Abstract
Objectives
To determine whether the risk of cardiovascular mortality associated with cardiorenal syndrome subtype 1 (CRS1) in patients who were hospitalized for acute coronary syndrome (ACS) was greater than the expected risk based on the sum of its components, to estimate the predictive value of CRS1, and to determine whether the severity of CRS1 worsens the prognosis.
Methods
Follow-up study of 1912 incident cases of ACS for 1 year after discharge. Cox regression models were estimated with time to event (in-hospital death, and readmission or death during the first year after discharge) as the dependent variable.
Results
The incidence of CRS1 was 9.2/1000 person-days of hospitalization (95% CI = 8.1–10.5), but these patients accounted for 56.6% (95% CI = 47.4–65.) of all mortality. The positive predictive value of CRS1 was 29.6% (95% CI = 23.9–36.0) for in-hospital death, and 51.4% (95% CI = 44.8–58.0) for readmission or death after discharge. The risk of in-hospital death from CRS1 (RR = 18.3; 95% CI = 6.3–53.2) was greater than the sum of risks associated with either acute heart failure (RR = 7.6; 95% CI = 1.8–31.8) or acute kidney injury (RR = 2.8; 95% CI = 0.9–8.8). The risk of events associated with CRS1 also increased with syndrome severity, reaching a RR of 10.6 (95% CI = 6.2–18.1) for in-hospital death at the highest severity level.
Conclusions
The effect of CRS1 on in-hospital mortality is greater than the sum of the effects associated with each of its components, and it increases with the severity of the syndrome. CRS1 accounted for more than half of all mortality, and its positive predictive value approached 30% in-hospital and 50% after discharge.
Introduction
The simultaneous appearance of heart failure and kidney injury has been termed cardiorenal syndrome (CRS) [1–3]. According to the proposed classification, CRS is divided into five subtypes depending on the organ that initiates the cascade and leads to failure of the other organ, and on whether organ failure is acute or chronic.1 In CRS subtype 1 (CRS1), acute heart failure (AHF) leads to acute kidney injury (AKI) [4]. The incidence of CRS1 varies widely among studies because of differences in the definitions used and heterogeneity in the populations studied to date [5]. It has been estimated that CRS1 occurs in approximately 25%-33% of patients admitted with acute decompensated heart failure [6].
Coronary heart disease is still the cause of more than two-thirds of all heart disease-related deaths in western societies [7], and patients who develop AKI after a coronary event have significantly higher morbidity and mortality, longer hospitalization and a greater incidence of readmission [8]. In patients with acute coronary syndrome (ACS) CRS worsens the prognosis [9]. and it is not surprising that those who develop both AHF and AKI have worse outcomes than those with just one. Clinical guidelines have classically treated cardiac and renal failure separately, but it is important to elucidate the characteristics of CRS in more detail in order to enhance the integrative clinical management of this syndrome [1]. Therefore, a better awareness of the importance of CRS1 in patients with ACS is needed, and we also need to know more about whether the risk of mortality associated with CRS1 is greater than the expected risk based on the sum of its components (AHF and AKI), and whether the severity of CRS1 matters.
The present study was designed to determine whether the risk of cardiovascular mortality associated with CRS1 in patients who were hospitalized for ACS was greater than the expected risk based on the sum of its components, to estimate the positive predictive value of CRS1, and to determine whether the severity of CRS1 worsens the prognosis.
Patients and Methods
This follow-up study was based on a cohort of incident cases of ACS in the general population. Between 1 January 2007 and 31 December 2010, we prospectively recruited all patients admitted for ACS at Nuestra Señora de Candelaria University Hospital, a tertiary reference center which serves a population of 600,000 inhabitants on the island of Tenerife (Canary Islands, Spain). The patients consented to take part of the study and provided written informed consent; and they were initially followed during their hospital stay, and then for 1 year after discharge. After discharge, patients were followed at their cardiology outpatient department appointments and primary care appointments. To avoid missing data, they were also followed by telephone interview. The sample size far exceeded the requirements for an alpha error of 1% and a beta error of 5%, assuming a ratio of 8 for unexposed/exposed to CRS in the sample. The study was approved by the hospital’s clinical research ethics committee.
The diagnosis of ACS was based on the presence of as at least 2 of the following criteria: 1) compatible clinical presentation; 2) dynamic changes in the ST segment, inverted T wave or the appearance of a new Q wave; 3) troponin I elevation above the upper limit of normality (0.6 ng/mL); 4) angiographic finding of a compatible coronary lesion.
Heart failure and kidney function: Cardiorenal syndrome definition and categorization
The Killip–Kimball classification was used to identify heart failure during the episode, and heart failure was recorded if the patient was considered class II or higher. Use of balloon dilatation or amines depended on the stage of evolution of ACS in each patient in Killip stage III and IV, but these treatments were not included in the present analysis since they have not been shown to modify survival [10].Chronic kidney disease (CKD) was recorded when diminished creatinine clearance was documented according to an estimated glomerular filtration rate <60 mL/min in the year prior to admission; patients on dialysis were specifically excluded from the analysis. AKI was defined, according to Acute Kidney Injury Network criteria, as an acute increase in serum creatinine on admission ≥0.3 mg/dL or 1.5-fold higher than normal [11]. CRS1 was defined as the sum of the two components: AHF followed by AKI [1,3]. Treatment of acute kidney failure in patients with heart failure ranged from hydration and avoiding potentially nephrotoxic medications to dialysis in the intensive care unit, in consultation with the nephrology department.
To assess the importance of CRS severity we empirically categorized patients in 4 grades according to Killip–Kimball score and the increase in serum creatinine on admission. The combination that performed best in predicting mortality (creatinine elevations ≥0.5) was used for t study. The four categories were: 1: Killip–Kimball = II, and increase in creatinine <0.5 mg/dL (n = 29); 2: Killip–Kimball = II, but increase in serum creatinine ≥0.5 mg/dL (n = 41); 3: Killip–Kimball > II, but increase in serum creatinine <0.5 mg/dL (n = 36); 4: Killip–Kimball > II, and increase in serum creatinine ≥0.5 mg/dL (n = 110. We accepted the fourth grade as equivalent to cardiogenic shock.
Other variables
Approximately 30% of the patients who had infarction with elevated ST segment received therapy with fibrinolytics. All these patients subsequently underwent coronary angiography and revascularization, if needed. Revascularization was done percutaneously or surgically according to the angiographic findings, and was classified as: none, surgical or percutaneous catheterization. Left ventricular dysfunction (LVD) was recorded when ejection fraction calculated from echocardiographic studies on admission was 40% or lower. Peripheral vascular disease (PVD) was diagnosed when confirmation was obtained from imaging studies for arterial disease in the lower extremities, abdominal aorta or carotid arteries, or when the patient had prior evidence of PVD (amputation or revascularization). Previous coronary artery disease was recorded when a diagnosis of angina or myocardial infarction was documented.
Antecedents of hypertension, diabetes mellitus, dyslipidemia, chronic obstructive pulmonary disease (COPD), smoking (non-smoker, former smoker or smoker) and alcohol consumption were recorded for all patients. A diagnosis of hypertension was noted when the patients were taking antihypertensive medication or if their blood pressure in hospital was ≥140/90 mmHg at least twice, and diabetes mellitus was recorded when the patients were receiving antidiabetic treatment or if their fasting serum glucose was >125 mg/dL at least twice. Dyslipidemia was noted if the patient was using statins or if serum cholesterol was >240 mg/dL. Anemia was defined as a plasma hemoglobin concentration <13 g/dL in men or <12 g/dL in women. Patients were considered smokers if they stated smoking at least 1 cigarette per day, and ex-smokers if they stated having quit at least 1 year previously. Excessive drinking was defined as alcohol consumption >40 g/day in men or >30 g/day in women. Obesity was defined as a body mass index ≥30.
Statistical analysis
Categorical variables are summarized as relative frequencies and 95% confidence intervals (95% CI), and continuous variables as the mean ± standard deviation or the median (P25–P75). We used ANOVA tests to find associations between continuous and categorical variables, and univariate Cox models for the variables time to death or time to readmission. For associations between categorical variables we used the Pearson chi-squared test. Incidence density was calculated as the quotient of the number of events divided by the sum of hospital stays, in days, for all patients (sum of days after discharge when the event studied was death or readmission). Incidence densities, comparisons of rates (rates ratios, 95% CI, p) and positive predictive values were calculated with the application available at http://www.openepi.com.
For the multivariate analysis we adjusted two Cox regression models (A and B) with time to event as the dependent variable in a backwards stepwise procedure (Wald), with exit criteria = 0.10 and confirmation of the proportional hazards assumption by log-log plotting. To estimate relatives risks (RR) of CRS1 for each event, a variable was included to detect a potential interaction (0 = no AHF and no AKI [reference category]; 1 = AKI but no AHF; 2 = AHF but no AKI; 3 = CRS1 [AHF and AKI]). In both models the RR was adjusted by age (in years), and the categorical variables considered (as described above) were sex, LVD, acute myocardial infarction with ST segment elevation (AMI-ST), diabetes mellitus, anemia, CKD, dyslipidemia, smoking, obesity, excessive alcohol consumption, PVD, COPD, previous coronary artery disease, arterial hypertension, elevated troponin, and revascularization (no revascularization as the reference). Later, two more models were built to adjust the RR associated with CRS1 severity for the same variables, with no CRS1 as the reference category.
Secondarily, we also carried out sensitivity analyses by restricting our study cohort to determine whether the association of CRS1 with in-hospital mortality persisted in: (1) patients with short hospitalization, excluding those hospitalized longer than 1 week, (2) patients with long hospitalization, excluding those hospitalized for 1 week or less, and (3) patients stratified by age at admission (≤65 and >65 years). All analyses were done with the IBM SPSS Statistics 21 software package.
Results
The study group included a total of 1912 incident cases of ACS. Of these patients, 113 (5.9%) died during the index hospitalization, and 396 (20.7%) were readmitted or died during the first year after discharge. On average women (n = 552) were 5 years older than men, and they had a higher frequency of CRS1 (14.5% versus 10.0%; p = 0.005).
Table 1 shows that CRS1 was associated with older age and shorter time to death or readmission (p = 0.011). The prevalence of CKD, anemia and PVD and the incidence of LVD were higher in patients with CRS1 (p<0.001). The incidence of CRS1 per 1000 person-days of hospital stay was higher in women (11.2 [95% CI = 9.3–14.5]) than in men (8.2 [95% CI = 6.8–9.7]; p = 0.012). More than half of the patients (56.6% [95% CI = 47.4–65.6]) who died during their hospital stay had CRS1. The positive predictive value of CRS1 was 29.6% (95% CI = 23.9–36.0) for death, and 51.4% (95% CI = 44.8–58.0) for readmission or death after discharge.
Table 1. Distribution of variables in patients with ACS according to sex and CRS1 status.
| No CRS1 n = 1696 | CRS1 n = 216 | P | |
|---|---|---|---|
| Age (years) | 63.1±12.5 | 70.3±12.1 | <0.001 |
| Sex (women) | 27.8 (25.7–29.9) | 37.2 (30.7–43.7) | 0.004 |
| In-hospital death | 2.9 (2.1–3.7) | 29.6 (23.9–36.0) | <0.001 |
| Days to in-hospital death* | 8.0 (6.0–13.0) | 10.0 (6.0–19.0) | <0.001 |
| Death or readmission after discharge | 16.8 (15.0–18.6) | 51.4 (44.8–58.0) | <0.001 |
| Days to death or readmission * | 93.0 (30.0, 189.0) | 41.5 (14.8, 93.5) | 0.011 |
| Current infarction with ST segment elevation | 39.7 (37.4–42.0) | 49.8 (43.1–56.5) | 0.005 |
| Left ventricular dysfunction | 15.7 (14.0–17.4) | 47.5 (40.8–54.2) | <0.001 |
| Cardiogenic shock | 1.2 (0.7–1.7) | 29.6 (23.9–36.0) | <0.001 |
| Troponin elevation | 72.1 (70.0–74.2) | 94.4 (91.3–97.5) | <0.001 |
| Chronic kidney disease | 8.7 (7.4–10.0) | 32.6 (26.3–38.9) | <0.001 |
| Smoker | 36.7 (34.4–39.0) | 27.0 (21.1–32.9) | <0.001 |
| Ex-smoker | 24.6 (22.6–26.7) | 19.5 (14.2–24.8) | |
| Excess alcohol consumption | 11.8 (10.3–13.3) | 11.2 (7.0–15.4) | 0.910 |
| Obesity | 34.9 (32.6–379.2) | 28.3 (22.3–34.3) | 0.594 |
| Hypertension | 62.5(60.2–64.8) | 70.7 (64.6–76.8) | 0.019 |
| Type 2 diabetes | 32.8 (30.6–359.0) | 53.5 (46.8–60.2) | <0.001 |
| Anemia | 20.0 (18.1–21.9) | 47.9 (41.2–54.6) | <0.001 |
| Dyslipidemia | 51.5 (49.1–53.9) | 51.2 (44.5–57.9) | 0.931 |
| Peripheral vascular disease | 7.1 (5.9–8.3) | 14.0 (9.4–18.6) | <0.001 |
| Chronic obstructive pulmonary disease | 4.4 (3.4–5.4) | 9.3 (5.4–13.2) | 0.002 |
| No revascularization | 27.3 (25.2–29.4) | 38.2 (31.7–44.7) | 0.004 |
| Percutaneous revascularization | 55.8 (53.4–58.2) | 45.9 (39.3–52.3) | |
| Surgical revascularization | 16.9 (15.1–18.7) | 15.9 (11.0–20.8) | |
| Angiography | 84.4 (82.7–86.1) | 75.5 (69.8–81.2) | 0.001 |
| No revascularization after angiography | 15.8 (14.1–17.5) | 20.5 (15.1–25.8) | 0.257 |
| Previous coronary disease (AMI with ST) | 10.5 (9.0–12.0) | 11.6 (7.3–15.9) | 0.254 |
| Previous coronary disease (AMI without ST) | 5.8 (4.7–6.9) | 6.5 (3.2–9.8) | |
| Previous coronary disease (Stable angina) | 2.2 (1.5–2.9) | 4.7 (1.9–7.5) | |
| Previous coronary disease (Unstable angina) | 5.8 (4.7–6.9) | 4.2 (1.5–6.9) |
Categorical variables: % (95% CI). Age: mean ± SD.
*Time variables: median (P25-P75).
Table 2 shows that the incidence rate of in-hospital death was higher in patients with CRS1 than in patients with AHF (p = 0.030) or AKI (p<0.001) alone. When the event studied was death or readmission after discharge, the incidence rate for CRS1 was significantly higher than for AKI (p<0.001). Furthermore, the incidence of in-hospital death became higher as the severity of CRS1 increased (Table 3).
Table 2. Incidence rates of the two events studied here among patients with CRS1, AHF and AKI.
Comparison of rates (RR, 95% CI; p).
| Event: Death during hospital stay | ||||
| No. events / Person-days to event | Incidence rate per 103 (95% CI) | RR CRS/AHF | RR CRS/AKI | |
| CRS1 | 64/2943 | 21.8 (16.8–27.8) | 1.8 (1.0–3.4) p = 0.030 | 8.5 (5.4–3.6) p<0.001 |
| AHF | 14/1173 | 11.9 (6.5–20.0) | ||
| AKI | 25/9714 | 2.6 (1.7–3.8) | ||
| Event: Death or readmission during the first year after discharge | ||||
| No. events / Person-days to event | Incidence rate per 103 (95% CI) | RR CRS/AHF | RR CRS/AKI | |
| CRS1 | 50/37043 | 1.4 (1.0–1.8) | 1.3 (0.8–2.4) p = 0.295 | 2.4 (1.7–2.3) p<0.001 |
| AHF | 17/16911 | 1.0 (0.6–1.6) | ||
| AKI | 122/214485 | 0.6 (0.5–0.7) | ||
Table 3. Incidence rates according to the severity of CRS1.
Comparison of rates (RR, 95% CI; p).
| Event: Death during hospital stay | ||||
| No. events / Person-days to event | Incidence rate per 103 (95% CI) | RR | p | |
| No CRS | 49 /20446 | 2.4 (1.8–3.2) | 1 | - |
| CRS1 severity 1 | 0/391 | 0.0 (0.0–9.4) | 0 (0.0–3.3) | 0.395 |
| CRS1 severity 2 | 7/549 | 12.8 (5.1–26.3) | 5.3 (2.2–11.2) | <0.001 |
| CRS1 severity 3 | 7/478 | 14.6 (5.9–30.1) | 6.1 (2.6–12.9) | <0.001 |
| CRS1 severity 4 | 50/1525 | 32.8 (24.3–43.2) | 13.7 (9.2–20.3) | <0.001 |
| Event: Death or readmission during the first year after discharge | ||||
| No. events / Person-days to event | Incidence rate per 103 (95% CI) | RR | p | |
| No CRS | 285/511184 | 5.6 (4.9–6.3) | 1 | - |
| CRS1 severity 1 | 8/7925 | 10.1 (4.3–19.9) | 1.8 (0.9–3.7) | 0.090 |
| CRS1 severity 2 | 17/8757 | 19.4 (11.3–31.1) | 3.5 (2.1–5.7) | <0.001 |
| CRS1 severity 3 | 18/6653 | 27.1 (16.0–42.8) | 4.9 (3.0–7.8) | <0.001 |
| CRS1 severity 4 | 68/14177 | 48.0 (37.3–60.8) | 8.6 (6.6–11.2) | <0.001 |
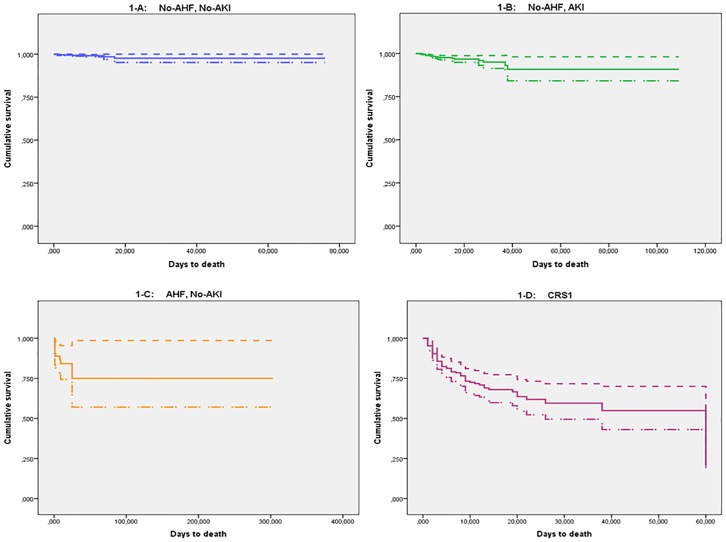
The multivariate Cox models found that the risk of in-hospital death in patients with CRS1 after adjustment for all other variables was greater than the sum of the effects of each component separately (Table 4, model A, RR = 18.3); for this Cox model, the linear hypothesis of no difference among the regression coefficients of CRS1 and the sum of the AHF+AKI coefficients was tested, obtaining a difference of 1.357 (p<0.001). Multivariate analysis also confirmed the direct association between the severity of CRS1 and the risk of events. The RR for in-hospital mortality increased up to 10.6 for the highest level of severity (Table 4, model C), and the highest RR for readmission or death after discharge was 4.7. Fig 1 illustrates the survival function based on model A for death during hospitalization (Fig 1A: No AHF and no AKI; Fig 1B: AKI but no AHF; Fig 1C: AHF but no AKI; Fig 1D = CRS1).
Table 4.
Models A and B adjust the associated risk of CRS1 for the two events studied. Models C and D adjust the severity of CRS1. Only the variables that remained in each model are shown.
| Model A. Dependent variable: Time to in-hospital death | RR | 95% CI | p | |
| CRS1 (heart failure + acute kidney injury) | 18.3 | 6.3 | 53.2 | <0.001 |
| Acute heart failure only (without acute kidney injury) | 7.6 | 1.8 | 31.8 | 0.006 |
| AMI-ST | 3.8 | 1.9 | 7.3 | <0.001 |
| Acute kidney injury only (without acute heart failure) | 2.8 | 0.9 | 8.8 | 0.076 |
| COPD | 2.6 | 1.2 | 5.6 | 0.017 |
| Left ventricular dysfunction | 2.1 | 1.2 | 3.6 | 0.012 |
| Age (years) | 1.0 | 1.0 | 1.1 | 0.056 |
| Percutaneous revascularization | 0.5 | 0.3 | 1.0 | 0.034 |
| Obesity | 0.3 | 0.1 | 0.8 | 0.017 |
| Surgical revascularization | 0.2 | 0.1 | 0.7 | 0.009 |
| Model B. Dependent variable: Time to death after discharge or readmission | RR | 95% CI | p | |
| CRS1 (heart failure + acute kidney injury) | 2.5 | 1.7 | 3.8 | <0.001 |
| Acute heart failure only (without acute kidney injury) | 2.3 | 1.2 | 4.3 | 0.008 |
| Diabetes | 1.7 | 1.3 | 2.2 | <0.001 |
| Anemia | 1.7 | 1.3 | 2.2 | <0.001 |
| Acute kidney injury only (without acute heart failure) | 1.4 | 1.0 | 1.8 | 0.043 |
| Sex (men) | 1.4 | 1.0 | 1.9 | 0.032 |
| Previous coronary artery disease | 1.3 | 1.0 | 1.8 | 0.041 |
| Age (years) | 1.0 | 1.0 | 1.0 | 0.089 |
| Percutaneous revascularization | 0.6 | 0.5 | 0.9 | 0.005 |
| Surgical revascularization | 0.6 | 0.4 | 0.9 | 0.011 |
| Model C. Dependent variable: Time to in-hospital death | RR | 95% CI | p | |
| CRS1 severity 4 | 10.6 | 6.2 | 18.1 | <0.001 |
| CRS1 severity 3 | 7.8 | 3.1 | 19.9 | <0.001 |
| CRS1 severity 2 | 4.8 | 1.6 | 14.6 | 0.006 |
| AMI-ST | 4.0 | 2.2 | 7.3 | <0.001 |
| Peripheral vascular disease | 3.0 | 1.5 | 5.9 | 0.002 |
| COPD | 2.0 | 0.1 | 4.1 | 0.070 |
| Age (years) | 1.0 | 1.0 | 1.1 | 0.003 |
| Hypertension | 0.6 | 0.4 | 1.0 | 0.053 |
| Percutaneous revascularization | 0.6 | 0.4 | 1.1 | 0.087 |
| Smoking | 0.4 | 0.2 | 0.7 | 0.002 |
| Obesidad | 0.4 | 0.2 | 0.8 | 0.009 |
| Surgical revascularization | 0.2 | 0.1 | 0.6 | 0.004 |
| CRS1 severity 1 | 0 | 0 | 1.9247 | 0.969 |
| Model D. Dependent variable: Time to death after discharge or readmission | RR | 95% CI | p | |
| CRS1 severity 4 | 4.7 | 3.4 | 6.5 | <0.001 |
| CRS1 severity 3 | 3.4 | 2.0 | 5.9 | <0.001 |
| CRS1 severity 2 | 2.5 | 1.4 | 4.5 | 0.002 |
| Peripheral vascular disease | 1.8 | 1.3 | 2.6 | 0.001 |
| AMI-ST | 1.6 | 1.2 | 2.0 | <0.001 |
| Diabetes | 1.4 | 1.1 | 1.8 | 0.003 |
| Anemia | 1.3 | 1.0 | 1.7 | 0.031 |
| Previous coronary artery disease | 1.3 | 1.0 | 1.6 | 0.058 |
| Age (years) | 1.0 | 1.0 | 1.0 | <0.001 |
| CRS1 severity 1 | 0.8 | 0.3 | 2.3 | 0.744 |
| Percutaneous revascularization | 0.6 | 0.5 | 0.8 | <0.001 |
| Surgical revascularization | 0.5 | 0.4 | 0.7 | <0.001 |
Fig 1. Survival curves, including confidence bands, for death during hospitalization for acute coronary syndrome in model A (Table 4).
Fig 1A: No AHF and no AKI (reference category); Fig 1B: AKI but no AHF; Fig 1C: AHF but no AKI; Fig 1D = CRS1.
In the sensitivity analysis the strong association of CRS1 with in-hospital mortality persisted, and was greater than the sum of the effects of each component separately in multivariate Cox models for: 1) patients with short hospitalization (n = 798; RR = 29.7 [95% CI = 6.3–140.5]; p<0.001), 2) patients with long hospitalization (n = 1114; RR = 15.5 [95% CI = 3.5–69.3]; p<0.001), 3) patients ≤65 years old on admission (n = 1006; RR = 37.8 [95% CI = 4.7–326.7]; p = 0.001), and 4) patients >65 years old on admission (n = 906; RR = 24.5 [95% CI = 5.8–103.9]; p<0.001).
Discussion
The effect of CRS1 on mortality during the hospital stay was greater than the sum of the effects of the components of this syndrome separately, and the severity of CRS1 was an important factor in the mortality. The incidence of CRS1 was approximately 10 cases per 1000 person-days of hospital stay for ACS, but this incidence accounted for more than half of all mortality. The positive predictive value of CRS1 for death approached 30% during the hospital stay and surpassed 50% in patients who were readmitted or died from cardiovascular causes during the first year after discharge.
Both heart failure and AKI are known to be important factors in the clinical course of ACS [12]. However, as in metabolic syndrome [13], the question arises as to whether the risk contributed by CRS differs from the risk estimated as the sum of the risks of each component separately. Our study provides an answer to this question: we calculated the independent effect of CRS1 per se and of each of its components, and found that the RR for in-hospital death from CRS1 was much greater than the sum of the risks contributed by either AHF or AKI alone. We found no previous studies that categorized the severity of CRS1 to analyze its importance in mortality; although there is no standard categorization, we have devised one to show that a combination of increasingly severe impairment in heart and kidney function is associated with increasing risk of events. The modification in clinical course detected in our analysis may thus reflect unique features of the pathophysiology of this entity rather than the combination of the independent effects of each of its components. A number of pathophysiological relationships have been described between CRS and ACS. Examples of potential connections include the acute hemodynamic effects of myocardial infarction [12], the toxic effects of iodinated contrast media used for coronary angiography [14] and drugs that modify the renin-angiotensin-aldosterone system [15–16]. Regarding the possible role of contrast-induced nephrotoxicity after angiography, in most patients with ACS hemodynamic deterioration is itself largely responsible for the development of CRS [17]; in fact, the proportion of patients who underwent angiography in our study was higher among those who did not have SCR1. In addition, histological and functional changes in the kidney may involve macrophage infiltration and interstitial fibrosis in the cortex [18]. Permeability of the microvessel endothelium and tubule cell apoptosis were increased in the kidneys of rats in which myocardial infarction was induced [19]. Together, these findings suggest the existence of specific mechanisms that make CRS1 a unique pathophysiological entity.
In contrast to our findings for death during hospitalization for ACS, our results did not confirm a higher risk of readmission or death after discharge when CRS1 is diagnosed than when the risks of each component of this syndrome are combined. Bivariate analysis showed that the incidence rate of readmission or death was high in patients with CRS1, but the multivariate analysis yielded no significant differences between the syndrome per se and the sum of heart failure and AKI during the first year after discharge. The severity of CRS1 is also important for this event, but the results may reflect a less important role for CRS1 in patients who survive the acute phase of ACS.
The incidence of CRS1 in patients with ACS in earlier studies varied across populations and depending on the diagnostic criteria used to define AKI [2,7,20]. In the present study we used the widely known Acute Kidney Injury Network criteria [11], and found that CRS1 was predictive of death in one third of the patients. We found no previous studies that analyzed the positive predictive value of CRS1, but even discreet increases in creatinine during the hospital stay are associated with an increased estimated incidence of CRS, and have been shown to have repercussions on the prognosis [11,21].Although a period of 48 hours has been proposed to confirm the acute nature of the deterioration in renal function [22], longer periods have also been found to have prognostic value and have also been suggested as diagnostic criteria for CRS [23]. The validity of different criteria notwithstanding, international consensus has been reached on the concept of CRS, which is defined as heart and kidney disorders in which dysfunction in one organ can induce acute or chronic dysfunction in the other [2].
Aside from CRS1 and its components, the other factors that had a significant effect on in-hospital death (AMI-ST, LVD, COPD, age and revascularization) are well known. Other factors that emerged as having a significant effect on the likelihood of readmission or death were diabetes, anemia, and previous coronary disease, whose effects have also been documented. Of particular interest was the greater protection associated with bypass versus percutaneous surgery, which agrees with the results of studies comparing the two procedures [24]. Regarding obesity, its paradoxical protective effect against death during the hospital stay is known [25] and can be attributed to residual confounding or to the high incidence of ACS in patients with an excess of adipose tissue, including some with a better initial prognosis. A similar phenomenon has also been described for the paradoxical association of tobacco smoking with lower inpatient mortality in acute ischemic stroke [26].
The main limitations of our study are those related with its observational research design. The appearance and clinical course of CRS1 may have been influenced by the use of amines and other drugs that affect the renin-angiotensin system, and this is a possibility that we cannot rule out. The use of these drugs depended on the management choices made for each individual patient by his or her doctor. Our data may be missing some baseline clinical variables and in-hospital complications, so we cannot completely exclude the possibility that unmeasured or residual confounding may explain some of our findings. It should also be noted that our findings may not be generalizable to other samples of patients in whom CRS was defined on the basis of different criteria. Specifically, we defined cases of AKI using only serum creatinine, but the criteria changed after our patients were recruited [27]. As a strength of our study, we note that the size of our cohort and our prospective follow-up design made it possible to study a large number of cases of CRS1 and final events.
We conclude that during the index hospital stay, the effect of CRS1 on mortality is greater than the sum of the effects associated to each component of this syndrome separately, and increasing severity of CRS1 worsens the prognosis. The incidence of CRS1 is approximately 10 cases per 1000 person-days of hospital stay among patients admitted because of ACS, but this incidence accounts for more than half of the mortality. The positive predictive value of CRS1 approached 30% for in-hospital death and was slightly higher than 51% after discharge.
Supporting Information
(XLS)
Acknowledgments
We thank K. Shashok for translating parts of the original manuscript into English.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Canary Islands Health Service and the Spanish Government (Instituto de Salud Carlos III, RD12/0042/0031 and 0013. FEDER).
References
- 1.McCullough PA, Kellum JA, Haase M, Müller C, Damman K, Murray PT, et al. Pathophysiology of the cardiorenal syndromes: executive summary from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013;182:82–98. 10.1159/000349966 [DOI] [PubMed] [Google Scholar]
- 2.Ronco C. Cardiorenal syndromes: definition and classification. Contrib Nephrol 2010;164:33–38. 10.1159/000313718 [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539. 10.1016/j.jacc.2008.07.051 [DOI] [PubMed] [Google Scholar]
- 4.Eren Z, Ozveren O, Buvukoner E, Kaspar E, Degertekin M, Kantarci G. A Single-Centre Study of Acute Cardiorenal Syndrome: Incidence, Risk Factors and Consequences. Cardiorenal Med 2012;2:168–176. 10.1159/000337714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virzì GM, Clementi A, Brocca A, de Cal M, Vescovo G, Granata A, et al. The hemodynamic and nonhemodynamic crosstalk in cardiorenal syndrome type 1. Cardiorenal Med. 2014;4:103–12 10.1159/000362650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy AK, Mc Gorrian C, Treacy C, Kavanaugh E, Brennan A, Mahon NG, et al. A Comparison of Traditional and Novel Definitions (RIFLE, AKIN, and KDIGO) of Acute Kidney Injury for the Prediction of Outcomes in Acute Decompensated Heart Failure. Cardiorenal Med 2013;3:26–37. 10.1159/000347037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haase M, Muller C, Damman K, Murray PT, Kellum JA, Ronco C, et al. Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013;182: 99–116. 10.1159/000349969 [DOI] [PubMed] [Google Scholar]
- 8.Gillespie CD, Wigington C, Hong Y; Centers for Disease Control and Prevention (CDC). Coronary heart disease and stroke deaths—United States, 2009. MMWR Surveill Summ. 2013. November 22;62 Suppl 3:157–60 [PubMed] [Google Scholar]
- 9.Ismail Y, Kasmikha Z, Green HL, McCullough PA. Cardio-renal syndrome type 1: epidemiology, pathophysiology, and treatment. Semin Nephrol 2012;32: 18–25. 10.1016/j.semnephrol.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37: 2129–00. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. 10.1093/eurheartj/ehs215 [DOI] [PubMed] [Google Scholar]
- 13.Kahn R, Buse J, Ferrannini E, Stern M; American Diabetes Association; European Association for the Study of Diabetes.The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005;28:2289–2304. [DOI] [PubMed] [Google Scholar]
- 14.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 15.Roubille F, Morena M, Leray-Moragues H, Canaud B, Cristol JP, Klouche K. Pharmacologic therapies for chronic and acute decompensated heart failure: specific insights on cardiorenal syndromes. Blood Purif. 2014;37 Suppl 2: 20–33. [DOI] [PubMed] [Google Scholar]
- 16.Marana I, Marenzi G, Kazory A. Extracorporeal ultrafiltration for heart failure: focus on organ cross talk and clinical trials. Nephrol Ther 2014;10:203–209. 10.1016/j.nephro.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 17.Shacham Y, Leshem-Rubinow E, Gal-Oz A, Arbel Y, Keren G, Roth A, et al. Acute Cardio-Renal Syndrome as a Cause for Renal Deterioration Among Myocardial Infarction Patients Treated With Primary Percutaneous Intervention. Can J Cardiol. 2015;31:1240–1244. 10.1016/j.cjca.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 18.Lekawanvijit S, Kompa AR, Zhang Y, Wang BH, Kelly DJ, Krum H. Myocardial infarction impairs renal function, induces renal interstitial fibrosis, and increases renal KIM-1 expression: implications for cardiorenal syndrome. Am J Physiol Heart Circ Physiol 2012;302: H1884–H1893. [DOI] [PubMed] [Google Scholar]
- 19.Cho E, Kim M, Ko YS, Lee HY, Song M, Kim MG, et al. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant 2013;28:2766–2778. 10.1093/ndt/gft376 [DOI] [PubMed] [Google Scholar]
- 20.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol 2011;7:201–208. 10.1038/nrneph.2011.14 [DOI] [PubMed] [Google Scholar]
- 21.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 2008;168:609–616. 10.1001/archinte.168.6.609 [DOI] [PubMed] [Google Scholar]
- 22.Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol 2000;36:1542–1548. [DOI] [PubMed] [Google Scholar]
- 23.Kellum JA, Mehta RL, Angus DC, Palevsky P, Ronco C; ADQI Workgroup.The first international consensus conference on continuous renal replacement therapy. Kidney Int 2002;62:1855–1863. 10.1046/j.1523-1755.2002.00613.x [DOI] [PubMed] [Google Scholar]
- 24.Marui A, Kimura T, Nishiwaki N, Mitsudo K, Komiya T, Hanyu M, et al. Five-year outcomes of percutaneous versus surgical coronary revascularization in patients with diabetes mellitus (from the CREDO-Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol. 2015;115:1063–72. 10.1016/j.amjcard.2015.01.544 [DOI] [PubMed] [Google Scholar]
- 25.Niedziela J, Hudzik B, Niedziela N, Gąsior M, Gierlotka M, Wasilewski J, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol. 2014;29:801–12. 10.1007/s10654-014-9961-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali SF, Smith EE, Bhatt DL, Fonarow GC, Schwamm LH. Paradoxical association of smoking with in-hospital mortality among patients admitted with acute ischemic stroke. J Am Heart Assoc. 2013;2: e000171 10.1161/JAHA.113.000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:4263–72. 10.1093/ndt/gfs375 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.