Abstract
The corn earworm Helicoverpa zea (Boddie) and the old world bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) are allopatric species and occur in important agricultural crops. In maize, both species tend to infest the ear. The introduction of H. armigera in Brazil has created a new scenario, where these Helicoverpa species might cohabit and interact with one another, affecting the prevalence of each species in the agroecosystem, integrated pest management, and insect resistance management. In this study, larval occurrence and proportion of these species in maize was assessed in three regions of Brazil during three crop seasons. Interaction between the species was evaluated in interspecific and intraspecific scenarios under laboratory and field conditions. Helicoverpa zea was predominant in Rio Grande do Sul and the Planaltina, DF (central Brazil). In western Bahia, H. zea was predominant in the first collection, but approximately equal in number to H armigera in the second crop season. Both species exhibit high cannibalism/predation rates, and larval size was the primary factor for larval survival in the interaction studies. Larva of H. zea had higher survival when interacting with H. armigera, indicating that H. zea has an advantage in intraguild interactions with H. armigera in maize. Overall, the results from this study indicate that maize might play a role as a source of infestation or a sink of insecticide or Bt protein unselected H. armigera populations, depending on the H. zea:H. armigera intraguild competition and adult movement in the landscape.
Introduction
The Heliothinae (Lepidoptera: Noctuidae) is a cosmopolitan subfamily of noctuid moths that comprises more than 400 species [1]. A review involving the corn earworm complex proposed the genera Helicoverpa [2]. One of the significant contributions of this review was to propose the species differentiation between Helicoverpa zea (Boddie) and Helicoverpa armigera (Hübner) based on male and female genitalia.
Helicoverpa zea and H. armigera are allopatric species that have a preference to feed on plant reproductive tissues, often causing economic damage [3]. Helicoverpa zea is only present in the American continents, and so is named the new old world bollworm. Helicoverpa armigera, the old world bollworm, was reported present in Asia, Africa, Europe and Australia. However, a new scenario of H. zea and H. armigera cohabiting in an American continent was reported with the detection of H. armigera in Brazil during the crop season of 2012/2013 [4–6]. Indeed, H. armigera was present in Brazil at least since 2008 [7], and the potential for it to spread throughout North and South America [8] has been confirmed by reports of its occurrence in other countries, such as Argentina [9], and in the U.S on 17 June 2015, where three H. armigera moths were collected in Florida [10].
Several concerns have been raised about the impact of the interaction between these two former allopatric species, with relevance to integrated pest management and insect resistance management. Maize is the major host of H. zea, and moths usually oviposit on silks, where the neonates feed, and then move to kernels after entering the tip of the husk. When larvae reach the third instar, they become cannibalistic and frequently only one larva survives per maize ear [11–14]. This species presents management challenges because of its biological and behavioral characteristics and rapid adaptation to chemical control (e.g. resistance to organophosphate and pyrethroid insecticides) [15–17]. One alternative to insecticide control has been the use of transgenic hybrids expressing active proteins of the bacterium Bacillus thuringiensis (Bt). However, cases of resistance of H. zea have already been reported on Bt maize expressing the proteins Cry1Ab [18]. Helicoverpa armigera is a polyphagous pest and prefers cotton and sorghum [11, 19, 20]. However, injury in maize is reported, and similarly to H. zea, H. armigera moths tend to oviposit on maize silks and larvae feed on kernels [19]. Considering this preference for reproductive tissues (silks and early ear development stages), maize supports one generation of H. armigera, and economic impact has been reported [21]. In addition, insecticide resistance of H. armigera has been reported to organophosphate, carbamate, and pyrethroid insecticides [22, 23].
In Brazil, besides maize, there is the possibility that these two species could cohabit in other economically important crops such as soybean, cotton, tomatoes, dry beans, among others [5, 6, 24]. However, maize represents a model crop to study the interaction between these two pests. The maize ear is the same feeding site for both species, where larvae are naturally confined [14, 19, 25] with less possibility of movement to other reproductive tissues on the same plant. Larval movement to other feeding sites is more likely in cotton [19] or soybean [26]. Thus, maize represents an appropriate arena for intraguild interaction studies. In addition, larvae of both species have cannibalistic behavior [12, 27, 28], which might intensify the intraguild competition.
Here we documented the larval occurrence and proportion of H. zea and H. armigera in maize ears, in three regions of Brazil, using data collected from the 2012/2013 and 2014/2015 crop seasons. The interaction between these former allopatric species was also investigated based on different interspecific and intraspecific scenarios, which were conducted under laboratory and field conditions.
Materials and Methods
Larval occurrence and percentage of H. zea and H. armigera in maize fields in Brazil
Maize fields were sampled in three states of Brazil (Bahia, Distrito Federal, Rio Grande do Sul) to document the occurrence and proportion of H. zea and H. armigera larvae in maize ears. Commercial fields of maize were selected based on their representative size for each region and when maize kernels were in the milk to dough stages (R3-R4) [29]. The field collections were authorized by Instituto Chico Mendes de Conservação da Biodiversidade, under SISBIO licenses n.38520-1, n.38520-2, n.38520-3, n.38520-4, and n.48218-1. In western Bahia, in the municipality of São Desidério (12°21’48”S and 44°58’24”W), larval sampling was conducted in the 2012/2013 and 2014/2015 crop seasons. In the crop season of 2012/2013, maize ears were sampled in Bt maize (Cry1Ab+Cry1F) and non-Bt maize. Each maize field was 200 hectares and approximately 300 plants were randomly inspected per field during maize stage R3 [29]. Helicoverpa sp. larvae at different instars were collected from maize ears, placed in 30 ml containers with artificial diet [30] and transported to the laboratory (25 ± 2°C, RH: 60 ± 10%; 14:10 [L:D]) at Embrapa Cerrados, Planaltina, DF. The larvae were held under laboratory conditions until adult emergence. In the crop season 2014/2015, Helicoverpa larvae were likewise collected and held under the same laboratory conditions until adult emergence.
In the experimental area of Embrapa Cerrados, municipality of Planaltina, DF (15°37’09”S and 47°39’09”W) in central Brazil, larval sampling was conducted in two non-Bt maize fields (5 hectares each field) during the 2014/2015 crop season. One field was sampled in November 2014 and approximately 80 larvae were collected. In April 2015, the other field was sampled with approximately 60 larvae collected. At both sampling dates, the maize fields were in stage R3-R4.
In the municipality of São Pedro da Serra (29°25’16”S and 51°30’48”W), Rio Grande do Sul, southern Brazil, larval sampling was conducted in two maize fields during the 2015/2016 crop season. Both fields were non-Bt maize and 5 hectares each. The collected larvae were transported to Embrapa Cerrados, Planaltina, DF and held under the same laboratory conditions previously described.
Insects that reached the adult stage in each collection were identified by genitalia dissection [31], and identification based on morphological characters described in the literature [2]. Male individuals of H. zea have three lobes at the base of the vesical, while H. armigera have one single lobe at the base of vesical [2].The female genitalia of H. zea has the appendix bursae less speculated than in the bursae of H. armigera. Moreover, the number of coils in the appendix bursae vary between the two species. Helicoverpa zea appendix bursae has nine to 11 dilations. Helicoverpa armigera appendix bursae has eight to nine dilations [2]. The characters described by Hardwick [2] to identify interspecific hybrid individuals were also considered during the genital examination, since cross-matting between H. zea and H. armigera under laboratory conditions has been reported [2, 32, 33]. All identified insects’ genitalia characters were typical of either H. zea or H. armigera. Maize ears infested with Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) were not considered or reported in the present study. The percentage of H. zea and H. armigera were calculated considering the total of adults of Helicoverpa emerged. The total of H. zea and H. armigera were grouped per each location and crop season.
Lepidoptera stock colony
During the years of 2013 to 2016, stock colonies of H. zea and H. armigera were maintained under laboratory conditions (25 ± 2°C, RH: 60 ± 10%; 14:10 [L:D]) on artificial diet [34]. Portions of cotton embedded in a solution of water and honey (10%) was provided for moths. The stock colony was reared based on established protocols [34, 35], enabling a supply of larvae for the laboratory and field experiments at the São Paulo State University, College of Agronomic Science, Department of Crop Protection, Botucatu, SP, Brazil. To maintain colony vigor, insects were collected from the field every two months, identified [2] and transferred to the colony of each species. The insects from the stock colony of H. zea and H. armigera had the species identification validated following the genital morphological characters, after it’s dead, described in the previews section.
Intraguild competition
Assays involving larvae of H. zea and H. armigera were performed to evaluate the intraguild competition. The assays were performed under laboratory conditions at LARESPI—Laboratory of Host Plant Resistance and Insecticidal Plants (25 ± 2°C, RH: 60 ± 10%; 14:10 [L:D]) and under field conditions during two crop seasons (May to August of 2015, and November of 2015 to February of 2016) at the São Paulo State University, Botucatu, SP, Brazil (22°82’48”S and 48°42’80”W, 720 m elevation). The competition assays were conducted in three arenas (plastic cups in laboratory; plastic tubes in laboratory; and maize ears in field); two types of diet (non-Bt maize silks and non-Bt maize ear); and in 16 competition scenarios as described in Table 1. The non-Bt hybrid maize (Pioneer 30F35) was used to eliminate the possible effect of Bt proteins on larval interactions.
Table 1. Helicoverpa armigera and Helicoverpa zea competition scenarios at different instar.
| Intraguild competition scenarios (16) a | |
|---|---|
| Treatments (instar) | Controlsb (instar) |
| H. armigera (2nd) vs. H. zea (2nd) | H. armigera (2nd) vs. H. armigera (2nd) |
| H. armigera (2nd) vs. H. zea (4th) | H. armigera (2nd) vs. H. armigera (4th) |
| H. armigera (4th) vs. H. zea (2nd) | H. armigera (4th) vs. H. armigera (2th) |
| H. armigera (4th) vs. H. zea (4th) | H. armigera (4th) vs. H. armigera (4th) |
| H. zea (2nd) vs. H. armigera (2nd) | H. zea (2nd) vs. H. zea (2nd) |
| H. zea (2nd) vs. H. armigera (4th) | H. zea (2nd) vs. H. zea (4th) |
| H. zea (4th) vs. H. armigera (2nd) | H. zea (4th) vs. H. zea (2nd) |
| H. zea (4th) vs. H. armigera (4th) | H. zea (4th) vs. H. zea (4th) |
a Larval development: 4–12 h after ecdysis
b Control treatments [36]
Laboratory intraguild competition
Two competition studies were conducted under laboratory conditions. In the first study, two larvae (Table 1) were placed into a transparent plastic cup (5 cm diameter, 100 mL) containing maize silks and enclosed with a plastic lid with holes to allow ventilation. For the second study, two larvae were placed on the silks of a maize ear, that was fixed on a base of polystyrene, supported by two wooden rods and held in place by a rubber band. The stabilized maize ear was then placed in a transparent plastic cylinder (8 cm height x 30 cm diameter), sealed on the top with organdy fabric attached with a rubber band to allow adequate ventilation. Each plastic cup or tube was considered one replicate, with 20 replicates per scenario for both arenas in a completely randomized design.
Maize silks (100 g) and maize ears were collected from non-Bt field-grown maize, which were not proximal to Bt maize fields to avoid possible cross-pollination with Bt maize [37, 38]. Maize silks were removed from the ears, sprayed with alcohol, followed by water, and then dried before being offered to larvae. Silks were changed daily to maintain quantity and tissue quality. The maize ears were cleaned with alcohol and a portion of paper towel was fixed at the base of the ear using a rubber band. The paper towel was moistened every two days to maintain ear turgidity. Ears in stage R1 were selected to obtain silks, and ears between stages R2 (blister stage) and R3 (milk stage) [29] were selected for the on-ear competition scenarios.
Larvae used in this study were removed from the artificial diet and kept individualized in plastic cups one hour to fast before each assay. For the competition study with maize silks, evaluations of larval survival were performed daily for a period of 10 days. For the competition study using maize ear, the evaluations of larval survival were performed 10 days after infestation due to the difficulty in accessing larvae in the ear every day.
Blue and red paint (Luminous Paints Kit, BioQuip Products, Rancho Dominguez, CA, USA) were utilized to identify larvae in the scenario of H. armigera and H. zea (2nd vs. 2nd or 4th vs. 4th) in plastic cups. Larvae were checked twice a day and repainted after each molt. In competition study conducted on maize ears (in the laboratory and in the field), the surviving larvae were kept individualized in laboratory on the same diet. After moth emergence, species identification was carried out through examination of the morphological characteristics of genitalia [31].
Field intraguild competition
The field competition studies were carried out in non-Bt maize fields grown using standard agronomic practices recommended for the region of Botucatu, SP, Brazil. Natural infestations of S. frugiperda, H. zea, and H. armigera were managed using the insecticide clorfluazuron (Atabron, ISK Ltd., Indaiatuba, Brazil) during vegetative stages. During the reproductive stages, insecticide applications ceased and eggs, larvae and moths were manually eliminated when detected.
For each planting date, an area of approximately 1500 m2 was divided into five blocks, evenly spaced, with 10 plots each (corresponding to the competition scenarios). Four replicates were established in each plot in a randomized complete block design. Each plot was 4 m long with three rows, spaced 0.70 m apart, corresponding to approximately 22 m2. In each plot, only the central row was used for the competition scenario evaluations.
When the plants reached R2-R3, each maize ear was infested with larvae, placed on the region of the silks (Table 1), and the maize covered with an organdy fabric bag (25 x 30 cm) [39]. The survival of larvae was assessed 10 days after infestation.
Statistical Analyses
The proportions of H. zea and H. armigera sampled in maize ears were calculated and expressed in percentage. Larval survival data from the different lab and field studies of larval competition were assessed for normality with Shapiro-Wilks tests. The data were tested using Chi-square test (x2) (P ≤ 0.05) (CHISQ option, PROC FREQ, SAS Institute 2001) between the survival in the competition treatment scenario and its corresponding control [40]. The control treatment for each competition scenario consisted of individuals of the same species, in the same instars as larvae from the corresponding treatments (Table 1).
Results
Occurrence and larval percentage of H. zea and H. armigera in maize fields in Brazil
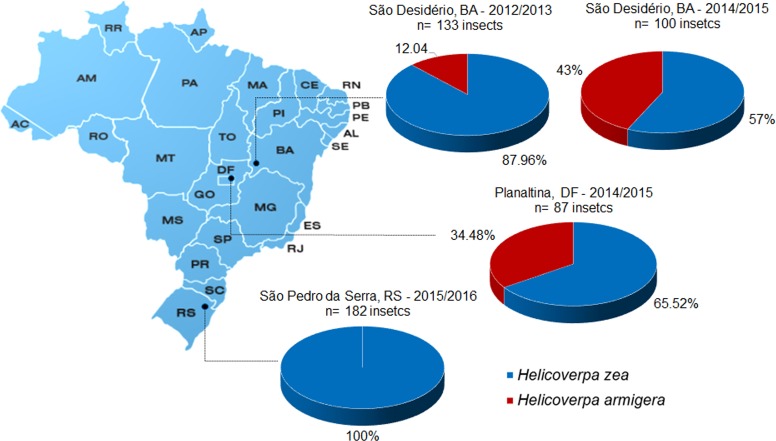
Overall, some larval mortality was observed during the transport of the larvae collected, in part because of parasitism by Diptera and wasps from the Ichenumonidae family [41]. In western Bahia, from the 200 larvae collected in the 2012/2013 crop season, 133 adults emerged. Examination of adult female and male genitalia indicated 117 H. zea (87.96%) and 16 H. armigera (12.04%) (Fig 1). In the 2014/2015 crop season, from 100 adults that emerged, 57 were identified as H. zea and 43 were H. armigera. In central Brazil, from 87 adults that emerged, 57 (65.52%) adults were H. zea and 30 (34.48%) were H. armigera. In the collection from southern Brazil, from 182 adults that emerged, 100% were H. zea. Helicoverpa armigera was not found in any collections from samples in the south. More than one larvae per ear was never observed in any collection.
Fig 1. Infestation of Helicoverpa zea and Helicoverpa armigera in maize ears, in three Brazilian regions.
Intraguild competition
In laboratory trials with maize silks, the survival of H. armigera did not differ significantly in competitions of 2nd vs. 2nd instar against H. zea or H. armigera, where the survival ranged between 20 and 35% (Table 2). There were no significant differences between the survival of H. armigera on maize silks when the competitions were between 2nd vs. 4th instar (0% survival) and 4th vs. 2nd instar (100% survival). In scenario 4th vs. 4th instar, with maize silks, survival of H. armigera was lower in intraspecific competition (0% survival), compared to the scenario against H. zea (25% survival).
Table 2. Survival of Helicoverpa armigera in intraguild competition against Helicoverpa zea and in intraspecific competition by instar, in maize silks and ears, in the laboratory and field.
| Site | Scenario a | Survival of H. armigera (%) | Scenario | Survival of H. armigera (%) | ||||
|---|---|---|---|---|---|---|---|---|
| H. armigera | H. zea | H. armigera | H. armigera | x2 | P b | |||
| Silks | 2nd | 2nd | 35 | 2nd | 2nd | 20 | 1.12 | 0.2881 |
| Silks | 2nd | 4th | 0 | 2nd | 4th | 0 | - | - |
| Silks | 4th | 2nd | 100 | 4th | 2nd | 100 | - | - |
| Silks | 4th | 4th | 0 | 4th | 4th | 25 | 5.71 | 0.0168 |
| Ear | 2nd | 2nd | 20 | 2nd | 2nd | 30 | 0.53 | 0.4652 |
| Ear | 2nd | 4th | 15 | 2nd | 4th | 20 | 0.17 | 0.6673 |
| Ear | 4th | 2nd | 100 | 4th | 2nd | 95 | 1.02 | 0.3112 |
| Ear | 4th | 4th | 25 | 4th | 4th | 30 | 0.12 | 0.7233 |
a First column, H. armigera (treatment); second column, competitor
b P value related to the comparison the survival in intraspecific and interspecific competition.
In trials with maize ears, the survival of H. armigera did not differ among the competition scenarios, with the percentage of survival from 95% in 4th vs. 2nd instar scenario and between 15 and 30% in the other scenarios.
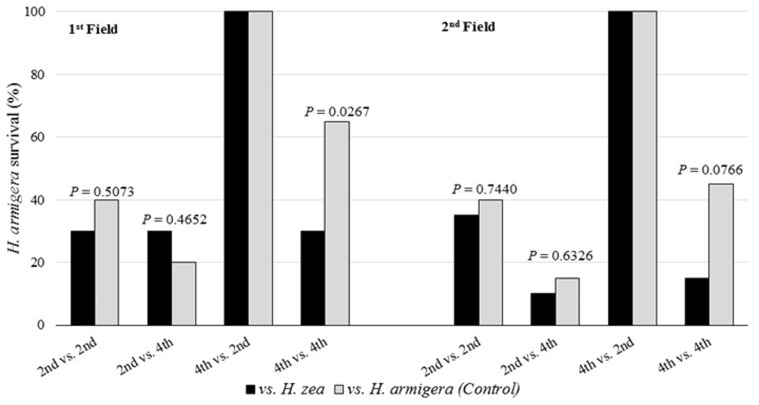
Regarding the first field competition study (Fig 2), there were no significant differences in H. armigera survival between the scenarios involving H. zea and the control, except in scenario 4th vs. 4th instar, where H. armigera had 65% survival in competition against its conspecific and 30% against H. zea (x2 = 4.91; df = 1; P = 0.0267). For the second field study, there were no significant differences in H. zea survival between the scenarios against H. zea or in intraspecific competition, except in scenario 4nd vs. 4th instar, where the survival of H. armigera was higher in intraspecific competition compared to the survival against H. zea (x2 = 4.29; df = 1; P = 0.0384), with 45 and 15% survival, respectively.
Fig 2. Survival of Helicoverpa armigera in intraspecific and interspecific interactions in field conditions in two crop seasons.
In laboratory trials with maize silks for H. zea, (Table 3), the survival of H. zea was significantly higher in competitions against H. armigera (75%) than against H. zea (0%) (x2 = 24.00; df = 1; P < 0.0001). There were no significant differences in survival of H. zea larvae in scenario 2nd vs. 4th instar and 4th vs. 2nd instar, with the larvae having 0% survival in the first case and 100% survival when larvae were larger than the competitor. In the scenario 4th vs. 4th instar, larvae of H. zea continued to show greater survival in competition against H. armigera (75%) than in competition against H. zea (0%) (x2 = 24.00; df = 1; P < 0.0001), similar to that observed in scenario 2nd vs. 2nd.
Table 3. Survival of Helicoverpa zea in intraguild competition against Helicoverpa armigera in intraspecific competition by instar, in maize silks and ears, in the laboratory and field.
| Site | Scenario a | Survival of H. zea (%) | Scenario | Survival of H. zea (%) | ||||
|---|---|---|---|---|---|---|---|---|
| H. zea | H. zea | H. zea | H. armigera | x2 | P b | |||
| Silks | 2nd | 2nd | 0 | 2nd | 2nd | 75 | 24.00 | <0.0001 |
| Silks | 2nd | 4th | 0 | 2nd | 4th | 0 | - | |
| Silks | 4th | 2nd | 100 | 4th | 2nd | 100 | - | - |
| Silks | 4th | 4th | 0 | 4th | 4th | 75 | 24.00 | <0.0001 |
| Ear | 2nd | 2nd | 30 | 2nd | 2nd | 80 | 10.10 | 0.0015 |
| Ear | 2nd | 4th | 10 | 2nd | 4th | 30 | 2.50 | 0.1138 |
| Ear | 4th | 2nd | 95 | 4th | 2nd | 100 | 1.02 | 0.3112 |
| Ear | 4th | 4th | 10 | 4th | 4th | 70 | 15.00 | 0.0001 |
a First column, H. zea (treatment); second column, competitor
b P value related to the comparison the survival in intraspecific and interspecific competition.
On maize ears, the results of H. zea survival were similar to those observed on maize silks (Table 3). There was no difference in the scenario 2nd vs. 4th instar and 4th vs. 2nd instar. In the scenario of 2nd vs. 2nd instar, H. zea larvae had 80% survival in competition against H. armigera (x2 = 10.10; df = 1; P = 0.0015), while in intraspecific competition, the survival was 30%. When competing in scenario 4th vs. 4th instar, the survival of H. zea remained high against H. armigera (70%), and low in intraspecific competition (10%) (x2 = 15.00; df = 1; P = 0.0001).
For both field competition studies, the results were similar for the scenarios of 2nd vs. 2nd instar and 4th vs. 4th instar (Fig 2). In the first scenario, H. zea survival was above 75% in competition against H. armigera, while in intraspecific competition the survival was below 10% (x2 = 19.79; 20.42; df = 1; P < 0.0001). In scenario 4th vs. 4th instar, survival of H. zea remained higher in competition against H. armigera (above 65%) in relation to survival against H. zea (0%) (x2 = 26.66; 19.26; df = 2; P < 0.0001). In the first field competition study, there were no significant differences in the scenario of 2nd vs. 4th instar and 4th vs. 2nd instar. In the second study, there was a significant difference in the scenario of 2nd vs. 4th instar, when the survival was higher in competition against H. armigera (35%) compared to the control (x2 = 8.48; df = 1; P = 0.0036). No difference was observed for the scenario 4th vs. 2nd instar in the second competition study.
Discussion
Larval survival in plastic cups with maize silks was lower than larval survival in ears (Tables 2 and 3). Although larval survival was slightly lower in the laboratory than in the field, in general the behaviors and relationships observed under both sites were similar. The field study used arenas that allowed the larvae in maize ears to be isolated from the external environment and natural predators, parasitoids or other food competitors, which can be significant causes of larval mortality [42]. This demonstrates the importance of the competition arena size and the food source. The higher survival in maize ears might be related to a greater quantity and higher quality of food for the larvae [43] compared to simply silks, which provide less nutritional value [44] than maize ears (i.e. silks and kernels). In addition, larval encounters in the maize ear may be less frequent due to the larger area available for escape, and therefore less chance of an aggressive encounter, which is a larval response during larval interaction [45, 46]. Overall, results indicate that the maize ear is an appropriate arena to study intraguild competition between H. zea and H. armigera, and among other ear feeding noctuid species.
Helicoverpa zea exhibited the highest level of cannibalism, mainly in plastic cups with maize silks (limited food and small arenas). Cannibalism is dependent on instar, with higher rates in later instars, and especially when larvae of different instars interact [47–49]. Cannibalism in H. zea may occur at rates of up to 75% [48, 50]. Cannibalism and intraguild predation are treated as direct benefits when food is limited, where one larva preying on others can result in fitness benefits and increase the rate of development, body mass and fecundity [12, 48, 49], or as an indirect benefit by the exclusion of a potential food competitor [47, 48]. Negative effects of cannibalism may be injury and subsequent death, or acquisition of pathogens or parasites and consequent reduction in larval fitness and development [49–54]. These results suggest the aggressive behavior of H. zea is higher than that of H. armigera in intraspecific competition scenarios (Tables 2 and 3) (Figs 2 and 3). The impact of aggressiveness and the cannibalism in H. zea populations help to explain why the occurrence of more than one larvae in a maize ear is rare [55], and was not observed during larval collections in maize in different regions in Brazil.
Fig 3. Survival of Helicoverpa zea in intraspecific and interspecific interactions in field conditions in two crop seasons.
The introduction of H. armigera in Brazil and other countries in the American continents, presents the opportunity and need to investigate the interaction of these former allopatric species. An initial result is the possible occurrence of hybrids between the two species, which until now has only been documented in the laboratory under artificial conditions [2, 56–58]. An examination of all specimens collected from the field did not reveal any variability in female and male genital characters as described by Hardwick (1995) that would indicate the occurrence of hybrids from cross-mating between H. zea and H. armigera under natural conditions.
Another aspect of H. armigera’s introduction is the possible interspecific competition between these two species. Previously, H. zea and H. armigera were considered one cosmopolitan species, referred to as either Heliothis obsolete or H. armigera [2]. Based on taxonomic investigations in different regions of the world, an actual complex of species was suggested under the new genus Helicoverpa, the armigera group represented by two species in the Old World and the zea group represented by eight species distributed in America continents [2]. This increasing acknowledgment of Heliothinae systematics [59, 60] indicated H. zea and H. armigera originated from a common ancestor and were geographically isolated and defined as allopatric species [2, 58, 59].
The maize ear is an appropriate arena for intraguild interaction studies. Natural infestation of both species occur during the reproductive stage [14, 19], and exhibit similar development [61, 62], which increases the possibility of interaction. Although a challenge in the competition studies was the precise identification of the species of the insect that survived, differentiation between H. zea and H. armigera is possible with the dissection and examination of genitalia characteristics [31].
The preliminary expectation of the interaction between H. zea and H. armigera was that H. armigera would be favored, since it was recently detected as an invasive species in Brazil [4–6] it would have all advantages that a new organism typically has in a new environment [63]. However, the intermediate to low survival of H. armigera in competition scenarios 2nd (vs. 2nd), 2nd (vs. 4th) and 4th (vs. 4th) (Table 2) (Fig 2) suggests that this species is negatively affected in intraspecific competition (with a considerable percentage of cannibalism), and in interspecific competition (intraguild predation) on non-Bt maize. In competition scenarios 2nd (vs. 2nd) and 4th (vs. 4th), the low survival of H. armigera in intraspecific competition and against H. zea indicates the presence of cannibalism and the disadvantage of larvae competing with H. zea of the same instar (Table 2) (Fig 2). The results of 4th vs. (4th) in the field support this disadvantage, where 80% survival of H. zea was observed (Fig 3). The low survival of H. amigera in 2nd (vs. 4th) competitions, with zero survival on maize silks, suggests the importance of the arena size and food source during an intraguild competition. This scenario also indicates the importance of larval stadia during a competition, where the smaller insect has considerable disadvantages. Similarly, in 4th vs. (2nd) competition, the high larval survival indicates the importance of the instar in intraguild competition between the species, and only in this case does larvae of H. armigera have a high probability of gaining advantage over H. zea. Some studies have reported similar development between these species [10, 61, 62, 64], however, a recent study conducted on artificial diet indicated that H. zea are larger than H. armigera, and have slower development [64]. Although the study was conducted on artificial diet, it indicates that biological differences between the species may occur, and along with host plant and other factors, influence intraguild competition.
Overall, in the interspecific competition, H. zea larval survival was higher when in competition against H. armigera, even when H. zea was smaller than H. armigera. The survival of H. zea indicates the potential of this species to gain advantage in intraguild competition, as was observed in the second crop season.
The percentage of H. zea:H. armigera in maize ears determined in natural infestations indicates the prevalence of H. zea, and supports the results observed in the competition studies. However, it is important to highlight that the prevalence of H. zea was variable in different regions. In Rio Grande do Sul, H. armigera was not detected in the maize ears (Fig 1), even though this species was reported as present in southern Brazil from at least 2011 [7]. In Planaltina, DF (central Brazil), the proportion of H. zea: H. armigera larvae in maize ears was approximately 2:1 (Fig 1). In western BA, two different infestation events and proportions of H. zea:H. armigera larvae in maize ears were documented. During the 2012/2013 crop season, when the first outbreak of the H. armigera in the region was reported [4–6], the proportion of H. zea: H. armigera larvae in maize ears was 7:1. In the following crop season (2013/2014), an increase of the proportion of H. armigera to 1:1 larvae in maize ears was observed (Fig 1).
These field data from natural infestations in maize indicate that besides the aggressive behavior of H. zea, the region-specific landscape, and especially the presence of cotton, may play a role in the presence and proportion of H. armigera in maize. Cotton is a preferred host of H. armigera [65–67]. In Rio Grande do Sul there is no cultivation of cotton. In Planaltina, DF (central Brazil), cotton is present, but not a prevalent crop in a diversified mosaic of several hosts, including soybean, maize, dry beans, and vegetables, among others. In western BA, the landscape is a mosaic formed by an ocean of soybean and patches of maize and cotton. It is possible that because of the higher proportion of cotton than maize in the western BA landscape, H. armigera has been able to successfully establish and build high populations. These populations feed in maize as an alternative host, especially in the beginning of the crop season because maize is the first crop planted in the region. Moreover, these results indicate a significant presence of H. armigera in maize even when in competition with the aggressive H. zea. This hypothesis is supported by the absence of H. armigera in maize in a state that does not cultivate cotton, and the increase of the proportion of H. armigera in maize in western BA from the 2012/2013 to the 2014/2015 crop seasons.
In an IPM perspective, these results indicate that maize and cotton could play a role as a reservoir of H. armigera in the landscape. Helicoverpa armigera has preference for reproductive plant stages and maize has a relatively short flowering stage. In consequence, maize supports only one generation of this species [19]. However, the cultivation of maize all year round in Brazil provides the opportunity for maize to be a source of infestation of H. armigera, even though competition with H. zea will in part regulate the population density of H. armigera on non-Bt hybrids.
On the other hand, these results should be also be considered with respect to insect resistance management to insecticides and to Bt technology. Maize may be considered a source of H. armigera unselected by insecticides commonly used in cotton, such as emamectin benzoate [68, 69]. Non-Bt maize may also support populations of H. armigera that are not exposed to Bt proteins. This is the case for Cry1Ac and Cry2Ae expressed in some Bt cotton events [70–72], and Cry1Ac in Bt soybean, which is cultivated in large areas in Brazil since the 2014/2015 crop season.
In the savanna areas of Brazil, with a prevalence of maize, soybean and cotton, it is important to investigate how the agroecosystem will define the prevalence and the impact of these two former allopatric species. In the same way, because of the recent detection of H. armigera in the United States [10], it is important to consider the variable agroecosystems of North America (e.g. Corn Belt, Cotton Belt) on how H. zea and H. armigera will ultimately impact IPM and IRM in the Americas.
Acknowledgments
We thank Fernando Martins and Henrique Medeiros for the help in the dissection of adult genitalia, Programa Fitossanitário da Associação Baiana dos Produtores de Algodão (ABAPA), and the group Horita, for the support in insect collection.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Council for Scientific and Technological Development (CNPq: 403376/2013-0): AS, SVP; Embrapa Project, MP2 (PA: 02.13.14.606.00.00 / 02.13.14.606.00.02): AS, SVP; Coordination for the Improvement of Higher Education Personnel (CAPES: 99999.002564/2014-09): JPFB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mitter C, Poole RW, Matthews M. Biosystematics of the Heliothinae (Lepidoptera: Noctuidae). Annu Rev Entomol. 1993; 38: 207–25. [Google Scholar]
- 2.Hardwick DF. The corn earworm complex. Mem Entomol Soc Can. 1965; 97: 5–247. [Google Scholar]
- 3.Kitching IJ, Rawlins JE. The Noctuoidea In: Kristensen NP, editors. Lepidoptera: moths and butterflies. New York: Walter de Gruyter; 1998. p. 355–401. [Google Scholar]
- 4.Embrapa. Nota técnica sobre resultado do trabalho inicial de levantamento da lagarta do gênero Helicoverpa–detecção da espécie Helicoverpa armigera no Brasil Planaltina: Embrapa Cerrados; 2013. 2p. [Google Scholar]
- 5.Czepack C, Albernaz KC, Vivan LM, Guimarães H. O., Carvalhais T. First reported occurrence of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil. Brasil Pesq Agropec Trop. 2013; 43: 110–113. [Google Scholar]
- 6.Specht A, Sosa-Gómez DR, Paula-Moraes SV, Yano SAC. Morphological and molecular identification of Helicoverpa armigera (Lepidoptera: Noctuidae) and expansion of its occurrence record in Brazil. Pesq Agropec Bras. 2013; 48: 689–692. [Google Scholar]
- 7.Sosa-Gómez DR, Specht A, Paula-Moraes SV, Lopes-Lima A, Yano SAC, Micheli A, et al. Timeline and geographical distribution of Helicoverpa armigera (Hübner) (Lepidoptera, Noctuidae: Heliothinae) in Brazil. Rev Bras Entomol. 2016; 60: 101–104. [Google Scholar]
- 8.Kriticos DJ, Ota N, Hutchison WD, Beddow J, Walsh T, Tay WT, et al. The potential distribution of invading Helicoverpa armigera in North America: Is it just a matter of time? PLOS One. 2015; 10: e0119618 10.1371/journal.pone.0119618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murúa MG, Scalora FS, Navarro FR, Cazado LE, Casmuz A, Villagrán ME, et al. First record of Helicoverpa armigera (Lepidoptera: Noctuidae) in Argentina. Fla Entomol. 2014; 97: 854–856. [Google Scholar]
- 10.Hayden JE, Brambila J. Helicoverpa armigera (Lepidoptera: Noctuidae), the Old World Bollworm. Pest Alert, Florida Department of Agriculture and Consumer Services, Division of Plant Industry; 2015 Jun. Report No.: FDACS-02039
- 11.Fitt GP. The ecology of Heliothis species in relation to agroecosystems. Annu Rev Entomol. 1989; 34: 17–52. [Google Scholar]
- 12.Kakimoto T, Fujisaki K, Miyatake T. Egg laying preference, larval dispersion, and cannibalism in Helicoverpa armigera (Lepidoptera: Noctuidae). Ann Entomol Soc Am. 2003; 96: 793–798. [Google Scholar]
- 13.Perkins LE, Cribb BW, Hanan J, Glaze E, Beveridge C, Zalucki MP. Where to from here? The mechanisms enabling the movement of first instar caterpillars on whole plants using Helicoverpa armigera (Hübner). Arthrop Plant Interact. 2008; 2: 197–207. [Google Scholar]
- 14.Burkness EC, Dively GP, Patton T, Morey AC, Hutchison WD. Novel Vip3A Bacillus thuringiensis (Bt) maize approaches high dose efficacy against Helicoverpa zea (Lepidoptera: Noctuidae) under field conditions: implications for resistance management. GM Crops. 2010; 1: 337–343. 10.4161/gmcr.1.5.14765 [DOI] [PubMed] [Google Scholar]
- 15.Hutchison WD, Burkness EC, Jensen B, Leonard BR, Temple J, Cook DR, et al. Evidence for decreasing Helicoverpa zea susceptibility to pyrethroid insecticides in the Midwestern United States. Plant Health Progress; 2007: [Google Scholar]
- 16.Jacboson A, Foster R, Krupke C, Hutchison W, Pittendrigh B, Weinzieri R. Resistance to pyrethroid insecticides in Helicoverpa zea (Lepidoptera: Noctuidae) in Indiana and Illinois. J Econ Entomol. 2009; 102: 2289–2295. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho RA, Omoto C, Field LM, Williamson MS, Bass C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLOS One. 2013; 8: e62268 10.1371/journal.pone.0062268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisig DD, Reay-Jones FPF. Inhibition of Helicoverpa zea (Lepidoptera: Noctuidae) growth by transgenic corn expressing Bt toxins and development resistance to Cry1Ab. Environ Entomol. 2015; 44: 1275–1285. 10.1093/ee/nvv076 [DOI] [PubMed] [Google Scholar]
- 19.Zalucki MP, Daglish G, Firempong S, Twine PH. The biology and ecology of Helicoverpa armigera (Hübner) and H. punctigera Wallengren (Lepidoptera: Noctuidae) in Australia: what do we know? Aust J Zool. 1986; 34: 779–814. [Google Scholar]
- 20.Cunningham JP, Zalucki MP. Understanding Heliothine (Lepidoptera: Heliothinae) pests: what is a host plant? J Econ Entomol. 2014; 107: 881–896. [DOI] [PubMed] [Google Scholar]
- 21.Keszthelyi S, Szentpéteri J, Pál-Fám F. Morphometrical and front wing abrasion analysis of a Hungarian cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) population. Biologia. 2011; 66: 340–348. [Google Scholar]
- 22.Kranthi KR, Jadhav D, Wanjari R, Kranthi S, Russell D. Pyrethroid resistance and mechanisms of resistance in field strains of Helicoverpa armigera (Lepidoptera: Noctuidae). J Econ Entomol. 2001; 94: 253–263. [DOI] [PubMed] [Google Scholar]
- 23.Torres-Vila LM, Rodríguez-Molina MC, Lacasa-Plasencia A, Bielza-Lino P, Rodríguez-del-Rincón Á. Pyrethroid resistance of Helicoverpa armigera in Spain: current status and agroecological perspective. Agric Ecosyst Environ. 2002; 93: 55–66. [Google Scholar]
- 24.Pratissoli D, Lima VLS, Pirovani VD, Lima WL. Occurrence of Helicoverpa armigera (Lepidoptera: Noctuidae) on tomato in the Espírito Santo state. Hortic Bras. 2015; 33: 101–105. 2015. [Google Scholar]
- 25.Siebert MW, Nolting SP, Hendrix W, Dhavala S, Craig C, Leonard BR, et al. Evaluation of corn hybrids expressing Cry1F, Cry1A.105, Cry2Ab2, Cry34Ab1/Cry35Ab1, and Cry3Bb1 against southern United States insect pests. J Econ Entomol. 2012; 105: 1825–1834. [DOI] [PubMed] [Google Scholar]
- 26.Duffield SJ, Chapple DG. Within-plant distribution of Helicoverpa armigera (Hübner) and Helicoverpa punctigera (Wallergren) (Lepidoptera: Noctuidae) eggs on irrigated soybean. Aust J Biol Sci. 2001; 40: 151–157. [Google Scholar]
- 27.Pierce NE. Predatory and parasitic Lepidoptera: carnivores living on plants. J Lepid Soc. 1995; 49: 412–453. [Google Scholar]
- 28.Chapman JW, Williams T, Martínez AM, Cisneros J, Caballero P, Cave RD, et al. Does cannibalism in Spodoptera frugiperda (Lepidoptera: Noctuidae) reduce the risk of predation? Behav Ecol Sociobiol. 2000; 48: 321–327. [Google Scholar]
- 29.Ritchie SW, Hanway JJ, Benson GO. How a corn plant develops. Iowa State University of Science and Technology, Ames, IA; 1993. Report No.: 48
- 30.Montezano DG, Specht A, Bortolin TM, Fronza E, Sosa-Gómez DR, Roque-Specht VF, et al. Immature stages of Spodoptera albula (Walker) (Lepidoptera: Noctuidae): developmental parameters and host plants. An Acad Bras Cienc. 2013; 85: 271–284. [DOI] [PubMed] [Google Scholar]
- 31.Pogue MG. A new synonym of Helicoverpa zea (Boddie) and differentiation of adult males of H. zea and H. armigera (Hübner) (Lepidoptera: Noctuidae: Heliothinae). Ann Entomol Soc Am. 2004; 97: 1222–1226. [Google Scholar]
- 32.Laster ML, Hardee DD. Intermating compatibility between North American Helicoverpa zea and Heliothis armigera (Lepidoptera: Noctuidae) from Russia. J Econ Entomol. 1995; 88: 77–80. [Google Scholar]
- 33.Laster ML, Sheng CF. Search for hybrid sterility for Helicoverpa zea in crosses between the North American H. zea and H. armigera (Lepidoptera: Noctuidae) from China. J Econ Entomol. 1995; 88: 1288–1291. [Google Scholar]
- 34.Jah RK, Chi H, Tang LC. A comparison of artificial diet and hybrid sweet corn for the rearing of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) based on life table characteristics. Environ Entomol. 2012; 41: 30–39. 10.1603/EN11206 [DOI] [PubMed] [Google Scholar]
- 35.Kao SS. Mass rearing of insects. In: Lee KC, editor. Taiwan Agricultural Chemicals and toxic substances research institute special report. Taichung: Taiwan; 1995. p. 1–8.
- 36.Dorhout DL, Rice ME. Intraguild competition and enhanced survival of western bean cutworm (Lepidoptera: Noctuidae) on transgenic Cry1Ab Bt corn. J Econ Entomol. 2010; 103: 54–62. [DOI] [PubMed] [Google Scholar]
- 37.Chilcutt CF, Tabashnik BE. Contamination of refuges by Bacillus thuringiensis toxin genes from transgenic maize. Proc Natl Acad Sci USA. 2004; 101: 7526–7529. 10.1073/pnas.0400546101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burkness EC, Hutchison WD. Bt pollen dispersal and Bt kernel mosaics: integrity of non-Bt refugia for lepidopteran resistance management in maize. J Econ Entomol. 2012; 105: 1773–1780. [DOI] [PubMed] [Google Scholar]
- 39.Paula-Moraes SV, Hunt TE, Wright RJ, Hein GL, Blankenship EE. Western bean cutworm survival and the development of economic injury levels and economic thresholds in field corn. J Econ Entomol. 2013; 106: 1274–1285. [DOI] [PubMed] [Google Scholar]
- 40.Sas Institute. SAS/STAT user’s guide, version 8.1 Cary: SAS Institute; 2001. [Google Scholar]
- 41.Luz PMC, Otanásio PN, Claudino VCM, Penteado-Dias AM, Pujol-Luz JR, Paula-Moraes SV, et al. Ocorrência de parasitoides associados com Helicoverpa amigera (Hübner, 1809) no Oeste da BA. XXVIII Congresso Brasileiro de Entomologia and IX Congresso Latino-Americano de Entomologia; 2016 Mar 13–17; Maceió, AL. Maceió: Brazil 2016.
- 42.Zalucki MP, Clarke AR, Malcom SB. Ecology and behavior of first instar larval Lepidoptera. Annu Rev Entomol. 2002; 47: 361–393. 10.1146/annurev.ento.47.091201.145220 [DOI] [PubMed] [Google Scholar]
- 43.Pannuti LER, Baldin ELL, Hunt TE, Paula-Moraes SV. On-plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages. Environ Entomol. 2015; 45: 192–200. 10.1093/ee/nvv159 [DOI] [PubMed] [Google Scholar]
- 44.Paula-Moraes SV, Hunt TE, Wright RJ, Hein GL, Blankenship EE. On-Plant movement and feeding of western bean cutworm (Lepidoptera: Noctuidae) early instars on corn. J Econ Entomol 2012; 41: 1494–1500. [DOI] [PubMed] [Google Scholar]
- 45.Dial CI, Adler PH. Larval behavior and cannibalism in Heliothis zea (Lepidoptera: Noctuidae). Ann Entomol Soc Am. 1990. 83: 258–263. [Google Scholar]
- 46.Horner TA, Dively GP, Herbert DA. Development, survival and fitness performance of Helicoverpa zea (Lepidoptera: Noctuidae) in MON-810 Bt field corn. J Econ Entomol. 2003; 96: 914–924. [DOI] [PubMed] [Google Scholar]
- 47.Polis G. The evolution and dynamics of intraspecific predation. Annu Rev Ecol Syst. 1981; 12: 125–151. [Google Scholar]
- 48.Joyner K, Gould F. Developmental consequences of cannibalism in Heliothis zea (Lepidoptera: Noctuidae). Ann Entomol Soc Am. 1985; 78: 24–28. [Google Scholar]
- 49.Chilcutt CF. Cannibalism of Helicoverpa zea (Lepidoptera: Noctuidae) from Bacillus thuringiensis (Bt) transgenic corn versus non-Bt corn. J Econ Entomol. 2006; 99: 728–732. [DOI] [PubMed] [Google Scholar]
- 50.Fox L. Cannibalism in natural populations. Annu Rev Ecol Syst. 1975; 6: 87–106. [Google Scholar]
- 51.Pfennig DW, Reeve HK, Sherman PW. Kin recognition and cannibalism in spadefoot toad tadpoles. Anim Behav. 1993; 46: 87–94. [Google Scholar]
- 52.Chapman JW, Williams T, Escribano A, Caballero P, Cave RD, Goulson D. Age-related cannibalism and horizontal transmission of a nuclear polyhedrosis virus in larval Spodoptera frugiperda. Ecol Entomol. 1999a; 24: 268–275. [Google Scholar]
- 53.Chapman JW, Williams T, Escribano A, Caballero P, Cave RD, Goulson D. Fitness consequences of cannibalism in the fall armyworm, Spodoptera frugiperda. Behav Ecol. 1999b; 10: 298–303. [Google Scholar]
- 54.Rudolf VH, Antonovics J. Disease transmission by cannibalism: rare event or common occurrence? Proc R Soc B Biol Sci. 2007; 274: 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mally FW. Report of progress in the investigation of the cotton bollworm. U.S. Dep Agric Div Entomol Bull. 1892; 26: 45–56. [Google Scholar]
- 56.Cho S, Mitchell A, Mitter C, Regier J, Matthews M, Robertson R. Molecular phylogenetics of heliothine moths (Lepidoptera: Noctuidae: Heliothinae), with comments on the evolution of host range and pest status. Syst Entomol. 2008; 33: 581–594. [Google Scholar]
- 57.Hardwick DF. A generic revision of the North American Heliothidinae (Lepidoptera: Noctuidae). Mem Entomol Soc Can. 1970; 73: 1–59. [Google Scholar]
- 58.Matthews M, Classification of the Heliothinae. Chatham, Kent: Natural Resources Institute; 1991. Report No.: 44
- 59.Matthews M. Heliothine moths of Australia: a guide to pest bollworms and related noctuid groups Melbourne: CSIRO; 1999. [Google Scholar]
- 60.Zhao XC, Berg BG. Arrangement of output information from the 3 macroglomerular units in the heliothine moth Helicoverpa assulta: morphological and physiological features of male-specific projection neurons. Chem Senses. 2010; 35: 511–521. 10.1093/chemse/bjq043 [DOI] [PubMed] [Google Scholar]
- 61.Butler GD Jr. Bollworm: development in relation to temperature and larval food. Environ Entomol. 1976; 5: 520–522. [Google Scholar]
- 62.Mironidis GK, Savopoulou-Soultani M. Development, survivorship, and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under constant and alternating temperatures. Environ Entomol. 2008; 37: 16–28. 10.1603/0046-225X(2008)37[16:DSAROH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 63.Pimentel D. Biological invasions. Economic and Environmental Costs of Alien Plant, Animal and microbe species. Boca Raton: CRC Press; 2002. [Google Scholar]
- 64.Barbosa TAN, Mendes SM, Rodrigues GT, Ribeiro PEA, Santos CA, Valicente FH, Oliveira CM. Comparasion of biology between Helicoverpa zea and Helicoverpa armigera (Lepidoptera: Noctuidae) reared on artificial diets. Flo Entomol. 2016; 99: 72–76. [Google Scholar]
- 65.Firempong S, Zalucki MP. Host plant preferences of populations of Helicoverpa armigera (Hübner) (Lepidoptera, Noctuidae) from different geographic locations. Aust J Zool. 1990; 37: 665–673. [Google Scholar]
- 66.Sharma HC. Cotton bollworm/legume pod borer, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): biology and management. Crop protection compendium Wallingford: CAB International; 2001. [Google Scholar]
- 67.Liu ZD, Gong PY, Wu KJ, Li DM. Effects of parental exposure to high temperature on offspring performance in the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): adaptive significance of the summer diapause. Appl Entomol Zool. 2004; 39: 373–379. [Google Scholar]
- 68.Downes S, Mahon R, Olsen K. Monitoring and adaptive resistance management in Australia for Bt-cotton: current status and future challenges. J Invertebr Pathol. 2007; 95: 208–213. 10.1016/j.jip.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 69.Yang Y, Li Y, Wu Y. Current status of insecticide resistance in Helicoverpa armigera after 15 years of Bt cotton planting in China. J Econ Entomol. 2013; 106: 375–381. [DOI] [PubMed] [Google Scholar]
- 70.Bird LJ, Akhurst RJ. Variation in susceptibility of Helicoverpa armigera (Hübner) and Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) in Australia to two Bacillus thuringiensis toxins. J Invertebr Pathol. 2007; 94: 84–94. 10.1016/j.jip.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 71.Luo S, Wu K, Tian Y, Liang G, Feng X, Zhang J, et al. Cross-resistance studies of Cry1Ac-resistant strains of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry2Ab. J Econ Entomol. 2007; 100: 909–915. [DOI] [PubMed] [Google Scholar]
- 72.Gouffon C, Van Rie J, Jansens S, Jurat-Fuentes JL. Binding sites for Bacillus thuringiensis Cry2Ae toxin on heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F, or Vip3A toxin. Appl Environ Microbiol. 2011; 77: 3182–3188. 10.1128/AEM.02791-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.