Abstract
Despite recent efforts to sample broadly across metazoan and insect diversity, current sequence resources in the Coleoptera do not adequately describe the diversity of the clade. Here we present deep, staged transcriptomic data for two coleopteran species, Atrachya menetriesi (Faldermann 1835) and Callosobruchus maculatus (Fabricius 1775). Our sampling covered key stages in ovary and early embryonic development in each species. We utilized this data to build combined assemblies for each species which were then analysed in detail. The combined A. menetriesi assembly consists of 228,096 contigs with an N50 of 1,598 bp, while the combined C. maculatus assembly consists of 128,837 contigs with an N50 of 2,263 bp. For these assemblies, 34.6% and 32.4% of contigs were identified using Blast2GO, and 97% and 98.3% of the BUSCO set of metazoan orthologs were present, respectively. We also carried out manual annotation of developmental signalling pathways and found that nearly all expected genes were present in each transcriptome. Our analyses show that both transcriptomes are of high quality. Lastly, we performed read mapping utilising our timed, stage specific RNA samples to identify differentially expressed contigs. The resources presented here will provide a firm basis for a variety of experimentation, both in developmental biology and in comparative genomic studies.
Introduction
The order Coleoptera is the most speciose clade of animals currently known. Despite the best efforts of generations of biologists, its species are only sparsely sampled and are yet to be comprehensively described, with approximately 90% of coleopteran diversity as yet uncategorized (e.g. [1,2]). A similar discrepancy exists at the molecular level; while several genomic resources are available in this clade, their number and phylogenetic distribution is only just beginning to accurately sample that of the Coleoptera as a whole. A wide range of transcriptomic data is available in whole organisms (for example [3–5], among others), specific body parts (such as [6,7]), and in several cases for staged embryos following RNA interference-mediated gene knock-down [8–10]. The i5K project [11] will also greatly advance our knowledge of the genomic complement of Coleopterans, with 69 species of this Order listed on that database as nominated for genomic sequencing as of the 07/04/16 (url: http://arthropodgenomes.org/wiki/i5K_nominations) and several genomes publically available [12–14]. However, the majority of this information is largely still outside of the public domain, the Coleoptera are still relatively undersampled compared to the Diptera and Hymenoptera, and, in particular, timed embryonic resources (e.g. [15]) are rare in the literature.
The true phylogeny of the Coleoptera is still under investigation but, in general, four suborders, 17 superfamilies, and 168 families are recognised [16]. The structure of the coleopteran clade can be seen summarised in Fig 1A. Coleopterans have long been used for research into embryology, and in the pre-molecular era, the Chrysomelidae (summarised phylogeny shown in Fig 1B) was one of the best studied superfamilies. For example, the first functional embryonic experiments in any insect were carried out in the Colorado potato beetle, Leptinotarsa decemlineata (sub-family Chrysomelinae, see Fig 1B), leading to the discovery of the function of pole cells and the existence of germ plasm in insects [17,18]. Further, the larval cuticle preparation technique, so vital for arthropod developmental biology, was first perfected in the bean beetle, Callosobruchus maculatus [19]. Chrysomelid beetles are also interesting from an ecological and economic viewpoint, as members of the group are usually pest species, perhaps most famously the aforementioned Colorado potato beetle, which is a major pest of potato crops in America, Asia and Europe [20].
Fig 1. Phylogenetic positions of A. menetriesi and C. maculatus.
A) Cladogram of Coleoptera simplified from that determined by [16]. Black asterisk indicates the superfamily in which the Chrysomelidae are located. B) Cladogram of Chrysomelidae simplified from that determined by [21]. Black and grey asterisks indicate the sub-families in which A. menetriesi and C. maculatus are located (respectively). C) A. menetriesi and D) C. maculatus adults.
The Chrysomelidae are represented in public databases by a number of ongoing transcriptomic and genomic projects. In particular, both L. decemlineata (Bioproject PRJNA171749) and C. maculatus [22] are the subject of ongoing genomic sequencing. However, while a number of transcriptomes are planned or published in this clade (e.g. [5,6,23–27]) none yet sample across embryonic time points in a fashion which would allow insight into the genetic mechanisms behind key developmental stages or deep sampling of developmentally important genes. Here we present transcriptomic sequences for two species of chrysomelid beetle, the false melon beetle, Atrachya menetriesi, and the aforementioned C. maculatus (each of which are pictured along with their relative phylogenetic positions in Fig 1).
Compared to C. maculatus, the false melon beetle A. menetriesi (Faldermann), is a relatively unknown and understudied species. It is native to Japan, where it is an agricultural pest, and feeds on a variety of plants such as clover and lettuce. Although there has only been a small amount of research carried out on this beetle, the work that has been done has highlighted several interesting developmental traits, including the possibility of generating twin embryos after egg bisection [28], or up to four, seemingly complete, embryos following treatment with low temperatures [28,29]. Another interesting trait exhibited by this beetle is that almost all eggs enter diapause at a certain stage, and this is only broken in the wild by winter conditions. However, a small proportion of eggs skip this diapause and continue to adulthood. The ratio of diapause to non-diapause eggs varies in different parts of Japan [30], and is a heritable trait [31]. Further, A. menetriesi embryos undergo a very short germ band mode of development [32], contrasting strongly with beetles such as C. maculatus (see below). As the last common ancestor of these two species is estimated to have existed only 80 million years ago [21], how their developmental mechanisms have diverged so greatly is a potentially fascinating area for future study. Research on these topics would greatly benefit from modern molecular studies.
C. maculatus (Fabricius) is native to West Africa [33] but is now found worldwide, and is a common pest of stored legumes. It is also known as the southern cowpea weevil, however, it is not a true weevil. As noted above, this beetle was the focus of active developmental research in the pre-molecular era, with special focus on segmentation [34,35]. Segmentation in C. maculatus has also been studied more recently via immunohistochemistry for the even-skipped protein [36]. This recent work confirmed previous reports that the embryos undergo the long germ mode of development, similar to dipterans like Drosophila melanogaster and hymenopterans like Nasonia vitripennis [37]. It is commonly believed that the long germ mode of development evolved independently in dipterans and hymenopterans, and given the phylogenetic distribution of short and intermediate germ development within the Coleoptera [38,39], it seems likely that the long germ mode of development also evolved independently in the clade to which C. maculatus belongs. A comparison of the molecular basis of development in C. maculatus and other long germ insects, plus with more closely related species that feature short germ development, such as well studied flour beetle Tribolium castaneum (super-family Tenebrionoidea, see Fig 1A) and A. menetriesi, would yield crucial information on how developmental pathways have evolved to generate the long germ mode of development. C. maculatus has also been studied in other fields and is a useful system for undergraduate lab teaching [22] which could be aided further with deeper sequence resources.
In order to facilitate research on A. menetriesi and C. maculatus, as well as wider investigations in the Coleoptera and beyond, we present here deep, multi-stage transcriptomic resources from a range of key time points in the development of these two beetle species. Using RSEM-based methods we have compared transcript abundance across these life stages, which will allow the investigation of genes that play key roles in developmental changes in these species, particularly at the maternal-zygotic transition. We have carried out extensive searches for key genes in developmental patterning and cell signalling pathways, and from our analyses we conclude that the transcriptomes for both species are of very high coverage, with almost all expected genes being present with long (likely full) average open reading frames. We have already made extensive use of these resources for our own studies on the embryonic development of these two beetles, and are confident that they will be of broad utility to a range of fields in genomics and developmental biology.
Materials and Methods
Animal Husbandry
A. menetriesi eggs were collected from the wild (from a garden near Matsubara-Nishi, Hirosaki, Japan) and kindly provided by Dr Yoshikazu Ando (no special permission was needed as the species is not endangered or protected), and were reared at 25°C on wet sand or soil and fed fresh lettuce. C. maculatus beetles were kindly provided by Dr Joel Savard, and were reared at 30°C on dry black-eyed peas.
RNA Extraction and Sequencing
RNA was extracted from dissected ovaries and timed embryonic samples using a TRIzol RNA extraction kit according to the manufacturer’s protocols. RNA quantity and quality was checked using a Thermo Scientific Nanodrop 2000C Spectrophotometer and 1 μg was sent for sequencing by the Cologne Centre for Genomics on a Illumina HiSeq 2000 sequencer after sample preparation using a TruSeq RNA Library Preparation Kit (Illumina). Samples were sequenced on one lane per species. Adaptor trimming and initial quality control was performed by the provider according to their proprietary standards, and after exclusion of complete reads, no orphan reads were kept. This cleaned data was then made available to us for download from an external server. Paired end read quality after sequencing was assessed using the FastQC program [40] and no residual adaptor was observed, as detailed in the Results.
Transcriptome Assembly and Comparative Expression Analyses
Assemblies used in our final analysis were made using Trinity version 2013_08_14 [41], with no changes to the default settings except for—min_contig_length 200. Trimmomatic [42] was assayed (Illuminaclip Leading:3 Trailing:3 Slidingwindow:4:15 Minlen:36) but not utilised for assemblies presented here, as described in results. Full assemblies were made using reads from all time points, and individual assemblies were then constructed using reads from each sampled time point individually. All assemblies are available from Figshare online (A. menetriesi DOI: 10.6084/m9.figshare.2056464.v2, C. maculatus DOI: 10.6084/m9.figshare.2056467.v2). DeconSeq standalone version 0.4.3 [43] was run on full assemblies with settings -i 95 -c 95, using the bact, fungi, hsref, and prot databases. Comparative expression analyses were performed by mapping reads from individual time points to the combined assemblies using RSEM [44] as packaged in the Trinity module (align_and_estimate_abundance.pl script,—est_method RSEM—aln_method bowtie) to compare staged RNA samples with the combined assembly. The abundance_estimates_to_matrix.pl script was then run (cross sample normalisation: Trimmed Mean of M-values). edgeR was then run using the run_DE_analysis.pl script and the most differentially expressed transcripts extracted and clustered using the analyze_diff_expr.pl script, p-value cut off for FDR of 0.001 and min abs(log2(a/b)) change of 2 (meaning 4 fold change in this case), with—gene_dist euclidean and—gene_clust.complete. Results shown here are the ‘as-isoform’ data, although ‘as-gene’ data is also provided in Supporting Information files. BUSCO v1.1b1 [45] was used to assess gene complement completeness, and the Ortholog Hit Ratio method was used to determine the proportion of best-hit ortholog sequences (compared to the T. castaneum Tcas3.31.pep.all.fa peptide set, with a BlastX cut off of E-5) as detailed in [46].
Functional Annotation
Our combined assemblies were automatically assigned homologs and annotated according to gene ontology (GO) terms using Blast2GO [47,48]. Initially, BLASTx was run using BLAST 2.2.29+ against the NCBI nr database as downloaded to a local server on the 17/01/2015, with settings -evalue 0.001 -max_target_seqs 5 -outfmt 5. GO term distribution within the D. melanogaster and H. sapiens genomes were downloaded from B2GO-FAR [49] and calculated using the Combined Graph function of Blast2GO 3.1.3. KEGG KAAS mapping was automatically performed using the KEGG KAAS tool (http://www.genome.jp/tools/kaas/), single-directional best hit with default BLAST settings, and with the eukaryote dataset as a basis for annotation.
Gene Identification
Gene sequences were manually identified and their homology confirmed by independently using tBLASTn [50] searches using gene sequences of known homology downloaded from the NCBI nr database as queries against standalone databases created on a local server using BLAST 2.2.29+ or the CLC Main workbench (version 7.6.2, function: “BLAST”). Genes putatively identified using this method were reciprocally BLASTed against the online NCBI nr database using BLASTx to confirm their identity. Where identity was uncertain, phylogenetic analysis was used to confirm identity.
Phylogenetic Tree Construction
Sequences were aligned using MAFFT 7 [51] unless otherwise stated under the L-INS-i strategy. Alignments were then saved and exported to MEGA 6, where regions of poor alignment were manually excluded and maximum likelihood phylogenetic trees were constructed using the LG model, 1000 bootstrap replicates as indicated, 4 gamma categories and invariant sites, and all other default prior settings [52].
Results and Discussion
RNA Extraction and Sample Selection
Ovaries and embryos were collected as described in Materials and Methods, and as seen in Fig 2. The chosen time-windows cover a variety of important stages in the development of these species, and when combined can reasonably be expected to contain the majority of embryonic transcripts in their expressed complement. Briefly, RNA was collected from dissected ovaries from each species and from four embryonic time windows for A. menetriesi and two embryonic time windows for C. maculatus, at time points and with results as seen in Fig 2.
Fig 2. Summary of RNA sources, life stages sampled and sequencing results.
A.menetriesi data is presented at left and C. maculatus data at right.
Read Quality and Assembly Metrics
Fig 2 summarises initial read information, and these reads are available from the NCBI SRA, Bioproject Accession numbers: PRJNA293391 and PRJNA293393. To confirm quality of read data, FastQC [40] was run on all read data. This showed Phred quality scores were high, with median scores always exceeding 28 through to the 101st base, and generally in the mid-30s. A slight bias was found in initial nucleotide sequence. To ensure that Illumina adaptors were removed in their entirety, Trimmomatic v.0.32 [42] was used to check for residual adaptor sequence, but none were observed. We therefore posit that this bias is due to known biases in Illumina hexamer binding [53] rather than sequencing-based artifacts.
Using Trinity [41] as an assembler, Trimmomatic-corrected read assemblies were then compared with assemblies based on uncorrected reads. Initial assays of Trimmomatic-treated read assemblies empirically found them to be less well assembled by some metrics than those using un-trimmed reads, with a shorter N50 and less total sequence recovery (total number of bp, of which some could result from read errors in both assemblies). By way of example, for the A. menetriesi 0–24 hour dataset, further trimming resulted in an average read length of 91 bp (c.f. 101 in the original sample, due largely to trimming of skewed initial sequence due to biases in hexamer binding as reported above), but no reads were removed in their entirety. The resulting assemblies from trimmed reads had a slightly smaller N50 (1,993 c.f. 2,005 bp) and a markedly smaller total sequence recovery (102,320,009 bp, compared with 109,463,362 bp in the untrimmed read set). Given the advantages of a longer N50 and a preference to preserve as much information as possible, trimmed assemblies were therefore discarded in favour of untrimmed assemblies given the reasonable coverage requirement, and these ‘untrimmed’ assemblies were used for all further analyses.
Preliminary BLAST searches of initial assemblies noted a small amount of fungal contamination in the data for both species. This contamination likely comes from the environments in which the beetles are cultured. To correct for this, DeconSeq [43] was run on the Trinity output for both species, with high stringency as noted in the methods. A total of 336 and 206 contigs with some homology to fungal sequence was removed from the A. menetriesi and C. maculatus total assemblies as a result, before read mapping was performed. In the A. menetriesi assembly, we observed particularly high contamination with the protists, notably Dictyostelium discoideum and Naegleria gruberi, and some of this almost certainly remains in the transcriptome, as is normal with most ‘omics’ experiments [54]. Metrics for final assemblies after the removal of contamination can be seen in Table 1, alongside the results of assemblies for individual time points.
Table 1. Assembly metrics for individual time point and combined transcriptomic resources.
| A. menetriesi | C. maculatus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ovary | 0–24 hpf | 24–48 hpf | 48–72 hpf | 72–100 hpf | Combined | Ovary | 0–7 hpf | 7–24 hpf | Combined | |
| Number of contigs | 100,084 | 106,548 | 120,497 | 140,868 | 126,400 | 228,096 | 98,082 | 57,879 | 80,665 | 128,837 |
| Number of ‘genes’ (as per Trinity) | 62,115 | 63,192 | 71,304 | 88,949 | 74,719 | 144,634 | 66,675 | 37,857 | 52,282 | 89,192 |
| Max contig length (bp) | 20,759 | 17,765 | 18,398 | 17,591 | 17,180 | 20,757 | 30,742 | 22,941 | 22,956 | 29,933 |
| Mean contig length (bp) | 983.95 | 1027.36 | 924.27 | 879.03 | 914.2 | 844.66 | 1074.86 | 1216.55 | 1106.56 | 991.04 |
| Median contig length (bp) | 471 | 490 | 453 | 426 | 448 | 401 | 416 | 526 | 474 | 391 |
| N50 contig length (bp) | 1,894 | 1,993 | 1,721 | 1,643 | 1,686 | 1,598 | 2,450 | 2,570 | 2,308 | 2,263 |
| # contigs in N50 | 14,196 | 15,200 | 17,511 | 19,814 | 18,105 | 31,196 | 11,695 | 7,874 | 10,978 | 15,155 |
| # contigs > 1kb | 28,255 | 31,944 | 32,307 | 34,520 | 33,002 | 52,217 | 27,763 | 19,919 | 25,529 | 33,250 |
| # bases, total | 98,477,173 | 109,463,362 | 111,371,655 | 123,827,226 | 115,555,146 | 192,664,213 | 105,424,220 | 70,412,726 | 89,260,499 | 127,682,507 |
| # bases in contigs > 1kb | 68,737,074 | 78,526,348 | 75,208,106 | 80,796,007 | 77,199,414 | 122,986,547 | 78,519,052 | 55,032,546 | 67,296,566 | 91,466,099 |
| GC Content % (2dp) | 33.54 | 33.14 | 33.14 | 33 | 33.17 | 32.82 | 39.26 | 39.21 | 38.59 | 38.7 |
The final, combined timepoint, contamination-removed assemblies, comprising 228,096 and 128,837 contigs for A. menetriesi and C. maculatus respectively, contain a large number of well-assembled transcripts, with the number of contigs greater than 1 kb in length (52,217 in A. menetriesi, 33,250 in C. maculatus) and a high N50 (1,598 bp A. menetriesi, 2,263 bp C. maculatus) indicating a very well assembled dataset. This size is sufficient to span most protein coding domains, allowing easy inference of homology, and would represent the full length of most transcripts. It is important to note that many of the contigs in our assembly will represent splice variants of single genes, and some genes will have multiple splice variants, which will affect these statistics. However, excellent recovery of splicing variation itself will be useful for a range of later analysis. A simple measure of the possible splice variation in our dataset can be gained by comparing the ‘isoform’ and ‘gene’ assignations provided by the Trinity assembler. For C. maculatus, 89,192 ‘genes’ are predicted from 128,837 total contigs, which would indicate that approximately 1 in every 3 contigs has a very similar isoform within the assembly. For A. menetriesi these figures are 144,634 ‘genes’ for 228,096 contigs, and thus approximately 1 in every 2 contigs has a closely related splice variant within our assembly. Further results for timed assembly can be seen in Table 1, and in all cases is reasonable, indicating that the variation recovered by Trinity is of true biological significance. GC content of the final assembled transcriptomes closely mirrors that of reads (32.82% in A. menetriesi, 38.7% in C. maculatus; reads 36–38%/40-43%, respectively, depending on library), and is therefore similar to that expected.
To gain an understanding of whether full-length transcripts were present in our data, we ran TransDecoder v 2.01 [55] to identify open reading frames (ORFs) and filtered for the results that were at least 100 amino acids long. For the A. menetriesi assembly, this analysis yielded 71,961 raw and 51,912 filtered contigs, while for the C. maculatus assembly, the analysis yielded 65,433 raw and 36,535 filtered contigs. The mean average length of the predicted polypeptides, after the filtering step, was 351 (A. menetriesi) and 448 (C. maculatus) amino acids. This is long enough for us to be confident that our assembly adequately spans coding regions, as the average eukaryotic protein length is 361 amino acids [56]. Together, these analyses suggest that we have recovered the vast majority of coding sequence in our combined assemblies, with sufficient length to adequately span ORFs, a conclusion further supported by gene annotation data as discussed further below. The numbers of ORFs presented here are considerably more than the 16,404 gene models observed in the T. castaneum genome (Tribolium Genome Sequencing Consortium, 2008), and this is likely due to both spurious ORFs in our dataset and to multiple splice variants.
Further information was gained using the Ortholog Hit Ratio method detailed in [46], which describes the proportion of best-hit ortholog sequence represented by a dataset. When compared to the T. castaneum Tcas3.31.pep.all.fa peptide set with a BlastX cut off of E-5, the average ratio of the full length ortholog present in all of our blast hits was 0.4369 (A. menetriesi) and 0.5079 (C. maculatus). These statistics compare very well with those found in other organisms and previous studies, such as that in [46], and further indicate that the C. maculatus transcriptome may be slightly better assembled than the A. menetriesi dataset. This difference is likely due to a major difference in the level of genetic heterogeneity between our samples from the two species, as C. maculatus have been cultured in our lab for many years, while A. menetriesi was recently sourced from the wild.
Timed RNA Expression—Differential Expression Analysis
Key developmental stages for both A. menetriesi and C. maculatus can be easily observed following fixation and nuclei staining (data not shown). Briefly, egg lay to uniform blastoderm stage takes approximately 24 hours in A. menetriesi, and 7 hours in C. maculatus. Germband formation, gastrulation and elongation occur from 24–100 hours in A. menetriesi, and from 7–24 hours in C. maculatus. The latter period was subdivided in A. menetriesi according to characteristic stages of short germ development pertinent to our research interests. These stages, along with assayed sample periods, are shown diagrammatically in Fig 2. As well as being used for combined assemblies as described earlier, RNA extracted from mature ovaries and from embryos collected during the aforementioned time periods was also individually assembled using Trinity, allowing this information to be used to find time/stage-specific transcripts within our dataset.
The timed transcriptome assemblies for each species often possess better assembly quality when compared to the combined assemblies by metrics such as N50 and mean contig length (Fig 2, Table 1). As a result of being made up of a subset of the total reads, the individual assemblies do not possess the breadth of the combined assemblies, with fewer contigs, especially at long contig length (e.g. greater than 1kb). As such, the reads from the staged RNA samples were used solely for comparison of expression levels across time (mapped to the combined total assemblies), while the combined assemblies themselves were used for gene family analyses. Individual assemblies for timed samples are provided purely for completeness as a resource. We emphasise that no technical replicate was performed for these comparisons, and any conclusions drawn from them should hold this consideration in regard. With this limitation in mind, we carried out differential expression analyses and observed broad trends in expression, as can be seen in Fig 3.
Fig 3. Overview of results of differential expression analysis performed by RSEM within the Trinity framework.
A) and B) show the sample correlation matrix for A. menetriesi and C. maculatus respectively. C) and D) show relative expression of each differentially expressed contig, considered as isoforms, across time.
First, we generated matrices from general comparison between time points in order to find the most similar samples (Fig 3A and 3B). Generally, these results are congruent with steady changes in gene expression across the course of development, with most time points being most similar to those immediately preceding and following them. However, a split can be seen in A. menetriesi (Fig 3A and 3B) between the ovary and 0–24 hour samples and all others, with the three later samples resembling each other more closely than the 0–24 hour dataset resembles the 24–48 hour sample. This could be due to the maternal-zygotic transition, which will occur at some point in this time frame. This cannot be seen for C. maculatus, and could be due to more admixture of RNA within the last 7–24 hour sample. Focused analysis on when the maternal-zygotic transition occurs in each species is required to resolve this question.
Next, we clustered the results of our differential expression calculations (Fig 3C and 3D). The results shown are those with RSEM considering each isoform separately (rather than taking into account clustering into genes performed by Trinity). Numerous up- and down-regulated contigs can be seen at each time point, with some time points more obviously possessing or lacking a subset of genes found in the combined transcriptomes.
While replicates have not been performed and we have not analysed up and down regulated transcripts in detail, these data are available to download from S6 and S7 Files attached to this document online. These data will act as a good initial guide for those interested in tracking differential expression of specific genes across development and will be useful for hypothesis building.
Basic Gene Annotation
To gain an understanding of the depth of coverage of our datasets we used the BUSCO library of well-annotated genes [45], which are known to be highly conserved in single copy across the Metazoa, as a basis for comparison with our combined, assembled transcriptomes. Of the BUSCO set of 843 metazoan orthologs, the A. menetriesi assembly possesses 801 (95%) complete (of which 225, 26%, appear to be duplicated), 16 fragmented (1.8%) and 26 missing genes (3.0%), for a total recovery of 97% of the BUSCO dataset. The C. maculatus assembly contains 815 (96%) complete (with 202, 23%, appear to be duplicated), 13 (1.5%) fragmented and 15 (1.7%) missing genes (98.3% recovery). This extremely high level of recovery gives us confidence that at least all housekeeping genes expected to be present in these species are found in our datasets, which strongly suggests that these transcriptomes contain the vast majority of the gene cassette of these species.
The number of potentially duplicated genes in our BUSCO analyses likely reflects the construction of our transcriptomes from mixed RNA samples, with the allelic variation that this implies. Discerning true duplicates from allelic and splice variant data is largely contingent on the availability of well-assembled genomes. However, the recovery of these putative duplicates in our assemblies underlines that our RNA sequencing and assembly was of good depth and quality (respectively). With this information in-hand, a range of investigations will be made possible, particularly into the regulation and expression of developmentally important genes.
A further understanding of the content of our assemblies was gained from Blast2GO analysis [47,48]. Genes were annotated using b2gpipe, on the basis of BLASTx (E value cutoff, 10−3) comparisons made against the nr database as downloaded on the 26 January 2015. Of 228,096 (A. menetriesi) and 128,837 (C. maculatus) contigs in each assembly, 78,879 (34.6%) and 41,744 (32.4%) possessed a hit in the nr databases above the threshold. After further annotation with ANNEX and Interproscan, a total of 36,315 (15.9%) and 13,096 (10.2%) contigs were assigned to one or more GO categories. These numbers, while only a fraction of the total number of contigs within our transcriptomes, more closely reflect the expected eukaryotic protein complement in number. Blast2GO annotations are given in S2 and S3 Files.
Fig 4A shows the distribution of species best hit by BLASTx comparison of contigs from A. mentriesi and C. maculatus with the nr database. For both species, T. castaneum is the best represented species—a reflection of both the phylogenetic position of this species and its well annotated genome. The fact that contigs in our transcriptomes match T. castaneum and other coleopteran and insect species more closely than those of other species suggests that gene orthology will be easy to assign in many cases, and that an abnormally high rate of molecular evolution is not observed in our species.
Fig 4. Blast2GO results.
A) Distribution of BLASTx best hits by species, showing metazoans only, for A. menetriesi in orange, C. maculatus in blue. B) Distribution of GO terms expressed as a percentage of annotated contigs which were assigned a term within each of the three (Molecular Function, Cellular Component, Biological Process) categories of GO ID.
The distribution of GO terms within our datasets are shown in Fig 4B, alongside those of the well-annotated D. melanogaster and Mus musculus proteomes. In general, our datasets resemble one another more than they mirror that of the two sequenced genomes noted. Our transcriptomic resources empirically seem under-represented relative to D. melanogaster and M. musculus in developmentally interesting categories such as ‘Protein Binding’ (Molecular Function), ‘Multicellular Organism Development’ and ‘Cell Differentiation’ (Biological Process). Given our results for more targeted investigations, found below, we feel this is likely a result of poor annotation of these, rather than true absence from the transcriptome.
At a gross level, in the intracellular ‘Cellular Component’ GO categories, our data appears more similar to that seen in the two ‘model’ species than in Molecular Function or Biological Process categories, although this has not been tested statistically. This would suggest these well-conserved structural components were more readily assigned GO categories than the developmentally interesting categories listed above. While not all GO categories are as well-annotated by this process as may be desired, the broad classification of our data into a wide range of GO categories of all levels of GO distribution demonstrates that Blast2GO annotations of our data are a useful starting point for more focussed investigations and identification of specific genes and pathways of interest.
Gene Family Recovery
To extend the semi-automated analyses presented above, we performed more targeted analysis of individual, developmentally important gene families. In many genes, we observed several copies with slight but marked sequence variation. Given our RNA sources are mixed embryonic samples with a heterogenous genetic background and from a range of stages, this could represent splice variation, gene duplications, allelic forms, or mis-assembly, although the latter is made less likely due to the minimum coverage requirements of the assembler.
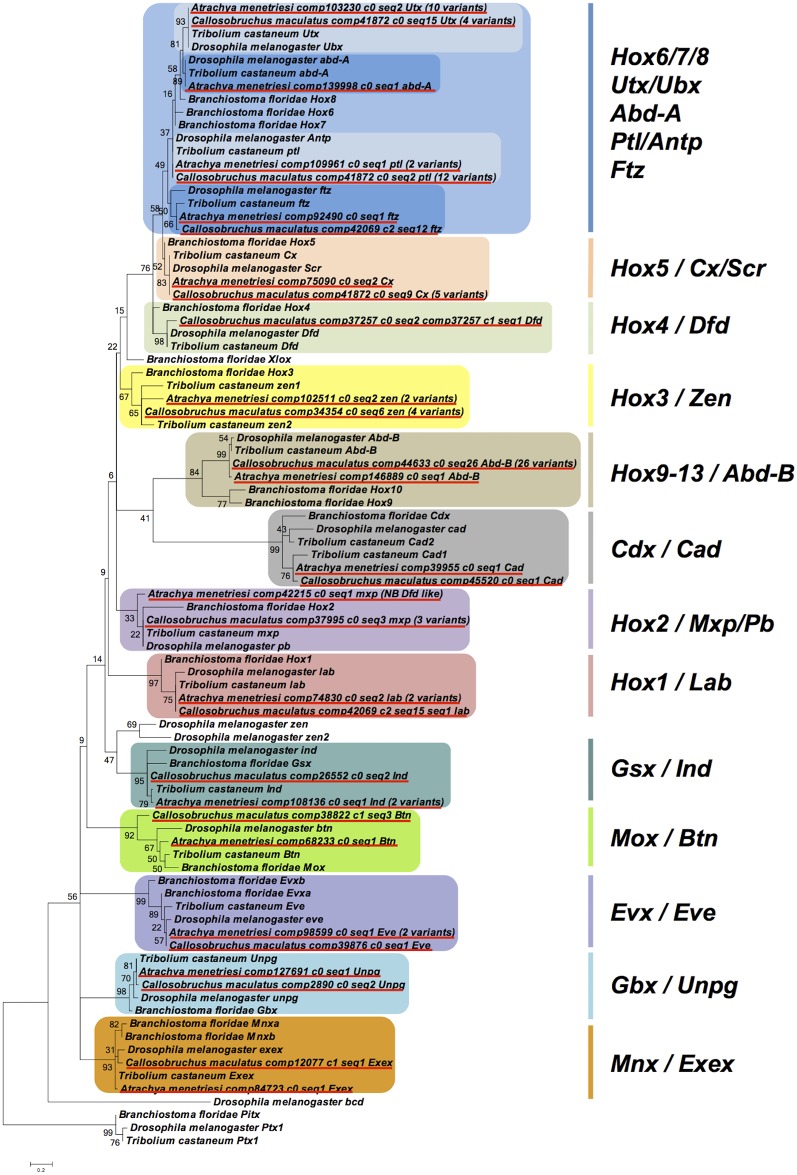
Both the Hox family of transcription factors and the TGF-β cassette were exceptionally well recovered in our dataset. The Hox genes, and in particular the ANTP HOXL class, which pattern the anterior-posterior body axis, are recovered almost in their entirety in both species when compared to well-catalogued databases (e.g. [57]). This can be seen in Fig 5, which shows the phylogenetic distribution of recovered ANTP HOXL class sequences from our transcriptomes alongside previously annotated members of this class.
Fig 5. Phylogenetic inter-relationships of ANTP HOXL class genes.
Phylogenetic relationships were reconstructed using MEGA 6 using the LG+Freqs model with 4 gamma categories and invariant sites, based on a 59 amino acid alignment spanning the homeodomain. Numbers at base of nodes represent bootstrap percentages of 1000 replicates. Scale bar at base of phylogeny gives substitutions per site at given unit distance. Red underline indicates A. menetriesi and C. maculatus sequences, coloured boxes are used to delineate known gene families (and in the case of Hox 6/7/8, a superfamily).
In A. menetriesi, sequences for all the ANTP HOXL class genes are recovered in our transcriptome, as can be seen in Fig 5, with sequences and alignments in S1 File. We note, however, that the A. menetriesi Hox 2 / maxillopedia (mxp)/ proboscipedia (pb) sequence bears some BLAST similarity with Hox 4 / Dfd sequences, while the putative Hox 4 / Deformed (Dfd) recovered does not include the homeodomain sequence—whether it has lost this crucial domain, or if this portion of the sequence is simply not recovered in our assembly is at present unknown. The putative A. menetriesi Hox 4 / Dfd sequence is given in S1 File, although it is not shown in the phylogeny in Fig 5 due to its truncated length.
While 2 (A. menetriesi) and 4 (C. maculatus) Hox 3 / zerknüllt (zen) variants are seen in our species, these are identical at the coding level, and therefore seem to be splice or allelic variants, rather than the two paralogous genes seen in T. castaneum [58]. Similarly, no evidence of the caudal (cad) duplication seen in T. castaneum [59] can be found in our transcriptomic assemblies, suggesting this is perhaps specific to the flour beetle lineage. Both zen and caudal are important embryonic patterning genes, and comparison of these genes in our two species and Tribolium would be an excellent situation in which to study how sub- and neo-functionalisation occurs. Likely allelic or splice variants are also observed for other HOXL genes in both A. menetriesi and C. maculatus. It should be noted that these could represent very recent duplications, or the effect of gene conversion, although this can only be tested fully with the advent of a complete genomic resource. No Pdx/Xlox gene was seen, adding further circumstantial evidence for the broad scale loss of this gene across the Insecta [60] and possibly the wider Arthropoda [61].
Our C. maculatus transcriptome also contains the full complement of ANTP HOXL genes, although, similar to the case of Hox 4 / Dfd in A. menetriesi, the 4 recovered abdominal-A (abd-A) homologs lack the whole homeodomain sequence, with several residues missing. These truncations excluded them from the phylogeny shown in Fig 5, but the sequences for these putative homologs are given in S1 File.
Even more so than in A. menetriesi, a remarkable diversity of potential splice/allelic variants are noted in C. maculatus, particularly for the Hox 6/8 superfamily and Hox 9–13 / Abdominal-B (Abd-B) gene family. Of the Hox 6/8 gene superfamily, normally represented by four genes in T. castaneum (prothoraxless (ptl), fushi tarazu (ftz), Ultrathorax (Utx) and abd-A), 12 ptl, 1 ftz, 4 Utx and 4 abd-A representatives were found in our analysis. Furthermore, up to 26 different potential allelic or splice variants of abdB are recorded. As our transcriptome is made of mixed embryonic samples of both differing developmental stages and genetically heterogenous background, it is perhaps not surprising that a diversity of putative splice/allelic variants are observed, but the excellent recovery of this data confirms the deep coverage provided by our sequencing and assembly given the coverage possessed by all isoforms.
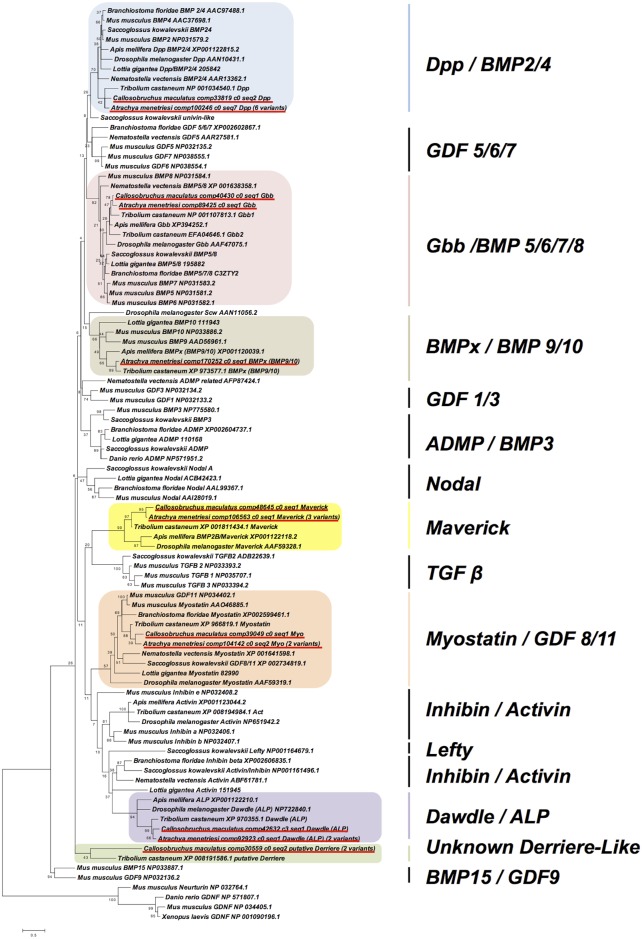
The TGF-β cassettes of the insects have been very well described previously (e.g. [62,63]). Our datasets recover almost the full expected complement of the Coleoptera. A slight exception to this is Activin (Act), a partial sequence of which is recovered for both species: a portion of the propeptide which does not span the mature signal peptide sequence. Whether this is a consequence of loss of the mature domain in these species or low levels of expression at the sampled timepoints remains to be established. A BMPx ortholog of clear homology to genes of that family can be found in C. maculatus, but has been excluded from the tree seen in Fig 6 as it is incomplete in length. Its sequence can be found in S1 File, and we have no doubt as to its identity due to high levels of sequence conservation between it and the T. castaneum and A. menetriesi orthologs of this gene.
Fig 6. Maximum Likelihood Phylogeny of TGF-β ligands.
Phylogenetic relationships were determined using MEGA under the LG+Freqs model with 4 gamma categories and invariant sites, on the basis of a 72 amino acid alignment of mature peptide sequences. The given scale depicts the number of substitutions per site per unit length. Bootstrap percentages (of 1000 replicates) are given at base of nodes. A. menetriesi and C. maculatus sequences are underlined in red. Coloured boxes represent known gene families with representatives in our transcriptomic resources, while all gene families, including those not found in our datasets, are indicated at right.
The glass bottom boat (gbb) duplication observed in T. castaneum cannot be found in our data, but we can recover a range of splice or allelic variants for other genes, especially in A. menetriesi. These do not differ in the protein coding regions, which leads us to suspect that these are not from gene duplications (unless the duplication(s) occurred very recently). The phylogeny shown in Fig 6 confirms the homology of all genes, and splice/allelic variant numbers observed are given there in brackets, with all sequences available in S1 File.
We also note the discovery of an additional putative TGF-β ligand in C. maculatus. This gene has been previously automatically annotated as derriere in T. castaneum, (XP_008191586.1) and if it is truly of this family, which is also known as GDF1/3/Univin/Vg1, this would be a surprise, as its presence outside the Deuterostomia is controversial [64]. If proof could be found for this being a bona fide GDF1/3/Univin/Vg1, the presence of this gene in more than one coleopteran could suggest that this might in fact be ancestrally present in all bilaterian species, but further investigation is warranted before strong conclusions can be drawn in this regard. We could gain no phylogenetic support for placing either of these beetle sequences in the GDF1/3 clade, and it may well be that these sequences instead represent a coleopteran novelty.
Our recovery of not only the full expected complements of these vital developmental genes, but also a remarkable diversity of alternative variants, demonstrates the depth of our assemblies as a resource, given the high coverage for each of these variants. Whether used as the basis for simple cloning or more sophisticated analysis of patterns of gene variation and diversification, these transcriptomes will be of wide utility to the field of coleopteran and insect developmental biology.
Pathway Recovery
As well as examining specific gene families, we investigated a number of broader pathways commonly studied in insects [65]. This allows us to both note how well-recovered such pathways are in our species as a measure of transcriptome utility, as well as note interesting differences between these pathways in our species when compared to others. We did this using both automated (KEGG KAAS mapping) and manual (BLAST based) methods. Some representative results of KEGG KAAS mapping can be seen in Fig 7, and all KEGG annotations can be downloaded from S4 and S5 Files.
Fig 7. KEGG style pathway maps showing recovery in our transcriptome resources.
A) the Wnt signalling pathway in canonical and non-canonical contexts, B) the Hedgehog signalling pathway and C) the Notch signalling pathway. Coloration of genes indicates presence, absence or ancestral absence from the Coleoptera as detailed in the key, which also gives other information as noted. All genes automatically annotated by KEGG KAAS server, with the exception of PAR-1, which was manually annotated.
Automated methods: KEGG KAAS mapping uses BLAST results to annotate known pathways, and gives a rapid overview of the recovery of these. Here we have shown the well-known Wnt, Notch and Hedgehog pathways to indicate the depth of our transcriptomes, and show how they may be useful for future research. However, these maps often use terminology based on vertebrate nomenclature, and contain genes known to be absent from particular clades. We have therefore indicated in Fig 7 (using unshaded boxes as shown in the Key) genes that may be absent ancestrally in the Coleoptera, based on their absence from the T. castaneum pathway as listed on the KEGG resource. Of genes expected to be present in the Coleoptera we find almost total recovery in our transcriptomes. In the three pathways examined, only three genes noted to be present in T. castaneum were noted as absent from both of our transcriptome datasets, all in the Wnt cascades (Fig 7A). The expected Hedgehog cassette was recovered in toto (Fig 7B) and in the Notch signalling cascade (Fig 7C), only APH-1 was noted to be absent, and only from the C. maculatus transcriptome. We must note that these may not be true absences—KEGG KAAS mapping is based on automatic BLAST assignation, and if these sequences are divergent in our transcriptomes they may have been missed by this analysis.
Manual methods: We also examined pathways manually, using reciprocal BLAST hits and closer manual investigation to confirm the identities of individual genes, the results of which can be seen in Table 2. The anterior-posterior patterning genes cad (mentioned earlier) and hunchback (hb) are present in both species. Of the germline establishment and localization genes examined, nanos was surprisingly absent in both species, while bruno (bru; also known as arrest), exuperantia (exu), tudor (tud; 2 copies in A. menetriesi), oskar (osk), vasa (vas) and valois (vls) were present. Interestingly, pumilio (pum) is present in C. maculatus in single copy (although it is divided across two contigs), while A. menetriesi possesses a total of seven copies. The different A. menetriesi pum copies varied both at the nucleotide level and in their amino acid sequences, strongly suggesting that they are in fact paralogs. In depth analysis of these genes is required to uncover why they have undergone several rounds of duplication. Orthologs of the Drosophila gene swallow (swa) could not be found in either of our transcriptome resources, nor is it present in several other insects (data not shown) and we suggest it may therefore be a schizophoran novelty.
Table 2. Genes identified by manual annotation.
| Pathway/Gene | A. menetriesi | C.maculatus | Pathway/Gene | A. menetriesi | C.maculatus |
|---|---|---|---|---|---|
| Maternal Effect: | Pair rule: | ||||
| caudal | present | present | even skipped | present | present |
| hunchback | present | present | fushi tarazu | present (see Hox) | present (see Hox) |
| hairy | present | present | |||
| Germline: | odd paired | present | present | ||
| bruno/arrest | present | present | odd skipped | present | present |
| exuperantia | present | present | paired | present | present |
| nanos | absent | absent | runt | present | present |
| oskar | present | present | sloppy paired 1 | present | present |
| pumilio | present—7 copies | present | |||
| swallow | absent | absent | Segment Polarity: | ||
| tudor | present—2 copies | present | armadillo | present | present |
| valois | present | present | cubitus interruptus | present | present |
| vasa | present | present | engrailed | present | present |
| fused | present | present | |||
| Gap genes: | gooseberry | present | present | ||
| buttonhead | present | present | gooseberry-neuro | absent | absent |
| empty spiracles | present | present | hedgehog | present | present (but on 3 contigs) |
| giant | present | present | pangolin | present | present |
| huckebein | present | present | patched | present | present |
| knirps | present | present | wingless | present | present |
| Krüppel | present | present | |||
| orthodenticle 1 | present | present | |||
| orthodenticle 2 | present | present | |||
| tailless | present | present—2 copies |
Canonical gap genes Krüppel (Kr), knirps (kni), giant (gt), huckebein (hkb), tailless (tll; 2 paralogs in C. maculatus), buttonhead (btd), empty spiracles (ems) and both orthodenticle orthologs (Otd and Otd2) were recovered in both species examined here. The C. maculatus paralogs of tll exhibited differences at both the nucleotide and amino acid level along their entire lengths (data not shown) confirming that they are paralogs. Given the important embryonic role of tailless in other insects (for example [66]), this duplication would be excellent opportunity to study gene duplication and evolution. The pair rule genes even skipped (eve), hairy (h), fushi tarazu (ftz), odd paired (opa), odd skipped (odd), paired (prd), runt (run) and sloppy paired 1 (slp1) were present in single copy. The segment polarity genes were also present in both species, with the notable absence of gooseberry-neuro (gsb-n) from our datasets. The genes armadillo (arm), cubitus interruptus (ci), engrailed (en), fused (fu), gooseberry (gsb), hedgehog (hh), pangolin (pan), patched (ptc) and wingless (wg) were all present, and their sequences can be found in S1 File.
All of these pathways are commonly studied in insects, and the annotations provided here, along with preliminary timed expression data, will provide a basis for a wide range of targeted investigations into the embryonic development of these two species, and how these pathways have changed over the course of evolution. Furthermore, the excellent recovery of these pathways by both automated (KEGG-KAAS) and manual annotation gives us high confidence in the completeness of our transcriptomic resources. This confirms the results of our BUSCO analysis, and our datasets are therefore likely to contain the vast majority of transcribed genes in these two species, with only lowly expressed and temporally restricted genes absent from these transcriptome resources.
Conclusions
Our generation of deep transcriptomic sequence data for A. menetriesi and C. maculatus will assist in the inference of character gain and loss across the Coleoptera, aid in future phylogenetic efforts, and allow a range of investigations into the embryonic development of these species at the molecular level. The status of these organisms as common agricultural pests also suggests that such resources may allow targeted control mechanisms to be developed for these species. This data will be another key building block in our understanding of the transcriptomic basis to embryological development, and provide a window into the basic biology of the most successful clade of animals.
Supporting Information
(XLSX)
(TXT)
(TXT)
(TXT)
(TXT)
(GZ)
(GZ)
Acknowledgments
The authors would like to thank the members of their laboratories for their support and discussions. In addition, we thank Dr Y Ando for providing A. menetriesi eggs and advice on establishing cultures, Dr J Savard for providing C. maculatus, and Dr F Marletaz for help in running BUSCO analyses. The efforts of editors and reviewers in considering this manuscript are gratefully acknowledged.
Data Availability
The datasets supporting the conclusions of this article are available in the NCBI SRA repository [Bioproject Accession numbers: PRJNA293391 and PRJNA293393] and in the Figshare repository [DOIs: 10.6084/m9.figshare.2056464.v2, 10.6084/m9.figshare.2056467.v2].
Funding Statement
MAB was supported by an Alexander von Humboldt (https://www.humboldt-foundation.de/web/home.html) Fellowship for Postdoctoral Researchers. KHC, SR and JAL were supported by the Deutsche Forschungsgemeinschaft (http://www.dfg.de/en/index.jsp) Collaborative Research Center Grant 680. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stork NE. Insect diversity—facts, fiction and speculation. Biol J Linn Soc. 24–28 OVAL RD, LONDON, ENGLAND NW1 7DX: ACADEMIC PRESS LTD; 1988;35: 321–337. [Google Scholar]
- 2.Grimaldi D, Engel M. Evolution of the Insects. Cambridge University Press; 2005. [Google Scholar]
- 3.1KITE: 1000 Insect Transcriptome Evolution [Internet]. 2015. http://www.1kite.org/
- 4.Keeling CI, Henderson H, Li M, Yuen M, Clark EL, Fraser JD, et al. Transcriptome and full-length cDNA resources for the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major insect pest of pine forests. Insect Biochem Mol Biol. England; 2012;42: 525–536. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Congiu L, Lindstrom L, Piiroinen S, Vidotto M, Grapputo A. Sequencing, De Novo assembly and annotation of the Colorado Potato Beetle, Leptinotarsa decemlineata, Transcriptome. PLoS One. United States; 2014;9: e86012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauchet Y, Wilkinson P, van Munster M, Augustin S, Pauron D, ffrench-Constant RH. Pyrosequencing of the midgut transcriptome of the poplar leaf beetle Chrysomela tremulae reveals new gene families in Coleoptera. Insect Biochem Mol Biol. England; 2009;39: 403–413. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Lin L, Xie M, Zhang G, Su W. De novo sequencing, assembly and characterization of antennal transcriptome of Anomala corpulenta Motschulsky (Coleoptera: Rutelidae). PLoS One. United States; 2014;9: e114238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberhofer G, Grossmann D, Siemanowski JL, Beissbarth T, Bucher G. Wnt/-catenin signaling integrates patterning and metabolism of the insect growth zone. Development. 2014;141: 4740–4750. 10.1242/dev.112797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs CGC, Braak N, Lamers GEM, van der Zee M. Elucidation of the serosal cuticle machinery in the beetle Tribolium by RNA sequencing and functional analysis of Knickkopf1, Retroactive and Laccase2. Insect Biochem Mol Biol. Elsevier Ltd; 2015;60: 7–12. [DOI] [PubMed] [Google Scholar]
- 10.Stappert D, Frey N, von Levetzow C, Siegfried R. Genome wide identification of Tribolium dorsoventral patterning genes. (Under review). Development. [DOI] [PubMed]
- 11.i5k-Consortium. The i5K Initiative: advancing arthropod genomics for knowledge, human health, agriculture, and the environment. J Hered. United States; 2013;104: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, et al. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452: 949–55. 10.1038/nature06784 [DOI] [PubMed] [Google Scholar]
- 13.Keeling CI, Yuen MM, Liao NY, Roderick Docking T, Chan SK, Taylor G a, et al. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 2013;14: R27 10.1186/gb-2013-14-3-r27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham CB, Ji L, Wiberg RAW, Shelton J, McKinney EC, Parker DJ, et al. The Genome and Methylome of a Beetle with Complex Social Behavior, Nicrophorus vespilloides (Coleoptera: Silphidae). Genome Biol Evol. 2015;7: 3383–96. 10.1093/gbe/evv194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin A, Pan L, Zhang X, Wang L, Yin Y, Jia S, et al. Transcriptomic study of the red palm weevil Rhynchophorus ferrugineus embryogenesis. Insect Sci. Australia; 2015;22: 65–82. [DOI] [PubMed] [Google Scholar]
- 16.Hunt T, Bergsten J, Levkanicova Z. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science (80-). 2007;438: 1–4. Available: http://www.sciencemag.org/content/318/5858/1913.short [DOI] [PubMed] [Google Scholar]
- 17.Hegner RW. Effects of Removing the Germ-Cell Determinants from the Eggs of Some Chrysomelid Beetles. Preliminary Report. 1908;16: 19–26. [Google Scholar]
- 18.Hegner RW. The origin and early history of the germ-cells in some chrysomelid beetles. J Morphol. 1909;20: 231–296. [Google Scholar]
- 19.Van Der Meer JM. Optical clean and permanent whole mount preparation for phase-contrast microscopy of cuticular structures of insect larvae. Dros Inf Serv. 1977;52. [Google Scholar]
- 20.Jolivet PH, Cox ML, Petitpierre E, editors. Novel aspects of the biology of Chrysomelidae. Kluwer Academic Publishers; 1994. [Google Scholar]
- 21.Gómez-Zurita J, Hunt T, Kopliku F, Vogler AP. Recalibrated tree of leaf beetles (Chrysomelidae) indicates independent diversification of angiosperms and their insect herbivores. PLoS One. 2007;2: e360 10.1371/journal.pone.0000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumer LS, Beck CW. Bean Beetles: A Model Organism for Inquiry-based Undergraduate Laboratories [Internet]. 2015. http://www.beanbeetles.org/
- 23.Pauchet Y, Wilkinson P, Chauhan R, Ffrench-Constant RH. Diversity of beetle genes encoding novel plant cell wall degrading enzymes. PLoS One. United States; 2010;5: e15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirsch R, Wielsch N, Vogel H, Svatos A, Heckel DG, Pauchet Y. Combining proteomics and transcriptome sequencing to identify active plant-cell-wall-degrading enzymes in a leaf beetle. BMC Genomics. England; 2012;13: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flagel LE, Bansal R, Kerstetter RA, Chen M, Carroll M, Flannagan R, et al. Western corn rootworm (Diabrotica virgifera virgifera) transcriptome assembly and genomic analysis of population structure. BMC Genomics. England; 2014;15: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss AS, Wang D, Stock M, Gretscher RR, Groth M, Boland W, et al. Tissue-specific transcript profiling for ABC transporters in the sequestering larvae of the phytophagous leaf beetle Chrysomela populi. PLoS One. United States; 2014;9: e98637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi YH, Salzman RA, Balfe S, Ahn J-E, Sun W, Moon J, et al. Cowpea bruchid midgut transcriptome response to a soybean cystatin—costs and benefits of counter-defence. Insect Mol Biol. 2009;18: 97–110. 10.1111/j.1365-2583.2008.00854.x [DOI] [PubMed] [Google Scholar]
- 28.Miya K, Kobayashi K. The embryonic development of Atrachya menetriesi. Faldermann (Coleoptera, Chrysomelidae). II. Analyses of early development by ligation and low temperature treatment. J Fac Agric Iwate Univ. 1974;12: 39–55. [Google Scholar]
- 29.Miya K, Ando Y, Kurihara M. Formation of duplicated embryos by treatment of low temperature in Atrachya menetriesi Faldermann (Chrysomelidae, Coleoptera). Proc 26th Ann Meet Ent Soc Japan. 1966;9. [Google Scholar]
- 30.Ando Y. Geographic Variation In The Incidence Of Non-Diapause Eggs Of The False Melon Beetle, Atrachya-Menetriesi Faldermann (Coleoptera, Chrysomelidae). Appl Entomol Zool. 1979;14: 193–202. Available: <Go to ISI>://A1979GZ01300008 [Google Scholar]
- 31.Ando Y, Miya K. Diapause character in the false melon beetle, Atrachya menetriesi Faldermann, produced by crossing between diapause and non diapause strains. Bull Fac Agri Iwate Univ. 1968;9: 87–96. [Google Scholar]
- 32.Miya K. The embryonic development of a Chrysomelid Beetle, Atrachya menetriesi. Faldermann (Coleoptera) I. The stages of development and changes of external form. J Fac Agric Iwate Univ. 1965;7: 155–166. [Google Scholar]
- 33.Tran BMD, Credland PF. Consequences of inbreeding for the cowpea seed beetle, Callosobruchus maculatus (F)(Coleoptera: Bruchidae). Biol J Linn Soc. 1995;56: 483–503. [Google Scholar]
- 34.van der Meer J. The specification of metameric order in the insect Callosobruchus maculatus Fabr. (Coleoptera) I. Incomplete segment patterns can result from constriction-induced cytological damage. J Embryol Exp. 1979;51: 1–26. Available: http://dev.biologists.org/content/51/1/1.short [PubMed] [Google Scholar]
- 35.Van Der Meer JM. Parameters influencing reversal of segment sequence in posterior egg fragments of Callosobruchus (Coleoptera). Roux’s Arch Dev Biol. 1984; 339–356. [DOI] [PubMed] [Google Scholar]
- 36.Patel NH, Condron BG, Zinn K. Pair-rule expression patterns of even-skipped are found in both short- and long-germ beetles. Nature. 1994;367: 429–434. 10.1038/367429a0 [DOI] [PubMed] [Google Scholar]
- 37.Lynch JA, Brent AE, Leaf DS, Pultz MA, Desplan C. Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature. 2006;439: 728–32. 10.1038/nature04445 [DOI] [PubMed] [Google Scholar]
- 38.Anderson DT. The Development of Holometabolous Insects In: Counce SJ, Waddinton CH, editors. Developmental systems Insects, Vol 1 New York: Academic Press; 1972. pp. 165–242. [Google Scholar]
- 39.Davis GK, Patel NH. SHORT, LONG, AND BEYOND: Molecular and Embryological Approaches to. Annu Rev Entomol. 2002; 669–99. [DOI] [PubMed] [Google Scholar]
- 40.Andrews S. FastQC: A quality control tool for high throughput sequence data [Internet]. 2010. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 41.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. United States; 2011;29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. England; 2014;30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmieder R, Edwards R. Fast identification and removal of sequence contamination from genomic and metagenomic datasets. PLoS One. United States; 2011;6: e17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. England; 2011;12: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simao FA, Waterhouse RM, Ioannidis P, Kriventseva E V, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. England; 2015;31: 3210–3212. [DOI] [PubMed] [Google Scholar]
- 46.O’Neil ST, Dzurisin JD, Carmichael RD, Lobo NF, Emrich SJ, Hellmann JJ. Population-level transcriptome sequencing of nonmodel organisms Erynnis propertius and Papilio zelicaon. BMC Genomics. 2010;11: 310 10.1186/1471-2164-11-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. England; 2005;21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- 48.Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. England; 2008;36: 3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gotz S, Arnold R, Sebastian-Leon P, Martin-Rodriguez S, Tischler P, Jehl M-A, et al. B2G-FAR, a species-centered GO annotation repository. Bioinformatics. England; 2011;27: 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. ENGLAND; 1990;215: 403–410. [DOI] [PubMed] [Google Scholar]
- 51.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. United States; 2013;30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. United States; 2013;30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen KD, Brenner SE, Dudoit S. Biases in Illumina transcriptome sequencing caused by random hexamer priming. Nucleic Acids Res. England; 2010;38: e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tosar JP, Rovira C, Naya H, Cayota A. Mining of public sequencing databases supports a non-dietary origin for putative foreign miRNAs: underestimated effects of contamination in NGS. RNA. 2014;20: 754–757. 10.1261/rna.044263.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. England; 2013;8: 1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brocchieri L, Karlin S. Protein length in eukaryotic and prokaryotic proteomes. Nucleic Acids Res. England; 2005;33: 3390–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong Y-F, Butts T, Holland PWH. HomeoDB: a database of homeobox gene diversity. Evol Dev. United States; 2008;10: 516–518. [DOI] [PubMed] [Google Scholar]
- 58.van der Zee M, Berns N, Roth S. Distinct Functions of the Tribolium zerknullt Genes in Serosa Specification and Dorsal Closure. Curr Biol. 2005;15: 624–636. 10.1016/j.cub.2005.02.057 [DOI] [PubMed] [Google Scholar]
- 59.Schulz C, Schröder R, Hausdorf B, Wolff C, Tautz D. A caudal homologue in the short germ band beetle Tribolium shows similarities to both, the Drosophila and the vertebrate caudal expression patterns. Dev Genes Evol. Springer; 1998;208: 283–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/9683744 [DOI] [PubMed] [Google Scholar]
- 60.Hui JH, Raible F, Korchagina N, Dray N, Samain S, Magdelenat G, et al. Features of the ancestral bilaterian inferred from Platynereis dumerilii ParaHox genes. BMC Biol. 2009;7: 43 10.1186/1741-7007-7-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kenny NJ, Shen X, Chan TFTH, Wong NWY, Chan TFTH, Chu KH, et al. Genome of the Rusty Millipede, Trigoniulus corallinus, Illuminates Diplopod, Myriapod, and Arthropod Evolution. Genome Biol Evol. 2015;7: 1280–1295. 10.1093/gbe/evv070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van der Zee M, da Fonseca RN, Roth S. TGFbeta signaling in Tribolium: vertebrate-like components in a beetle. Dev Genes Evol. Germany; 2008;218: 203–213. [DOI] [PubMed] [Google Scholar]
- 63.Ozuak O, Buchta T, Roth S, Lynch JA. Ancient and diverged TGF-beta signaling components in Nasonia vitripennis. Dev Genes Evol. Germany; 2014;224: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kenny NJ, Namigai EKO, Dearden PK, Hui JHL, Grande C, Shimeld SM. The Lophotrochozoan TGF-beta signalling cassette—diversification and conservation in a key signalling pathway. Int J Dev Biol. Spain; 2014;58: 533–549. [DOI] [PubMed] [Google Scholar]
- 65.Gilbert SF, editor. Developmental Biology. 10th ed Sunderland, MA: Sinauer Associates, Inc; 2013. [Google Scholar]
- 66.Wilson MJ, Dearden PK. Tailless patterning functions are conserved in the honeybee even in the absence of Torso signaling. Dev Biol. Elsevier Inc.; 2009;335: 276–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(TXT)
(TXT)
(TXT)
(TXT)
(GZ)
(GZ)
Data Availability Statement
The datasets supporting the conclusions of this article are available in the NCBI SRA repository [Bioproject Accession numbers: PRJNA293391 and PRJNA293393] and in the Figshare repository [DOIs: 10.6084/m9.figshare.2056464.v2, 10.6084/m9.figshare.2056467.v2].