Abstract
Aging is associated with sarcopenia, which is a loss of skeletal muscle mass and function. Coenzyme Q10 (CoQ10) is involved in several important functions that are related to bioenergetics and protection against oxidative damage; however, the role of CoQ10 as a determinant of muscular strength is not well documented. The aim of the present study was to evaluate the determinants of muscular strength by examining hand grip force in relation to CoQ10 status, gender, age and body mass index (BMI) in two independent cohorts (n = 334, n = 967). Furthermore, peak flow as a function of respiratory muscle force was assessed. Spearman’s correlation revealed a significant positive association between CoQ10/cholesterol level and hand grip in the basic study population (p<0.01) as well as in the validation population (p<0.001). In the latter, we also found a negative correlation with the CoQ10 redox state (p<0.01), which represents a lower percentage of the reduced form of CoQ10 (ubiquinol) in subjects who exhibit a lower muscular strength. Furthermore, the age of the subjects showed a negative correlation with hand grip (p<0.001), whereas BMI was positively correlated with hand grip (p<0.01), although only in the normal weight subgroup (BMI <25 kg/m2). Analysis of the covariance (ANCOVA) with hand grip as the dependent variable revealed CoQ10/cholesterol as a determinant of muscular strength and gender as the strongest effector of hand grip. In conclusion, our data suggest that both a low CoQ10/cholesterol level and a low percentage of the reduced form of CoQ10 could be an indicator of an increased risk of sarcopenia in humans due to their negative associations to upper body muscle strength, peak flow and muscle mass.
Introduction
Sarcopenia is a common problem in the Western world. It is characterized by progressive and generalized loss of skeletal muscle mass and strength with the risk of adverse outcomes, such as physical disability, poor quality of life and death [1,2]. Furthermore, impairment in skeletal muscle function is age-related and associated with a decrease in fiber number and an increase in extramyocyte space [3]. The fiber cross-sectional area and amount of connective tissue undergo significant age-related changes [4].
Coenzyme Q10 (CoQ10) plays a crucial role in mitochondrial bioenergetics, including ATP production [5,6]. Furthermore, CoQ10 could act as an antioxidant, preventing oxidative damage of lipids, proteins and DNA [7,8]. Moreover, CoQ10 has been identified as a modulator of gene expression [9, 10] and is necessary for the function of uncoupling proteins [11] and pyrimidine biosynthesis [12]. The percentage of the oxidized form of CoQ increases with age, indicating a decreased anti-oxidative capacity of aged individuals [13]. On the other hand, we have found that the phenotypic characteristics of senescence in SAMP1 mice can be partly counteracted by supplementation with the reduced form of CoQ10 [14]. Previous reports have documented different contributors to sarcopenia (including TNF-α-dependent apoptotic signaling), which could be potential targets for CoQ10. Hence, pro-apoptotic responses to TNF-α are mediated by activation of the plasma membrane neutral sphingomyelin phosphodiesterase (SMPD2) [15]. Navas and coworkers have previously demonstrated that SMPD activity is regulated by CoQ through noncompetitive inhibition of the enzyme [16,17]. Dietary supplementation with CoQ abolished age-related increases of SMPD activity in the rat liver plasma membrane [18]. Moreover, it was shown that a higher expression of muscle peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a major factor that controls mitochondrial biogenesis and respiration, protects from sarcopenia during aging [19] and that PGC-1α expression was increased in SAMP1 mice by dietary supplementation of reduced CoQ10 (ubiquinol) [20].
In the present study, we aimed to investigate the determinants of muscular strength with a particular focus on CoQ10 in two independent cohorts that compromise a total of n = 1301 subjects. The European Working Group on Sarcopenia in Older People (EWGSOP) has recommended hand grip strength as a robust and simple measure of muscle strength [21]. Furthermore, reduced grip strength has been associated with an increased risk of all-cause and cardiovascular mortality and is even a stronger predictor than systolic blood pressure [22,23]. Thus, in our study, the values for hand grip were correlated with the CoQ10 status, age, BMI and peak expiratory flow (PEF) as a measure of respiratory muscle function, which could also be affected in age-associated alterations in skeletal muscles [24]. Accordingly, the EWGSOP identified PEF as an alternative measure of muscle strength. To evaluate the influence of the variables that predict the outcome of muscular strength independently, analysis of covariance (ANCOVA) was performed with hand grip as the dependent variable. Because cholesterol is the main transport vehicle for CoQ10 in serum [7,25] and cholesterol and CoQ10 share parts of a common synthetic pathway [26], the plasma CoQ10/cholesterol ratio was used in ANCOVA. Additionally in the basic study population, the muscle mass, creatinine content and creatine kinase activity were determined.
Materials and Methods
Participants and Ethical Statement
The basic study population consisted of 334 apparent healthy blood donors and is part of the PopGen control cohort [27,28]. The validation population (n = 967) is part of the FoCus cohort [29]. The participants in this European study collective were recruited in cooperation with the University Hospital Schleswig-Holstein (UKSH), Kiel, Germany as healthy blood donors or in the adiposity ambulance of UKSH. Both populations have been described recently [30] and raw data of participants are given in S1 Table
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all of the procedures that involve human subjects were approved by the ethics committee of the Medical Faculty of the Christian-Albrechts University of Kiel. All participants gave their written informed consent before participation.
CoQ10 and blood parameters
Analysis of ubiquinol-10 and ubiquinone-10 was based on the method of HPLC with electrochemical detection after hexane extraction and has been described before [31]. Ubiquinol-9 and ubiquinone-9 were used as internal standards.
Blood samples were taken from every participant after an overnight fast. Serum concentrations of total cholesterol were measured by an enzymatic colorimetric assay (Hitachi Modular; Roche). Creatinine and creatine kinase were analyzed by standard clinical chemistry.
Physiological measurements
Standing height and weight were measured in light clothing without shoes. Body mass index (BMI) was calculated by dividing the weight (kg) by the square of the height (m2). Total plasma creatinine values were used to calculate the muscle mass in the basic study population [32,33]. Beforehand, the plasma volume was determined by using the equation by Sprenger et al. [34], which considers the height, weight and hematocrit of the participants.
Upper muscular strength (hand grip strength) was characterized with a digital dynamometer (MAP 80K1, Kern, Balingen, Germany). The participants maintained the standard bipedal position during the entire test with the arm in complete extension. Each participant performed the test three times with each hand. The highest value was chosen as the test score for each hand (dominant and non-dominant hand), and an average score for both hands was computed as the bimanual hand grip score according to [8]. Peak flow was measured by having the person exhale as forcefully as possible through the peak flow meter (Mini Wright Standard) after a maximum inspiration.
Statistical analysis
Statistical analysis was performed using IBM SPSS 23.0 software (Armonk, New York, USA) and GraphPad Prism 4.0 package (La Jolla, California, USA). The data are expressed as the mean ± SD, and statistical significance was set at p<0.05. After testing for normality (Kolmogorov-Smirnov), significant differences between sexes were analyzed by a two-sided, unpaired Student’s t-test in the case of normally distributed parameters or by the Mann-Whitney-U-Test in the case of non-normally distributed parameters. Correlation analysis (Spearman’s or Pearson’s correlation, dependent on the distributions of the parameters) was conducted for all of the measured variables in both of the study populations and separately for both genders. Analysis of covariance (ANCOVA) was performed on both cohorts, with “hand grip” as the dependent variable. Gender was taken as an independent factor and BMI, age and CoQ10/cholesterol as independent covariates. Prior to ANCOVA, the different terms were controlled for the significance of interaction.
Results
Characterization of the basic study population
The age, body composition (weight, height, BMI, muscle mass), and biochemical profile, including the CoQ10 status and functional status (hand grip, peak flow), of the study population, separated by gender, are shown in Table 1. The total study population (n = 178 males, n = 156 females) had a mean age of 40.4 ± 11.2 years (age range 19–61 years), and all of the parameters evaluated were within the normal range, including BMI, total CoQ10, CoQ10 redox state (% oxidized CoQ10 in total), peak flow and hand grip. As expected, men had a significantly higher body weight, height, BMI, muscle mass, peak flow and hand grip compared to females. With regard to the biochemical profile, men also showed higher serum levels of total CoQ10, CoQ10/cholesterol, creatine kinase and creatinine.
Table 1. Characterization of the basic study population (n = 334).
| Males (n = 178) | Females (n = 156) | Total (n = 334) | ||||
|---|---|---|---|---|---|---|
| Parameter | r vs. hand gripa | r vs. hand gripa | r vs. hand gripa | |||
| Age (years) | 41.0 ± 10.9 | 0.008 | 39.8 ± 11.5 | −0.258 | 40.4 ± 11.2 | −0.015 |
| p = 0.918 | p<0.01 | p = 0.780 | ||||
| Weight (kg) | 87.6 ± 14.7 | 0.245 | 73.1 ± 14.7*** | 0.271 | 80.8 ± 16.4 | 0.525 |
| p<0.01 | p<0.01 | p<0.001 | ||||
| Height (cm) | 182.1 ± 7.3 | 0.207 | 168.9 ± 6.75*** | 0.242 | 175.9 ± 9.6 | 0.672 |
| p<0.01 | p<0.01 | p<0.001 | ||||
| Muscle massb (kg) | 32.6 ± 5.15 | 0.172 | 19.8 ± 3.92*** | 0.306 | 26.7 ± 7.89 | 0.744 |
| p<0.05 | p<0.001 | p<0.001 | ||||
| Peak flow (L/min) | 525.3 ± 98.5 | 0.316 | 388.2 ± 55.1*** | 0.274 | 461.3 ± 106.2 | 0.525 |
| p<0.001 | p<0.01 | p<0.001 | ||||
| BMI (kg/m2) | 26.4 ± 4.08 | 0.138 | 25.6 ±4.79** | 0.175 | 26.0 ± 4.4 | 0.212 |
| p = 0.067 | p<0.05 | p<0.001 | ||||
| CoQ10 (μg/mL) | 0.978 ± 0.37 | 0.004 | 0.872 ± 0.29** | -0.099 | 0.929 ± 0.34 | 0.103 |
| p = 0.955 | p = 0.217 | p = 0.061 | ||||
| CoQ10 redox (%) | 12.1 ± 2.53 | 0.073 | 12.4 ± 2.20 | −0.067 | 12.3 ± 2.4 | −0.066 |
| p = 0.333 | p = 0.407 | p = 0.232 | ||||
| CoQ10/cholesterol (μmol/mol) | 193.7 ± 53.4 | 0.004 | 177.3 ± 54.1** | -0.025 | 186.0 ± 54.4 | 0.154 |
| p = 956 | p = 0.756 | p<0.01 | ||||
| Cholesterol (mmol/L) | 5.01 ± 1.03 | -0.015 | 4.91 ± 0.77 | -0.148 | 4.96 ± 0.917 | 0.013 |
| p = 0.844 | p = 0.066 | p = 0.812 | ||||
| Creatine kinase (U/L) | 166.1 ± 94.1 | 0.013 | 106.7 ±62.7*** | 0.018 | 138.4 ± 86.2 | 0.365 |
| p = 0.865 | p = 0.827 | p<0.001 | ||||
| Creatinine (mg/dL) | 0.904 ± 0.12 | 0.039 | 0.713 ± 0.11*** | 0.063 | 0.815 ±0.151 | 0.570 |
| p = 0.604 | p = 0.436 | p<0.001 | ||||
| Hand grip Dominant hand (kg) | 52.4 ± 8.63 | 32.5 ± 5.88*** | 43.1 ± 12.4 | |||
Data are presented as the mean ± SD. BMI: body mass index; CoQ10: total Coenzyme Q10; CoQ10 redox: % oxidized CoQ10 in total.
ar = Spearman’s correlation coefficient between the hand grip and the evaluated parameter in the total study population. In the case of a normal distribution (muscle mass, cholesterol), Pearson’s correlation was applied.
*p<0.05
**p<0.01
***p<0.001 significant differences between the sexes, evaluated by the Mann-Whitney-U-Test after testing for normality (Kolmogorov-Smirnov). In case of a normal distribution (muscle mass; cholesterol), Student’s t-test was applied.
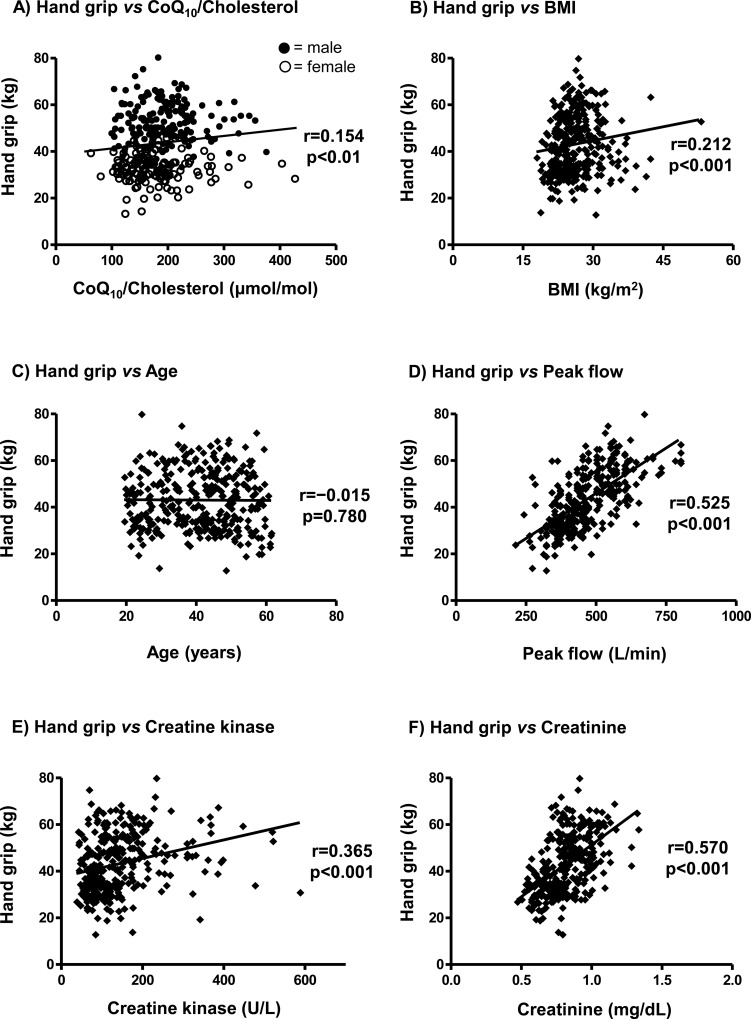
We hypothesized that the selected parameters (as age, BMI and CoQ10 status) are determinants of hand grip, a valid proxy of upper-body muscular strength (Table 1, Fig 1). Correlation analysis of the total study population to hand grip revealed significant positive associations between hand grip and CoQ10/cholesterol (r = 0.154, p<0.01, Fig 1A), BMI (r = 0.212, p<0.001, Fig 1B), peak flow (r = 0.525, p<0.001, Fig 1D), creatine kinase (r = 0.365, p<0.001, Fig 1E) and creatinine (r = 0.570, p<0.001, Fig 1F). The highest correlation coefficient was evident between hand grip and muscle mass (r = 0.744, p<0.001). No significant association was evident between hand grip and age (r = −0.015, p = 0.780, Fig 1C), total CoQ10 (r = 0.103, p = 0.061), CoQ10 redox (r = -0.066, p = 0.232) and total cholesterol (r = 0.013, p = 0.812). Except for the parameters BMI (r = 0.138, p = 0.067) and age (r = 0.008, p = 0.918), which were only significantly correlated to hand grip in females, similar outcomes were found when both genders were correlated separately.
Fig 1. Scatterplots of correlations between hand grip and CoQ10/cholesterol, body mass index, age, peak flow, creatine kinase and creatinine in the basic study population (n = 334).
Spearman’s correlation analysis revealed a significant relationship (p<0.01) between hand grip and CoQ10/cholesterol (A), BMI (B), peak flow (C), creatine kinase (E) and creatinine (F), whereas the correlation between hand grip and age (C) was statistically not significant. Spearman’s correlation coefficient (r), p-values and regression lines are given. CoQ10: Coenzyme Q10; BMI: body mass index.
Characterization of the validation population
To further evaluate the determinants of muscular strength, we validated our results derived from the basic study population on a second, independent cohort. The characteristics of this validation population are given in Table 2. This population consists of 341 males and 626 females for a total of 967 subjects; they exhibited a mean age of 52.6 ± 14.1 years (age range: 20–85 years). Thus, this population was, on average, 10 years older than the basic study population. Furthermore, among the validation population, 468 obese study subjects (BMI >30, mean BMI: 40.8 ± 8.52 kg/m2) were incorporated to provide a broader BMI range. In total, the population showed a mean BMI of 32.2 ± 10.4 kg/m2, which is considered to be obese. The remaining parameters, however, were within the normal range, including the total CoQ10,CoQ10 redox state (oxidized CoQ10 in total) and hand grip. Similarly, males had a significantly higher body weight, height, total CoQ10 levels and hand grip and were on average 6 years older than females.
Table 2. Characterization of the validation population (n = 967), including 658 overweight/obese study subjects.
| Males (n = 341) | Females (n = 626) | Total (n = 967) | ||||
|---|---|---|---|---|---|---|
| Parameter | r vs. hand gripa | r vs. hand gripa | r vs. hand gripa | |||
| Age (years) | 56.1 ± 13.5 | -0.427 | 50.6 ± 14.5*** | -0.416 | 52.6 ± 14.1 | -0.137 |
| p<0.001 | p<0.001 | p<0.001 | ||||
| Weight (kg) | 102.3 ± 33.2 | 0.195 | 92.6 ± 32.1*** | 0.230 | 96.0 ± 32.8 | 0.270 |
| p<0.001 | p<0.001 | p<0.001 | ||||
| Height (cm) | 180.5 ± 8.5 | 0.331 | 168.0 ± 6.7*** | 0.434 | 172.4 ± 9.5 | 0.695 |
| p<0.001 | p<0.001 | p<0.001 | ||||
| BMI (kg/m2) | 31.3 ± 9.0 | 0.115 | 32.8 ± 11.0 | 0.131 | 32.2 ± 10.4 | 0.056 |
| p<0.05 | p<0.01 | p = 0.082 | ||||
| BMI <25 (normal) | 23.2 ± 1.59 | 0.301 | 22.1 ± 2.07 | 0.170 | 22.4 ± 2.01 | 0.170 |
| (n = 88) | p<0.01 | (n = 221) | p<0.01 | (n = 309) | p<0.01 | |
| BMI 25–30 (overweight) | 27.3 ± 1.13 | −0.144 | 27.3 ± 1.19 | −0.110 | 27.3 ± 1.16 | −0.110 |
| (n = 112) | p = 0.131 | (n = 78) | p = 0.131 | (n = 190) | p = 0.131 | |
| BMI >30 (obese) | 39.4 ± 8.68 | -0.044 | 41.4 ± 8.39 | 0.002 | 40.8 ± 8.52 | 0.002 |
| (n = 141) | p = 0.601 | (n = 327) | p = 0.973 | (n = 468) | p = 0.973 | |
| CoQ10 (μg/mL) | 0.852 ± 0.30 | -0.071 | 0.801 ± 0.28** | -0.120 | 0.819 ± 0.29 | -0.002 |
| p = 0.193 | p<0.01 | p = 0.946 | ||||
| CoQ10 redox (%) | 13.4 ± 2.31 | -0.035 | 13.6 ± 2.08* | -0.059 | 13.5 ± 2.17 | -0.082 |
| p = 0.525 | p = 0.143 | p<0.01 | ||||
| CoQ10/cholesterol (μmol/mol) | 193.1 ± 56.0 | -0.088 | 168.8 ± 49.3*** | -0.029 | 177.3 ± 53.0 | 0.131 |
| p = 0.104 | p = 0.476 | p<0.001 | ||||
| Cholesterol (mmol/L) | 4.41 ± 0.887 | 0.046 | 4.76 ± 0.989 | -0.207 | 4.63 ± 0.968 | -0.185 |
| p = 0.393 | p<0.001 | p<0.001 | ||||
| Hand grip Dominant hand (kg) | 42.5 ± 9.4 | 25.0 ± 6.4*** | 31.2 ± 11.3 | |||
Data are presented as the mean ± SD. BMI: body mass index; CoQ10: total Coenzyme Q10; CoQ10 redox: % oxidized CoQ10 in total.
ar = Spearman’s correlation coefficient between the hand grip and the evaluated parameter in the total study population. In the case of a normal distribution (cholesterol), Pearson’s correlation was applied.
*p<0.05
**p<0.01
***p<0.001 significant differences between the sexes, evaluated by the Mann-Whitney U-Test after testing for normality (Kolmogorov-Smirnov). In the case of a normal distribution (hand grip; cholesterol), Student’s t-test was applied.
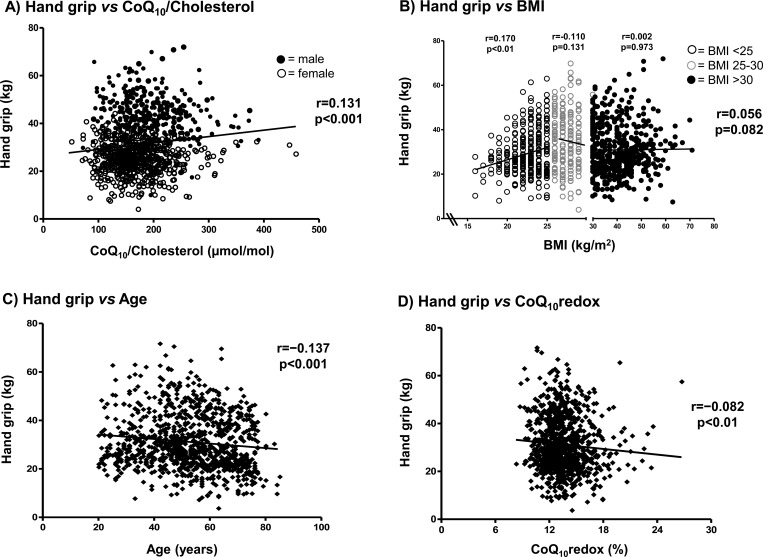
To substantiate our results in the basic study population, we correlated the parameters that were available with the values of the hand grip measurements (Table 2, Fig 2). Accordingly, correlation analysis revealed a significant positive association between hand grip and the CoQ10/cholesterol ratio (r = 0.131, p<0.001, Fig 2A) and a negative significant association to cholesterol itself (r = -0.185, p<0.001). Hand grip correlated with BMI in the total validation population and revealed no significant relationship (r = 0.056, p = 0.082). However, when we examined the BMI classes separately (Fig 2B), we found a significant correlation in subjects who exhibited a normal BMI (<25 kg/m2, r = 0.170, p<0.01), but not in overweight subjects (BMI 25–30 kg/m2, r = -0.110, p = 0.131) or obese subjects (BMI >30, r = 0.002, p<0.973). Furthermore, hand grip was negatively correlated with age (r = -0.137, p<0.001, Fig 2C) and the CoQ10 redox state (r = -0.082, p<0.01, Fig 2D), which represents a lower percentage of the reduced form of CoQ10 (ubiquinol) in subjects who exhibited lower muscular strength. In the validation population, similar outcomes were found when males and females were correlated separately with hand grip, with the exception of total CoQ10 (r = -0.071, p = 0.193) and cholesterol (r = -0.046, p = 0.393), which were only significantly correlated in females.
Fig 2. Scatterplots of correlations between hand grip and CoQ10/cholesterol ratio, body mass index, age and CoQ10 redox in the validation population (n = 967).
Spearman’s correlation analysis revealed a significant relationship (p<0.01) between hand grip and CoQ10/cholesterol (A), normal BMI (<25 kg/m2, B), age (C) and CoQ10 redox (D). The correlations between hand grip and overweight (BMI 25–30 kg/m2) and obese subjects (BMI >30) were statistically not significant. Spearman’s correlation coefficient (r), p-values and regression lines are given. CoQ10: Coenzyme Q10; CoQ10 redox: % oxidized coenzyme Q10 in total; BMI: body mass index.
CoQ10 status and muscular strength stratified according to Gender, Age and BMI
To independently evaluate the influence of the variables that predict the outcome of the upper body muscular strength (hand grip) in the basic study population, analysis of covariance (ANCOVA) was performed with hand grip as the dependent variable. In a model, including the gender of the study subjects as the independent factor and CoQ10/cholesterol, age and BMI as the independent covariates, gender was found to be the main effector (p<0.001, partial η2 = 0.636, data not shown). Next, we analyzed the remaining variables independent of gender, which revealed a significant effect on hand grip according to BMI (p<0.05, η2 = 0.017), whereas CoQ10/cholesterol (p = 0.099) and age (p = 0.563) were not significant predictors of hand grip in this study population (Table 3A).
Table 3. Analysis of covariance (ANCOVA) between hand grip, CoQ10/cholesterol ratio, age and BMI in A) the basic study population (n = 334) and B) the validation population (n = 967), including 658 overweight/obese subjects.
| Source | Type III sum of Squares | df | F | Significance | Partial η2 |
|---|---|---|---|---|---|
| A) Basic study population | |||||
| Corrected Model | 1,477a | 3 | 3.24 | 0.022 | 0.029 |
| Intercept | 6491 | 1 | 42.7 | <0.001 | 0.115 |
| CoQ10/cholesterol | 416 | 1 | 2.74 | 0.099 | 0.008 |
| Age | 50.9 | 1 | 0.336 | 0.563 | 0.001 |
| BMI | 872 | 1 | 5.74 | 0.017 | 0.017 |
| B) Validation population | |||||
| Corrected Model | 4,049b | 3 | 10.8 | <0.001 | 0.033 |
| Intercept | 28,617 | 1 | 230.1 | <0.001 | 0.193 |
| CoQ10/cholesterol | 2,266 | 1 | 18.2 | <0.001 | 0.019 |
| Age | 2164 | 1 | 17.4 | <0.001 | 0.018 |
| BMI | 2.13 | 1 | 0.017 | 0.896 | <0.001 |
aR2 = 0.033 (Adjusted R2 = 0.030), dependent variable: hand grip, independent covariate: age, CoQ10/cholesterol, BMI
bR2 = 0.029 (Adjusted R2 = 0.020), dependent variable: hand grip, independent covariate: age, CoQ10/cholesterol, BMI
BMI = body mass index; df = degrees of freedom, F = variance ratio
Thus, we intended to test our hypothesis in an independent validation study population, which was characterized, on average, by a higher number of subjects, higher mean age and higher mean BMI values. When we analyzed the influence of the variables that predict the outcome of upper body muscular strength (hand grip, dependent factor) in ANCOVA with gender as an independent factor and CoQ10/cholesterol and BMI as independent covariates, gender was found to be the strongest effector of hand grip (p<0.001, η2 = 0.601, data not shown). However, when we analyzed the remaining variables independent of gender, we found a significant effect on hand grip by CoQ10/cholesterol (p<0.001, η2 = 0.019) and age (p<0.001, η2 = 0.018), whereas in this study population, which is characterized by a higher percentage of obese subjects, BMI was no longer a significant predictor of hand grip (p = 0.896, Table 3B).
Discussion
Aging is associated with sarcopenia, which is a loss of skeletal muscle mass and function [35]. CoQ10 plays a crucial role in mitochondrial bioenergetics and can act as an antioxidant [7,36]. However, CoQ10 as a determinant of muscular strength is not well documented. Therefore, this study is, to the best of our knowledge, the first to examine the determinants of muscular strength with a special focus on the serum CoQ10 status in two independent cohorts. We showed that the CoQ10/cholesterol ratio was significantly correlated with a lower hand grip and a predictor of hand grip outcome. Gender was found to be the main influencing factor. Age was also significantly negatively correlated with muscle strength, but only in older subjects (males and females) of the validation population, which exhibited a mean age of 52.6 ± 14.1 years in males or 39.8 ± 11.5 years in females, of the basic study population. Interestingly, the positive association between hand grip and BMI was dependent on the BMI classes because we found a significant correlation in the subjects of the validation population who exhibited a normal BMI, but not in overweight or obese subjects (Fig 2B). This finding was irrespective of gender. We conclude that in a normal BMI range, an increase in BMI equals an increase in muscle mass, whereas in obese subjects (BMI>30), an increase in BMI possibly equals an increase in fat mass and hence does not necessarily lead to an increase in muscle strength. In our basic study population, which had a mean BMI of 26.0 ± 4.4, BMI was significantly correlated with hand grip strength (Fig 1B) and also with muscle mass (r = 0.205, p<0.001), which agrees with our hypothesis. Similarly, in a population of elderly women, higher BMI values were associated with a greater probability of a functional limitation [37]. Here, the isometric leg strength was significantly lower in subjects with sarcopenia and sarcopenic obesity. In an observational cross-sectional study, older men and women with weak muscle strength and higher BMI had considerably poorer performance than others [38]. The authors suggested a very likely benefit of early assessment and interventions to reduce fat mass and improve muscle strength in the prevention of future functional limitations.
The relationship of obesity and health and CoQ10 as a major lipophilic antioxidant and mitochondrial respiratory chain redox coupler [39] is currently a matter of debate. Recently, the impact of aging, BMI and physical capacity on the CoQ10 levels in human blood was investigated [8]. In a cohort of community-dwelling people (n = 43, mean age = 71.01 ± 6.22 years), the impact of hand grip measurements and further parameters of physical activity on the CoQ10 plasma level was studied. The authors found that people who exhibited higher levels of functional capacity presented lower levels of cholesterol, which was accompanied by higher levels of CoQ10 in plasma. On the other hand, obesity was related to lower CoQ10 levels. They concluded that physical activity at advanced ages can increase the levels of CoQ10 and reduce the levels of lipid peroxidation in plasma, probably reducing the progression of cardiovascular diseases. In accordance with these findings, in our validation population, cholesterol itself as well as the CoQ10 redox state were significantly negative correlated with hand grip. For the observed lower level of CoQ10/cholesterol in the validation population, we cannot fully rule out that it could be partly caused by normalization to the significantly different cholesterol level. Hence, we showed that in this older and obese study group, the subjects who exhibited a lower level of muscular strength presented a higher level of cholesterol and a lower percentage of reduced CoQ10 (ubiquinol). In this context, it is worthwhile to mention that physical activity has been shown to generate reactive oxygen species (ROS) [40]; however, on the other hand, it has also been demonstrated to be a preventive mechanism against oxidative stress. Different training degrees have been suggested to be beneficial in humans by enhancing the antioxidant capacity [41]. Importantly, it has also been shown by Ristow and coworkers [42] that dietary antioxidants could counteract the health-promoting effects of physical exercise due to a suppression of endogenous antioxidant defense mechanisms (the so-called “hormesis” effect). In addition to upper body muscular strength (hand grip), we also indicated that peak expiratory flow (PEF) is a function of respiratory muscle, which could also be affected in age-associated alterations in skeletal muscles, which has been shown to be correlated with hand grip strength [24]. In a study comprising data from 960 older individuals, pulmonary function was suggested to partially account for the association of muscle strength and mortality [43]. In the present study, PEF was also significantly correlated with hand grip strength in the basic study population. Furthermore, when we correlated CoQ10/cholesterol versus PEF, a significant Spearman correlation coefficient (r = 0,138, p<0.05) was evident (data not shown). Similarly, CoQ10/cholesterol was significantly correlated with muscle mass (r = 0.1425, p<0.01).
CoQ10 supplementation increases plasma levels of CoQ10 [44–46]. Nevertheless, short-term dosing of CoQ10 in middle-aged, untrained men did not improve the aerobic capacity of forearm exercise metabolism [47]. On the other hand, a placebo controlled study with supplementation of 300 mg of ubiquinol for 6 weeks significantly enhanced physical performance, which was measured as the maximum power output in young healthy trained Olympic athletes [48]. A systematic review to analyze the influence and effect of CoQ10 supplementation on parameters related to exercise in healthy humans revealed that CoQ10 has the potential to be used as a nutritional supplement to improve exercise capacity; however, the current literature shows a disparity and inconsistency, possibly due to differences in the CoQ10 formulations used, dosage, timing of the supplement, exercise tests performed and study participants [49].
In conclusion, the present data suggest that gender and CoQ10/cholesterol are determinants of muscular strength, with gender being the main influencing factor. In older subjects, age is an additional inverse determinant of muscular strength, whereas the body mass of a person ascertains muscular strength only when in the normal range. A lower CoQ10/cholesterol level could be a predictor of an increased risk of sarcopenia in humans due to its association to upper body muscle strength, peak flow and muscle mass. The present study provides further evidence that a higher muscular strength or physical activity in risk groups, including older and obese subjects, could lead to a more a favored outcome in the cholesterol metabolism, CoQ10 and CoQ10 redox status.
Supporting Information
Raw data of the A) Basic study population and the B) Validation population.
(XLSX)
Acknowledgments
We thank all of the participants of the cohorts for their invaluable contribution to the study. We are also very grateful to all of those who were involved in this comprehensive data collection. Furthermore, the excellent statistical support of Mario Hasler is also gratefully acknowledged.
Data Availability
Due to ethical restrictions imposed by the Ethic Committee of the Medical Faculty of the Christian-Albrechts University of Kiel as well as legal restrictions imposed by German legislation, all data cannot be made publicly available. A minimal dataset is provided as a supplemental file. Further data is available upon request.
Funding Statement
Data from the basic study population presented in this paper derived from the PopGen control cohort, a population-based sample of mainly Caucasian individuals, identified through official population registries in Kiel (Germany). Participants were recruited into the PopGen biobank between June 2005 and February 2006. The study was funded by the Federal Ministry for Education and Research (01EY1103). Subjects of the validation population were randomly recruited via registry office of Kiel, Germany or via the adiposity ambulance of UKSH, Kiel, Germany. Members are part of the Focus Cohort, which was supported by a grant of the Federal Ministry for Education and Research (0315540A). Determining and analyzing the CoQ10 status was financially supported by Kaneka Corporation, Japan. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. Journal of the American Geriatrics Society. 2007;55(5):769–74. Epub 2007/05/12. 10.1111/j.1532-5415.2007.01140.x [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2006;61(10):1059–64. Epub 2006/11/02. [DOI] [PubMed] [Google Scholar]
- 3.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Experimental Gerontology. 2010;45(2):138–48. Epub 2009/11/12. 10.1016/j.exger.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Experimental Gerontology. 2008;43(4):317–29. Epub 2008/03/04. 10.1016/j.exger.2007.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentinger M, Tekle M, Dallner G. Coenzyme Q—biosynthesis and functions. Biochemical and Biophysical Research Communications. 2010;396(1):74–9. 10.1016/j.bbrc.2010.02.147 [DOI] [PubMed] [Google Scholar]
- 6.Chai W, Novotny R, Maskarinec G, Le Marchand L, Franke AA, Cooney RV. Serum coenzyme Q(1)(0), alpha-tocopherol, gamma-tocopherol, and C-reactive protein levels and body mass index in adolescent and premenopausal females. Journal of the American College of Nutrition. 2014;33(3):192–7. Epub 2014/05/09. 10.1080/07315724.2013.862490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Molecular Biotechnology. 2007;37(1):31–7. Epub 2007/10/05. [DOI] [PubMed] [Google Scholar]
- 8.Del Pozo-Cruz J, Rodriguez-Bies E, Navas-Enamorado I, Del Pozo-Cruz B, Navas P, Lopez-Lluch G. Relationship between functional capacity and body mass index with plasma coenzyme Q10 and oxidative damage in community-dwelling elderly-people. Experimental Gerontology. 2014;52:46–54. Epub 2014/02/12. 10.1016/j.exger.2014.01.026 [DOI] [PubMed] [Google Scholar]
- 9.Fischer A, Klapper M, Onur S, Menke T, Niklowitz P, Doring F. Dietary restriction decreases coenzyme Q and ubiquinol potentially via changes in gene expression in the model organism C. elegans. Biofactors. 2015;41(3):166–74. Epub 2015/05/06. 10.1002/biof.1210 [DOI] [PubMed] [Google Scholar]
- 10.Schmelzer C, Doring F. Identification of LPS-inducible genes downregulated by ubiquinone in human THP-1 monocytes. Biofactors. 2010;36(3):222–8. 10.1002/biof.93 [DOI] [PubMed] [Google Scholar]
- 11.King MS, Sharpley MS, Hirst J. Reduction of hydrophilic ubiquinones by the flavin in mitochondrial NADH:ubiquinone oxidoreductase (Complex I) and production of reactive oxygen species. Biochemistry. 2009;48(9):2053–62. 10.1021/bi802282h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traut TW, Payne RC, Jones ME. Dependence of the aggregation and conformation states of uridine 5'-phosphate synthase on pyrimidine nucleotides. Evidence for a regulatory site. Biochemistry. 1980;19(26):6062–8. [DOI] [PubMed] [Google Scholar]
- 13.Onur S, Niklowitz P, Fischer A, Metges CC, Grune T, Menke T, et al. A comparative study into alterations of coenzyme Q redox status in ageing pigs, mice, and worms. Biofactors. 2014;40(3):346–54. Epub 2014/03/01. 10.1002/biof.1160 [DOI] [PubMed] [Google Scholar]
- 14.Schmelzer C, Kubo H, Mori M, Sawashita J, Kitano M, Hosoe K, et al. Supplementation with the reduced form of Coenzyme Q10 decelerates phenotypic characteristics of senescence and induces a peroxisome proliferator-activated receptor-alpha gene expression signature in SAMP1 mice. Molecular Nutrition and Food Research. 2010;54(6):805–15. 10.1002/mnfr.200900155 [DOI] [PubMed] [Google Scholar]
- 15.Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, et al. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mechanisms of Ageing and Development. 2009;130(4):272–80. Epub 2009/04/29. 10.1016/j.mad.2008.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navas P, Manuel Villalba J. Regulation of ceramide signaling by plasma membrane coenzyme Q reductases. Methods in Enzymology. 2004;378:200–6. Epub 2004/03/25. 10.1016/S0076-6879(04)78016-7 [DOI] [PubMed] [Google Scholar]
- 17.Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7 Suppl:S34–40. Epub 2007/05/08. [DOI] [PubMed] [Google Scholar]
- 18.Bello RI, Gomez-Diaz C, Buron MI, Navas P, Villalba JM. Differential regulation of hepatic apoptotic pathways by dietary olive and sunflower oils in the aging rat. Experimental Gerontology. 2006;41(11):1174–84. Epub 2006/10/20. 10.1016/j.exger.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 19.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proceedings of the National Academy of Science U S A. 2009;106(48):20405–10. Epub 2009/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Tian G, Sawashita J, Kubo H, Nishio SY, Hashimoto S, Suzuki N, et al. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxidants and Redox Signaling. 2014;20(16):2606–20. Epub 2013/10/16. 10.1089/ars.2013.5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and Ageing. 2010;39(4):412–23. Epub 2010/04/16. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr., Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–73. Epub 2015/05/20. 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
- 23.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2002;57(10):B359–65. Epub 2002/09/21. [DOI] [PubMed] [Google Scholar]
- 24.Bahat G, Tufan A, Ozkaya H, Tufan F, Akpinar TS, Akin S, et al. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. The Aging Male: The Official Journal of the International Society for the Study of the Aging Male. 2014;17(3):136–40. Epub 2014/07/06. [DOI] [PubMed] [Google Scholar]
- 25.Kalyan S, Huebbe P, Esatbeyoglu T, Niklowitz P, Cote HC, Rimbach G, et al. Nitrogen-bisphosphonate therapy is linked to compromised coenzyme Q10 and vitamin E status in postmenopausal women. The Journal of Clinical Endocrinology and Metabolism. 2014;99(4):1307–13. Epub 2014/01/16. 10.1210/jc.2013-3648 [DOI] [PubMed] [Google Scholar]
- 26.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochimica et Biophysica Acta. 1995;1271(1):195–204. Epub 1995/05/24. [DOI] [PubMed] [Google Scholar]
- 27.Krawczak M, Nikolaus S, von Eberstein H, Croucher PJ, El Mokhtari NE, Schreiber S. PopGen: population-based recruitment of patients and controls for the analysis of complex genotype-phenotype relationships. Community Genetics. 2006;9(1):55–61. 10.1159/000090694 [DOI] [PubMed] [Google Scholar]
- 28.Nothlings U, Krawczak M. [PopGen. A population-based biobank with prospective follow-up of a control group]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(6–7):831–5. Epub 2012/06/28. PopGen. Eine populationsbasierte Biobank mit Langzeitverfolgung der Kontrollkohorte. 10.1007/s00103-012-1487-2 [DOI] [PubMed] [Google Scholar]
- 29.Müller N, Türk K, Freitag-Wolf S, Hampe J, Zeuner, Schröder JO, et al. Interleukin-6 blockade by monoclonal antibodies as potential therapeutic option to treat elevated lipoprotein(a) serum levels in human subjects. Journal of Lipid Research 2015;56(5):1034–42. Epub 2015/02/21. 10.1194/jlr.P052209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onur S, Niklowitz P, Fischer A, Jacobs G, Lieb W, Laudes M, et al. Determination of the coenzyme Q10 status in a large Caucasian study population. Biofactors. 2015;41(4):211–21. Epub 2015/08/01. 10.1002/biof.1216 [DOI] [PubMed] [Google Scholar]
- 31.Menke T, Niklowitz P, Adam S, Weber M, Schluter B, Andler W. Simultaneous detection of ubiquinol-10, ubiquinone-10, and tocopherols in human plasma microsamples and macrosamples as a marker of oxidative damage in neonates and infants. Analytical Biochemistry. 2000;282(2):209–17. Epub 2000/06/30. 10.1006/abio.2000.4579 [DOI] [PubMed] [Google Scholar]
- 32.Schutte JE, Longhurst JC, Gaffney FA, Bastian BC, Blomqvist CG. Total plasma creatinine: an accurate measure of total striated muscle mass. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1981;51(3):762–6. Epub 1981/09/01. [DOI] [PubMed] [Google Scholar]
- 33.Lukaski HC. Methods for the assessment of human body composition: traditional and new. The American Journal of Clinical Nutrition. 1987;46(4):537–56. Epub 1987/10/01. [DOI] [PubMed] [Google Scholar]
- 34.Sprenger KB, Huber K, Kratz W, Henze E. Nomograms for the prediction of patient's plasma volume in plasma exchange therapy from height, weight, and hematocrit. Journal of Clinical Apheresis. 1987;3(3):185–90. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 35.Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. Journal of the American Geriatrics Society. 2010;58(11):2055–62. Epub 2010/11/09. 10.1111/j.1532-5415.2010.03145.x [DOI] [PubMed] [Google Scholar]
- 36.Acosta MJ, Vazquez Fonseca L, Desbats MA, Cerqua C, Zordan R, Trevisson E, et al. Coenzyme Q biosynthesis in health and disease. Biochimica et Biophysica Acta. 2016. Epub 2016/04/10. [DOI] [PubMed] [Google Scholar]
- 37.Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A, Guariento S, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004;28(2):234–41. Epub 2004/01/07. [DOI] [PubMed] [Google Scholar]
- 38.Hardy R, Cooper R, Aihie Sayer A, Ben-Shlomo Y, Cooper C, Deary IJ, et al. Body mass index, muscle strength and physical performance in older adults from eight cohort studies: the HALCyon programme. PLOS One. 2013;8(2):e56483 Epub 2013/02/26. 10.1371/journal.pone.0056483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenfeldt FL, Pepe S, Linnane A, Nagley P, Rowland M, Ou R, et al. Coenzyme Q10 protects the aging heart against stress: studies in rats, human tissues, and patients. Annals of the New York Academy of Sciences. 2002;959:355–9; discussion 463–5. Epub 2002/04/27. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Molecular and Cellular Biochemistry. 2003;253(1–2):307–12. Epub 2003/11/19. [DOI] [PubMed] [Google Scholar]
- 41.Corbi G, Conti V, Russomanno G, Rengo G, Vitulli P, Ciccarelli AL, et al. Is physical activity able to modify oxidative damage in cardiovascular aging? Oxidative Medicine and Cellular Longevity. 2012;2012:728547 Epub 2012/10/03. 10.1155/2012/728547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Science U S A. 2009;106(21):8665–70. Epub 2009/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchman AS, Boyle PA, Wilson RS, Gu L, Bienias JL, Bennett DA. Pulmonary function, muscle strength and mortality in old age. Mechanisms of Ageing and Development. 2008;129(11):625–31. Epub 2008/08/30. 10.1016/j.mad.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regulatory Toxicology and Pharmacology: RTP. 2007;47(1):19–28. Epub 2006/08/22. 10.1016/j.yrtph.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 45.Yoritaka A, Kawajiri S, Yamamoto Y, Nakahara T, Ando M, Hashimoto K, et al. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson's disease. Parkinsonism and Related Disorders. 2015;21(8):911–6. Epub 2015/06/10. 10.1016/j.parkreldis.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 46.Schmelzer C, Niklowitz P, Okun JG, Haas D, Menke T, Doring F. Ubiquinol-induced gene expression signatures are translated into altered parameters of erythropoiesis and reduced low density lipoprotein cholesterol levels in humans. International Union of Biochemistry and Molecular Biology Life. 2011;63(1):42–8. 10.1002/iub.413 [DOI] [PubMed] [Google Scholar]
- 47.Porter DA, Costill DL, Zachwieja JJ, Krzeminski K, Fink WJ, Wagner E, et al. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. International Journal of Sports Medicine. 1995;16(7):421–7. Epub 1995/10/01. 10.1055/s-2007-973031 [DOI] [PubMed] [Google Scholar]
- 48.Alf D, Schmidt ME, Siebrecht SC. Ubiquinol supplementation enhances peak power production in trained athletes: a double-blind, placebo controlled study. Journal of the International Society of Sports Nutrition. 2013;10:24 Epub 2013/05/01. 10.1186/1550-2783-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarmiento A, Diaz-Castro J, Pulido-Moran M, Kajarabille N, Guisado R, Ochoa JJ. Coenzyme Q10 Supplementation and Exercise in Healthy Humans: A Systematic Review. Current Drug Metabolism. 2016;17(4):345–58. Epub 2015/11/04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data of the A) Basic study population and the B) Validation population.
(XLSX)
Data Availability Statement
Due to ethical restrictions imposed by the Ethic Committee of the Medical Faculty of the Christian-Albrechts University of Kiel as well as legal restrictions imposed by German legislation, all data cannot be made publicly available. A minimal dataset is provided as a supplemental file. Further data is available upon request.