Abstract
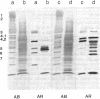
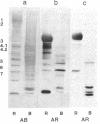
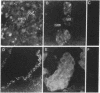
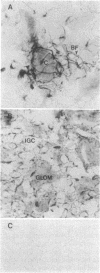
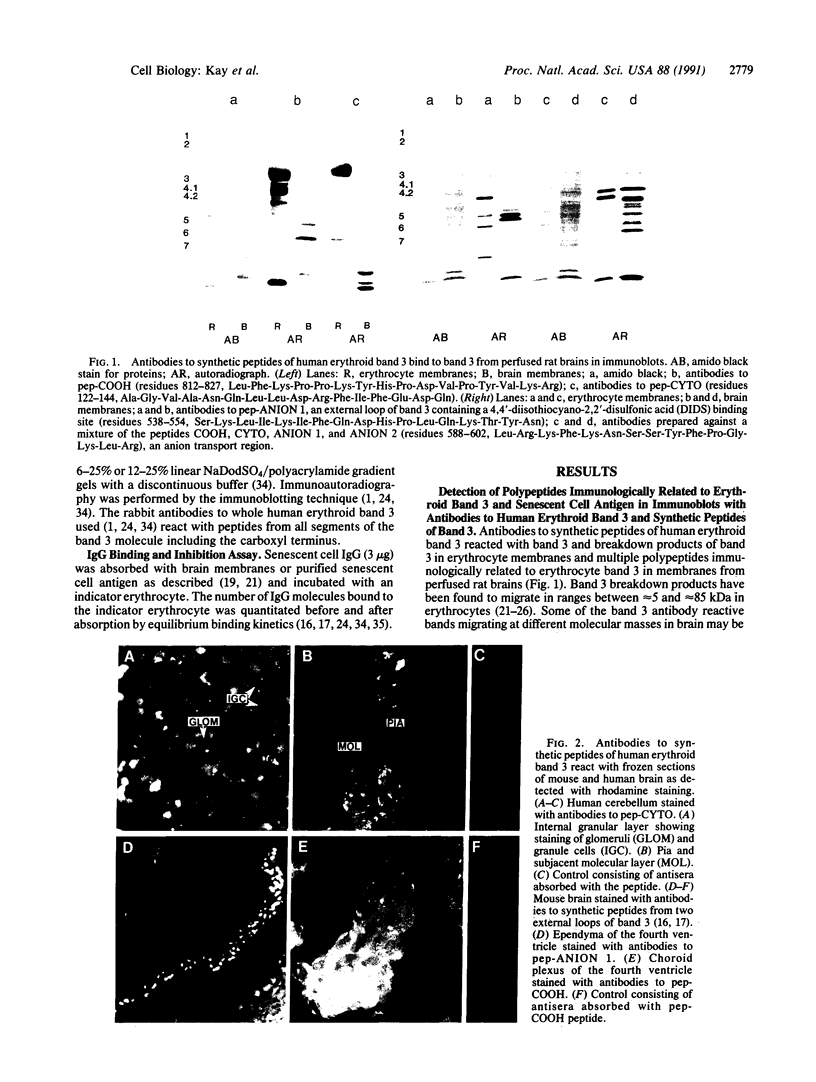
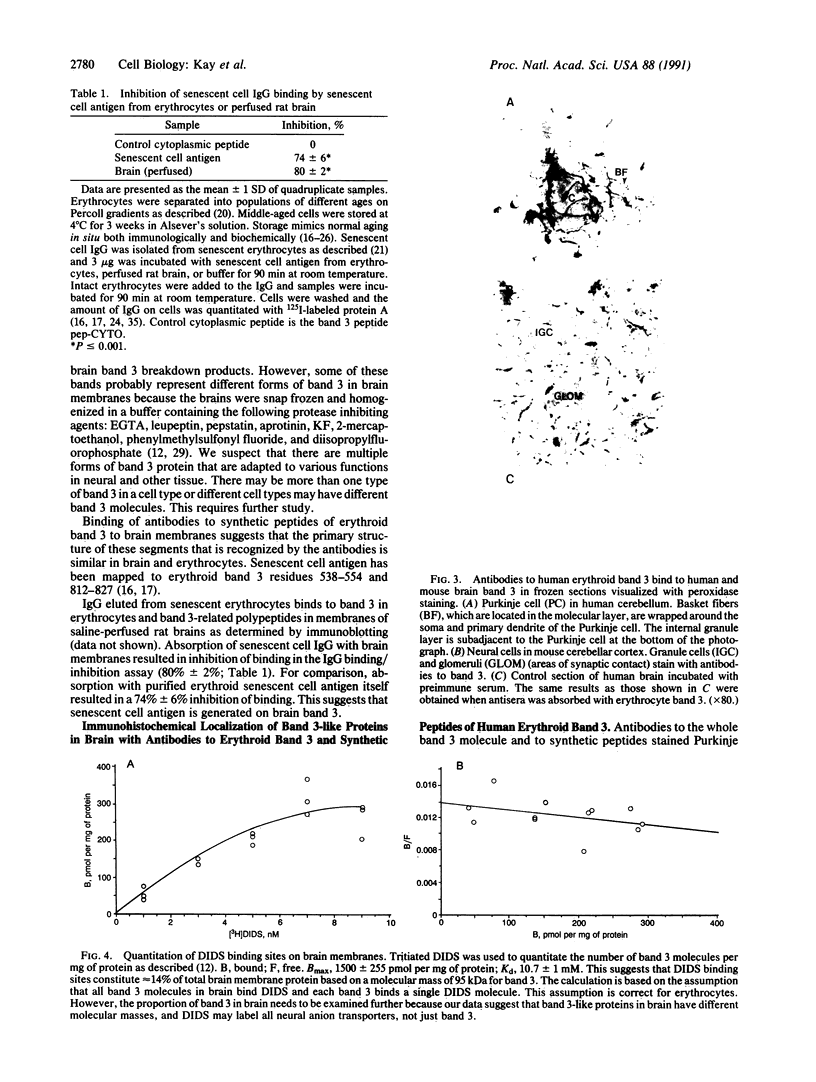
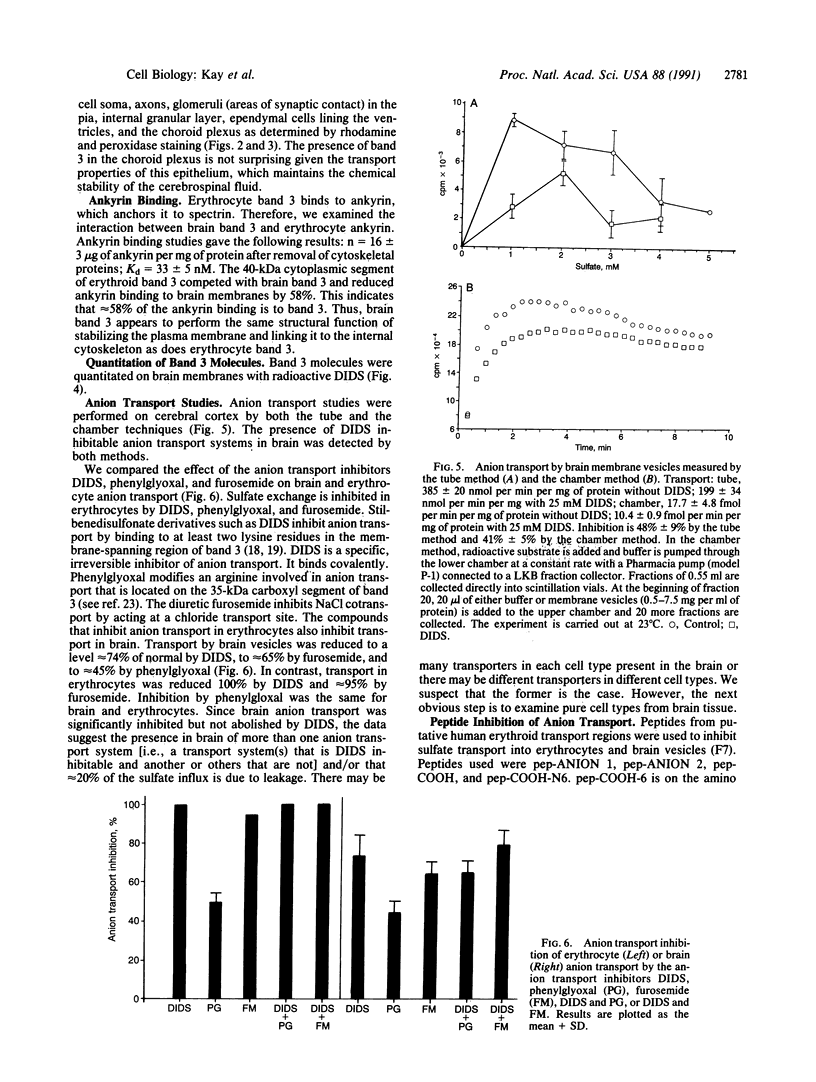
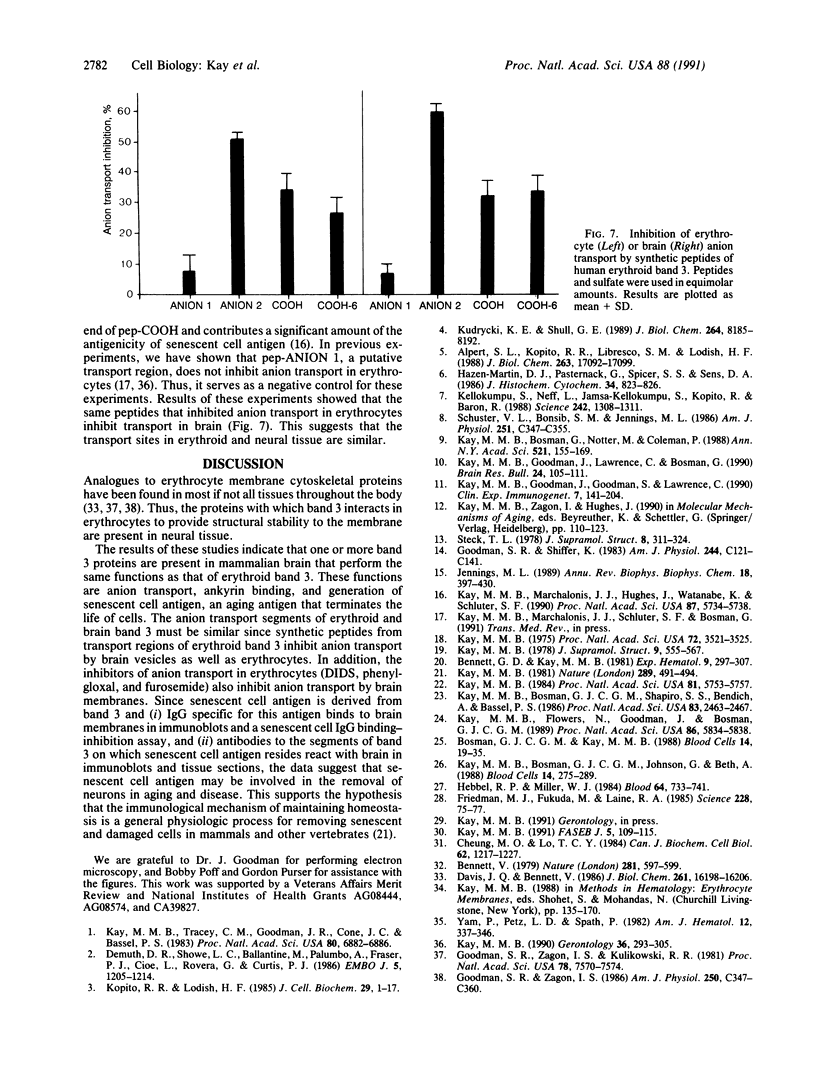
We report the presence of band 3 protein(s) in mammalian brain that performs the same functions as those of erythroid band 3. These functions are anion transport, ankyrin binding, and generation of senescent cell antigen, an aging antigen that terminates the life of cells. Structural similarity of brain and erythroid band 3 is suggested by the reaction of antibodies to synthetic peptides of erythroid band 3 with brain band 3, the inhibition of anion transport by the same inhibitors, and an equal degree of inhibition of brain and erythrocyte anion transport by synthetic peptides of erythroid band 3 (pep-ANION 2, residues 588-602; pep-COOH, residues 812-827; pep-COOH-N6, residues 813-818). One of these segments, pep-COOH, contains antigenic determinants of senescent cell antigen. These findings suggest that the transport domains of erythroid and neural band 3 are similar functionally and structurally and support the hypothesis that the immunological mechanism of maintaining homeostasis is a general physiologic process for removing senescent and damaged cells in mammals and other vertebrates.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L., Kopito R. R., Libresco S. M., Lodish H. F. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. J Biol Chem. 1988 Nov 15;263(32):17092–17099. [PubMed] [Google Scholar]
- Bennett G. D., Kay M. M. Homeostatic removal of senescent murine erythrocytes by splenic macrophages. Exp Hematol. 1981 Mar;9(3):297–307. [PubMed] [Google Scholar]
- Bennett V. Immunoreactive forms of human erythrocyte ankyrin are present in diverse cells and tissues. Nature. 1979 Oct 18;281(5732):597–599. doi: 10.1038/281597a0. [DOI] [PubMed] [Google Scholar]
- Bosman G. J., Kay M. M. Erythrocyte aging: a comparison of model systems for simulating cellular aging in vitro. Blood Cells. 1988;14(1):19–46. [PubMed] [Google Scholar]
- Cheung M. O., Lo T. C. Hexose transport in plasma membrane vesicles of rat myoblast L6. Can J Biochem Cell Biol. 1984 Nov;62(11):1217–1227. doi: 10.1139/o84-156. [DOI] [PubMed] [Google Scholar]
- Davis J. Q., Bennett V. Association of brain ankyrin with brain membranes and isolation of active proteolytic fragments of membrane-associated ankyrin-binding protein(s). J Biol Chem. 1986 Dec 5;261(34):16198–16206. [PubMed] [Google Scholar]
- Demuth D. R., Showe L. C., Ballantine M., Palumbo A., Fraser P. J., Cioe L., Rovera G., Curtis P. J. Cloning and structural characterization of a human non-erythroid band 3-like protein. EMBO J. 1986 Jun;5(6):1205–1214. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. J., Fukuda M., Laine R. A. Evidence for a malarial parasite interaction site on the major transmembrane protein of the human erythrocyte. Science. 1985 Apr 5;228(4695):75–77. doi: 10.1126/science.3883494. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. The spectrin membrane skeleton of normal and abnormal human erythrocytes: a review. Am J Physiol. 1983 Mar;244(3):C121–C141. doi: 10.1152/ajpcell.1983.244.3.C121. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Zagon I. S., Kulikowski R. R. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7570–7574. doi: 10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. R., Zagon I. S. The neural cell spectrin skeleton: a review. Am J Physiol. 1986 Mar;250(3 Pt 1):C347–C360. doi: 10.1152/ajpcell.1986.250.3.C347. [DOI] [PubMed] [Google Scholar]
- Hazen-Martin D. J., Pasternack G., Spicer S. S., Sens D. A. Immunolocalization of band 3 protein in normal and cystic fibrosis skin. J Histochem Cytochem. 1986 Jun;34(6):823–826. doi: 10.1177/34.6.3517151. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Miller W. J. Phagocytosis of sickle erythrocytes: immunologic and oxidative determinants of hemolytic anemia. Blood. 1984 Sep;64(3):733–741. [PubMed] [Google Scholar]
- Jennings M. L. Structure and function of the red blood cell anion transport protein. Annu Rev Biophys Biophys Chem. 1989;18:397–430. doi: 10.1146/annurev.bb.18.060189.002145. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Johnson G. J., Beth A. H. Band-3 polymers and aggregates, and hemoglobin precipitates in red cell aging. Blood Cells. 1988;14(1):275–295. [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Shapiro S. S., Bendich A., Bassel P. S. Oxidation as a possible mechanism of cellular aging: vitamin E deficiency causes premature aging and IgG binding to erythrocytes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2463–2467. doi: 10.1073/pnas.83.8.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Bosman G., Notter M., Coleman P. Life and death of neurons: the role of senescent cell antigen. Ann N Y Acad Sci. 1988;521:155–169. doi: 10.1111/j.1749-6632.1988.tb35274.x. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Flowers N., Goodman J., Bosman G. Alteration in membrane protein band 3 associated with accelerated erythrocyte aging. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5834–5838. doi: 10.1073/pnas.86.15.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Goodman J., Lawrence C., Bosman G. Membrane channel protein abnormalities and autoantibodies in neurological disease. Brain Res Bull. 1990 Jan;24(1):105–111. doi: 10.1016/0361-9230(90)90293-9. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981 Feb 5;289(5797):491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Lin F. B. Molecular mapping of the active site of an aging antigen: senescent cell antigen requires lysine(s) for antigenicity and is located on an anion-binding segment of band 3 membrane transport protein. Gerontology. 1990;36(5-6):293–305. doi: 10.1159/000213214. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Localization of senescent cell antigen on band 3. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Marchalonis J. J., Hughes J., Watanabe K., Schluter S. F. Definition of a physiologic aging autoantigen by using synthetic peptides of membrane protein band 3: localization of the active antigenic sites. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5734–5738. doi: 10.1073/pnas.87.15.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Molecular mapping of human band 3 anion transport regions using synthetic peptides. FASEB J. 1991 Jan;5(1):109–115. doi: 10.1096/fasebj.5.1.1991578. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Tracey C. M., Goodman J. R., Cone J. C., Bassel P. S. Polypeptides immunologically related to band 3 are present in nucleated somatic cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6882–6886. doi: 10.1073/pnas.80.22.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellokumpu S., Neff L., Jämsä-Kellokumpu S., Kopito R., Baron R. A 115-kD polypeptide immunologically related to erythrocyte band 3 is present in Golgi membranes. Science. 1988 Dec 2;242(4883):1308–1311. doi: 10.1126/science.2461589. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Structure of the murine anion exchange protein. J Cell Biochem. 1985;29(1):1–17. doi: 10.1002/jcb.240290102. [DOI] [PubMed] [Google Scholar]
- Kudrycki K. E., Shull G. E. Primary structure of the rat kidney band 3 anion exchange protein deduced from a cDNA. J Biol Chem. 1989 May 15;264(14):8185–8192. [PubMed] [Google Scholar]
- Schuster V. L., Bonsib S. M., Jennings M. L. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol. 1986 Sep;251(3 Pt 1):C347–C355. doi: 10.1152/ajpcell.1986.251.3.C347. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The band 3 protein of the human red cell membrane: a review. J Supramol Struct. 1978;8(3):311–324. doi: 10.1002/jss.400080309. [DOI] [PubMed] [Google Scholar]
- Yam P., Petz L. D., Spath P. Detection of IgG sensitization of red cells with 125I staphylococcal protein A. Am J Hematol. 1982 Jun;12(4):337–346. doi: 10.1002/ajh.2830120405. [DOI] [PubMed] [Google Scholar]