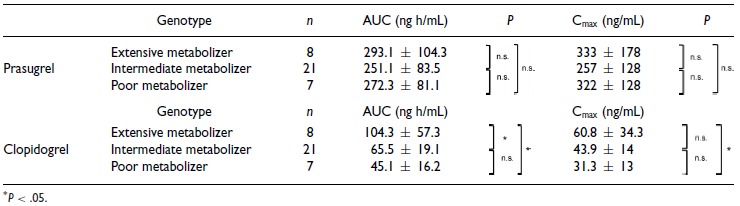
|
Pharmacodynamic Response
The IPA of prasugrel showed a rapid increase and reached its maximum inhibition 1 hour after administration as measured by LTA, with continued inhibition thereafter. The IPA of clopidogrel, on the other hand, showed a gradual increase and reached its maximum inhibition 2 hours after administration. The IPA of prasugrel was significantly greater than that of clopidogrel (Figure 2a). Furthermore, the IPA of clopidogrel in the poor metabolizers was significantly lower (∼30% inhibition) than that in the extensive metabolizers (Figure 2b). However, the IPA of prasugrel was not affected by CYP2C19 polymorphisms. The IPA 10 days after prasugrel and clopidogrel administration was 9.0% ± 12.7% and 1.1% ± 20.6%, respectively, at which time the antiplatelet action of both drugs was undetectable. The time to reach 80% of the MPA was 68.2 ± 44.2 and 123.5 ± 56.2 minutes for prasugrel and clopidogrel, respectively.
Figure 2.
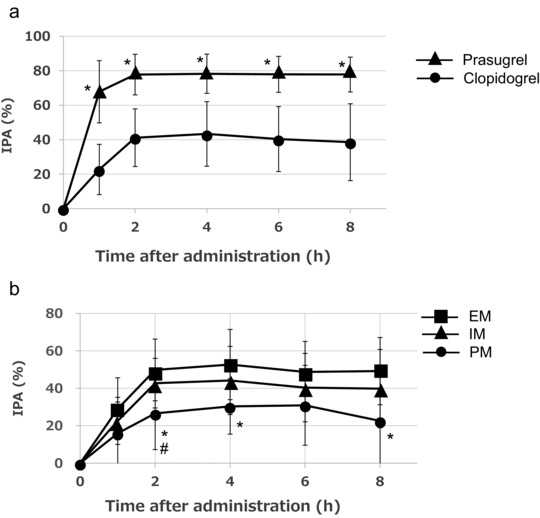
The time course of the inhibition of platelet aggregation induced by 20 μM ADP in response to prasugrel or clopidogrel in 36 subjects as determined by the light transmission aggregometry (LTA) assay (a). The inhibition of platelet aggregation by clopidogrel in relation to the respective CYP2C19 polymorphism (b). The data represent the mean ± SD. IPA, inhibition of platelet aggregation. (a) *P ≤ .05 versus clopidogrel. (b) *P ≤ .05 versus the extensive metabolizers and # P ≤ .05 versus the intermediate metabolizers.
The PRU as determined in the VerifyNow assay and the PRI as determined by the VASP phosphorylation ratio were rapidly reduced by prasugrel, reaching maximum inhibition 1 hour after prasugrel administration. In contrast, the PRU and PRI gradually decreased and reached maximum inhibition 2 hours after the administration of clopidogrel. The PRU and PRI after prasugrel administration were significantly lower than those after clopidogrel administration (Figures 3a and 4a). The PRU and PRI in the poor metabolizers were significantly higher than those in the extensive metabolizers after clopidogrel administration (Figures 3b and 4b). The PRU and PRI after prasugrel administration were not affected by CYP2C19 polymorphisms.
Figure 3.
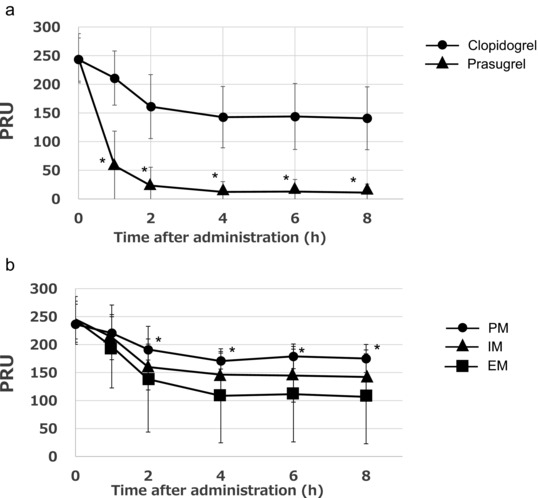
The time course of the P2Y12 reaction unit (PRU) after treatment with prasugrel or clopidogrel in 36 subjects as determined by the VerifyNow assay (a). The PRU after treatment with clopidogrel in relation to the respective CYP2C19 polymorphism (b). The data represent the mean ± SD. PRU, P2Y12 reaction units. (a) *P ≤ .05 versus clopidogrel. (b) *P ≤ .05 versus the extensive metabolizers.
Figure 4.
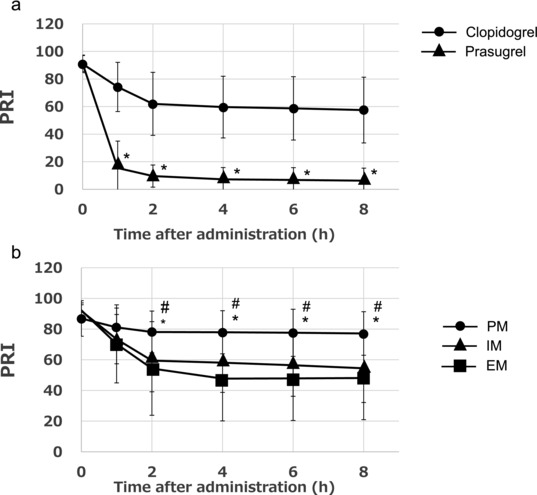
The time course of the platelet reactivity index (PRI) after treatment with prasugrel or clopidogrel in 36 subjects as determined by the vasodilator‐stimulated phosphoprotein (VASP) phosphorylation ratio (a). The PRI after treatment with clopidogrel in relation to the CYP2C19 polymorphism (b). The data represent the mean ± SD. PRI, platelet reactivity index. (a) *P ≤ .05 versus clopidogrel. (b) *P ≤ .05 versus the extensive metabolizers and # P ≤ .05 versus the intermediate metabolizers.
The relationship between the antiplatelet effects and the AUC was investigated. A significant (P < .01) correlation was observed between the AUC and the maximum IPA, PRU, and PRI in the clopidogrel group (Figure 5). In contrast, prasugrel at a dose of 20 mg had only a submaximal effect with small variations in the IPA, PRU, and PRI, indicating the absence of a correlation between the antiplatelet effects and AUC in the prasugrel group.
Figure 5.
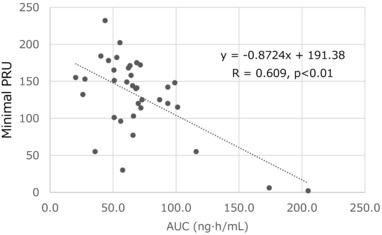
The relationship between the P2Y12 reaction unit (PRU) and area under the curve (AUC) in the clopidogrel treatment group. A significant correlation existed between the PRU and AUC.
Discussion
In this study a prasugrel loading dose of 20 mg and maintenance dose of 3.75 mg, which was adjusted for Japanese patients with acute coronary syndrome undergoing PCI and for patients undergoing elective PCI, were associated with a low incidence of major adverse cardiovascular events. This was similar to the results of the TRITON‐TIMI 38 study and was indicative of a low risk of clinically serious bleeding.2, 3 The loading dose of 20 mg prasugrel in Japanese patients is less than that used in Americans and Europeans and is similar to the 3.75‐mg maintenance dose, approved based on phase 1–3 clinical trial data. However, in this study, prasugrel at this relatively low dose of 20 mg showed a platelet aggregation of about 80% in the LTA assay. It was reported that in healthy European subjects, prasugrel at a dose of 60 mg showed about 80% platelet aggregation inhibition, which was almost the same as that in Japanese subjects receiving 20 mg prasugrel.19 This might be related to the plasma concentration of the active metabolite of prasugrel in Asian groups being found to be higher than that in white groups.20 The lower mean body weight of Asian subjects compared with white subjects may contribute to the higher concentration of the active metabolite in Asians.
In the present study, the concentration of the active metabolite of prasugrel increased rapidly, reaching its peak 30 minutes after administration. The IPA reached its maximum 1 hour after prasugrel administration. In contrast, the IPA of clopidogrel reached its maximum 2 hours after administration. Clopidogrel's platelet aggregation inhibition efficacy in the poor metabolizers showed a gradual increase and reached its maximum 4 hours after administration. In Japanese and other Asian subjects, CYP2C19 polymorphisms in poor metabolizers are present in about 20% of the population; therefore, the efficacy of agents affected by CYP2C19 polymorphisms is highly variable, which could explain why there was low responsiveness to clopidogrel in some patients.
The speed of onset of platelet inhibition after receiving antiplatelet agents may be relevant in the setting of acute coronary syndrome or PCI, as rapid onset of antiplatelet activity may inhibit stent thrombosis in PCI. A number of large‐scale pharmacogenomic trials have demonstrated that the response to clopidogrel and interindividual variability in this response were related to CYP2C19 polymorphisms and that poor or nonresponsiveness to clopidogrel was possibly associated with an increased risk of recurrent ischemic events and stent thrombosis in patients receiving drug‐eluting stents.10, 11, 12, 21, 22 Prasugrel is a more effective inhibitor of platelet aggregation than clopidogrel because of its rapid onset of action with low variability. Therefore, in the TRITON‐TIMI 38 study, prasugrel significantly reduced the incidence of ischemic events (cardiovascular death, fatal myocardial infarction, and nonfatal stroke), urgent target vessel revascularization, and stent thrombosis compared with the results obtained with clopidogrel.1 Similar results were found in Japanese patients in the PRASFIT‐ACS2 and PRASFIT‐Elective3 studies.
Now, clopidogrel as a generic drug can be available, so the cost of clopidogrel is much lower than that of prasugrel. When the drug for treatment is chosen, the cost is one of the major factors.
Conclusions
Prasugrel inhibited platelet aggregation more rapidly and more effectively in healthy Japanese subjects than was observed for clopidogrel. The efficacy of prasugrel was not affected by CYP2C19 polymorphisms; therefore, prasugrel's inhibition of platelet aggregation showed less variability than that achieved by clopidogrel.
Declaration of Conflicting Interests
The article has been edited and proofread by Editage (www.editage.com), a professional scientific editing company.
Funding
This study was funded by Daiichi Sankyo Co., Ltd. (Tokyo, Japan). This study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (https://upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr_view.cgi?recptno=R000016494).
Acknowledgments
The authors highly appreciate the valuable assistance of the staff involved in the present study.
References
- 1. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. [DOI] [PubMed] [Google Scholar]
- 2. Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted‐dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT‐ACS study. Circ J. 2014;78(7):1684–1692. [DOI] [PubMed] [Google Scholar]
- 3. Isshiki T, Kimura K, Ogawa H, et al. Prasugrel, a third‐generation P2Y12 receptor antagonist, in patients with coronary artery diseases undergoing elective percutaneous coronary intervention: A Phase III, randomized, double‐blind study. Circ J. 2014;78(12):2926–2934. [DOI] [PubMed] [Google Scholar]
- 4. Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force on the management of ST‐segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J. 2012;33(20):2569–2619. [DOI] [PubMed] [Google Scholar]
- 5. O'Gara PT, Kushner FG, Ascheim DD, et al. ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82(1):E1–E27. [DOI] [PubMed] [Google Scholar]
- 6. Weerakkody GJ, Jakubowski JA, Brandt JT, et al. Comparison of speed of onset of platelet inhibition after loading doses of clopidogrel versus prasugrel in healthy volunteers and correlation with responder status. Am J Cardiol. 2007;100(2):331–336. [DOI] [PubMed] [Google Scholar]
- 7. Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss‐of‐function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108(7):2244–2247. [DOI] [PubMed] [Google Scholar]
- 8. Umemura K, Furuta T, Kondo K. The common gene variants of CYP2C19 affect pharmacokinetics and pharmacodynamics to an active metabolite of clopidogrel in healthy subjects. J Thromb Haemost. 2008;6(8):1439–1441. [DOI] [PubMed] [Google Scholar]
- 9. Schrör K, Siller‐Matula JM, Huber K. Pharmacokinetic basis of the antiplatelet action of prasugrel. Fundam Clin Pharmacol. 2012;26(1):39–46. [DOI] [PubMed] [Google Scholar]
- 10. Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119(19):2553–2560. [DOI] [PubMed] [Google Scholar]
- 11. Shuldiner AR, O'Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373(9660):309–317. [DOI] [PubMed] [Google Scholar]
- 13. Farid NA, McIntosh M, Garofolo F, et al. Determination of the active and inactive metabolites of prasugrel in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(2):169–179. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi M, Pang H, Kawabata K, Farid NA, Kurihara A. Quantitative determination of clopidogrel active metabolite in human plasma by LC–MS/MS. J Pharm Biomed Anal. 2008;48(4):1219–1224. [DOI] [PubMed] [Google Scholar]
- 15. Cattaneo M, Cerletti C, Harrison P, et al. Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J Thromb Haemost. 2013;11:1183–1189. [DOI] [PubMed] [Google Scholar]
- 16. Gremmel T, Koppensteiner R, Panzer S. Comparison of Aggregometry with flow cytometry for the assessment of agonists´‐induced platelet reactivity in patients on dual antiplatelet therapy. PLoS One. 2015;10(6):e0129666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jakubowski JA, Bourguet N, Boulay‐Moine D, et al. Comparison of a new ELISA assay with the flow cytometric assay for platelet vasodilator‐associated stimulated phosphoprotein (VASP) phosphorylation in whole blood to assess P2Y12 inhibition. Thromb Haemost. 2012;107(2):388–395. [DOI] [PubMed] [Google Scholar]
- 18. Patnaik M, Dlott JS, Fontaine RN, et al. Detection of genomic polymorphisms associated with venous thrombosis using the invader biplex assay. J Mol Diagn. 2004;6(2):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am Heart J. 2007;153(1):66.e9–66.e16. [DOI] [PubMed] [Google Scholar]
- 20. Small DS, Kothare P, Yuen E, et al. The pharmacokinetics and pharmacodynamics of prasugrel in healthy Chinese, Japanese, and Korean subjects compared with healthy Caucasian subjects. Eur J Clin Pharmacol. 2010;66(2):127–135. [DOI] [PubMed] [Google Scholar]
- 21. Giusti B, Gori AM, Marcucci R, et al. Relation of cytochrome P450 2C19 loss‐of‐function polymorphism to occurrence of drug‐eluting coronary stent thrombosis. Am J Cardiol. 2009;103(6):806–811. [DOI] [PubMed] [Google Scholar]
- 22. Mega JL, Close SL, Wiviott SD, et al. Cytochrome p‐450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354–362. [DOI] [PubMed] [Google Scholar]


