Abstract
The importance of Notch signaling in colorectal cancer (CRC) carcinogenesis and progression has previously been presented. Increased expression of Jagged‐1 (JAG1), a Notch ligand, in CRC has been revealed, but the detailed prognostic significance of JAG1 in CRC has not been determined. Protein expression of JAG1 was examined using immunohistochemistry in 158 CRC specimens. Expression of JAG1 and E‐cadherin and their associations with clinicopathologic characteristics, overall survival (OS) and relapse‐free survival (RFS) were evaluated. In vitro studies using compounds to regulate intracellular signaling and small interfering RNA to silence JAG1 were performed in a colon cancer cell line. JAG1 expression in cancerous tissues was weak, moderate or strong in 32%, 36% and 32% of specimens, respectively, and correlated with histologic type and T stage. In multivariate analysis, JAG1 expression, histologic type and lymphatic invasion independently correlated with OS and RFS. The combination of high JAG1 expression and low E‐cadherin expression had an additive effect toward poorer OS and RFS compared with the low JAG1/high E‐cadherin expression subtype. A significant correlation between JAG1 expression and KRAS status was detected in groups stratified as high E‐cadherin expression. In vitro studies suggested that RAS‐MEK‐MAP kinase and the Wnt pathways positively regulated JAG1 expression. Gene silencing with siJAG1 indicated that JAG1 promotes the transition from epithelial to mesenchymal characteristics and cell growth. High expression of JAG1 is regulated by various pathways and is associated with poor prognosis through promoting the epithelial to mesenchymal transition and cell proliferation or maintaining cell survival in CRC.
Keywords: Colorectal cancer, epithelial–mesenchymal transition, JAG1, Notch, survival rate
The Notch signaling pathway is important for intestinal epithelial stem/progenitor cell self‐renewal and differentiation.1 Four Notch receptors (Notch 1–4) and five Notch ligands (Delta‐like 1 [DLL1], DLL3, DLL4, Jagged‐1 [JAG1] and JAG2) have been identified.2 JAG1, like the other ligands, binds to Notch receptors and induces activation through the cleavage of Notch receptors by γ‐secretase and subsequent release of the Notch intracellular domain (NICD). NICD can translocate to the nucleus, where it forms a complex with a transcriptional regulator and activates transcription of target genes such as the hairy and enhancer of split (HES) gene family.2
Accumulating evidence has shown that dysregulation of the Notch pathway has a significant role in the progression of several malignancies. Furthermore, high expression levels of JAG1 are associated with increased progression and metastatic potential, recurrence and poor overall survival (OS) in prostate cancer, breast cancer, glioma, head and neck cancers, and gastric cancer.3, 4, 5, 6, 7, 8
Additionally, it has been shown that Notch signaling is strongly activated in primary human colorectal cancer (CRC) and has an important role in the initiation and progression of CRC through the regulation of apoptosis, proliferation, angiogenesis and cell migration.9, 10, 11, 12, 13, 14 Recent reports have also indicated that JAG1 mediates the activation of Notch signaling in CRC and induces CRC progression.15, 16, 17, 18, 19 Thus, the JAG1‐Notch pathway has been regarded an attractive target for CRC therapy.
Although high expression of JAG1 and the prognostic implications of Notch receptors in cancer cells have been described,11, 12, 13, 14, 16, 17, 18, 19, 20 the prognostic significance of high JAG1 expression in CRC has not been determined. Therefore, we investigated the association of JAG1 protein expression with survival and recurrence in CRC by immunohistochemistry (IHC) using postoperative specimens and survey information on CRC prognosis collected in our research institute. We also examined E‐cadherin expression as a marker of epithelial–mesenchymal transition (EMT) to evaluate a possible relationship between JAG1 and EMT in the prognostic role of these factors in CRC. To our knowledge, the detailed clinical results of this study provide the first report of the poor prognostic implication of high JAG1 expression in CRC patients.
Materials and Methods
Patients and specimens
A total of 158 consecutive patients with CRC who underwent surgical resection at the Department of Surgery and Science, Kyushu University Hospital between 1995 and 2002 were analyzed in this study. Histologic diagnosis was based on the World Health Organization Classification of Colorectal Carcinoma.21 Pathologic staging was performed by the Department of Anatomic Pathology, Pathological Sciences, Kyushu University, Fukuoka, Japan, according to the tumor‐node‐metastasis classification system, as revised in 2002.22 Written informed consent was obtained from each patient prior to tissue acquisition. All fresh specimens were fixed in 10% formalin and embedded in paraffin. This study was conducted with the approval of the Ethics Committee of Kyushu University Hospital, Fukuoka, Japan in accordance with the Declaration of Helsinki (Approval No. 27‐193).
Immunohistochemistry
Tumor sections were assessed immunohistochemically using rabbit polyclonal antibody against an intracellular region of JAG1 (sc‐8303, 1:200; Santa Cruz Biotechnology, CA, USA), rabbit monoclonal antibody against an extracellular region of JAG1 (2155, 1:100; Cell Signaling Technology, MA, USA) and mouse monoclonal antibody against E‐cadherin (M106, 1:1000; Takara Bio; Kyoto, Japan) with the HRP‐labeled polymer secondary antibody Envision+ system (Dako, CA, USA). Briefly, 4‐μm sections were deparaffinized and dehydrated. For antigen retrieval, the specimens were pretreated in an autoclave at 120°C for 15 min in 0.01‐M citrate buffer, pH 6.0. The sections were incubated for 30 min in 0.3% hydrogen peroxidase in absolute methanol to deactivate endogenous peroxidases. After blocking of nonspecific binding with 10% goat serum, the specimens were incubated at 4°C with primary antibodies overnight. After washing with TBS, the sections were incubated with the Envision+ system (Dako) for 1 h at room temperature. Color was developed with liquid DAB chromogen in Tris‐buffered saline (pH 7.4) containing hydrogen peroxide. The sections were counterstained with hematoxylin. The immunoreactivity score was determined as described by Allred et al.23 Scoring was performed by the study investigators, including general pathologists. The score for JAG1 was determined by three grades of intensity (“weak” for no or weak staining; “moderate”; or “strong”). The score for E‐cadherin was determined by adding the grades for intensity (1 for no or weak; 2 for moderate; 3 for strong) and the percentage of positive cells (1: 0–1%; 2: 1–10%; 3: 10–33%; 4: 33–66%; and 5: 66–100%).
Statistical analysis
All statistical calculations were performed using JMP Pro 10 statistical software (SAS Institute Japan, Tokyo, Japan). Relationships among the clinicopathologic factors and JAG1 and E‐cadherin staining were analyzed using χ2‐tests. Survival curves were plotted using the Kaplan–Meier method, and the log‐rank test was used to determine associations between individual variables and survival. OS and relapse‐free survival (RFS) were evaluated using the univariate or multivariate Cox proportional hazard model. Recurrence rate was evaluated using the multivariate logistic regression model. Differences were considered significant at P < 0.05.
Additional experimental information
Additional information is available in the Supporting informations.
Results
JAG1 immunohistochemistry
Antibody against the intracellular region of JAG1 was used for immunohistochemical staining of CRC specimens. This antibody was relatively selective for JAG1 protein and had appropriate characteristics for analyzing the prognostic significance of JAG1 expression in the cancerous tissue and the endothelium by IHC, as shown in the supporting informations (Figs S1,S2).
Immunohistochemical analysis indicated that JAG1 was expressed by cancer cells and the endothelium (Fig. 1). Weak (jcIHC‐W), moderate (jcIHC‐M) and strong (jcIHC‐S) staining of cancerous tissues was detected in 51 (32%), 57 (36%) and 50 (32%) samples, respectively (Fig. 1, Table 1). Weak (jeIHC‐W), moderate (jeIHC‐M) and strong (jeIHC‐S) staining of endothelium was detected in 61 (39%), 54 (34%) and 43 (27%) samples, respectively (Fig. 1, Table 1).
Figure 1.
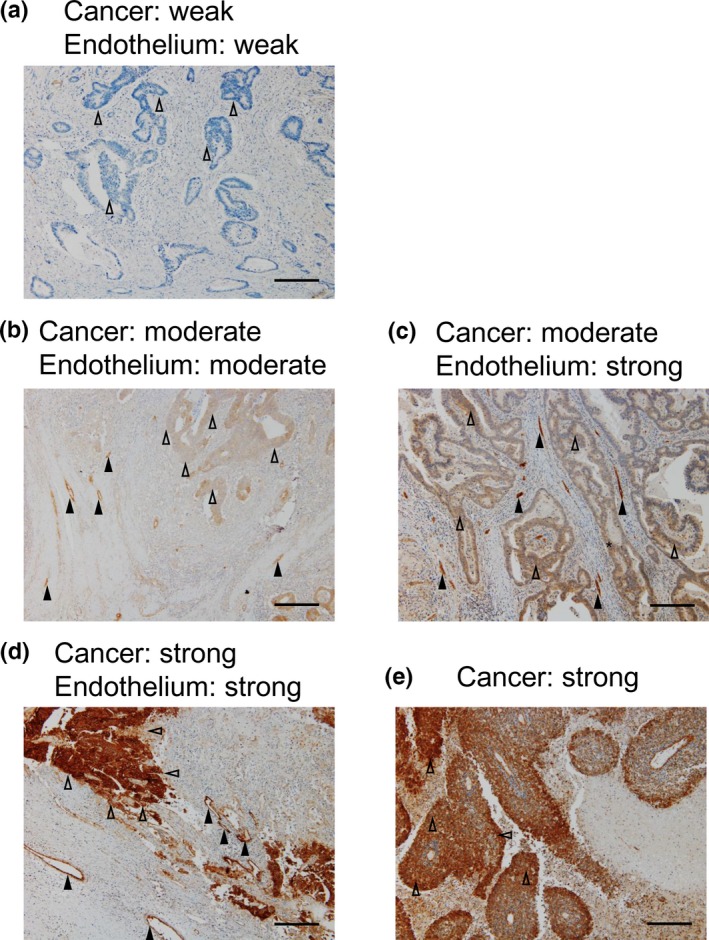
Representative immunohistochemical staining of JAG1 expression in human colorectal cancer tissues (original magnification ×100, scale bars represent 0.25 mm). (a) Example of cancer and endothelium tissue with weak intensity of staining (jcIHC‐W, jeIHC‐W). (b) Example of cancer and endothelium with moderate intensity of staining (jcIHC‐M, jeIHC‐M). (c) Example of cancer and endothelium with moderate and strong intensity of staining, respectively (jcIHC‐M, jeIHC‐S). (d) Example of cancer and endothelium with strong intensity of staining (jcIHC‐S, jeIHC‐S). (e) Example of poorly differentiated carcinoma with a strong intensity of staining. Representative each five regions in cancer or endothelium were indicated by open or filled arrow‐heads, respectively.
Table 1.
Association between clinical characteristics and JAG1 expression in cancer cells and endothelium
| Characteristics | JAG1 (cancer cells) | P‐value | JAG1 (endothelium) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| Weak | Moderate | Strong | Weak | Moderate | Strong | |||
| Total | 51 (32) | 57 (36) | 50 (32) | 61 (39) | 54 (34) | 43 (27) | ||
| Sex | 0.352 | 0.877 | ||||||
| Male | 31 (61) | 36 (63) | 25 (50) | 36 (59) | 30 (56) | 26 (60) | ||
| Female | 20 (39) | 21 (37) | 25 (50) | 25 (41) | 24 (44) | 17 (40) | ||
| Age (years) | NS | NS | ||||||
| Mean ± SD | 64.7 ± 10.8 | 64.6 ± 11.8 | 62.1 ± 13.9 | 63.0 ± 12.3 | 63.1 ± 10.1 | 65.9 ± 14.3 | ||
| Histologic type | 0.031* | 0.495 | ||||||
| Well differentiated | 38 (79) | 34 (61) | 26 (55) | 41 (71) | 32 (62) | 25 (61) | ||
| Moderate/Poorly | 10 (21) | 22 (39) | 21 (45) | 17 (29) | 20 (38) | 16 (39) | ||
| Others | 3 | 1 | 3 | 3 | 2 | 2 | ||
| T stage | 0.003** | 0.080 | ||||||
| T1 | 14 (27) | 8 (14) | 2 (4) | 15 (25) | 6 (11) | 3 (7) | ||
| T2 | 6 (12) | 7 (12) | 8 (16) | 5 (8) | 6 (11) | 10 (23) | ||
| T3 | 29 (57) | 31 (55) | 26 (53) | 32 (54) | 32 (59) | 22 (51) | ||
| T4 | 2 (4) | 11 (19) | 13 (27) | 8 (13) | 10 (19) | 8 (19) | ||
| Unknown | 1 | 1 | ||||||
| Lymph node metastasis | 0.242 | 0.035* | ||||||
| Negative | 33 (65) | 28 (49) | 26 (53) | 40 (67) | 23 (43) | 24 (56) | ||
| Positive | 18 (35) | 29 (51) | 23 (47) | 20 (33) | 31 (57) | 19 (44) | ||
| Unknown | 1 | 1 | ||||||
| Stage of tumor | 0.126 | 0.114 | ||||||
| 0–I | 18 (35) | 12 (21) | 7 (14) | 20 (33) | 6 (11) | 11 (25) | ||
| II | 12 (24) | 14 (25) | 15 (30) | 16 (26) | 13 (24) | 12 (28) | ||
| III | 15 (29) | 23 (40) | 15 (30) | 17 (28) | 22 (41) | 14 (33) | ||
| IV | 6 (12) | 8 (14) | 13 (26) | 8 (13) | 13 (24) | 6 (14) | ||
| Lymphatic invasion | 0.136 | 0.436 | ||||||
| Negative | 39 (76) | 34 (60) | 31 (62) | 43 (70) | 32 (59) | 29 (67) | ||
| Positive | 12 (24) | 23 (40) | 19 (38) | 18 (30) | 22 (41) | 14 (33) | ||
| Venous invasion | 0.818 | 0.039* | ||||||
| Negative | 32 (63) | 33 (58) | 27 (54) | 39 (64) | 24 (44) | 29 (67) | ||
| Positive | 19 (37) | 24 (42) | 23 (46) | 22 (36) | 30 (56) | 14 (33) | ||
| JAG1 (Endothelium) | <0.001** | JAG1 (Cancer) | <0.001** | |||||
| Weak | 35 (69) | 17 (30) | 9 (18) | 35 (57) | 14 (26) | 2 (5) | ||
| Moderate | 14 (27) | 24 (42) | 16 (32) | 17 (28) | 24 (44) | 16 (37) | ||
| Strong | 2 (4) | 16 (28) | 25 (50) | 9 (15) | 16 (30) | 25 (58) | ||
| Recurrence | 0.009** | 0.180 | ||||||
| Absent | 45 (90) | 42 (76) | 29 (64) | 47 (78) | 42 (84) | 27 (68) | ||
| Present | 5 (10) | 13 (24) | 16 (36) | 13 (22) | 8 (16) | 13 (32) | ||
| Unknown | 1 | 2 | 5 | 1 | 4 | 3 | ||
χ2‐test: *P < 0.05, **P < 0.01. Each value is presented as number (%) of specimens. NS, not significant; SD, standard deviation.
Correlation of JAG1 expression in cancer cells or endothelium with clinicopathologic characteristics and recurrence
The correlation between JAG1 expression and clinicopathologic characteristics is shown in Table 1. JAG1 expression in cancer cells was correlated with histologic type (P = 0.031) and T stage (P = 0.003). JAG1 expression in cancer cells was also significantly associated with JAG1 expression in endothelium (P < 0.001) and rate of recurrence (P = 0.009). Moreover, JAG1 expression in endothelium was correlated with lymph node metastasis (P = 0.035) and venous invasion (P = 0.039). Moderate intensity of staining in the endothelium tended to be associated with poorer characteristics than the other staining groups.
Analysis of the association between JAG1 expression and survival outcome
The association between JAG1 expression in cancer cells and OS was evaluated in all patients (Fig. 2a). JAG1 expression was significantly associated with OS (P = 0.006). Evaluation of the association between JAG1 expression and RFS in 131 patients with stage 0–III CRC (ST_0‐III) showed that JAG1 expression was also significantly associated with RFS (Fig. 2b; P = 0.010). Analysis of the prognostic significance of JAG1 expression as indicated by the 5‐year survival rate calculated by Kaplan–Meier estimates and hazard ratios determined using the Cox proportional hazard model revealed that higher expression of JAG1 is associated with a poorer survival rate and larger hazard ratio (Fig. S3).
Figure 2.
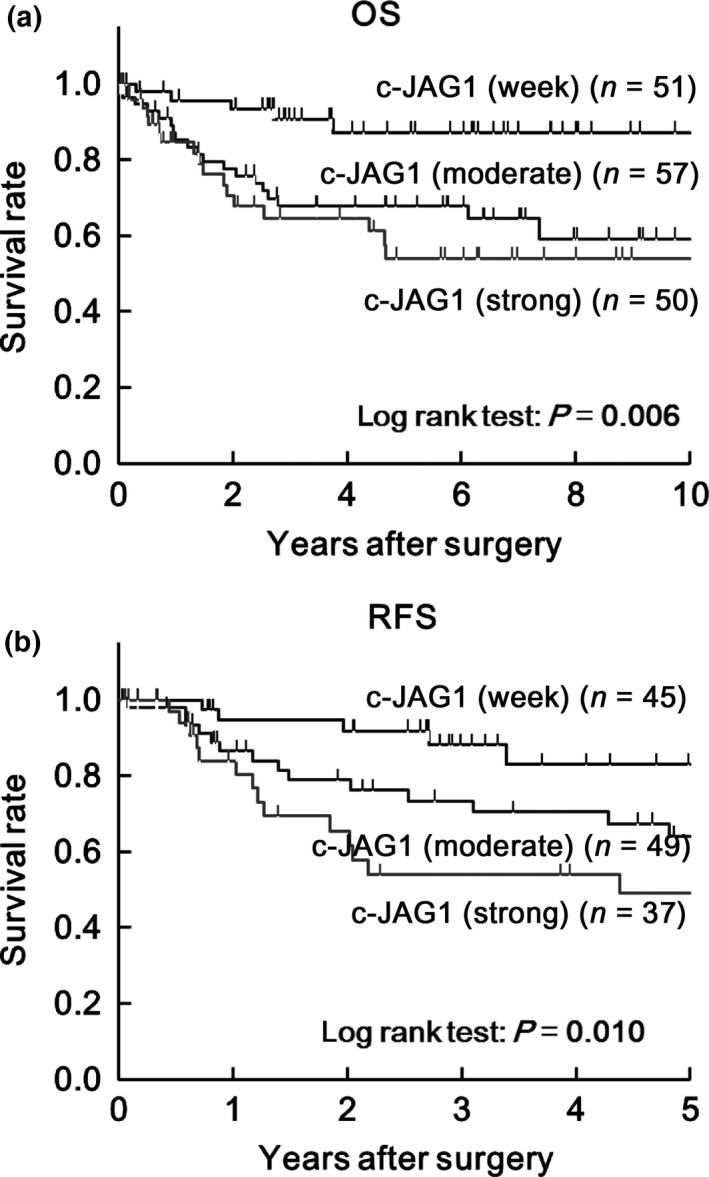
Prognostic significance of JAG1 expression in cancer cells by analysis of Kaplan–Meier estimates. (a) Kaplan–Meier estimates of 10‐year overall survival (OS) in all CRC patients and (b) 5‐year recurrence‐free survival (RFS) in patients except for Stage IV CRC according to staining intensity. Mod indicates moderate.
In univariate analysis of OS in all patients and for RFS in the ST_0‐III subgroup, JAG1 expression was significantly correlated with both OS and RFS (Table 2). In multivariate analysis, JAG1 expression, histologic type and lymphatic invasion showed independent association with OS and RFS (Table 2). Univariate analysis of the rate of recurrence in all patients using the logistic model (Table 2) revealed a significant correlation of JAG1 expression with recurrence. Multivariate analysis showed that tumor stage and JAG1 expression were independently associated with recurrence (Table 2).
Table 2.
Univariate and multivariate analysis of factors associated with overall survival or relapse‐free survival
| Characteristics | Parameters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||
| Overall survival (All patients, Cox model) | |||||||
| Sex | M vs F | 1.37 | 0.73–2.54 | 0.316 | 1.35 | 0.70–2.57 | 0.366 |
| Age | < 65 vs ≥65 | 0.96 | 0.52–1.78 | 0.906 | 0.99 | 0.51–1.93 | 0.980 |
| Histologic type | Well vs Others | 4.51 | 2.38–9.00 | <0.001** | 2.59 | 1.19–5.94 | 0.016* |
| T stage | T1,2 vs T3,4 | 3.91 | 1.56–13.07 | 0.002** | 1.47 | 0.51–5.31 | 0.497 |
| Lymph node metastasis | − vs + | 3.93 | 2.02–8.23 | <0.001** | 2.04 | 0.61–12.68 | 0.283 |
| Stage of tumor | 0/I/II vs III/IV | 4.08 | 2.03–9.08 | <0.001** | 1.02 | 0.14–4.31 | 0.980 |
| Lymphatic invasion | − vs + | 5.09 | 2.72–9.88 | <0.001** | 3.88 | 1.81–8.63 | <0.001** |
| Venous invasion | − vs + | 2.18 | 1.18–4.09 | 0.013* | 1.69 | 0.78–3.65 | 0.184 |
| JAG1 (Cancer) | W vs Mod/S | 3.99 | 1.71–11.62 | <0.001** | 2.62 | 1.10–7.74 | 0.027* |
| Relapse‐free survival (Patients besides Stage IV, Cox model) | |||||||
| Sex | M vs F | 1.58 | 0.78–3.13 | 0.226 | 1.21 | 0.54–2.63 | 0.643 |
| Age | <65 vs ≥65 | 1.66 | 0.83–3.42 | 0.138 | 2.10 | 0.99–4.62 | 0.055 |
| Histologic type | Well vs Others | 4.74 | 2.34–10.15 | <0.001** | 2.71 | 1.09–6.91 | 0.032* |
| T stage | T1,2 vs T3,4 | 2.40 | 1.06–6.44 | 0.035* | 0.92 | 0.33–2.77 | 0.870 |
| Lymph node metastasis | − vs + | 3.04 | 1.52–6.37 | 0.002** | 1.60 | 0.25–31.32 | 0.660 |
| Stage of tumor | 0/I/II vs III | 3.16 | 1.56–6.76 | 0.001** | 1.35 | 0.07–8.07 | 0.793 |
| Lymphatic invasion | − vs + | 4.56 | 2.29–9.20 | <0.001** | 4.47 | 1.76–11.57 | 0.002** |
| Venous invasion | − vs + | 2.07 | 1.03–4.12 | 0.033* | 0.51 | 0.19–1.37 | 0.184 |
| JAG1 (Cancer) | W vs Mod/S | 2.38 | 1.17–4.74 | 0.017* | 2.65 | 1.16–5.95 | 0.021* |
| Recurrence (All patients, Logistic model) | |||||||
| Sex | M vs F | 0.838 | 0.628 | ||||
| Age | <65 vs ≥65 | 0.369 | 0.080 | ||||
| Histologic type | Well vs Others | 0.003** | 0.082 | ||||
| T stage | T1,2 vs T3,4 | 0.272 | 0.354 | ||||
| Lymph node metastasis | − vs + | 0.006** | 0.712 | ||||
| Stage of tumor | 0/I/II vs III/IV | 0.005** | 0.035* | ||||
| Lymphatic invasion | − vs + | 0.031* | 0.471 | ||||
| Venous invasion | − vs + | 0.191 | 0.846 | ||||
| JAG1 (Cancer) | W/Mod vs S | 0.011* | 0.033* | ||||
*P < 0.05, **P < 0.01. CI, confidence interval; F, female; HR, hazard ratio; M, male; Mod, moderate; S, strong; W, weak.
The association between JAG1 expression in endothelium and OS or RFS was also analyzed (Fig. S4a,b). The 5‐year survival rate calculated by the Kaplan–Meier estimate is shown in Figure S4c and prognostic analysis of JAG1 expression by the Cox proportional hazard model is shown in Figure S4d. High expression of JAG1 in endothelium was more strongly associated with RFS than with OS.
Analysis of the association between JAG1 and E‐cadherin expression
To investigate whether JAG1 expression is associated with the transition between epithelial and mesenchymal characteristics, E‐cadherin expression was analyzed as an epithelial marker by IHC (Fig. S5). E‐cadherin expression was categorized by staining intensity (eIHC‐In1–3) or the proportion of positive cells (eIHC‐Pr1–5), as shown in Figure S5 and Table 3. High JAG1 expression (jcIHC‐S versus jcIHC‐W/M) was significantly correlated with low E‐cadherin expression in subgroup stratification by proportion (eIHC‐Pr1‐3 versus eIHC‐Pr4/5) (Table 3, P = 0.023).
Table 3.
Associations between E‐cadherin expression and clinical characteristics or JAG1 expression in cancer cells
| Characteristics | E‐cadherin (Intensity) | P value | E‐cadherin (Proportion) | P value | ||
|---|---|---|---|---|---|---|
| 1 | 2/3 | 1/2/3 | 4/5 | |||
| Total | 39 (25) | 119 (75) | 44 (28) | 114 (72) | ||
| Sex | ||||||
| Male | 22 (56) | 70 (59) | 0.791 | 18 (41) | 48 (42) | 0.891 |
| Female | 17 (44) | 49 (41) | 26 (59) | 66 (58) | ||
| Age (years) | ||||||
| <65 | 18 (46) | 63 (53) | 0.462 | 24 (55) | 57 (50) | 0.608 |
| ≥65 | 21 (54) | 56 (47) | 20 (45) | 57 (50) | ||
| Histologic type | ||||||
| Well differentiated | 17 (44) | 81 (68) | 0.007** | 20 (45) | 78 (68) | 0.008** |
| Others | 22 (56) | 38 (32) | 24 (55) | 36 (32) | ||
| T stage | ||||||
| T1, 2 | 1 (3) | 44 (37) | <0.001** | 4 (9) | 41 (36) | <0.001** |
| T3, 4 | 38 (97) | 74 (63) | 40 (91) | 72 (64) | ||
| Unknown | 1 | 1 | ||||
| Lymph node metastasis | ||||||
| Negative | 15 (38) | 72 (61) | 0.014* | 19 (43) | 68 (60) | 0.055 |
| Positive | 24 (62) | 46 (39) | 25 (57) | 45 (40) | ||
| Unknown | 1 | 1 | ||||
| Stage of tumor | ||||||
| 0/I/II | 10 (26) | 68 (57) | <0.001** | 13 (30) | 65 (57) | 0.002** |
| III/IV | 29 (74) | 51 (43) | 31 (70) | 49 (43) | ||
| Lymphatic invasion | ||||||
| Negative | 18 (46) | 86 (72) | 0.003** | 23 (52) | 81 (71) | 0.028* |
| Positive | 21 (54) | 33 (28) | 21 (48) | 33 (29) | ||
| Venous invasion | ||||||
| Negative | 15 (38) | 77 (65) | 0.004** | 21 (48) | 71 (62) | 0.100 |
| Positive | 34 (62) | 42 (35) | 23 (52) | 43 (38) | ||
| JAG1 (Cancer) | ||||||
| Weak | 11 (28) | 40 (34) | 0.527 | 14 (32) | 37 (32) | 0.939 |
| Moderate/Strong | 28 (72) | 79 (66) | 30 (68) | 77 (68) | ||
| Weak/moderate | 26 (67) | 82 (69) | 0.795 | 24 (55) | 84 (74) | 0.023* |
| Strong | 13 (33) | 37 (31) | 20 (45) | 30 (26) | ||
χ2‐test: *P < 0.05, **P < 0.01. Each value is presented as number (%) of specimens.
Analysis of JAG1 expression in patient samples stratified by E‐cadherin expression
A significant poor prognosis of low E‐cadherin expression for OS was detected by log‐rank test in the analysis according to staining intensity of 1 vs 2/3 (Fig. S6a, P = 0.038). In this stratification, no correlation between JAG1 and E‐cadherin expression was detected (Table 3); therefore, an additional prognostic impact of combined JAG1 and E‐cadherin expression was expected. To investigate the significance of JAG1 expression on prognosis in samples stratified by intensity of E‐cadherin staining, patients were divided into six groups as follows: (i) jcIHC‐W/eIHC‐In2/3; (ii) jcIHC‐M/eIHC‐In2/3; (iii) jcIHC‐S/eIHC‐In2/3; (iv) jcIHC‐W/eIHC‐In1; (v) jcIHC‐M/eIHC‐In1; and (vi) jcIHC‐S/eIHC‐In1 (Table 4). The P‐value of the log‐rank test for OS and RFS was 0.011 and 0.001, respectively (Fig. 3a,b). OS and RFS in jcIHC‐M/eIHC‐In2/3, jcIHC‐S/eIHC‐In2/3, jcIHC‐M/eIHC‐In1 and jcIHC‐S/eIHC‐In1 groups were significantly shorter than those of the jcIHC‐W/eIHC‐In2/3 group (Fig. 3a–d). Specifically, the jcIHC‐S/eIHC‐In1 group showed the poorest outcome of all groups: 3‐year OS = 34.9% (HR = 10.08, 95% CI = 2.64–47.97, P = 0.001 [vs jcIHC‐W/eIHC‐In2/3]); 3‐year RFS = 33.3% (HR = 8.27, 95% CI = 2.49–31.67, P = 0.001 [vs jcIHC‐W/eIHC‐In2/3]). The relationship between JAG1 expression and RFS was notable in the group with moderate or strong intensity of E‐cadherin staining (eIHC‐In2‐3). Namely, the 3‐year RFS rate in jcIHC‐S/eIHC‐In2/3 (45.6%) was much lower than that in jcIHC‐M/eIHC‐In2/3 (68%), whereas the 3‐year OS rate in jcIHC‐S/eIHC‐In2/3 (74.3%) was almost the same as that for jcIHC‐M/eIHC‐In2/3 (71.9%). This may indicate that high JAG1 expression is associated with shorter duration of recurrence rather than survival in the group with moderate or strong intensity of E‐cadherin staining (eIHC‐In2/3).
Table 4.
JAG1 expression in cancer cells stratified by E‐cadherin expression (based on intensity of staining) and correlation with clinicopathologic characteristics, JAG1 expression in endothelium, and KRAS status
| Characteristics | E‐cadherin intensity (1) | P‐value | E‐cadherin intensity (2/3) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|
| JAG1 | JAG1 | |||||||
| Weak | Moderate | Strong | Weak | Moderate | Strong | |||
| Total | 11 (28) | 15 (38) | 13 (33) | 40 (34) | 42 (35) | 37 (31) | ||
| Sex | 0.899 | 0.149 | ||||||
| Male | 6 (55) | 8 (53) | 8 (62) | 25 (62) | 28 (67) | 17 (46) | ||
| Female | 5 (45) | 7 (47) | 5 (38) | 15 (38) | 14 (33) | 20 (54) | ||
| Age (years) | 0.321 | 0.683 | ||||||
| <65 | 3 (27) | 8 (53) | 7 (54) | 22 (55) | 20 (48) | 21 (57) | ||
| ≥65 | 8 (73) | 7 (47) | 6 (46) | 18 (45) | 22 (52) | 16 (43) | ||
| Histologic type | 0.475 | 0.045* | ||||||
| Well differentiated | 5 (45) | 8 (53) | 4 (31) | 33 (83) | 26 (62) | 22 (59) | ||
| Others | 6 (55) | 7 (47) | 9 (69) | 7 (17) | 16 (38) | 15 (41) | ||
| T stage | 0.377 | 0.110 | ||||||
| T1, T2 | 0 (0) | 1 (7) | 0 (0) | 20 (50) | 14 (33) | 10 (28) | ||
| T3, T4 | 11 (100) | 14 (93) | 13 (100) | 20 (50) | 28 (67) | 26 (72) | ||
| Unknown | 1 | |||||||
| Lymph node metastasis | 0.480 | 0.348 | ||||||
| Negative | 5 (45) | 4 (27) | 6 (46) | 28 (70) | 24 (57) | 20 (56) | ||
| Positive | 6 (55) | 11 (73) | 7 (54) | 12 (30) | 18 (43) | 16 (44) | ||
| Unknown | 1 | |||||||
| Stage of tumor | 0.800 | 0.226 | ||||||
| 0/I/II | 3 (27) | 3 (20) | 4 (31) | 27 (68) | 23 (55) | 18 (49) | ||
| III/IV | 8 (73) | 12 (80) | 9 (69) | 13 (32) | 19 (45) | 19 (51) | ||
| Lymphatic invasion | 0.998 | 0.070 | ||||||
| Negative | 5 (45) | 7 (47) | 6 (46) | 34 (85) | 27 (64) | 25 (68) | ||
| Positive | 6 (55) | 8 (53) | 7 (54) | 6 (15) | 15 (36) | 12 (32) | ||
| Venous invasion | 0.822 | 0.724 | ||||||
| Negative | 5 (45) | 5 (33) | 5 (38) | 27 (68) | 28 (67) | 22 (59) | ||
| Positive | 6 (55) | 10 (67) | 8 (62) | 13 (32) | 14 (33) | 15 (41) | ||
| JAG1 (Endothelium) | 0.052 | <0.001** | ||||||
| Weak/moderate | 10 (91) | 10 (67) | 6 (46) | 39 (98) | 31 (74) | 19 (51) | ||
| Strong | 1 (9) | 5 (33) | 7 (54) | 1 (2) | 11 (26) | 18 (49) | ||
| KRAS status | 0.527 | 0.048* | ||||||
| Total | 6 | 8 | 9 | 12 | 22 | 21 | ||
| Wild type | 3 (50) | 5 (63) | 7 (78) | 10 (83) | 17 (77) | 11 (52) | ||
| Mutant | 3 (50) | 3 (37) | 2 (22) | 2 (17) | 5 (23) | 10 (48) | ||
| BRAF status | 0.111 | 0.863 | ||||||
| Total | 5 | 8 | 9 | 11 | 22 | 21 | ||
| Wild type | 4 (80) | 8 (100) | 6 (67) | 10 (91) | 21 (95) | 20 (95) | ||
| Mutant | 1 (20) | 0 (0) | 3 (33) | 1 (9) | 1 (5) | 1 (5) | ||
| MSI status | 0.007** | 0.977 | ||||||
| Total | 10 | 12 | 11 | 22 | 33 | 29 | ||
| MSS or MSI‐L | 10 (100) | 12 (100) | 7 (64) | 19 (86) | 29 (88) | 25 (86) | ||
| MSI‐H | 0 (0) | 0 (0) | 4 (36) | 3 (14) | 4 (12) | 4 (14) | ||
Each value is presented as number (%) of specimens. χ2‐test: *P < 0.05, **P < 0.01.
Figure 3.
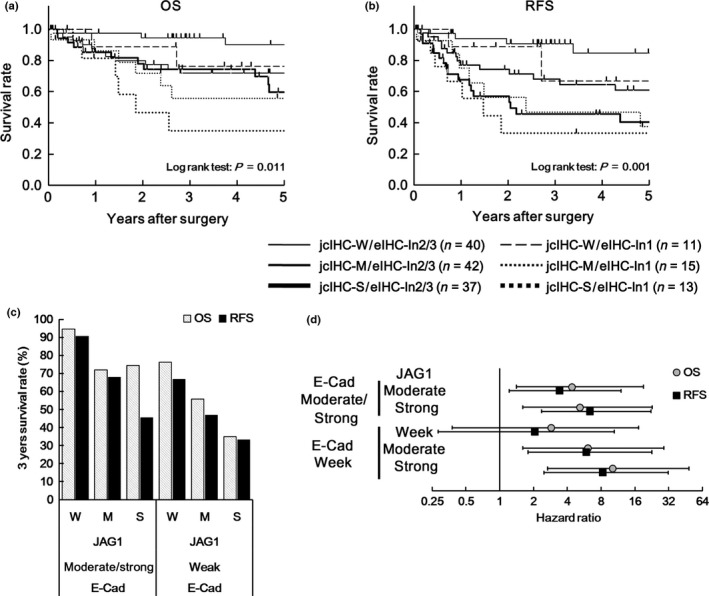
Prognostic significance of JAG1 expression in cancer cells stratified by E‐cadherin expression (based on intensity of staining) shown by analysis of Kaplan–Meier estimates and Cox proportional hazards model. (a, b) Kaplan–Meier estimate of 5‐year OS (a) and 5‐year RFS (b) in CRC patients according to staining intensity of JAG1 expression in cancer cells stratified by E‐cadherin expression. jcIHC‐W, ‐M, ‐S indicate weak, moderate, and strong intensity of staining of JAG1 expression in cancer cells, respectively. eIHC‐In2/3 and eIHC‐In1 indicate staining intensity of 2/3 and 1 for E‐cadherin expression, respectively. (c) 3‐year survival rate calculated by analysis of the Kaplan–Meier estimates shown in (a) and (b). (d) Hazard ratio (HR) and 95 % confidence interval (CI) of JAG1 and E‐cadherin expression analyzed by Cox proportional hazards model versus jcIHC‐W/eIHC‐In2/3 group.
The correlation of JAG1 expression in cancer cells stratified by E‐cadherin expression with clinicopathologic characteristics, JAG1 expression in endothelium, KRAS, BRAF and MSI status was evaluated (Tables 4,S1 and Fig. S7). Because of the retrospective analysis, data on KRAS, BRAF and MSI status were available for only 78, 76 and 117 specimens of the 158 CRC patients, respectively. JAG1 expression in cancer cells stratified as high intensity of E‐cadherin staining or large proportion of E‐cadherin expression (eIHC‐In2/3 or eIHC‐Pr4/5) was significantly correlated with histologic type (Tables 4,S1). A higher rate of KRAS mutation was observed in the jcIHC‐S/eIHC‐In2/3 group (48%) compared with the other groups (17% for jcIHC‐W/eIHC‐In2/3 and 23% for jcIHC‐M/eIHC‐In2/3) (Table 4, Fig. S7). A significant correlation between JAG1 expression and KRAS status (P = 0.037, data not shown) was also observed in the group with eIHC‐Pr5 in stratification by eIHC‐Pr1‐4 (n = 79) versus eIHC‐Pr5 (n = 79) based on the proportion of E‐cadherin expression. These results suggested that one of the mechanisms for high JAG1 expression in CRC was the enhancement of KRAS and its downstream pathway. There was not a significant correlation between JAG1 expression and BRAF or MSI status in the group stratified as strong and moderate intensity of E‐cadherin staining (eIHC‐In2/3). In contrast, in the group of the other stratification in E‐cadherin (eIHC‐In1), strong intensity of JAG1 staining seemed to associate with MSI or BRAF status. However, we could not determine whether these associations for MSI or BRAF status were really significant because the sample size was small.
Mechanism of increasing JAG1 expression and JAG1‐dependent promotion of epithelial–mesenchymal transition and proliferation in a colon cancer cell line
To investigate whether the KRAS‐MEK‐MAP kinase pathway regulates JAG1 expression and the transition between epithelial and mesenchymal status, the effect of the MEK inhibitor PD325901 was examined in the HCT‐116 colon cancer cell line. Treatment with MEK inhibitor decreased expression of JAG1 and the mesenchymal marker SNAIL (Fig. 4a,b). Conversely, E‐cadherin expression was increased after inhibition of MEK (Fig. 4a,b). The effect of the MEK inhibitor PD325901 on JAG1 expression and transition toward mesenchymal phenotype was also investigated by western blotting in the HCT‐116 (KRAS G13D) and Caco‐2 (KRAS wild) colon cancer cell lines (Fig. S8). HCT‐116 cells have a lower level of E‐cadherin and a higher level of JAG1, SNAIL, phosphorylated ERK1/2 in the control condition than in Caco‐2 cells. Inhibition of MEK kinase lead to suppression of phosphorylated ERK1/2, JAG1 and SNAIL level in HCT‐116. Conversely, increasing E‐cadherin expression was observed by inhibition of MEK in HCT‐116. In contrast, there was no or little change for JAG1, E‐cadherin and SNAIL expression with inhibition of MEK in Caco‐2.
Figure 4.
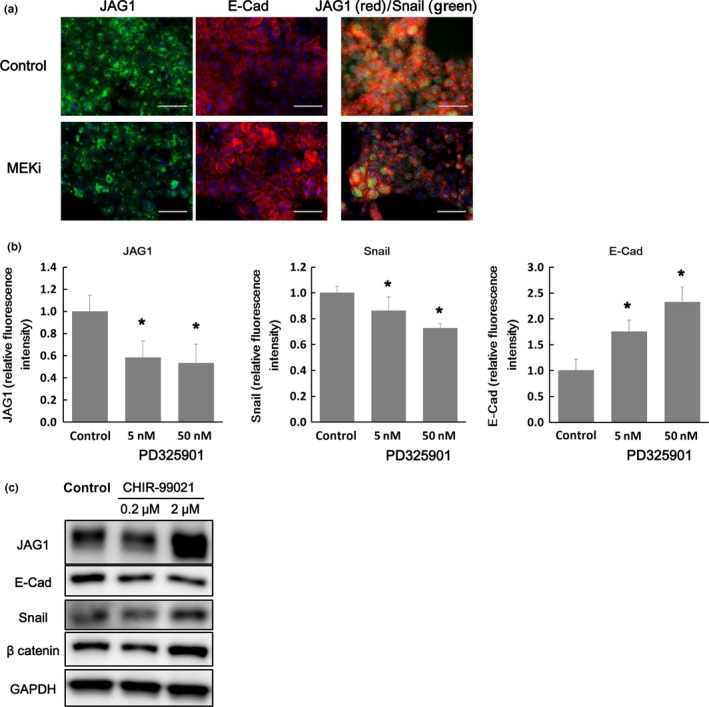
Mechanisms of regulation of JAG1 expression based on in vitro study. (a) The effect of inhibition of RAS‐MEK‐MAP kinase pathway on JAG1 expression and induction of EMT‐like phenotype was examined using the MEK inhibitor PD325901 in the colon cancer cell line HCT‐116. In the left panel, blue staining indicates the nucleus and green staining indicates JAG1 protein. In the middle panel, blue staining indicates the nucleus and red staining indicates E‐cadherin protein. In the right panel, blue, green, and red staining indicate the nucleus, SNAIL, and JAG1 protein, respectively. Scale bars represent 50 µm. (b) Fluorescence intensity of JAG1, Snail, and E‐cadherin was analyzed. Data are presented as mean ± SD of 20 fields of view. *, P < 0.05, Student's t‐test. (c) Effects of GSK3β inhibitor on JAG1 protein expression and EMT‐like phenotype. JAG1, E‐cadherin, and SNAIL protein expression was analyzed by western blotting.
We also detected the positive regulation of JAG1 expression and the EMT resulting from Wnt‐β catenin pathway activation by the GSK3β inhibitor, CHIR‐99021, in HCT‐116 cells. Namely, treatment with CHIR‐99021 increased JAG1 and SNAIL expression and decreased E‐cadherin expression (Fig. 4c).
The effect of JAG1 gene silencing on EMT in the colon cancer cell line was investigated using siRNA for JAG1 (siJAG1). Western blot (Fig. 5a), qRT‐PCR (Fig. 5b) and immunofluorescence (Fig. S9) analyses indicated that siJAG1 treatment increased expression of E‐cadherin protein/mRNA, decreased expression of Snail protein and decreased expression of the mesenchymal marker vimentin mRNA compared with control non‐targeting siRNA (siNON) treatment. These results suggested that the colon cancer cell line transited into a more epithelial and less mesenchymal phenotype upon JAG1 gene silencing.
Figure 5.
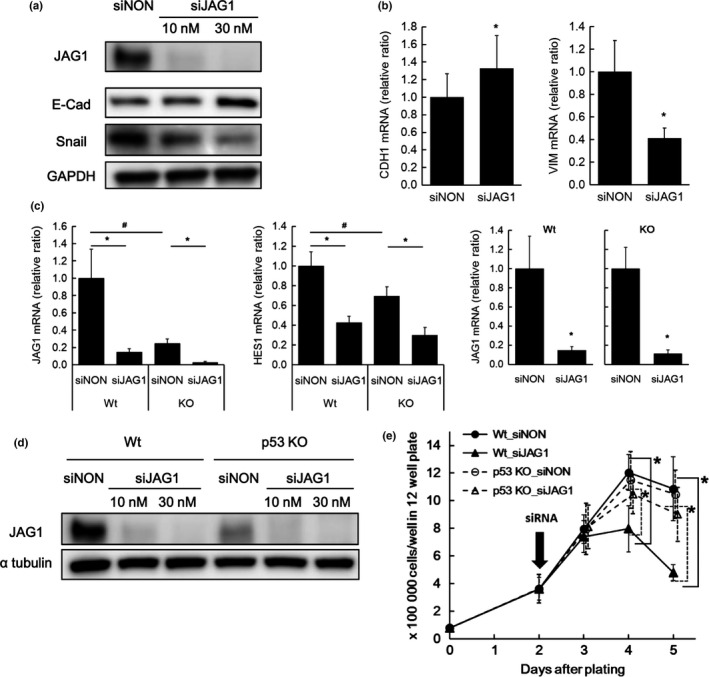
Effect of JAG1 gene silencing on proliferation and epithelial mesenchymal transition (EMT)‐like phenotype based on in vitro study. (a, b) Effect of small interfering RNA for JAG1 (siJAG1) on JAG1 expression and EMT‐like phenotype. E‐cadherin and SNAIL protein expression (a) or E‐cadherin (CDH1) and vimentin (VIM) mRNA expression (b) were analyzed by western blotting and qRT‐PCR respectively. Non‐targeting siRNA (siNON) was used as a negative control. Expression levels of mRNA are indicated relative to expression with siNON treatment. Data are mean ± SD of nine wells. (c) Effect of siJAG1 on JAG1 and HES1 mRNA expression in p53 −/− and wild type (Wt) HCT‐116 cells. mRNA expression was presented as a ratio relative to expression in Wt cells treated with siNON in the left two panels. JAG1 mRNA expression in Wt or p53 −/− cells treated with siJAG1 was also presented as a ratio relative to expression in Wt or p53‐/‐ cells treated with siNON in the right panels. Data are mean ± SD of nine wells. (d) Effect of siJAG1 on JAG1 protein expression in p53 −/− and Wt cells analyzed by western blotting. (e) Effect of siJAG1 on cell growth in p53 −/− and Wt cells. siJAG1 treatment was initiated 2 days after plating. Data are presented as mean ± SD of six wells for each time point (result from three independent experiments). Statistical analysis was performed by Student's t‐test. * or #, P < 0.05.
We also examined the effect of siJAG1 on proliferation of HCT‐116 cells and explored the possibility of crosstalk between the JAG1‐Notch pathway and p53‐related signaling by investigating the effects in HCT‐116 p53 −/− cells (p53KO). siJAG1 at the concentration of 30 nM decreased JAG1 mRNA levels by more than 80% compared with siNON in both wild type (Wt) and p53KO cells (Fig. 5c). mRNA expression of HES1, one of the Notch signal target genes, was also decreased, suggesting suppression of intracellular Notch signaling (Fig. 5c). JAG1 and HES1 mRNA expression was significantly lower in p53KO than in Wt cells (Fig. 5c). JAG1 protein level was also suppressed by siJAG1 compared with siNON treatment (Fig. 5d). The lower expression level of JAG1 protein in p53KO than in Wt was concordant with the results of qRT‐PCR analysis (Fig. 5c,d). Suppressed proliferation in Wt cells treated with siJAG1 compared with cells treated with siNON was evident 2 days after initiation of treatment (Fig. 5e). No obvious effect of siJAG1 on proliferation in p53 KO cells was observed (Fig. 5e).
Discussion
To our knowledge, this study is the first report of the prognostic significance of JAG1 expression in cancer cells in patients with CRC. Moreover, our data indicated a relationship between JAG1 and E‐cadherin expression in the prognosis of CRC. Furthermore, we provide novel insight into the correlation between KRAS status and JAG1 expression in CRC patients. Various studies previously reported that a high expression level of JAG1 was detected in cancer cells16, 17, 18, 19, 24 and was correlated with tumor grade in human patients.18 However, the prognostic significance of JAG1 expression in cancer cells of CRC has not been determined. Our study demonstrated that higher JAG1 expression in cancer cells of CRC patients is associated with a poorer survival rate and increased risk of recurrence and that combination of high JAG1 expression with low E‐cadherin expression leads to severely poor outcome.
Three causes of the poor survival rate and increased risk of recurrence associated with high JAG1 expression have been proposed: increased cell proliferation or maintenance of survival, acquisition of stem cell‐like phenotype and induction of an EMT‐like phenotype in cancer cells. Our study indicated that siRNA‐mediated JAG1 gene silencing delayed cell proliferation, with a subsequent decrease in cell number, in a colon cancer cell line. Similar results have been reported previously.18, 19 Evidence from our in vitro study and previous reports support the poor prognostic significance of high JAG1 expression in CRC.
The second possibility involves JAG1 protein expression associated with endothelium. We found that high expression of JAG1 protein in endothelium was associated with high expression of JAG1 protein in cancer cells and a poor prognosis, especially an increased recurrence risk. This result may be associated with acquisition of a stem cell‐like phenotype in cancer cells through Notch pathway activation by JAG1 secreted from the endothelium in CRC, as described previously.15 While our study did not address to explore mechanisms of high JAG1 expression in endothelium, some mechanisms were speculated from previous reports.25, 26 Namely, proinflammatory cytokines such as TNF‐α and IL6 are possible inducer for JAG1 expression in endothelium. In addition, JAG1 upregulation in colon cancer cell lines induced by forced Notch pathway activation promotes stemness in the cancer cells themselves through positive feedback.27 Our study indicated that JAG1 expression in cancer cells was strongly correlated with JAG1 expression in endothelium and that a stronger intensity of JAG1 staining in endothelium and cancer cells was associated with poor prognosis. Namely, JAG1 secreted from endothelium stimulates Notch pathway in cancer cells, and JAG1 expression in cancer cells may be upregulated dependent on the activity of Notch pathway itself in cancer cells. Then, JAG1‐Notch signaling in cancer cells may be amplified through positive feedback. These findings might mean that the transition toward a cancer stem cell‐like phenotype in cancer cells is promoted thorough interaction between endothelium and cancer cells mediated by JAG1, leading to a poorer prognosis in CRC patients.
Our study also suggests the significance of the JAG1‐Notch pathway in EMT in human CRC. We detected a significant association between low E‐cadherin and high JAG1 expression in CRC clinical samples and found that high JAG1 expression in cancer cells of CRC correlated with histologic type (decreased differentiation status) and T stage (deep invasion) among clinicopathologic characteristics. This correlation may be caused by an EMT‐like phenomenon induced by JAG1‐Notch pathway activation. Induction of an EMT‐like phenotype in cancer cells might allow these cells to exit from their original site, migrate to distant locations, and survive in a new microenvironment.28, 29 It was reported that Notch signaling mediates EMT thorough upregulation of Snail protein.30 Moreover, forced Notch pathway activation was shown to increase JAG1 expression and promote EMT through positive feedback in a colorectal cancer cell line.27 Our in vitro study demonstrated that although siRNA‐mediated JAG1 gene silencing induced the transition to a more epithelial phenotype, Wnt pathway activation by inhibition of GSK‐3β not only induced the transition to a more mesenchymal phenotype but also increased JAG1 expression, as predicted from circumstantial evidence reported previously.16, 17, 24, 31 Previous studies and our in vitro study support the significant role of JAG1‐Notch pathway activation in poor prognosis in human CRC through induction of EMT.
It was recently reported that concomitant Notch activation and p53 deletion triggers EMT and metastasis in a genetically engineered mouse model.32 We demonstrated that siJAG1 delayed or inhibited proliferation in the Wt cell line but had a less potent effect in the p53 −/− cell line. Moreover, whereas siJAG1 increased expression of E‐cadherin and decreased Snail protein in the Wt cell line, a similar effect could not be detected in the p53 −/− cell line (data not shown). These results may indicate that JAG1‐Notch signaling is important for induction of an EMT‐like phenotype as well as proliferation through suppression of a p53‐related pathway.33 Alternatively, lower expression of JAG1 mRNA and protein in the p53 −/− cell line compared with Wt might indicate a reciprocal relationship between JAG1 protein expression and p53 status.33 This is an unexpected result, and our studies could not validate a model in which concomitant Notch activation and p53 deletion triggers EMT.32 To address this issue, the effect of exogenous treatment with JAG1 in p53 −/− cancer cells should be examined in the future. In the aspect of low JAG1 expression induced by p53 KO, our preliminary analysis in human clinical specimens indicated that low JAG1 expression was significantly associated with the high proportion of loss of heterogeneity in p53 status (data not shown). Thus, future study of the association between JAG1 expression and p53 status in CRC patients might reveal the reciprocal relationship between p53 status and JAG1 expression.
Our findings also suggested that EMT is induced by mediators other than JAG1‐Notch signaling, as indicated by little correlation between the low staining intensity of E‐cadherin and high JAG1 expression. Consequently, we demonstrated an additive impact on prognosis of a combination of high JAG1 and low E‐cadherin expression, and the poorest survival rate for both OS and RFS was indicated for this combination. We could not find any association between JAG1 expression and clinicopathologic characteristics in groups stratified as low E‐cadherin expression, and, therefore, it might be important to identify which downstream pathway of JAG1‐Notch leads to poorer prognosis in these groups.
Our study indicated a shorter recurrence free interval after surgery in patients with high JAG1 expression among the subgroup with intensity 2/3 of E‐cadherin staining (Fig. 3), and showed that this phenomenon might be associated with a high rate of mutation in KRAS (Table 4 and Fig. S7). It was previously reported that MEK inhibitor suppresses JAG1 expression induced by growth factors in head and neck squamous cell carcinoma.34 In our study, MEK inhibitor suppressed JAG1 expression as well as SNAIL expression and upregulated E‐cadherin in a colon cancer cell line that has a KRAS mutation and is predicted to have activation of the MAP kinase pathway.35 Therefore, activation of the MAP kinase pathway by KRAS mutation might be partially upstream of JAG1 expression in CRC.
While this study presented the novel finding of the association among high JAG1 expression, KRAS status and prognostic significance in CRC, there are some limitations of the present study, such as the retrospective study design, the sample size, the various stages, and the lack of information for molecular status. Multicenter prospective study that enable investigation of the large sample size possibly verify the validity of our findings in this study.
In conclusion, to our knowledge this is the first report demonstrating the poor prognostic significance of high JAG1 expression in CRC. Moreover, our study also revealed that low expression of E‐cadherin plays an additive role for poor prognosis associated with high JAG1 expression in CRC. The results of our in vitro study support the poor prognostic impact associated with high JAG1 expression in CRC and suggest the clue of potential mechanisms for the complicated regulation of JAG1 expression and JAG1‐Notch pathway‐induced cancer development, as shown by the model illustrated in Figure 6. Furthermore, this study implicates JAG1 and its related signaling as a potential target for the development of new therapeutic approaches to reduce recurrence risk and cancer death after surgery for CRC.
Figure 6.
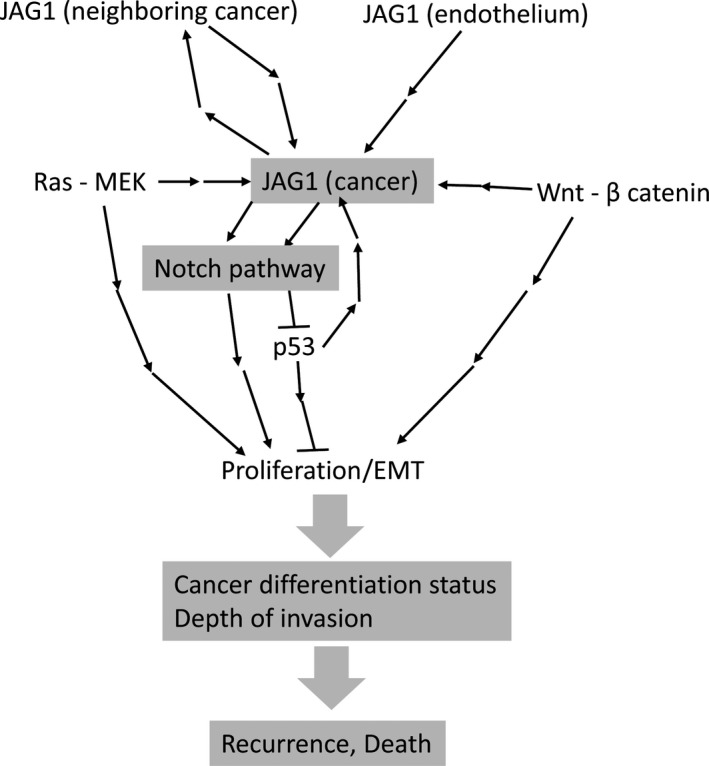
Hypothesized mechanisms of cancer recurrence or death induced by JAG1‐Notch pathway activation following increased JAG1 expression regulated by various factors.
Disclosure Statement
E.O. received lecture fees from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Merck Japan, Yakult Honsha K.K., Takeda Pharmaceutical Co., Ltd. and Eli Lilly Japan K.K. The first author (M.S.) and N.O. are employees of Chugai Pharmaceutical Co., Ltd. There are no other personal interests to declare for any of the other authors.
Supporting information
Fig. S1. Verification of anti‐JAG1 antibody for immunohistochemical staining to analyze the prognostic value of JAG1 expression in human CRC.
Fig. S2. Comparison between anti‐JAG1 antibodies for immunohistochemical staining in human CRC.
Fig. S3. Prognostic significance of JAG1 expression in CRC cancer cells in all patients and patients with stage 0–III disease.
Fig. S4. Prognostic significance of JAG1 expression in endothelium by analysis of Kaplan–Meier estimates and Cox proportional hazards model.
Fig. S5. Representative immunohistochemical staining of E‐cadherin expression in human CRC tissues.
Fig. S6. Prognostic significance of E‐cadherin expression by analysis of Kaplan–Meier estimates.
Fig. S7. Correlation between KRAS status and JAG1 expression in CRC patients with staining intensity 2/3 for E‐cadherin expression.
Fig. S8. Effect of inhibition of RAS‐MEK‐MAP kinase pathway on JAG1, SNAIL and E‐cadherin protein level in the cancer cell lines HCT‐116 and Caco‐2.
Fig. S9. Effect of JAG1 gene silencing on epithelial–mesenchymal transition in the cancer cell line HCT‐116.
Table S1. JAG1 expression in cancer cells stratified by E‐cadherin expression (based on proportion of positive staining) and correlation with clinicopathologic characteristics, JAG1 expression in endothelium, and KRAS status.
Acknowledgments
We would like to thank Dr Makoto Iimori for scientific advice, especially regarding the experimental technique. We thank Ms Yuko Kubota, Takako Shishino, Miki Nakashima, Saori Tsurumaru and Ruriko Aoki for their expert technical assistance. We would also like to thank Ms Shigemi Takami at The Research Support Center, Research Center for Human Disease Modelling, Kyushu University Graduate School of Medical Sciences for technical assistance. This study was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M. Kakenhi; Grant Number 15H05792).
Cancer Sci 107 (2016) 1705–1716
Funding Information
Ministry of Education, Culture, Sports, Science and Technology of Japan (M. Kakenhi; Grant Number 15H05792).
References
- 1. Sancho R, Cremona CA, Behrens A. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep 2015; 16: 571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hori K, Sen A, Artavanis‐Tsakonas S. Notch signaling at a glance. J Cell Sci 2013; 126: 2135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santagata S, Demichelis F, Riva A et al JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res 2004; 64: 6854–7. [DOI] [PubMed] [Google Scholar]
- 4. Purow BW, Haque RM, Noel MW et al Expression of Notch‐1 and its ligands, Delta‐like‐1 and Jagged‐1, is critical for glioma cell survival and proliferation. Cancer Res 2005; 65: 2353–63. [DOI] [PubMed] [Google Scholar]
- 5. Reedijk M, Odorcic S, Chang L et al High‐level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 2005; 65: 8530–7. [DOI] [PubMed] [Google Scholar]
- 6. Yeh TS, Wu CW, Hsu KW et al The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase‐2. Cancer Res 2009; 69: 5039–48. [DOI] [PubMed] [Google Scholar]
- 7. Lin JT, Chen MK, Yeh KT et al Association of high levels of Jagged‐1 and Notch‐1 expression with poor prognosis in head and neck cancer. Ann Surg Oncol 2010; 17: 2976–83. [DOI] [PubMed] [Google Scholar]
- 8. Sethi N, Dai X, Winter CG, Kang Y. Tumor‐derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011; 19: 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sikandar SS, Pate KT, Anderson S et al NOTCH signaling is required for formation and self‐renewal of tumor‐initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res 2010; 70: 1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sonoshita M, Aoki M, Fuwa H et al Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell 2011; 19: 125–37. [DOI] [PubMed] [Google Scholar]
- 11. Chu D, Li Y, Wang W et al High level of Notch1 protein is associated with poor overall survival in colorectal cancer. Ann Surg Oncol 2010; 17: 1337–42. [DOI] [PubMed] [Google Scholar]
- 12. Serafin V, Persano L, Moserle L et al Notch3 signalling promotes tumour growth in colorectal cancer. J Pathol 2011; 224: 448–60. [DOI] [PubMed] [Google Scholar]
- 13. Ozawa T, Kazama S, Akiyoshi T et al Nuclear Notch3 expression is associated with tumor recurrence in patients with stage II and III colorectal cancer. Ann Surg Oncol 2014; 21: 2650–8. [DOI] [PubMed] [Google Scholar]
- 14. Arcaroli JJ, Tai WM, McWilliams R et al A NOTCH1 gene copy number gain is a prognostic indicator of worse survival and a predictive biomarker to a Notch1 targeting antibody in colorectal cancer. Int J Cancer 2016; 138: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu J, Ye X, Fan F et al Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged‐1. Cancer Cell 2013; 23: 171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodilla V, Villanueva A, Obrador‐Hevia A et al Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci USA 2009; 106: 6315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guilmeau S, Flandez M, Mariadason JM, Augenlicht LH. Heterogeneity of Jagged1 expression in human and mouse intestinal tumors: implications for targeting Notch signaling. Oncogene 2010; 29: 992–1002. [DOI] [PubMed] [Google Scholar]
- 18. Kim MH, Kim HB, Yoon SP et al Colon cancer progression is driven by APEX1‐mediated upregulation of Jagged. J Clin Invest 2013; 123: 3211–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dai Y, Wilson G, Huang B et al Silencing of Jagged1 inhibits cell growth and invasion in colorectal cancer. Cell Death Dis 2014; 5: e1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paiva TF Jr, de Jesus VH, Marques RA et al Angiogenesis‐related protein expression in bevacizumab‐treated metastatic colorectal cancer: NOTCH1 detrimental to overall survival. BMC Cancer 2015; 15: 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jass JR, Sobin LH. Histological typing of intestinal tumors WHO International Histological Classification of Tumours, No. 15, 2nd edn Berlin: Springer, 1989. [Google Scholar]
- 22. Sobin LH, Wittekind C. TMN Classification of Malignant Tumors, No. 15, 2nd edn New York: Wiley‐Liss, 2002. [Google Scholar]
- 23. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68. [PubMed] [Google Scholar]
- 24. Pannequin J, Bonnans C, Delaunay N et al The wnt target jagged‐1 mediates the activation of notch signaling by progastrin in human colorectal cancer cells. Cancer Res 2009; 69: 6065–73. [DOI] [PubMed] [Google Scholar]
- 25. Johnston DA, Dong B, Hughes CC. TNF induction of jagged‐1 in endothelial cells is NFkappaB‐dependent. Gene 2009; 435: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gopinathan G, Milagre C, Pearce OM et al Interleukin‐6 stimulates defective angiogenesis. Cancer Res 2015; 75: 3098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fender AW, Nutter JM, Bertrand FE, Sigounas G. Notch‐1 promotes stemness and epithelial to mesenchymal transition in colorectal cancer. J Cell Biochem 2015; 116: 2517–27. [DOI] [PubMed] [Google Scholar]
- 28. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415–28. [DOI] [PubMed] [Google Scholar]
- 29. Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest 2009; 119: 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia‐induced tumor cell migration and invasion. Proc Natl Acad Sci USA 2008; 105: 6392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou BP, Deng J, Xia W et al Dual regulation of Snail by GSK‐3beta‐mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol 2004; 6: 931–40. [DOI] [PubMed] [Google Scholar]
- 32. Chanrion M, Kuperstein I, Barriere C et al Concomitant Notch activation and p53 deletion trigger epithelial‐to‐mesenchymal transition and metastasis in mouse gut. Nat Commun 2014; 5: 5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dotto GP. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat Rev Cancer 2009; 9: 587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng Q, Li S, Chepeha DB et al Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell 2005; 8: 13–23. [DOI] [PubMed] [Google Scholar]
- 35. Ahmed D, Eide PW, Eilertsen IA et al Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013; 2: e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Verification of anti‐JAG1 antibody for immunohistochemical staining to analyze the prognostic value of JAG1 expression in human CRC.
Fig. S2. Comparison between anti‐JAG1 antibodies for immunohistochemical staining in human CRC.
Fig. S3. Prognostic significance of JAG1 expression in CRC cancer cells in all patients and patients with stage 0–III disease.
Fig. S4. Prognostic significance of JAG1 expression in endothelium by analysis of Kaplan–Meier estimates and Cox proportional hazards model.
Fig. S5. Representative immunohistochemical staining of E‐cadherin expression in human CRC tissues.
Fig. S6. Prognostic significance of E‐cadherin expression by analysis of Kaplan–Meier estimates.
Fig. S7. Correlation between KRAS status and JAG1 expression in CRC patients with staining intensity 2/3 for E‐cadherin expression.
Fig. S8. Effect of inhibition of RAS‐MEK‐MAP kinase pathway on JAG1, SNAIL and E‐cadherin protein level in the cancer cell lines HCT‐116 and Caco‐2.
Fig. S9. Effect of JAG1 gene silencing on epithelial–mesenchymal transition in the cancer cell line HCT‐116.
Table S1. JAG1 expression in cancer cells stratified by E‐cadherin expression (based on proportion of positive staining) and correlation with clinicopathologic characteristics, JAG1 expression in endothelium, and KRAS status.


