Abstract
This study was aimed to measure the dietary effects of probiotic Bacillus subtilis strain fmbj (BS fmbj) on antioxidant capacity and oxidative stability of chicken breast meat during storage. Treatment groups were fed the basal diet with BS fmbj at 0 g/kg (CON), 0.2 g/kg (BS-1), 0.3 g/kg (BS-2), or 0.4 g/kg (BS-3) doses without antibiotics. During 8 days of storage at 4°C, BS-2 group showed a significant improvement (P < 0.05) on meat quality (pH, Drip loss, Cooking loss, Shear force, color L*, a*, b*), free radical scavenging activity (DPPH, ABTS+, H2O2), tissues antioxidant enzyme capacity (SOD, CAT, GSH-Px, GSH, T-SH), mitochondria antioxidant enzyme capacity (MnSOD, GPx, GSH), mRNA expression of antioxidant genes (Nrf2, HO-1, SOD, CAT, GSH-Px) and mitochondrial function genes (avUCP, NRF1, NRF2, TFAM, PGC-1α), oxidative damage index (MDA, ROS, PC, 8-OHdG), and MMP level in chicken breast meat as compared to the CON group. These results indicate that dietary BS fmbj in broiler diets can protect breast meat against the storage-induced oxidative stress by improving their free radical scavenging capacity and antioxidant activity during 8 days of storage at 4°C.
Introduction
Oxidative stress occurs when the balance of the antioxidant defense system and the free radicals generation system is impaired, and is considered an important factor in various diseases [1]. Reactive oxygen species (ROS) and free radicals generated either endogenously or exogenously, may contribute to various pathological effects, including DNA damage and cellular degeneration [2]. Lipid peroxidation plays a major role in generating oxygen free radicals, and is responsible for the devaluation of food quality. Antioxidant enzymes and synthetic antioxidants, which have the ability to suppress oxidative stress, seem promising. However, their toxicity and unwanted side effects impede their extensive use in food industry. With these safety concerns, there is an increasing attention to find natural sources to be used in food industry that can improve food quality, enhance the capacity of scavenging excess free radicals, and provide additional health benefits to consumers [3]. Probiotics are beneficial in animal health through suppressing the oxidative stress, and are applied as feed supplements to improve performance and antioxidant capacity of animals [4,5]. Among probiotics, Bacillus subtilis is well known for its resistance to harsh environments and beneficial role in improving the meat quality and antioxidant capacity of animals [6,7].
The increasing understanding on the relationship between poultry diets and health has led to new research areas. In recent years, the effect of functional foods on physiological and metabolic functions has gradually become the hot topic [8]. Consumers are in urgent need of these functional foods more than considering their nutritional value, which is beneficial to the development of functional foods [9]. It has been shown that some negative health changes are always associated with the intake of some meat products [10]. However, as mentioned earlier, meat products are a very commonly bought food, and have vital nutritional value to human beings. Therefore, meat products may be regarded as a key target for nutritional-profile modification and to reversal of the potential negative impact on an individual’s health. In this sense, adding suitable additives in animal diet to improve the meat products’ antioxidant capacity is of great interest.
The goal in poultry is producing meat that is safe for human consumption, taking into consideration the welfare of animal and respect for the environment. Animal meat is a crucial part of the human diet due to its high-quality proteins, essential amino acids, and various essential micronutrients such as unsaturated fatty acids, vitamins, and minerals [11]. Meat is susceptible to oxidative stress during storage at 2–5°C due to its chemical composition and physical structure [12]. Lipid peroxidation plays a major role in generating oxygen free radicals, and is responsible for the devaluation of food quality [13]. Endogenous antioxidant systems are made up of non-enzymatic hydrophilic and lipophilic compounds like vitamin E, vitamin C, and enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. Both enzymatic and non-enzymatic antioxidant systems can suppress oxidative stress in muscle tissue not only in living animals but also in the meat produced [14]. This study was aimed to measure the dietary effects of probiotic Bacillus subtilis strain fmbj on antioxidant capacity and oxidative stability of chicken breast meat during storage.
Materials and Methods
Bacterial Strain
The BS fmbj (CGMCCN 0943) used in this study was a wild-type strain originally isolated and characterized at the College of Food Science and Technology, Nanjing Agricultural University, and was procured from Heng Zeyuan Biological Technology Co., Ltd, Wuxi, People’s Republic of China. This product was determined to contain at least 1.0 × 10 11 cfu/g BS fmbj, and was kept in a sterilized container before use.
Experimental design
A total of 240 one day old male Arbor Acres broiler chickens were provided by a local commercial hatchery (Kangxin Poultry Co, Nanjing, People's Republic of China), and randomly assigned to four treatments. Each treatment had six replicate pens of ten birds in each pen. Treatment groups were fed the basal diets with BS fmbj at 0 g/kg (CON), 0.2 g/kg (BS-1), 0.3 g/kg (BS-2), or 0.4 g/kg (BS-3) without antibiotics. The basal diet was formulated to meet or exceed the nutritional requirements of broilers according to the NRC, 1994. All birds were raised in an environmentally controlled room (at 34–36°C) during 1 to 14 d, where the temperature was gradually decreased to 26°C until the end of this experiment. All birds were kept under a constant lighting of 24 h, and allowed to take food and water ad libitum. This study was approved and conducted under the supervision of Animal Care and Use Committee, Nanjing Agriculture University, Nanjing, People's Republic of China, and adopted the Animal Care and Use Guidelines for all the animals used in this experimental procedures. In this study, all efforts are taken to minimize suffering when these birds meet our euthanasia criteria. Progressive deterioration of the animals' health leading to death is not allowed. The humane endpoint is set to decide when to sacrifice them, which includes that body temperature and physical activity are significantly worse than the active one, and are decreased or not increase in a few hours. The birds are no response to intermittent stimulation 3 times in half an hour, or the respiratory rate of them are rapidly or slowly apparently. The broilers used in this study were taken care by trained workers in Nanjing Agriculture University, Nanjing, People's Republic of China. They monitored the health of each one every 6 h and strictly performed the rules of humane endpoints to determine when they should be euthanized by electrically stunned.
Sample preparation
At 42 day of raising, 24 chickens (one chicken per pen) were individually weighed, electrically stunned, and slaughtered. Individual carcasses were trimmed for breast meat tissue by removing feathers, bones, connective tissues, and stored at 4°C. During 8 days of storage, meat tissue samples (one sample per chicken breast meat at 0, 2, 4, 6, 8 day of storage) were collected for further study.
Meat quality analysis
The pH value was measured directly at three different locations within the same meat tissue using a spear type pH meter, and the average was used for statistical analysis. Drip loss was measured by suspending the meat tissue standardized for surface area in cups at 4°C for 48 h. Cooking loss was measured by weighing the differences of pre- and post- meat tissue after cooked to a final core temperature of 75°C. After cooking, three cores of each one (1.27 cm diameters) paralleled to longitudinal orientation of muscle fibers were used for the shear force measurement. The color of meat tissue were measured, and the average were obtained as C.I.E. (Commission Internationale de l’Eclairage) lightness (L*), redness (a*), and yellowness (b*).
Antioxidant capacity and oxidative stability analysis
One gram of chicken breast meat tissue sample (one sample per chicken breast meat at 0, 2, 4, 6, 8 day of storage) was homogenized at 8000 rpm for 10 s in 9 ml of 0.9% sodium chloride buffer on ice, and centrifuged at 4000 rpm at 4°C for 15 min. Then collected the supernatant respectively for further study.
DPPH radical scavenging activity
1,1-diphenyl-2-pierylhydrazy (DPPH) radical scavenging activity was measured following the procedure of Moon [15] with some modifications. Breifly, DPPH was dissolved into ethanol to a solution of 0.1 mM, and kept in dark. Then, this solution and meat tissue supernatant mixed vigorously, and incubated in darkness at 25°C for 30 min before determining the absorbance at 517 nm. DPPH radical scavenging activity was calculated on basis of protein content (mg/ml) of meat tissue using the following equation:
where, Acontrol was the absorbance of the control, and Atissue was the absorbance of meat tissue supernatant under the same conditions.
ABTS+ radical scavenging activity
2,2'-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS+) radical scavenging activity was measured according to the method described by Siddhuraju [16]. ABTS+ was produced by mixing 7 mM of ABTS+ stock solution in water with 2.45 mM K2S2O8, then incubated in darkness at 25°C for 12–16 h. After that, the ABTS+ solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm before usage. 1 ml of this ABTS+ solution, 3 ml of meat tissue supernatant were incubated at 30°C for 30 min, and the absorbance was measured at 534 nm. ABTS+ radical scavenging activity was calculated on basis of protein content (mg/ml) of meat tissue using the following equation:
where, Acontrol was the absorbance of the control, and Atissue was the absorbance of meat tissue supernatant under the same conditions.
H2O2 scavenging activity
Hydrogen peroxide (H2O2) scavenging assay was performed by the method of Hseu [17]. Briefly, 3.4 ml of meat tissue supernatant in phosphate buffer (0.1 M, pH = 7.4) were mixed with 0.6 ml of 43 mM H2O2 solution before the absorbance was recorded at 230 nm. H2O2 scavenging activity was calculated on basis of protein content (mg/ml) of meat tissue using the following equation:
where, Acontrol was the absorbance of the control, and Atissue was the absorbance of meat tissue supernatant under the same conditions.
Antioxidant enzyme activity of chicken breast meat tissue analysis
Meat tissue supernatant was individually to measure the activity of Superoxide dismutase (SOD), Hydrogen peroxidase (CAT), Glutathione peroxidase (GSH-Px), glutathione (GSH), and Total mercapto (T-SH) using corresponding diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) according to the instructions of the manufacturer.
Isolation of chicken breast meat tissue mitochondria
Meat tissue mitochondria were prepared according to the method described by Tang [18]. Namely, meat tissue sample (one sample per chicken breast meat at 0, 2, 4, 6, 8 day of storage) was homogenized in ice-chilled Dounce homogenizers (1:10, w/v) using isolation buffer containing 10 mM MOPS pH 7.4, 250 mM sucrose, 5 mM KH2PO4, 2 mM MgCl2, 1 mM EGTA, 0.1% fatty acid-free BSA, and centrifuged at 1000 g for 5 min at 4°C. Remove the supernatants and resuspend the mitochondria-enriched pellets gently, and washed with the isolation buffer, then obtained the pelleted by centrifugation at 12000 g for 5 min. Mitochondria were lysed and the protein was measured using the Micro BCA protein assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) according to the manufacturers’ instructions.
Antioxidant enzyme activity of chicken breast meat tissue mitochondria analysis
Manganese superoxide dismutase (MnSOD), Glutathione peroxidase (GPx), Glutathione (GSH) activity, and protein concentrations of meat tissue mitochondria were measured using corresponding diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) according to the instructions of the manufacturer.
Lipid peroxidation analysis
Lipid peroxidation, expressed as malondialdehyde concentration, was determined using a MDA assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) according to the instructions of the manufacturer. Briefly, meat tissue homogenates (0.9% sodium chloride buffer to produce a 10% tissue lysate) were used to calculate MDA levels by the method of thiobarbituric acid (TBA). The MDA-TBA mixture produced during the reaction of MDA in meat tissue with TBA was measured at 535 nm (UV-2401PC, Shimadzu, Japan).
ROS analysis
ROS concentrations in meat tissue mitochondria were detected using a ROS assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) according to the manufacturers’ instructions. Briefly, the meat tissue mitochondria was incubated with DCFH-DA (10 μM) and DNA stain Hoechst 33342 (10 mmol/L) at 37°C for 30 min. Then, the DCFH fluorescence of mitochondrial was measured at an emission wavelength of 530 nm and an excitation wavelength of 485 nm with a FLX 800 microplate fluorescence reader (Biotech Instruments Inc., USA). The results were expressed as the mean DCFH-DA fluorescence intensity over that of the control.
Protein oxidation and 8-OHdG analysis
Protein oxidation of meat tissue mitochondria was calculated using the concentrations of Protein carbonyls (PC), which was measured by using a previously described method [19], and presented in nmol/mg protein. 8-hydroxy-2-deoxyguanosine (8-OHdG) content in meat tissue mitochondria was calculated using a ELSA assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, P. R. China) according to the manufacturers’ instructions, and presented in ng/mg protein.
Mitochondrial membrane potential analysis
Mitochondrial membrane potential (MMP) changes in meat tissue mitochondria was detected using a MMP assay kit (Beyotime Institute of Biotechnology, Jiangsu, China) according to the instructions of the manufacturer. Namely, meat tissue mitochondria were loaded with 1×JC-1 at 37°C for 20 min, then washed, and analyzed by flow cytometry (FACS Aria III, BD, New Jersey, US). MMP can be calculated as an increasing green fluorescent/red fluorescent intensity ratio. When MMP levels are low, JC-1 exists mainly as a monomer, which emits green fluorescence (excitation wavelength of 490 nm and emission wavelength of 540 nm). However, when MMP levels are high, JC-1 exists mainly as a polymer, which emits red fluorescence (excitation wavelength of 525 nm and emission wavelength of 590 nm). The results were calculated as a fluorescence ratio of aggregates (red) to monomers (green).
Quantitative real-time PCR analysis
Total RNA was obtained from meat tissue using Trizol Reagent (TaKaRa, Dalian, China), and then reverse-transcribed using a commercial kit (Perfect Real Time, SYBR® PrimeScript™ TaKaRa, China) following the instructions of the manufacturer. The mRNA expression level of specific genes were quantified via real-time PCR, using SYBR® Premix Ex Taq™ II (Tli RNaseH Plus), and an ABI 7300 Fast Real-Time PCR detection system (Applied Biosystems, USA). The SYBR Green PCR reaction mixture consisted of 10 μl SYBR® Premix Ex Taq (2X), 0.4 μl of the forward and reverse primers, 0.4 μl of ROX reference dye (50X), 6.8 μl of ddH2O, and 2 μl of cDNA template. Each meat tissue was amplified in triplicate. The fold-expression of each gene was calculated according to the 2-ΔΔCt method [20], in which β-Actin gene was used as an internal standard. The primer sequences used are given in Table 1.
Table 1. Primer sequences used for Real-time PCR assay.
| Name1 | Sequence (5’→3’)2 | Genbank3 |
|---|---|---|
| Nrf2 | GATGTCACCCTGCCCTTAG | NM_205117.1 |
| CTGCCACCATGTTATTCC | ||
| HO-1 | GGTCCCGAATGAATGCCCTTG | HM237181.1 |
| ACCGTTCTCCTGGCTCTTGG | ||
| SOD | CCGGCTTGTCTGATGGAGAT | NM_205064.1 |
| TGCATCTTTTGGTCCACCGT | ||
| CAT | GGTTCGGTGGGGTTGTCTTT | NM_001031215.1 |
| CACCAGTGGTCAAGGCATCT | ||
| GPx | GACCAACCCGCAGTACATCA | NM_001277853.1 |
| GAGGTGCGGGCTTTCCTTTA | ||
| β-Actin | TGCTGTGTTCCCATCTATCG | NM_205518.1 |
| TTGGTGACAATACCGTGTTCA | ||
| avUCP | ACAACGTCCCCTGTCACTTC | AB088685.1 |
| ATGAACATCACCACGTTCCA | ||
| NRF1 | AAGAACACGGCGTGACTCAA | NM_001030646.1 |
| TCGCTTCCGTTTCTTACCCG | ||
| NRF2 | GAGCCCATGGCCTTTCCTAT | NM_001007858.1 |
| CACAGAGGCCCTGACTCAAA | ||
| TFAM | GTGAAAGCCTGGCGAAACTG | NM_204100.1 |
| CACAGCTCAGGTTACACCGT | ||
| PGC-1α | GATTCTTCACCTGGGTGGCA | NM_001006457.1 |
| TCAGCCCGAATTTCCTGGTC |
1 Nuclear factor erythroid 2-related factor 2 (Nrf2); Heme oxygenase 1 (HO-1); Superoxide dismutase (SOD); Hydrogen peroxidase (CAT); Glutathione peroxidase (Gpx); Avian uncoupling protein (avUCP); Nuclear respiratory factor 1 (NRF1); Nuclear respiratory factor 2 (NRF2); Mitochondrial transcription factor A (TFAM); peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α).
2 Shown as forward primer followed by reverse primer.
3 GenBank Accession Number.
Statistical Analysis
All data were analyzed by ANOVA using the General Linear Model procedures of SAS 2003 (v. 9.1, SAS Institute Inc., Cary, NC, USA). Duncan's multiple-range test was used for significance of difference (P < 0.05 or P < 0.01) of two factors (BS fmbj and storage day) and interaction between this two factors. Values on the same storage day with different letters (a—e) were significantly different (P < 0.05), and values in same group with different letters (A—E) were significantly different (P < 0.05). Data were expressed as the mean ± SEM.
Results
Meat quality analysis
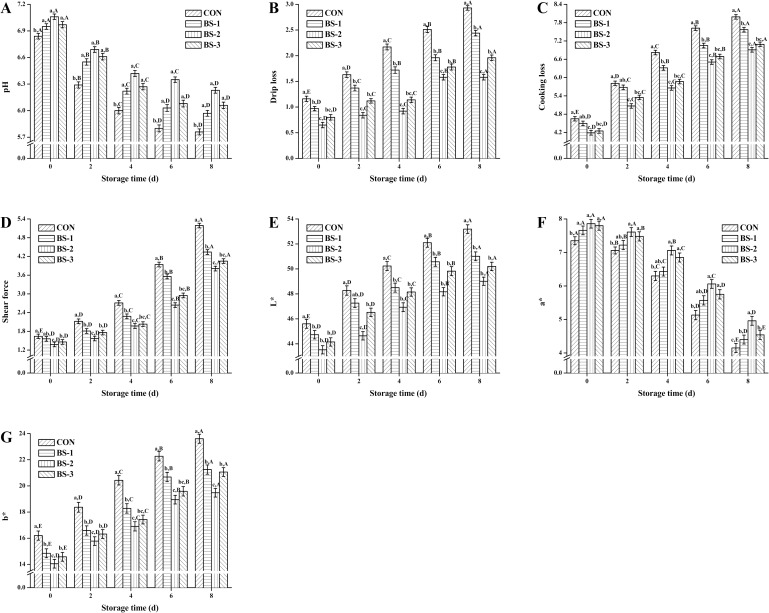
Fig 1 shows the pH (A), drip loss (B), cooking loss (C), shear force (D), L* (E), a* (F), and b* (G) value of chicken breast meat tissue during 8 days of storage at 4°C. At 42 d of raising, meat quality was significantly improved (P < 0.05) in BS group, and was gradually attenuated during storage in all groups. Meat quality was significantly improved (P < 0.05) in BS-2 group compared with that in CON group during storage. At 8 d of storage, pH and a* value were significantly decreased (P < 0.05), while, drip loss, cooking loss, shear force, L*, and b* value were significantly increased (P < 0.05) in BS-2 group compared with the CON group.
Fig 1.
Dietary effects of probiotic Bacillus subtilis strain fmbj on pH (A), drip loss (B), cooking loss (C), shear force (D), L* (E), a* (F), and b* (G) value of chicken breast meat tissue during 8 days of storage at 4°C. Values are mean ± SEM (n = 6). Values on the same storage day with different letters (a—e) were significantly different (P < 0.05), and values in same group with different letters (A—E) were significantly different (P < 0.05). CON, birds fed the basal diet without BS fmbj and antibiotics; BS-1, birds fed the basal diet with 0.2 g/kg BS fmbj without antibiotics; BS-2, birds fed the basal diet with 0.3 g/kg BS fmbj without antibiotics; BS-3, birds fed the basal diet with 0.4 g/kg BS fmbj without antibiotics.
Antioxidant capacity and oxidative stability analysis
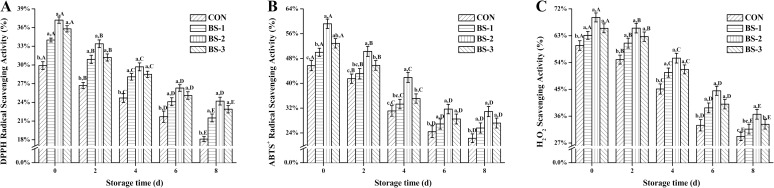
During 8 days of storage at 4°C, DPPH (A), ABTS+ (B), and H2O2 (C) radical scavenging activity of chicken breast meat tissue are presented in Fig 2. At 42 d of raising, free radicals scavenging activity were significantly increased (P < 0.05) in BS group, and were gradually decreased during storage in all groups. Free radicals scavenging activity were significantly increased (P < 0.05) in BS-2 group compared with that in CON group during storage. At 8 d of storage, free radicals scavenging activity were significantly enhanced (P < 0.05) in BS-2 group compared with those in CON group.
Fig 2.
Dietary effects of probiotic Bacillus subtilis strain fmbj on DPPH (A), ABTS+ (B), and H2O2 (C) scavenging activity of chicken breast meat tissue during 8 days of storage at 4°C. Values are mean ± SEM (n = 6). Values on the same storage day with different letters (a—e) were significantly different (P < 0.05), and values in same group with different letters (A—E) were significantly different (P < 0.05). 1,1-diphenyl-2-pierylhydrazy (DPPH); 2,2'-Azinobis- (3-ethylbenzthiazoline -6-sulphonate) (ABTS+); Hydrogen peroxide (H2O2). CON, birds fed the basal diet without BS fmbj and antibiotics; BS-1, birds fed the basal diet with 0.2 g/kg BS fmbj without antibiotics; BS-2, birds fed the basal diet with 0.3 g/kg BS fmbj without antibiotics; BS-3, birds fed the basal diet with 0.4 g/kg BS fmbj without antibiotics.
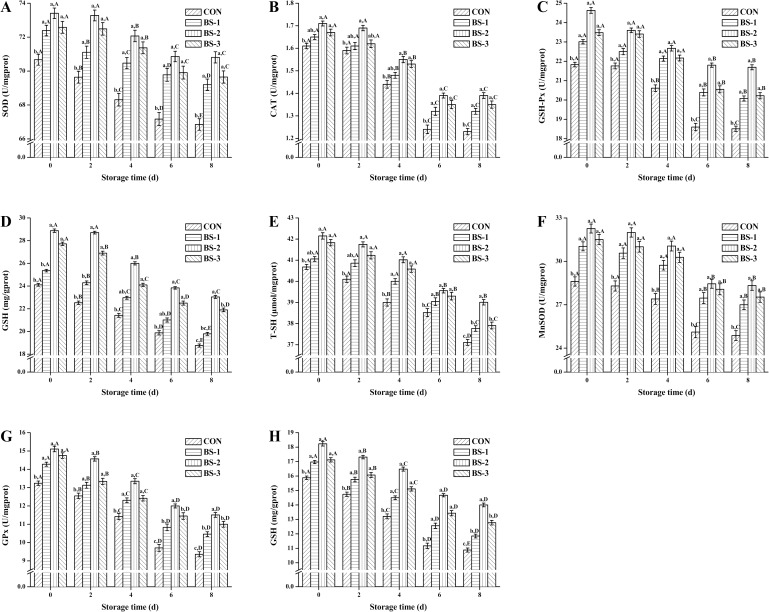
During 8 days of storage at 4°C, SOD (A), CAT (B), GSH-Px (C), GSH (D), T-SH (E) activity of chicken breast meat tissue, and MnSOD (F), GPx (G), GSH (H) activity of chicken breast meat tissue mitochondria are shown in Fig 3. At 42 d of raising, antioxidant enzyme activity of tissue and tissue mitochondria were significantly increased (P < 0.05) in BS group, and were gradually decreased during storage in all groups. Antioxidant enzyme activity of tissue and tissue mitochondria were significantly increased (P < 0.05) in BS-2 group compared with that in CON group during storage. At 8 d of storage, antioxidant enzyme activity of tissue and tissue mitochondria were still significantly higher (P < 0.05) in BS-2 group than that in the CON group.
Fig 3.
Dietary effects of probiotic Bacillus subtilis strain fmbj on SOD (A), CAT (B), GSH-Px (C), GSH (D), T-SH (E) activity of chicken breast meat tissue, and on MnSOD (F), GPx (G), GSH (H) activity of chicken breast meat tissue mitochondria during 8 days of storage at 4°C. Values are mean ± SEM (n = 6). Values on the same storage day with different letters (a—e) were significantly different (P < 0.05), and values in same group with different letters (A—E) were significantly different (P < 0.05). Superoxide dismutase (SOD); Hydrogen peroxidase (CAT); Glutathione peroxidase (GSH-Px); Glutathione (GSH); Total mercapto (T-SH); Manganese Superoxide dismutase (MnSOD); Glutathione peroxidase (GPx). CON, birds fed the basal diet without BS fmbj and antibiotics; BS-1, birds fed the basal diet with 0.2 g/kg BS fmbj without antibiotics; BS-2, birds fed the basal diet with 0.3 g/kg BS fmbj without antibiotics; BS-3, birds fed the basal diet with 0.4 g/kg BS fmbj without antibiotics.
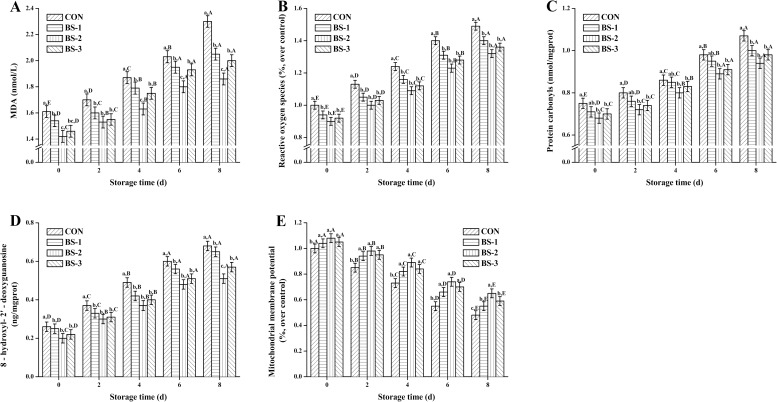
As seen in Fig 4, MDA (A), ROS (B), PC (C), 8-OHdG (D), and MMP level (E) of chicken breast meat tissue during 8 days of storage at 4°C are presented. At 42 d of raising, oxidative damage index were significantly decreased (P < 0.05), and MMP level was significantly increased (P < 0.05) in BS group, and they were gradually deteriorated during storage in all groups. Oxidative damage index and MMP level were significantly improved (P < 0.05) in BS-2 group compared with that in CON during storage. At 8 d of storage, values of MDA, ROS, PC, and 8-OHdG were significantly decreased (P < 0.05), and MMP level was significantly increased (P < 0.05) in BS-2 group compared with the CON group.
Fig 4.
Dietary effects of probiotic Bacillus subtilis strain fmbj on MDA (A), ROS (B), PC (C), 8-OHdG (D), and MMP level (E) of chicken breast meat tissue mitochondria during 8 days of storage at 4°C. Values are mean ± SEM (n = 6). Values on the same storage day with different letters (a—e) were significantly different (P < 0.05), and values in same group with different letters (A—E) were significantly different (P < 0.05). Malondialdehyde (MDA); Reactive oxygen species (ROS); Protein carbonyls (PC); 8-hydroxy-2- deoxyguanosine (8-OHdG); Mitochondrial membrane potential (MMP). CON, birds fed the basal diet without BS fmbj and antibiotics; BS-1, birds fed the basal diet with 0.2 g/kg BS fmbj without antibiotics; BS-2, birds fed the basal diet with 0.3 g/kg BS fmbj without antibiotics; BS-3, birds fed the basal diet with 0.4 g/kg BS fmbj without antibiotics.
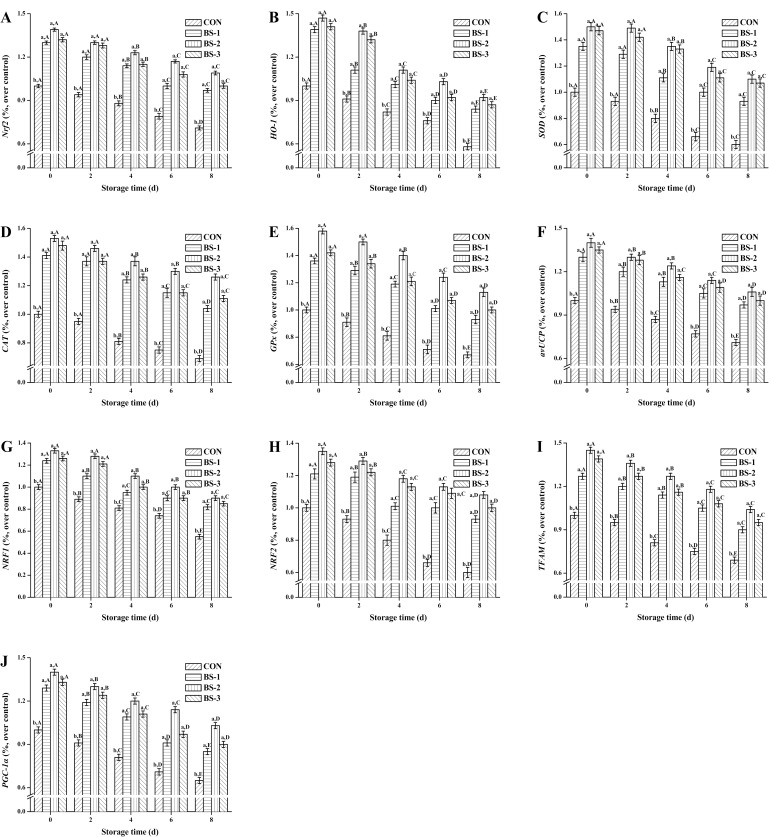
During 8 days of storage at 4°C, mRNA expression level of Nrf2 (A), HO-1 (B), SOD (C), CAT (D), GSH-Px (E), avUCP (F), NRF1 (G), NRF2 (H), TFAM (I), and PGC-1α (J) in chicken breast meat tissue are shown in Fig 5. At 42 d of raising, mRNA expression level of antioxidant genes and mitochondrial function genes were significantly increased (P < 0.05) in BS group, and were gradually decreased during storage in all groups. The mRNA expression level were significantly increased (P < 0.05) in BS-2 group compared with that in CON group during storage. At 8 d of storage, mRNA expression level of antioxidant genes and mitochondrial function genes were significantly enhanced (P < 0.05) in BS-2 group compared with those in CON group.
Fig 5.
Dietary effects of probiotic Bacillus subtilis strain fmbj on mRNA expression of Nrf2 (A), HO-1 (B), SOD (C), CAT (D), GSH-Px (E). avUCP (F), NRF1 (G), NRF2 (H), TFAM (I), and PGC-1α (J) of chicken breast meat tissue during 8 days of storage at 4°C. Values are mean ± SEM (n = 6). Values on the same storage day with different letters (a—e) were significantly different (P < 0.05), and values in same group with different letters (A—E) were significantly different (P < 0.05). Nuclear factor erythroid 2-related factor 2 (Nrf2); Heme oxygenase 1 (HO-1); Superoxide dismutase (SOD); Hydrogen peroxidase (CAT); Glutathione peroxidase (Gpx); Avian uncoupling protein (avUCP); Nuclear respiratory factor 1 (NRF1); Nuclear respiratory factor 2 (NRF2); Mitochondrial transcription factor A (TFAM); peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α). CON, birds fed the basal diet without BS fmbj and antibiotics; BS-1, birds fed the basal diet with 0.2 g/kg BS fmbj without antibiotics; BS-2, birds fed the basal diet with 0.3 g/kg BS fmbj without antibiotics; BS-3, birds fed the basal diet with 0.4 g/kg BS fmbj without antibiotics.
Discussion
Meat quality analysis
In recent years, meat quality has become a major concern for its consumers. The pH value is associated with the amount of free hydrogen ions (H+) in a given sample. It has been reported that adding probiotics in water and poultry feed together could improve the pH value of the meat [21]. Recent study also suggests that the pH value of the meat tends to be lower after storage [22]. Storage also alters the moisture content and distribution in meat tissues. Moisture loss from chicken meat can be measured in several ways, for example based on the drip loss and the cooking loss. Moisture loss from the meat is inevitable for the pH decrease after slaughter. It has been found that dietary B. subtilis strain B2A could increase the drip loss from the chicken meat after just one day of storage [23,24]. Mahajan et al. found that dietary probiotics could significantly improve the quality of meatballs during refrigerated storage for up to 14 days [25]. Shear force is often expressed in terms of the tenderness or the meat texture, and is one of the most important sensory qualities that influence consumer satisfaction. A study reported that dietary probiotics exerted beneficial effects on the shear force of chicken meat [26]. However, some studies observed no significant differences in the shear force between chicken breast meat from dietary probiotics group and a control group after 35 days of raising [27]. Almost 85% of consumers consider color as the primary decision factor for buying meat. It has been reported that dietary probiotics could improve chicken meat quality by altering the values of pH, tenderness, and color [21,28]. However, Park found that dietary B. subtilis B2A in broiler diets exerted little effect on the pH and color of breast meat after 1 day of storage [23]. These unconformities might be due to strains of probiotics, administered dosage, methods of preparation, bird age, diet composition, and hygiene status.
Antioxidant capacity and oxidative stability analysis
Free radical scavenging activities against DPPH, ABTS+, and H2O2 are widely used in the calculation of the antioxidant activity. DPPH is a stable nitrogen free radical [29]. Antioxidant reaction with DPPH can neutralize the excessive free radicals by transfer of either an electron or a hydrogen atom to DPPH. The addition of antioxidants to the DPPH solution induces a rapid change of color, which indicates the formation of a stable diamagnetic molecule [30]. Similarly, ABTS+ radical scavenging assay can be used to calculate the antioxidant activity of a broad diversity of substances with color changes after the reduction of ABTS+ radicals [31]. Some unpredictable factors may influence these two radical scavenging activity assays. Thus, another assay, such as the H2O2 scavenging activity measurement, should be considered. O2- is quite unreactive compared to some other free radicals, but it is the most abundant free radical in body, and is converted to more reactive species in biological systems such as H2O2 radicals [32]. The dismutation of O2- also leads to the formation of H2O2 radicals that can decompose into OH- radicals, which is highly damaging. In the current study, chicken breast meat tissue from BS-2 group showed significantly higher DPPH, ABTS+, and H2O2 radical scavenging activities than the CON group during 8 days of storage at 4°C, which is suggestive of a higher antioxidant capacity when dietary BS fmbj in broiler diets. Similar to our results, it has been reported that dietary probiotics exerts beneficial effect on the radical scavenging activity in tissue [33].
Chicken breast meat was selected for this study because it is the most economically valuable cut from the bird, and many antioxidative minerals may help to improve the oxidative stability. Oxidative stress occurs when the balance between the production and degradation of ROS, such as hydrogen peroxide and lipid peroxides is disturbed. Level of antioxidant enzymes assay can provide an indication of the antioxidant status of a tissue, and can act as a biomarker of oxidative stress. The body antioxidant defense system is composed of a mixture of antioxidants. Three main antioxidant enzymes: SOD, CAT, and GSH-Px, each with a unique mechanism, are crucial in antioxidant system. They can inhibit the excess ROS build up in muscle tissue and help avoid the oxidative damage to the body. SOD and CAT can directly react with free radicals. SOD can also promote the production of O2 and H2O2 from O2-, which in turn are decomposed to water by GSH-Px and CAT enzymes, thus avoiding the formation of the damaging OH- free radicals. GSH-Px is the most crucial among all antioxidant enzymes in most cells due to its capacity to regenerate oxidized antioxidants, and reduce hydrogen peroxide to water and lipid peroxides to their respective alcohols [34]. GSH is the most powerful intracellular antioxidant, and plays a great role in the detoxification of various peroxides via catalysis by GSH-Px [35]. Total sulfhydryl (T-SH) is the most common structure of many antioxidant enzymes, and has a strong affinity for free radicals, after which resulting a decrease in activity of antioxidant enzymes, and even influence cellular redox metabolic [36]. Previous study suggested that the probiotics exert beneficial effects on improving anti-oxidation and homeostasis [37]. In the present study, prolonged storage time causes oxidative damage as evidenced by increasing the level of oxidative damage index (MDA, ROS, PC, 8-OHdG), decreasing the activity of antioxidant enzymes, and their corresponding gene expressions in both the breast meat tissue and its mitochondria. We report that the antioxidant enzyme activity is significantly increased in the probiotic-treated groups compared to that in the control group with optimum dose of BS fmbj being 0.3 g/kg. Our findings were in agreement with the previous studies, who reported that some probiotics could help in the oxidation resistance, scavenge hydroxyl radical, and increase antioxidant capacity [38,39]. The important components of the antioxidative enzymes are SOD, CAT, GSH-Px, and T-SH contents, which play key roles in endogenous defense mechanism [7]. Probiotics were described as a natural source to enhance the anti-oxidation capacity of animals [33]. Rajput et al. also indicated that supplementation of S. boulardii and B. subtilis B10 could be applied to enhance the antioxidant capacity of broiler chickens [7]. In recent years, increasing attention has been directed to the development of safe and effective functional foods. The probiotic-treated chicken breast meat with an improved antioxidant capacity can be used as the functional food in that regard.
Lipid oxidation is one of major deteriorative processes during food storage, leading to the changes in food quality and production of toxic reaction products. Free radicals, generated by oxidative stress, are crucial in various health disorders including inflammation, ageing, and cancer [40]. Thus, it is important to suppress the oxidative stress and inhibit the formation of free radicals in the body and food produces [41]. The endogenous antioxidant defense mechanism of animals also rely on the external sources, such as the probiotics, which are the natural source to avoid the unfavorable effects caused by the oxidative stress. MDA is one of the most common final products of peroxidation of unsaturated fatty acids in phospholipids and damage cell membranes. Generation of ROS is important for many signal transduction pathways that are required for essential cell functions, however, excess ROS generated by inflammatory cells contributes to host defense mechanisms and tissue damages. The PC assay is widely used to study oxidative protein damage in body. Species able to generate PCs include free radicals and ROS [42]. Oxidative damage is also suggested by the increase of mitochondria 8-OHdG, which is an oxidized nucleoside of DNA. Numerous studies have found that 8-OHdG is not only a biomarker of cellular oxidative stress, but also a factor for diseases, such as cancer and diabetes [43]. Oxidative damage is also suggested by the increases in lipid peroxidation and in 8-OHdG [44]. Under oxidative stress, ROS usually attack the mitochondria membranes leading to the potential damage and increase in MDA contents. The decrease in MMP may be an early event in the oxidative stress, which has been demonstrated to induce depolarization of the transmembrane potential, and loss of oxidative phosphorylation. In the current study, MDA, ROS, PC, and 8-OHdG content were negatively correlated with antioxidant capacity and MMP level. During 8 days of storage at 4°C, chicken breast meat tissue in BS-2 group showed a high meat quality as compared to the control group, which might be explained by the ability of probiotic to provide adequate antioxidants against lipid peroxidation in broiler chickens. There is little study on the effects of dietary BS fmbj in diets and its role in oxidative stability of chicken meat during storage. It has been found that dietary materials with radical scavenging activity can minimize the oxidative instability of lipids, and the protection may be linked to the improved activity of antioxidant enzymes [45]. Ahmed et al. reported a significant relieve in oxidative damage of breast meat in response to the increasing concentrations of dietary probiotics in diets [46]. As a major component of muscle tissue, proteins play an important role in meat regarding the maintenance of cell function. Other components, such as unsaturated lipids, heme pigments, and oxidative enzymes, are potential precursors for the generation of ROS, and play a relevant role in the initiation of protein damage in muscle tissue [47]. During storage, the in vivo antioxidant mechanisms collapsed gradually, and meat proteins are exposed to oxidative stress resulting in the formation of ROS, structural and melting point changes [48–51], and decreasing the antioxidant capacity. Some studies have focused on the modifications of muscle proteins structural and melting point associated with the protein function by the formation of ROS during meat storage [52, 53]. According to previous study, meat proteins can be protected against this oxidative damage during storage through enhancing their antioxidant capacity by dietary additives with antioxidant function [54, 55]. It has been accepted that the oxidative stability of meat can be improved by dietary diets with potential antioxidant, and the protective role of dietary antioxidant would remain during long-term processing of meats [56]. In the present study, we were focused on the dietary effects of BS fmbj on suppressing the oxidative damage during storage and extending the meat preservation time through inhibition of the excessive ROS, and improving their antioxidant capacity. The future study should pay more attention on the structural and melting point changes during meat storage.
Under normal circumstances, cells cannot generate the mitochondria. However, under oxidative stress, cells can activate mitochondrial biogenesis, thereby increasing the proliferation of healthy cells and inhibiting the damaged or inefficient mitochondria [57]. The redox state of mitochondria can influence the chicken performance and its products quality. A study reported that chicken breast meat mitochondria in low performance group could produce high level of ROS, which indicated the tissues mitochondria is indeed involved in the performance of broiler chickens [58]. The mitochondrial biogenesis is mainly associated with the gene expression of PGC-1α, NRF1, NRF2, and TFAM [59]. PGC-1α is one of major factor in regulating mitochondrial biogenesis, and its low expression can directly result in the dysfunction of mitochondria. NRF1 and NRF2 can regulate the gene expression of mitochondria respiratory chain. TFAM is mainly involved in the mitochondrial DNA replication and transcription. PGC-1α can also increase mitochondrial biosynthesis through enhancing the expression of NRF1/2 and TFAM [60]. The avUCP, belonging to mitochondrial transporter protein in broiler chickens, is located on the inner mitochondrial membrane and involved in a variety of physiological functions, including the regulation of ROS [61]. It has been reported that the increasing expression of avUCP in tissues usually lead to the uncoupling of mitochondrial respiratory chain, and reduce the ROS level [62]. In the present study, the oxidative stress is gradually enhanced with the storage time in breast meat, leading to a decreasing gene expression of PGC-1α, NRF1, NRF2, TFAM, and avUCP, which is consistent with the results of ROS content in chicken breast meat. During storage, BS-2 group showed a better antioxidative stress effect among various treatment groups, which may be associated with the relatively high gene expression of PGC-1α, NRF1, NRF2, TFAM, and avUCP, and low ROS content. Further studies are needed to stress on the effects of dietary probiotic on the oxidative stress in muscle tissue. Nonetheless, the results from this study on the effects of probiotics on meat quality and antioxidant capacity during storage are beneficial in industrial applications and therapeutics.
Conclusion
Taken together, our results show that dietary probiotic BS fmbj in broiler diets in addition to enhance the antioxidant capacity of chicken breast meat at 42 day of raising, but also improve the oxidative stability of chicken breast meat during storage through targeting meat quality, free radical scavenging activity, tissue antioxidant enzyme activity, tissue mitochondrial antioxidant enzyme activity, mRNA expression of antioxidant genes and mitochondrial function genes, oxidative damage indexs, and MMP level. Moreover, antioxidant enzyme activity of chicken breast meat tissue in BS-2 group are still relatively higher than those in CON group at the end of storage suggesting that supplementation with BS fmbj at 0.3 g/kg in broiler diets could suppress the oxidative damage in chicken breast meat during storage at 4°C.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry & Cell Biology. 2007, 39 (1), 44–84. [DOI] [PubMed] [Google Scholar]
- 2.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. Journal of Nutritional Biochemistry. 2007, 18 (9), 567–79. 10.1016/j.jnutbio.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 3.Subash A, Adoor Gopalan S, Veeraraghavan G, Ganapathy R, Vasanthi HR. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food & Chemical Toxicology. 2010, 48 (1), 187–192. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M, Sakayori M, Takahashi S, Kato T, Kaji M, Kawahara M, et al. A novel germline mutation of the LKB1 gene in a patient with Peutz-Jeghers syndrome with early-onset gastric cancer. J. Gastroenterol. 2004, 39: 1210–1214. 10.1007/s00535-004-1474-y [DOI] [PubMed] [Google Scholar]
- 5.Bai K, Qiang H, Zhang J, He J, Zhang L, Tian W. Supplemental effects of probiotic bacillus subtilis fmbj on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poultry Science. 2016. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Yan H, Lv L, Xu Q, Yin C, Zhang K, et al. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. AsianAustral. J. Anim. Sci. 2012, 25:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajput IR, Li YL, Xu X, Huang Y, Zhi WC, Yu DY, et al. Supplementary effects of saccharomyces boulardii and bacillus subtilis b10 on digestive enzyme activities, antioxidation capacity and blood homeostasis in broiler. International Journal of Agriculture & Biology. 2013,15(3), 1560–8530. [Google Scholar]
- 8.Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? Journal of Nutrition. 2012, 142 (3), 614S–625S. 10.3945/jn.111.149633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baboota RK, Bishnoi M, Ambalam P, Kondepudi KK, Sarma SM, Boparai RK, et al. Functional food ingredients for the management of obesity and associated co-morbidities–A review. Journal of Functional Foods. 2013, 5 (3), 997–1012. [Google Scholar]
- 10.Pérez-Granados AM, Navas-Carretero S, Schoppen S, Vaquero MP. Reduction in cardiovascular risk by sodium-bicarbonated mineral water in moderately hypercholesterolemic young adults. Journal of Nutritional Biochemistry. 2010, 21 (10), 948–53. 10.1016/j.jnutbio.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 11.Tornberg E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Science. 2005, 70 (3), 493–508. 10.1016/j.meatsci.2004.11.021 [DOI] [PubMed] [Google Scholar]
- 12.Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, et al. Fat deposition, fatty acid composition and meat quality: A review. Meat Science. 2008, 78 (4), 343–358. 10.1016/j.meatsci.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 13.Gray J, Gomaa E, Buckley D. Oxidative quality and shelf life of meats. Meat Science. 1996, 43, 111–123. [DOI] [PubMed] [Google Scholar]
- 14.Chan KM, Decker EA. Endogenous skeletal muscle antioxidants. Critical Reviews in Food Science & Nutrition. 1994, 34 (4), 403–26. [DOI] [PubMed] [Google Scholar]
- 15.Moon JK, Shibamoto T. Antioxidant assays for plant and food components. Journal of Agricultural and Food Chemistry. 2009, 57 (5), 1655–1666. 10.1021/jf803537k [DOI] [PubMed] [Google Scholar]
- 16.Siddhuraju P, Manian S. The antioxidant activity and free radical-scavenging capacity of dietary phenolic extracts from horse gram (macrotyloma uniflorum, (lam.) verdc.) seeds. Food Chemistry. 2007, 105 (3), 950–958. [Google Scholar]
- 17.Hseu YC, Chang WH, Chen CS, Liao JW, Huang CJ, Lu FJ, et al. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food & Chemical Toxicology. 2008, 46 (1), 105–14. [DOI] [PubMed] [Google Scholar]
- 18.Tang X, Gao J, Wang Y, Fan YM, Xu LZ, Zhao XN, et al. Effective protection of Terminalia catappa L. leaves from damage induced by carbon tetrachloride in liver mitochondria. Journal of Nutritional Biochemistry. 2006, 17 (3), 177–182. 10.1016/j.jnutbio.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 19.Wei QY, Chen WF, Zhou B, Yang L, Liu ZL. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochimica Et Biophysica Acta. 2006, 1760 (1), 70–77. 10.1016/j.bbagen.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 20.Liu J. Intrauterine growth retardation increases the susceptibility of pigs to high-fat diet-induced mitochondrial dysfunction in skeletal muscle. Plos One 2012, 7 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelicano ERL, De Souza P, De Souza H, Oba A, Norkus E, Kodawara L, et al. Effect of different probiotics on broiler carcass and meat quality. Revista Brasileira de Ciência Avícola. 2003, 5 (3), 207–214. [Google Scholar]
- 22.Leygonie C, Britz TJ, Hoffman LC. Protein and lipid oxidative stability of fresh ostrich M. Iliofibularis, packaged under different modified atmospheric packaging conditions [J]. Food Chemistry. 2011, 127 (4), 1659–1667. [Google Scholar]
- 23.Park JH, Kim IH. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poultry Science. 2014, 93 (8), 2054–2059. 10.3382/ps.2013-03818 [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Kim IH. The effects of the supplementation of Bacillus subtilis RX7 and B2A strains on the performance, blood profiles, intestinal Salmonella concentration, noxious gas emission, organ weight and breast meat quality of broiler challenged with Salmonella typhimurium. Journal of animal physiology and animal nutrition. 2015, 99 (2), 326–34. 10.1111/jpn.12248 [DOI] [PubMed] [Google Scholar]
- 25.Mahajan P, Sahoo J. Effect of probiotic (Lacto-Sacc) feeding, packaging methods and seasons on the microbial and organoleptic qualities of chicken meat balls during refrigerated storage. Journal of Food Science and Technology (Mysore). 2000, 37 (1), 67–71. [Google Scholar]
- 26.Zhou X, Wang Y. Effect of dietary probiotic, Bacillus coagulans, on growth performance, chemical composition, and meat quality of Guangxi Yellow chicken. Poultry Science. 2010, 89 (3), 588–593. 10.3382/ps.2009-00319 [DOI] [PubMed] [Google Scholar]
- 27.Zhang AW, Lee BD. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poultry Science. 2005, 84 (7), 1015–1021. [DOI] [PubMed] [Google Scholar]
- 28.Endo T, Nakano M. Influence of a Probiotic on Productivity, Meat Components, Lipid Metabolism, Caecal Flora and Metabolites, and Raising Environment in Broiler Production. Nihon Chikusan Gakkaiho. 1999, 70, 207–218. [Google Scholar]
- 29.Wettasinghe M, Shahidi F. Scavenging of reactive-oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chemistry. 2000, 70 (1), 17–26. [Google Scholar]
- 30.Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry. 2007, 105 (3), 940–949. [Google Scholar]
- 31.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology & Medicine. 1996, 20 (7), 933–56. [DOI] [PubMed] [Google Scholar]
- 32.Winterbourn CC, Kettle AJ. Radical–radical reactions of superoxide: a potential route to toxicity. Biochemical & Biophysical Research Communications. 2003, 305 (3), 729–736. [DOI] [PubMed] [Google Scholar]
- 33.Li WF, Huang Q, Li YL, Rajput IR, Huang Y, Hu CH. Induction of probiotic strain Enterococcus faecium EF1 on the production of cytokines, superoxide anion and prostaglandin E2 in a macrophage cell line. Pak. Vet. J. 2012, 32: 530–534 [Google Scholar]
- 34.Sies H. Glutathione and its role in cellular functions. Free Radical Biology & Medicine. 1999, 27 (9–10), 916–921. [DOI] [PubMed] [Google Scholar]
- 35.Fraternale A, Paoletti MF, Casabianca A, Nencioni L, Garaci E, Palamara AT, et al. GSH and analogs in antiviral therapy. Molecular Aspects of Medicine. 2009, 30 (1–2), 99–110. 10.1016/j.mam.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 36.Kayali R, Çakatay U, Uzun H, Genç H. Gender difference as regards myocardial protein oxidation in aged rats: male rats have increased oxidative protein damage. Biogerontology. 2007, 8 (8), 653–61. [DOI] [PubMed] [Google Scholar]
- 37.Sanders ME. Summary of conclusions from a panel of experts on health attributes of lactic cultures: Significance to fluid milk products containing cultures. J. Dairy. Sci. 1993, 76: 1819–1828 10.3168/jds.S0022-0302(93)77514-1 [DOI] [PubMed] [Google Scholar]
- 38.Capcarova M, Weiss J, Hrncar C, Kolesarova A, Pal G. Effect of Lactobacillus fermentum and Enterococcus faecium strains on internal milieu, antioxidant status and body weight of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2010, 94: 215–224 [DOI] [PubMed] [Google Scholar]
- 39.Wen J, Sun JA, Zhou XX, Li WF. Effects of Enterococcus faecium on growth performance, immune and antioxidant function of piglets. Acta Agric. Zhejiang. 2011, 23: 70–73 [Google Scholar]
- 40.Butterfield D, Castegna A, Pocernich C, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. Journal of Nutritional Biochemistry. 2002, 13 (8), 444–461. [DOI] [PubMed] [Google Scholar]
- 41.Halliwell B, Murcia MA, Chirico S, Aruoma OI. Aruoma S. Free radicals and antioxidants in food and in vivo: what they do and how they work. Critical Reviews in Food Science & Nutrition. 1995, 35 (1–2), 7–20. [DOI] [PubMed] [Google Scholar]
- 42.Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochemical Journal. 1997, 324 (Pt 1) (324 (Pt 1)), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilger A, Germadnik D, Riedel K, Megerkossien I, Scherer G, Rüdiger HW. Longitudinal study of urinary 8-hydroxy-2′-deoxyguanosine excretion in healthy adults. Free Radical Research. 2009, 35 (3), 273–80. [DOI] [PubMed] [Google Scholar]
- 44.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, et al. Oxidative DNA Damage in the Parkinsonian Brain: An Apparent Selective Increase in 8-Hydroxyguanine Levels in Substantia Nigra. Journal of Neurochemistry. 1997, 69 (3), 1196–203. [DOI] [PubMed] [Google Scholar]
- 45.Delles RM, Xiong YL, True AD, Ao T, Dawson KA. Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity. Poultry Science. 2014, 93 (6), 1561–1570. 10.3382/ps.2013-03682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed ST, Mun HS. Effects of Citrus junos by-products fermented with multistrain probiotics on growth performance, immunity, caecal microbiology and meat oxidative stability in broilers. British Poultry Science. 2014, 55 (4), 540–547. 10.1080/00071668.2014.938021 [DOI] [PubMed] [Google Scholar]
- 47.Xiong YL. Protein oxidation and implications for muscle foods quality In Decker E. A., Faustman C., Lopez-Bote C. J. (Eds.), Antioxidants in muscle foods (pp. 85–111), 2000. New York: Wiley. [Google Scholar]
- 48.Hosainzadeh A, Gharanfoli M, Saberi M, Chamani J. Probing the interaction of human serum albumin with bilirubin in the presence of aspirin by multi-spectroscopic, molecular modeling and zeta potential techniques: insight on binary and ternary systems. Journal of Biomolecular Structure & Dynamics. 2012, 29(5), 1013–50. [DOI] [PubMed] [Google Scholar]
- 49.Chamani JK, Vahedian-Movahed H, Saberi MR. Lomefloxacin promotes the interaction between human serum albumin and transferrin: a mechanistic insight into the emergence of antibiotic's side effects. Journal of Pharmaceutical & Biomedical Analysis. 2011, 55(1), 114–24. [DOI] [PubMed] [Google Scholar]
- 50.Housaindokht MR, Chamani J, Saboury AA, Moosavi-Movahedi AA, Bahrololoom M. Three binding sets analysis of α-lactalbumin by interaction of tetradecyl trimethyl ammonium bromide. Bulletin- Korean Chemical Society. 2001, 22(2), 145–148. [Google Scholar]
- 51.Moosavi-Movahedi AA, Hakimelahi S, Chamani J, Khodarahmi GA, Hassanzadeh F, Luo FT. Design, synthesis, and anticancer activity of phosphonic acid diphosphate derivative of adenine-containing butenolide and its water-soluble derivatives of paclitaxel with high antitumor activity. Bioorganic & Medicinal Chemistry. 2003, 11(20), 4303–13. [DOI] [PubMed] [Google Scholar]
- 52.Kazemi S, Ngadi MO, Gariépy C. Protein denaturation in pork longissimus muscle of different quality groups. Food and Bioprocess Technology. 2011, 4, 102–106. [Google Scholar]
- 53.Fuentes V, Ventanas J, Morcuende D, Estévez M, Ventanas S. Lipid and protein oxidation and sensory properties of vacuum-packaged dry-cured ham subjected to high hydrostatic pressure. Meat Science. 2010, 85, 506–514. 10.1016/j.meatsci.2010.02.024 [DOI] [PubMed] [Google Scholar]
- 54.Mercier Y, Gatellier Ph, Via M, Remignon H, Renerre M. Effect of fat and vitamin E on colour stability and lipid and protein oxidation in turkey meat during storage. Meat Science. 1998, 48, 301–318. [DOI] [PubMed] [Google Scholar]
- 55.Ventanas S, Estévez M, Tejeda JF, Ruiz J. Protein and lipid oxidation in Longissimus dorsi and dry cured loin from Iberian pigs as affected by crossbreeding and diet. Meat Science. 2006, 72, 647–655. 10.1016/j.meatsci.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 56.Decker E, Faustman C, Lopez-Bote C. Antioxidants in muscle foods. nutritional strategies to improve quality New York, NY: Wiley and Sons, Inc, 2000. [Google Scholar]
- 57.Kowald A, Kirkwood TBL. Evolution of the mitochondrial fusion-fission cycle and its role in aging. Proceedings of the National Academy of Sciences of the United States of America. 2011, 108 (25), 10237–42. 10.1073/pnas.1101604108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bottje W, Iqbal M, Tang ZX, Cawthon D, Okimoto R, Wing T, et al. Association of mitochondrial function with feed efficiency within a single genetic line of male broilers. Poultry Science. 2002, 81 (4), 546–55. [DOI] [PubMed] [Google Scholar]
- 59.Piantadosi CA, Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radical Biology & Medicine. 2012, 53 (11), 2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hota KB, Hota SK, Chaurasia OP, Singh SB. Acetyl- L -carnitine-mediated neuroprotection during hypoxia is attributed to ERK1/2-Nrf2-regulated mitochondrial biosynthesis. Hippocampus. 2012, 22 (4), 723–736. 10.1002/hipo.20934 [DOI] [PubMed] [Google Scholar]
- 61.Dridi S, Onagbesan O, Swennen Q, Buyse J, Decuypere E, Taouis M. Gene expression, tissue distribution and potential physiological role of uncoupling protein in avian species. Comparative Biochemistry & Physiology Part A Molecular & Integrative Physiology. 2004, 139 (3), 273–83. [DOI] [PubMed] [Google Scholar]
- 62.Kikusato M, Toyomizu M. Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. Nucleic Acids Research. 2005, 33 (2), 6953–6960(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.