Abstract
Various animal models of stroke have been developed to simulate the human stroke with the development of the ischemic method facilitates preclinical stroke research. The photothrombotic ischemia model, based on the intravascular photochemical reaction, is widely used for in vivo studies. However, this study has limitations, which generated a relatively small‐sized infarction model on superficial cortex compared to that of the MCAO stroke model. In this study, the photothorombosis mouse model is adapted and the optimum conditions for generation of cell death and deficits with high reproducibility is determined. The extent of damage within the cortex was assessed by infarct volume and cellular/behavioral analyses. In this model, the neural cell death and inflammatory responses is detected; moreover, the degree of behavioral impairment is correlated with the brain infarct volume. Further, to enhance the understanding of neural repair, the effect of neural differentiation by transplantation of human bone marrow‐derived mesenchymal stem cells (BM‐MSCs) is analyzed. The authors demonstrated that transplantation of BM‐MSCs promoted the neural differentiation and behavioral performance in their photothrombosis model. Therefore, this research was meaningful to provide a stable animal model of stroke with low variability. Moreover, this model will facilitate development of novel MSC‐based therapeutics for stroke.
Keywords: Cerebral ischemic stroke, Human bone marrow‐derived mesenchymal stem cells (hBM‐MSCs), Infarct volume, Neural differentiation, Photothrombotic mouse model
Abbreviations
- BM‐MSC
bone marrow‐derived mesenchymal stem cells
- EDTA
ethylenediaminetetraacetic acid
- hBM‐MSC
human bone marrow‐derived mesenchymal stem cells
- H&E
hematoxylin and eosin
- MAPK
MAP kinase
- MCAO
middle cerebral occlusion
- MSC
mesenchymal stem cells
- NF‐kB
nuclear factor kappa B
- NF
neurofilament
- NH
non‐hematopoietic
- PAGE
polyacrylamide gel electrophoresis
- PARP
poly(ADP‐ribose) polymerase
- PBS
phosphate‐buffered saline
- ROS
reactive oxidative stress
- SDS
sodium dodecylsulfate
See accompanying commentary by I‐Ming Chu DOI 10.1002/biot.201600414
1. Introduction
Stroke is a common cause of disability and death worldwide. Ischemic stroke, a major cause of brain damage, results from cerebral artery occlusion and induces a series of alterations in the penumbra with neurochemical events including release of glutamate, calcium channel dysfunction, membrane disruption, inflammatory changes and necrotic/apoptotic cell death triggering 1, 2.
Over the past few decades, several in vivo preclinical trials used various animal models of stroke. Rodent models of stroke mimic the ischemic conditions in humans in terms of cerebral vasculature and physiology, and have advantages of lower cost and moderate body size, which facilitates monitoring 3.
Middle cerebral occlusion (MCAO) surgery is widely used to induce ischemic stroke in animal models. However, permanent MCAO produces significant edema and hemorrhage within the forebrain cortex and the lesions are variable in size, which can result in unexpected behaviors 4. In addition, differences among mouse strains in susceptibility to the effects of focal ischemia were identified, for example, the C57BL/6 strain is unsuitable for MCAO model 5. In this study, we used a photothrombotic ischemia model that has been widely used as an experimental model. Photothrombosis models have the advantages of reproducibility, low mortality and low invasiveness with no mechanical damage to the endothelium. This model also can produce easily the well‐defined infarcts in specific regions of the cortex based on photochemical reaction, and can be readily controlled by adjusting the intensity and field of light/laser 6, 7.
However, the photothrombosis‐induced method has the limitation that it generally produces small‐sized ischemic penumbra. The size and location of ischemia can be altered by modifying the duration and intensity of the light source. The biochemical responses are altered and degree of tissue injury differed according to volume of ischemic injury, accompanied by histological and behavioral deficits 8. This allows the location and size of the infarct region to be precisely controlled in the photothrombosis animal model.
According to many clinical researches, bone marrow‐derived mesenchymal stem cells (BM‐MSCs) have been suggested to have potential for treatment of ischemic stroke. Early studies have reported the efficacy of MSCs for the treatment of stroke both in vitro and in vivo 9, 10, 11, 12. Transplanted BM‐MSCs secreted bioactive factors and mediated diverse endogenous processes, including angiogenesis, apoptosis, and inflammation, within the deleterious post‐ischemia environment 13, 14, 15.
In the present study, we adapted the mouse photothrombosis model and performed a histological, cellular and behavioral assessment to determine the optimal ischemic condition for generation of severe ischemic penumbra. In addition, we analyzed the neurogenic effect of transplanted human BM‐MSCs in our photothrombosis model.
2. Materials and methods
2.1. Animals and cerebral ischemic surgery
Male C57BL/6 mice at eight weeks of age were used (Samtako Bio Korea, Osan, Korea). The Institutional Animal Ethics Committee of Dongguk University approved all animal experiments. Mice were anesthetized by intraperitoneal (i.p.) injection of tiletamine/zolazepam (Zoletile; Virbac Lab., Carros, France) and xylazine (Rompun; Bayer, Seoul, South Korea). The ischemic surgery procedure was described by 16, 17. Before surgery, animals were maintained at a body temperature of 37°C on a heating pad. After mice were placed on a stereotaxic apparatus, the skin of mice was incised and craniotomy made on the midline scalp. The photosensitive dye Rose Bengal (Sigma‐Aldrich; 0.1 mL of a 10 mg/mL solution in sterile saline) was injected into the penile vein for 5 min before illumination. To induce photothrombosis, the brain was exposed to illumination with distance of 1‐mm from a fiber‐optic bundle connected to a cold light source (wave length 560 nm, KL 1500 LCD, Schott, Germany) on mouse left frontal cortex, focused at 0.5 mm anterior to the bregma and 2.5 mm lateral from the midline for 15 min.
To generate ischemia of various sizes, injured mice were randomly divided into three groups according to output level. The aperture size of irradiated area was fixed at position C (5 mm diameter). The intensity of light source emitted by halogen lamp was set to three types of output level which can be controlled by turning knob. The light intensity is following; C‐4 (3200 K), C‐5 (3250 K) and C‐6 (3300 K) (step width 50 K).
After surgery, the scalp was sutured and injured mice were returned to their cages to recover.
2.2. Transplantation of human BM‐MSCs
Human BM‐MSCs were cultured and expanded in non‐hematopoietic (NH) stem cell medium (Miltenyi Biotech, Bergisch‐Gladbach, Germany) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA). At 24 h after surgery, mice were anesthetized and the human BM‐MSCs (1 × 105 cells) suspended in 1 mL Phosphate‐buffered saline (PBS) were transplanted intravenously. The control groups were performed as same procedures with an equal volume of saline. For immunosuppression, all animals were injected with cyclosporine A daily (10 µg/g body weight, intraperitoneally).
2.3. Hematoxylin and eosin staining and measurement of infarct volume
The brains were removed from the skull and embedded in paraffin after ischemia. Brain tissue was dissected laterally into 2 mm slices on the mouse brain matrix using a surgical blade. For staining, slices including the perilesional ischemic core on the cortex were picked up and stained with 10% formalin for 30 min and then washed three times with 10 mM Tris‐HCL (pH 7.2). The sections were stained with Mayer's hematoxylin, and washed in tap water to mature the hematoxylin staining. Then the sections were stained with eosin followed by immersion in maturation baths containing 95% ethanol and 100% ethanol, followed by xylene. Cover slides were directly mounted using permanent mounting medium (Richard‐Allan Scientific, Kalamazoo, USA).
Infarct volume was measured by result of hemotoxylin and eosin (H&E) staining of brain sections. The loss areas of cerebral infarction were analyzed by multiplying each area in the consecutive sections by the thickness of the sections.
2.4. Protein extraction and Western blotting
On day 30 after ischemia, cells were washed with PBS, lysed in sample buffer (2% SDS, 5% 2‐mercaptoethanol, 10% glycerol, and 0.1 mg/mL bromphenol blue in Tris–HCl, pH 6.8), and heated at 100°C for 10 min. Brain tissue was washed twice with cold PBS, and homogenized in ice cold RIPA buffer containing a protease inhibitor cocktail comprising aprotinin (serine protease inhibitor), leupeptin (serine and cysteine proteases inhibitor), bestatin (aminopeptidase inhibitor), and ethylenediaminetetraacetic acid (EDTA) (metalloprotease inhibitor). Protein concentrations of total lysates were determined by BCA Protein Assay (Thermo Scientific, Rockford, IL, USA). For Western blot analysis, 20 µg of lysates were electrophoresed by in a 10% SDS–PAGE gel and transferred onto nitrocellulose membranes. Membranes were blocked with 5% skim milk in TBS‐T (pH 7.4) for 30 min at room temperature, followed by incubation with neural and synaptic‐related antibodies as well as anti‐β‐actin antibody at the appropriate dilutions overnight at 4°C. After washing, membranes were incubated with anti‐mouse and anti‐rabbit secondary antibodies for 1 h at room temperature, and visualized using the ECL system (Thermo Fisher Scienctific, USA). Images were obtained using the ChemiDoc XRS+ Imaging System (Bio‐Rad, Hercules, CA, USA).
2.5. Behavioral tests
All animals were subjected to the rotarod test by a blinded investigator. Pre‐training conducted daily for three days before ischemia. Rotarod tests were performed at 1, 7, 14, 21 and 28 days after surgery as described previously 18 with some modifications. Mice were placed on the rotarod cylinder and the speed was slowly increased to 20 rpm, at which the mice walked stable. All animals were subjected to three trials of a maximum duration of 3 min. The maximum speed and time were selected so that uninjured mice would not fall off from the cylinder. The results were recorded as the time in seconds at which the mice fell off from the rung of the cylinder.
2.6. Statistical analysis
Data were reported as mean ± standard deviation (SD) and analyzed using the SPSS 10.0 software (SPSS, Inc., Chicago, IL). Differences between groups were assessed by Student's t‐test. A value of p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Changes in the size of infarcts on mouse cortex
Photothrombotic ischemia in the mouse cortex was induced using a cold‐light source after injection of the photo‐sensitive dye, Rose Bengal. We used about 5 mm diameter of aperture size with three types of output level from halogen lamp (Phillips, USA) to generate infarcts. Infarct lesions were made on the left frontal cortex by forming a 5 mm diameter infarct core. After one day and 30 days after ischemia, each mouse was sacrificed and all brain slices were stained with H&E to evaluate infarct volume. One day after ischemia, the average infarct volume was 2.11 mm3 in the C‐4 group, 2.225 mm3 in the C‐5 group, and 3.128 mm3 in the C‐6 group; the differences were not significant. Infarct lesions had similar patterns that were located in the parietal and occipital cortex and showed pale‐staining, surrounded by curvilinear border. On day 30, the average of infarct volume in the C‐6 group (0.835 mm3) was significantly greater than that in the other groups. In contrast, infarct lesions in brains in the C‐4 group recovered gradually (Fig. 1).
Figure 1.
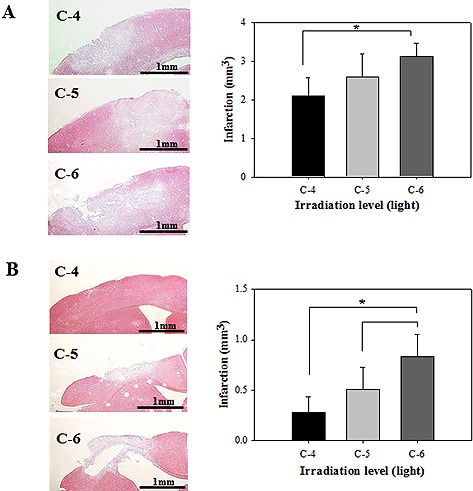
Infarct size of irradiated area on mouse cortex on day 1 (A) and day 30 (B). Brain sections were stained with H&E and the infarcted area was revealed with pale‐staining, surrounded by curvilinear border (left). Different sizes of infarct areas were generated by different irradiation level, C‐4, C‐5 and C‐6 (right). On day 30 after ischemia, the volume of infarction in C‐6 was significantly greater than other groups. Each bar represents the mean ± SD of independent experiments performed (n = 5; *P < 0.05).
3.2. Changes in the levels of apoptosis‐ and inflammation‐related proteins
We performed Western blot analysis of ischemic lesion to assess the cellular response. On day 30 after ischemia, apoptosis‐related proteins in infarct lesions were analyzed by Western blotting because apoptosis is a fundamental and important process accompanied by ischemic events. Caspase‐3 and poly(ADP‐ribose) polymerase (PARP) are crucial mediators of apoptosis in response to cellular stress 19, 20. As shown in Fig. 2, the cleaved caspase‐3 and PARP protein levels were higher after ischemia. In particular, mice treated with C‐6 irradiation exhibited the greatest increases in the levels of these proteins. Interestingly, the C‐6 irradiation treated‐mice also showed significant phosphorylation of p38 and MAPKAPK‐2, which regulate cellular responses to extracellular stress stimuli. The p38 MAP kinase (MAPK) family and its downstream signaling molecule MAPKAPK‐2 are known as signaling cascades which can control the cellular responses to cytokines and stresses 21.
Figure 2.
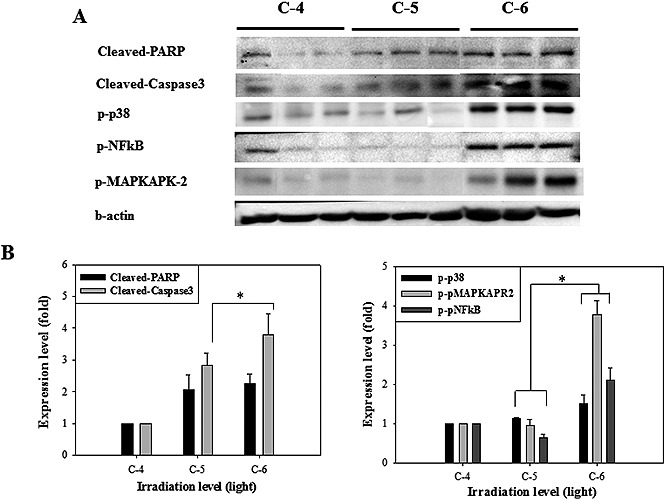
(A) The result of Western blot analysis on infarct lesion induced by C‐4, C‐5 and C‐6 irradiation. On 30 days, each mouse brain was lysed and detected using apoptosis and inflammatory response‐related proteins. The proteins were significantly phosphorylated in C‐6 irradiation group. (B) Quantitative analysis of expression level in comparison to β‐actin, an internal control. Each bar represents the mean ± SD of independent experiment performed in triplicate (n = 3; *P < 0.05).
In addition, we analyzed the protein level of the transcription nuclear factor kappa B (NF‐kB) to evaluate the inflammatory response. NF‐kB plays a major role in several inflammatory mechanisms in response to ischemic injury 22, 23. Phosphorylated NF‐kB expression was significantly higher in the C‐6 group.
These results showed that the levels of proteins associated with endogenous cellular responses were increased following ischemic injury, particularly C‐6 irradiation.
3.3. Changes in behavioral ability
The behavior ability was investigated using a rotarod test in detecting motor deficits and recovery. Before surgery, all animals showed similar behavior function, actively walking on the rod for 3 min. At 24 h after surgery, all mice could not use their paws (both right fore‐ and hind‐limbs) so that they immediately fell off from the device. On day 7, all mice showed increase in spontaneous limbs rather than day 1. However, by 28 days, the functional ability of mice in the C‐4 and C‐5 groups had improved (time of walking on the rotarod) to a great extent than mice in the C‐6 group. The group of C‐6 showed slightly recovery on behavioral performance. On day 21 and 28, there were significant (p < 0.5) deficits in performance in the C‐6 group (Fig. 3).
Figure 3.
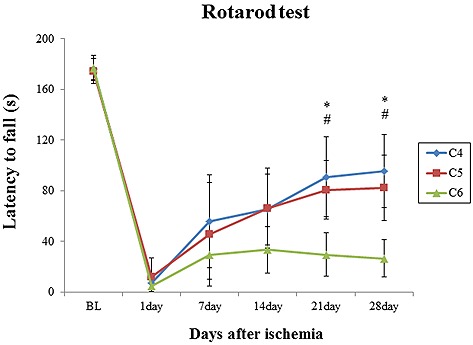
Behavioral performance after photothrombotic ischemia by 28 days. After one day of ischemia, the mice have a significant deficit that could not well use their forepaws. Mice in C‐4 and C‐5 groups was gradually improved the functional ability (time of walking on the rotarod), however, C‐6 mice showed slightly recovery on behavioral performance. Data are expressed as mean ± SD (n = 10; BL, internal baseline average before surgery). *p < 0.05, between C‐4 group and C‐6 group. #p < 0.05, between C‐5 group and C‐6 group.
These data suggested that mice in the C‐6 group were significantly impaired, and their behavioral performance improved slowly after ischemia.
3.4. The effect of neural differentiation on ischemic lesion treatment with human BM‐MSCs
We obtained results that the C‐6 irradiation induced severe ischemia by generating large infarct lesions in the mouse cortex and deficits in behavioral performance. To confirm neurogenesis by human BM‐MSCs in the C‐6 group, we performed a further in vivo experiment using the same photothrombosis‐induced method.
As shown in Fig. 4 and Fig. 5, we performed Western blotting, H&E staining and rotarod test in mice treated or not with human BM‐MSCs. On day 30 after surgery, we observed that neural and synaptic‐related proteins were more expressed after transplantation of BM‐MSCs compared with non‐transplanted. The human‐specific proteins, NF (neurofilament) and NeuroD1 protein levels were also up‐regulated in the hBM‐MSCs transplanted mice. Further, the relative infarct volume was significantly lower in the MSC‐treated groups (0.52 mm3) than the control group (0.885 mm3).
Figure 4.
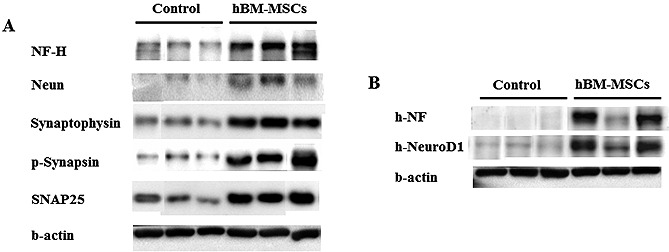
Western blot analysis after transplantation of human BM‐MSCs in photothrombosis model on day 30. (A) The hBM‐MSCs treated groups were more expressed neural and synaptic‐related proteins than control group (non‐transplanted hBM‐MSCs). (B) The human specific proteins, NF and NeuroD1, were expressed after transplantation of MSCs.
Figure 5.
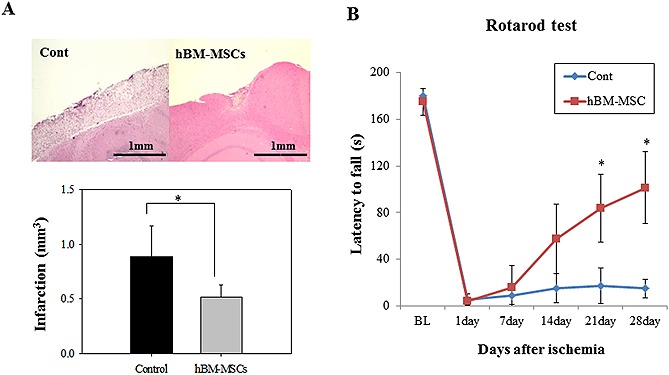
(A) Histological analysis of infarct lesion on mice cortex after photothrombotic ischemia (H&E) on day 30 after surgery. Infarct size was significantly reduced after transplantation of hBM‐MSCs. Each bar represents the mean ± SD (n = 10). (B) Behavioral performance (rotarod test) by 28 days after transplantation of hBM‐MSCs. Behavioral performance was significantly recovered 14 days after ischemia. Control mice have a severe deficit, so they couldn't recover by four weeks. Data are expressed as mean ± SD (n = 10; BL, internal baseline average before surgery). *p < 0.05.
No significant difference was observed in behavioral ability between the control and BM‐MSCs treated group at one week. However, the significant behavioral improvement (p < 0.5) appeared in the BM‐MSCs‐treated group after the day 7.
4. Discussion
Ischemic stroke is a leading cause of morbidity and disability, and results in damage to neural cells, massive tissue infarction and loss of brain function 1, 2, 24. The ischemic stroke induced by photothrombosis has emerged recently as an alternative MCAO surgery, which has poor reproducibility and small infarct size. Photothrombosis is based on photochemical reaction between light and a photo‐sensitive dye such as Rose Bengal. It was injected intravenously and activated by light, generating thrombotic events including peroxidative damage, platelet aggregation and coagulation cascades, which lead to microvascular occlusion 8. This model can be easily changed by different infarct sizes and regions on focal brain. Several studies have focused on design and development of the photothrombotic ischemic model by regulating the various light sources and intensities.
Here, we made three experimental ischemic groups on mouse focal cortex. The infarct volume was controlled by varying the light intensity and aperture size. As shown in Fig. 1, infarct size in the C‐6 irradiation group was greater on day 30. In contrast, the C‐4 and C‐5 groups showed small‐sized infarct lesions that recovered spontaneously from injury.
These results were verified by Western blotting and a behavioral test shown in Fig. 2 and Fig. 3. We performed a Western blot analysis of proteins related to apoptosis and the inflammatory‐response. Many reports have been already shown that inflammatory cascades are activated after ischemic events or brain injury because the ischemia closely mediates with various pathophysiological mechanisms such as disruption of glucose metabolism, reactive oxidative stress (ROS) production, necrosis and apoptosis 21, 22, 23, 25, 26. In infarcted lesions, we detected significantly increased inflammatory responses, particularly in the C‐6 irradiation group.
Ischemic mice had behavioral deficits and their motor performance had not fully recovered by day 28, especially in the C‐6 group. We detected that the C‐6 irradiation level was optimal for making the photothrombotic mouse model, which can maintain an ischemic state for a prolonged period. Further, our results also indicated that behavioral ability was closely correlated with the appearance and volume of infarct lesions on the focal cortex.
Regarding behavioral results, some mice showed minimal recovery and stabilized after 14 days of ischemia. These observations are consistent with previous reports that the behavioral performance of ischemic animals tended to recover spontaneously after surgery, in particular after photothrombosis 3, 6, 27, 28. The photothrombotic model showed a smaller ischemia penumbra on the superficial cortex compared to the MCAO model, so that infarct lesions by photothrombosis tend to readily mediate axonal reorganization and functional recovery within the brain. The exact mechanism underlying spontaneous recovery is unclear. This phenomenon is generally known due to brain plasticity with cortical reconnection and axonal sprouting in adjacent cortical areas 29, 30. After ischemia, few damaged neurons are replaced by neurogenesis, and these express markers of developing and mature of new neurons 31, 32. Therefore, to make severe ischemia penumbra with large and deep infarction like MCAO model is an important point in terms of design of animal stroke model. Recently, many studies have been focused on modifying the photothrombotic method to induce internal damage and produce severe motor deficit 33, 34, 35. Therefore, our photothrombotic mouse model has possibility to replace the MCAO model as it shows long‐lasting neurological severity and behavioral deficit.
To confirm that C‐6 is optimal for neural differentiation, we performed an additional in vivo experiment using human BM‐MSCs. After transplantation of BM‐MSCs, the expression of neural and synaptic‐related protein levels was increased. In particular, the human specific‐proteins were detected and their expression levels increased in MSCs‐treated mice. As shown in Fig. 4, we observed that the cells not only were well incorporated into the injured site but also enhanced endogenous neurogenesis. These results were confirmed in an additional experiment, Prussian blue staining. After transplantation of hBM‐MSCs incorporated with nanoparticle, the cells were well detected in infarct lesion with blue dots (data not shown). Further, infarct volume and behavioral performance also recovered significantly (Fig. 5). Therefore, human BM‐MSCs were successfully transplanted at the lesion site and promoted endogenous neural differentiation in our ischemia‐induced mouse model.
This tendency for behavioral recovery and neurogenesis is in agreement with many previous in vivo studies. The method of BM‐MSCs transplantation was well known as excellent and used for neural cell therapy and for treatment of experimental ischemic stroke 9, 10, 11, 12. However, most studies used the MCAO model. To date, few studies using the photochemically‐induced mouse model have been reported. Wang et al. demonstrated that in the photothrombosis model, the size of the irradiated area affected infarct volume and neuronal death within the cortex 34. Li et al. reported microglia proliferation and behavioral deficits after photothrombosis‐induced brain ischemia in adult mice 36. Ma et al. and Hou et al. reported that transplantation of neural stem cells enhanced neurological function in a photothrombosis stroke model, showing decreased infarct areas 37, 38. However, there were no reports on the effects of transplantation of MSCs on neurogenesis in the photothrombosis stroke model. Our study is meaningful as it is the first report of the neurogenic effect of BM‐MSCs in a photothrombosis mouse model, which has not been demonstrated before.
Our model, which involved transplantation of BM‐MSCs, significantly enhanced the effect of neurogenesis with low mortality and low variability. This model will contribute to provide novel methods for developing a stable animal model of stroke. Therefore, it is reasonable to expect that our study has the potential to improve understanding of the photothrombotic stroke model. Moreover, we proposed a new parameter for MSC‐based stroke therapy. To establish the clinical trials, further studies will be required to understand the underlying mechanisms and pathophysiological processes after ischemic stroke.
Acknowledgement
This research was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (Grant number: 2009‐0082941).
The authors declare no financial or commercial conflictof interest.
References
- 1. Donnan, G. A. , Fisher, M. , Macleod, M. , Davis, S. M. , Stroke. Lancet 2008, 371, 1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Lipton, P. , Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [DOI] [PubMed] [Google Scholar]
- 3. Fluri, F. , Schuhmann, M. K. , Kleinschnitz, C. , Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howells, D. W. , Porritt, M. J. , Rewell, S. S. , O'Collins, V. et al., Different strokes for different folks: The rich diversity of animal models of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 1412–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barone, F. C. , Knudsen, D. J. , Nelson, A. H. , Feuerstein, G. Z. , Willette, R. N. , Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J. Cereb. Blood Flow Metab. 1993, 13, 683–692. [DOI] [PubMed] [Google Scholar]
- 6. Lee, J. K. , Park, M. S. , Kim, Y. S. , Moon, K. S. et al., Photochemically induced cerebral ischemia in a mouse model. Surg. Neurol. 2007, 67, 620–625. [DOI] [PubMed] [Google Scholar]
- 7. Labat‐gest, V. , Tomasi, S. , Photothrombotic ischemia: A minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J. Visualized Exp. 2013, 76, e50370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boquillon, M. , Boquillon, J. P. , Bralet, J. , Photochemically induced, graded cerebral infarction in the mouse by laser irradiation evolution of brain edema. J. Pharmacol. Toxicol. Methods 1992, 27, 1–6. [DOI] [PubMed] [Google Scholar]
- 9. Bao, X. , Wei, J. , Feng, M. , Lu, S. et al., Transplantation of human bone marrow‐derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011, 1367, 103–113. [DOI] [PubMed] [Google Scholar]
- 10. Mitkari, B. , Nitzsche, F. , Kerkelä, E. , Kuptsova, K. et al., Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra‐arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav. Brain Res. 2014, 259, 50–59. [DOI] [PubMed] [Google Scholar]
- 11. Ma, X. L. , Liu, K. D. , Li, F. C. , Jiang, X. M. et al., Human mesenchymal stem cells increases expression of α‐tubulin and angiopoietin 1 and 2 in focal cerebral ischemia and reperfusion. Curr. Neurovasc. Res. 2013, 10, 103–11. [DOI] [PubMed] [Google Scholar]
- 12. Nam, H. S. , Kwon, I. , Lee, B. H. , Kim, H. et al., Effects of mesenchymal stem cell treatment on the expression of matrix metalloproteinases and angiogenesis during ischemic stroke recovery. PLoS One 2015, 10, e0144218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang, P. , Gebhart, N. , Richelson, E. , Brott, T. G. et al., Mechanism of mesenchymal stem cell‐induced neuron recovery and anti‐inflammation. Cytotherapy 2014, 16, 1336–1344. [DOI] [PubMed] [Google Scholar]
- 14. Gu, N. , Rao, C. , Tian, Y. , Di, Z. et al., Anti‐inflammatory and antiapoptotic effects of mesenchymal stem cells transplantation in rat brain with cerebral ischemia. J. Stroke Cerebrovasc. Dis. 2014, 23, 2598–2606. [DOI] [PubMed] [Google Scholar]
- 15. Ma, S. , Zhong, D. , Chen, H. , Zheng, Y. et al., The immunomodulatory effect of bone marrow stromal cells (BMSCs) on interleukin (IL)‐23/IL‐17‐mediated ischemic stroke in mice. J. Neuroimmunol. 2013, 257, 28–35. [DOI] [PubMed] [Google Scholar]
- 16. Watson, B. D. , Dietrich, W. D. , Busto, R. , Wachtel, M. S. , Ginsberg, M. D. , Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985, 17, 497–504. [DOI] [PubMed] [Google Scholar]
- 17. Liguz‐Lecznar, M. , Ziemka‐Nalecz, M. , Aleksy, M. , Kossut, M. et al., Comparison of matrix metalloproteinase activation after focal cortical ischemia in young adult and aged mice. J. Neurosci. Res. 2012, 90, 203–212. [DOI] [PubMed] [Google Scholar]
- 18. Hamm, R. J. , Pike, B. R. , O'Dell, D. M. , Lyeth, B. G. , Jenkins, L. W. , The rotarod test: An evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 1994, 11, 187–196. [DOI] [PubMed] [Google Scholar]
- 19. Garnier, P. , Ying, W. , Swanson, R. A. , Ischemic preconditioning by caspase cleavage of poly(ADP‐ribose) polymerase‐1. J. Neurosci. 2003, 23, 7967–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaitanya, G. V. , Babu, P. P. , Differential PARP cleavage: An indication of heterogeneous forms of cell death and involvement of multiple proteases in the infarct of focal cerebral ischemia in rat. Cell Mol. Neurobiol. 2009, 29, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butler, T. L. , Kassed, C. A. , Pennypacker, K. R. , Signal transduction and neurosurvival in experimental models of brain injury. Brain Res. Bull. 2003, 59, 339–351. [DOI] [PubMed] [Google Scholar]
- 22. Ridder, D. A. , Schwaninger, M. , NF‐kappaB signaling in cerebral ischemia. Neuroscience 2009, 158, 995–1006. [DOI] [PubMed] [Google Scholar]
- 23. Desai, A. , Singh, N. , Raghubir, R. , Neuroprotective potential of the NF‐κB inhibitor peptide IKK‐NBD in cerebral ischemia‐reperfusion injury. Neurochem. Int. 2010, 57, 876–883. [DOI] [PubMed] [Google Scholar]
- 24. Chen, P. , Goldberg, D. E. , Kolb, B. , Lanser, M. , Benowitz, L. I. , Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc. Natl. Acad. Sci. USA 2002, 99, 9031–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta, S. L. , Vemuganti, R. , Mechanisms of stroke induced neuronal death: Multiple therapeutic opportunities. Adv. Anim. Vet. Sci. 2014, 2, 438–446. [Google Scholar]
- 26. Crack, P. J. , Taylor, J. M. , Reactive oxygen species and the modulation of stroke. Free Radical Biol. Med. 2005, 38, 1433–1444. [DOI] [PubMed] [Google Scholar]
- 27. Clarkson, A. N. , Overman, J. J. , Zhong, S. , Mueller, R. et al., AMPA receptor‐induced local brain‐derived neurotrophic factor signaling mediates motor recovery after stroke. J. Neurosci. 2011, 31, 3766–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carmichael, S. T. , Plasticity of cortical projections after stroke. Neuroscientist 2003, 9, 64–75. [DOI] [PubMed] [Google Scholar]
- 29. Clarkson, A. N. , López‐Valdés, H. E. , Overman, J. J. , Charles, A. C. et al., Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J. Cereb. Blood Flow Metab. 2013, 33, 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrison, T. C. , Silasi, G. , Boyd, J. D. , Murphy, T. H. , Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke 2013, 44, 2300–2306. [DOI] [PubMed] [Google Scholar]
- 31. Arvidsson, A. , Collin, T. , Kirik, D. , Kokaia, Z. , Lindvall, O. , Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [DOI] [PubMed] [Google Scholar]
- 32. Parent, J. M. , Vexler, Z. S. , Gong, C. , Derugin, N. , Ferriero, D. M. , Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002, 52, 802–813. [DOI] [PubMed] [Google Scholar]
- 33. Carmichael, S. T. , Rodent models of focal stroke: Size, mechanism, and purpose. NeuroRx 2005, 2, 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tiannan, W. , Wenju, C. , Yicheng, X. , Weiping, Z. , Shinghua, D. , Controlling the volume of the focal cerebral ischemic lesion through photothrombosis. Am. J. Biomed. Sci. 2010, 2, 33–42. [Google Scholar]
- 35. Qian, C. , Li, PC. , Jiao, Y. , Yao, H. H. et al., Precise characterization of the penumbra revealed by MRI: A modified photothrombotic stroke model study. PLoS One 2016, 11, e0153756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li, H. , Zhang, N. , Lin, H. Y. , Yu, Y. et al., Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis‐induced ischemia in adult mice. BMC Neurosci. 2014, 15, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma, J. , Gao, J. , Hou, B. , Liu, J. et al., Neural stem cell transplantation promotes behavioral recovery in a photothrombosis stroke model. Int. J. Clin. Exp. Pathol. 2015, 8, 7838–7848. [PMC free article] [PubMed] [Google Scholar]
- 38. Hou, B. , Ma, J. , Guo, X. , Ju, F. et al., Exogenous neural stem cells transplantation as a potential therapy for photothrombotic ischemia stroke in Kunming mice model. Mol. Neurobiol. 2016, DOI: 10.1007/s12035‐016‐9740‐6. [DOI] [PubMed] [Google Scholar]


