Abstract
To assess the association of the programmed cell death ligand 1 (PD‐L1) with cisplatin‐based neo‐adjuvant chemotherapy (NAC) response, we investigated the level of PD‐L1 and found increased PD‐L1 expression in chemo‐resistant tumors compared with chemo‐sensitive tumors according to RNA‐Seq analysis. In a cohort of 92 patients with NAC, the positive staining of PD‐L1 was correlated with TNM stage, lower sensitive‐response rates and shorter overall survival rates. In another 30 paired tumor specimens pre‐ and post‐chemotherapy, the patients with high PD‐L1 expression post‐chemotherapy had a worse outcome and higher stable disease rate. CD8+ tumor‐infiltrating lymphocytes were found to be related to chemosensitive response and better prognosis and negative PD‐L1 expression. Furthermore, in two patient‐derived xenograft models and cell lines A549 and PC‐9, cisplatin upregulated PD‐L1 expression, and the enhancement of PD‐L1 in cancer cell lines was in a drug dose‐dependent manner. Moreover, the depletion of PD‐L1 significantly reduced cisplatin resistance. When phosphatidylinositol 3‐kinase/protein kinase B signaling was inhibited by corresponding inhibitors, PD‐L1 expression was downregulated and apoptosis was upregulated in the cisplatin‐treated cancer cells. These results suggest that the upregulation of PD‐L1 promotes a resistance response in lung cancer cells that might be through activation of the phosphatidylinositol 3‐kinase/protein kinase B pathway and suppression of tumor‐infiltrating lymphocytes. The high expression of PD‐L1 after NAC could be an indication of therapeutic resistance and poor prognosis in patients with non‐small‐cell lung cancer.
Keywords: Chemoresistance, neoadjuvant therapy, non‐small‐cell lung cancer, PD‐L1, tumor infiltrating lymphocyte
Lung cancer is the most common cause of death from malignant cancer worldwide.1 Surgical resection is the primary and effective option for patients with NSCLC, which accounts for 85% of all lung cancers, but only 20–25% of patients are eligible for surgery.2 Neo‐adjuvant chemotherapy benefits NSCLC patients, particularly those with advanced cancer, for the chance to obtain surgical resection and better prognosis. Despite a consistent rate of initial responses, the chemotherapy treatment often results in the development of chemoresistance, leading to therapeutic failure. Recently, the immune responses after chemotherapy that could indicate treatment response and prognosis for cancer patients were widely studied in melanoma and lung cancer.3
Investigating the potential molecular mechanisms for monitoring host response to cancer cells has led to the identification of oncogenic drivers in tumor cells and checkpoint molecules involved in the anticancer immune response.4, 5, 6, 7 Programmed cell death ligand 1, a 40‐kDa transmembrane protein, the major ligand for PD‐1, is a cell surface protein in the B7 family that is involved in the modulation of immune response through the inhibition of T‐cell function.8 The PD‐L1 protein is highly expressed in various solid tumors, such as melanoma, head and neck, esophagus, ovary, breast, and lung cancers to trigger immune evasion.9 Recent studies have shown that the induction of PD‐L1 was also associated with drug resistance and an intrinsic proliferative advantage in myeloma cells or multiple myeloma.10 Increasing evidence also demonstrated that PD‐L1 overexpression was found to predict a worse prognosis of patients with esophageal, gastric, renal, and lung carcinomas.11, 12, 13, 14 In addition, the existence of TILs in the cancerous microenvironment may indicate immune‐mediated host defense against the tumor. Several studies reported that strong TILs like CD8+ cytotoxic T cells were related to better prognosis of NSCLC.15, 16
Although some reports showed that conventional chemotherapeutics can induce the expression of PD‐L117 and TILs may increase chemotherapy‐induced cell death,18 little is known about the role of PD‐L1 and TILs in the chemotherapy response and prognosis for NSCLC patients who received NCA treatment.
The effective prediction of chemotherapeutic response and understanding the resistance mechanism are important for monitoring disease progression and improving the prognosis of lung cancer patients.19, 20, 21 Herein, we focused on assessing of the predictive value of PD‐L1 expression in NSCLC patients with NAC and identifying the possible mechanism for the chemoresistance of lung cancer cells. We found that PD‐L1 levels were increased in clinical samples from NSCLC patients with chemoresistance using RNA‐Seq analysis and IHC staining. High level of PD‐L1 or low TILs alone after NAC was significantly correlated with poor chemotherapeutic outcome and prognosis. It was found that PD‐L1 was negatively related to TILs in these NSCLC samples. Further investigations indicated that PD‐L1 expression was stimulated by cisplatin treatment in an NSCLC PDX model and cancer cell lines. The depletion of PD‐L1 could significantly inhibit resistance in cisplatin‐treated lung cancer cells. The upregulation of PD‐L1 might be through the activation of PI3K/AKT signaling and/or increase of TILs. Therefore, PD‐L1 could be a promising indicator for chemotherapeutic resistance and prognosis in patients with NSCLC.
Materials and Methods
Cell lines and cell culture
Human NSCLC cell lines A549 and PC‐9 were cultured in RPMI‐1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 g/mL streptomycin (Invitrogen, Grand Island, NY, USA) in a humidified atmosphere of 5% CO2 at 37°C. The PI3K inhibitor LY294002 and AKT inhibitor AT13148 were purchased from Selleck Chemical (Houston, TX, USA). The A549 and PC‐9 cisplatin‐resistant sublines A549/CIS and PC‐9/CIS were established according to a previous study.22 The cisplatin‐resistant sublines were developed by 12 months of exposure to cisplatin, starting at 100 nM and increasing stepwise to 100 μM. The cisplatin IC50 value for A549/CIS cells was 17.2 μmol/L, which was 5.7‐fold higher than that for the parental line (A549 IC50 3.04 μmol/L). Similarly, the IC50 of the drug‐resistant PC‐9/CIS cells (IC50 10.39 μmol/L) was significantly higher than that of PC‐9 parental cells (IC50 2.076 μmol/L).
Patients and specimens
In this study, we retrospectively obtained 92 pathologically confirmed primary lung cancer specimens from patients who underwent surgery at Peking University Cancer Hospital (Beijing, China), to examine the PD‐L1 protein level and CD8 expression by IHC analysis. In addition, 30 patients were recruited with matched biopsy and surgical resection samples before and after neoadjuvant treatment, to confirm chemotherapy‐related PD‐L1 status changes. In this cohort, patients with lung cancer were regularly followed, and the clinical outcomes of all the cases were obtained. Of 92 NSCLC patients, 72 received two cycles of adjuvant chemotherapy and/or mediastinal radiation (for N2 positive disease) after resection. The remaining 20 cases did not undergo any adjuvant therapy after surgery due to poor Eastern Cooperative Oncology Group status or based on physician or patient preference. This study was approved by the Ethics Committee of Peking University Hospital, and all participants provided informed consent to participate in this research.
Immunohistochemical staining for PD‐L1 and CD8
Formalin‐fixed and paraffin‐embedded primary lung cancer samples were acquired from the Department of Pathology, Peking University, under approval from the Ethics Committee. The PDX model tumor tissues and primary lung cancer samples were stained using rabbit anti‐PD‐L1 (1:500 dilution; Abcam, Cambridge, UK) and the tissues from the 92 cases after NAC treatment were added with rabbit anti‐CD8 (1:200 dilution; Abcam, Cambridge, UK), followed by incubation with HRP‐conjugated goat anti‐rabbit secondary antibody (Sigma‐Aldrich, Poole, UK).
Evaluation of IHC variables
The score used for all subsequent analyses was the average across the available scores. Staining was graded based on the intensity of staining (1, weak; 2, moderate; and 3, strong) and the percentage of cells stained (0, <5%; 1, 5–25%; 2, 26–50%; and 3, >50%) based on the method by Allred et al.23 When combining these two parameters, 0–1 and >1 were considered negative and positive staining, respectively.
Scoring was reviewed in parallel by two experienced pathologists who were blinded to all clinical data.
Flow cytometry and apoptosis analyses
Expression of PD‐L1 was assessed by flow cytometric analyses of anti‐PD‐L1–phycoerythrin (eBioscience/Affymetrix, San Diego, CA, USA). Cells were incubated with anti‐PD‐L1–phycoerythrin for 50 min before FACS analysis.
Apoptotic cells were evaluated in A549 and PC‐9 cells with cisplatin treatment by FACS. Briefly, the cells were suspended and incubated with propidium iodide and annexin V (FITC Annexin V Apoptosis Detection Kit 1; BD Biosciences, Frank Lake, NY, USA) for 30 min at 4°C in the dark. The cells were resuspended in PBS through a 70‐μm nylon mesh and analyzed on a flow cytometer (BD Biosciences, Frank Lake, NY, USA) within 30 min.
Reverse transcription and quantitative real‐time PCR
RNA was extracted using TRIzol following the manufacturer's protocol. Two micrograms of total RNA was added and reverse transcribed with Moloney murine leukemia virus (Invitrogen, Grand Island, NY, USA). Polymerase chain reactions were carried out using a LightCycler 480 SYBR Green I Master on a Light‐Cycler 480 Real‐Time PCR System (Roche, Mannheim, Germany). Cycling conditions were 5 min at 95°C, followed by 45 cycles each consisting of 10 s at 95°C, 20 s at 60°C, and 30 s at 72°C. The relative amount of genes was normalized to GADPH. Fold change was calculated by the method.
Cell transfection
The PD‐L1 shRNA was constructed by GenePharma (Suzhou GenePharma, Suzhou, China) and transfected into PC‐9 cells. The sequence for shPD‐L1 was 5′‐GGAGAATGATGGATGTGAA‐3′. Cells were allowed to grow for 2 days before drug treatments.
Western blot analysis
Proteins were extracted from cells using RIPA buffer containing complete protease inhibitor cocktail (Roche, Mannheim, Germany). Proteins were separated by SDS‐PAGE and transferred to PVDF membranes. Membranes were blocked with 5% non‐fat dried milk in TBST followed by Western blot analysis with the following specific antibodies: rabbit monoclonal anti‐human PD‐L1 antibody (1:500 dilution; Abcam, Cambridge, UK); rabbit monoclonal anti‐human phosphate‐AKT, AKT, GAPDH (1:5000 dilution; Cell Signaling Technology, Houston, TX, USA), and goat anti‐rabbit secondary antibody (1:2000). Signals were visualized using chemiluminescence (Millipore, Boston, MA, USA).
Patient‐derived xenograft mice (PDX model) and drug treatment
NOD/SCID mice were injected with tumor tissues from patients after surgery at Beijing Cancer Hospital by s.c. flap incisions. PDX1 and PDX2 were passaged as tumor tissue volume reached approximately 100 mm3. After validation of the successful generation of the PDX lung cancer model, we injected equal amounts of PDX tissues into NOD/SCID mice. When the tumors reached approximately 100 mm3 in volume, mice were divided into different groups and treated with PBS or cisplatin (5 mg/kg/week) by i.p. injection. After 4 weeks, the mice were killed and tumor tissues were excised. The dissected tumors were collected and prepared for subsequent analyses. All animal experiments were approved by the animal center of the Beijing Cancer Hospital.
Statistical analysis
The IHC expression and clinicopathological data were summarized using standard frequency tabulations. Associations between the markers’ expressions and patients’ clinical variables were assessed using the χ2‐test and Fisher's exact test. Survival rates were estimated using the Kaplan–Meier method. The prognostic value of PD‐L1 was studied using a Cox model, which was adjusted for significant and available prognostic factors of survival. All statistical analyses were undertaken using spss 17.0 statistical software (IBM, Armonk, NY, USA). Data are presented as the means ± SEM. All experiments, comprising three replicates, were performed at least twice independently. P < 0.05 was considered significant difference.
Results
Programmed cell death ligand 1 induced in NSCLC patients with chemoresistance
To explore potential immune checkpoint genes’ functioning in the course of chemotherapy, we carried out RNA‐Seq analysis on the primary tumor tissues from NSCLC with NAC including one group with four chemoresistant patients and another group with four chemosensitive cases. Interestingly, PD‐L1 was increased 6.88‐fold in tumor tissues from chemoresistant patients compared to chemosensitive patients (Fig. 1a). Subsequently, we verified PD‐L1 protein expression in the eight samples from the same patients used for RNA‐Seq analysis by IHC. The results showed that PD‐L1 was located on the membrane, in the cytoplasm, or at both locations of NSCLC tissues (Fig. 1b). Consistently, higher expression of PD‐L1 was observed in patients who were chemoresistant (SD) compared to those who were sensitive to chemotherapy with PR (Fig. 1b).
Figure 1.
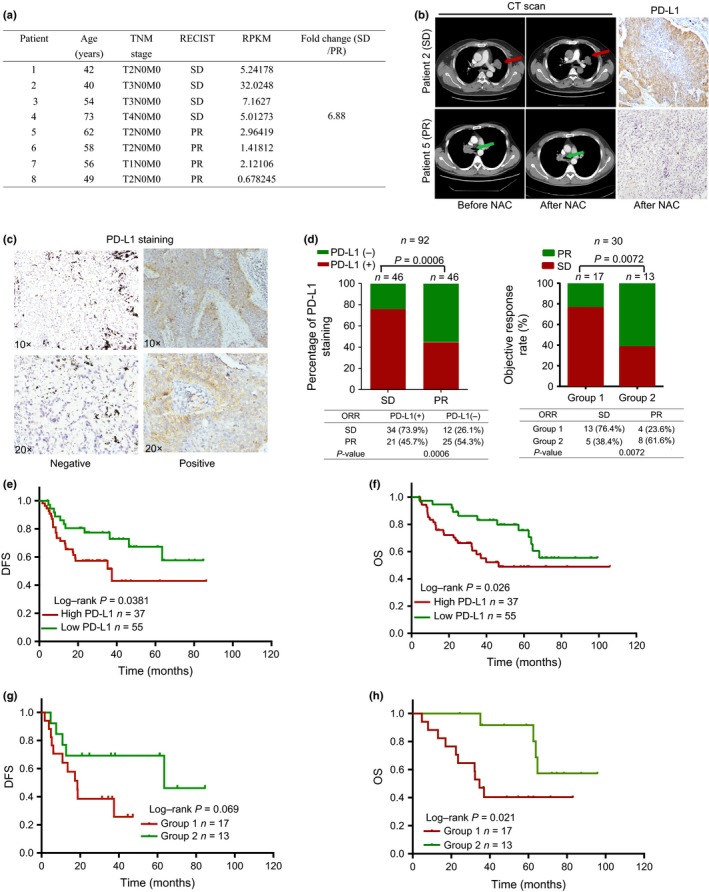
Expression of programmed cell death ligand 1 (PD‐L1) is associated with chemoresistance in patients with non‐small‐cell lung carcinoma (NSCLC). (a) Comparison of gene expression in four chemoresistant and four chemosensitive patients using RNA sequencing analysis. The expression levels of PD‐L1 in chemoresistant patients were higher than those in chemosensitive patients. (b) Left, computed tomography (CT) scan images of patients with stable disease (SD, red arrows) and partial response (PR, green arrows). Right, matched immunohistochemistry results of the patients. (c) Representative images of PD‐L1 immunohistochemical staining on tumor cells among patients with NSCLC. Images were taken at ×10 and ×20 magnification. (d) Left, significant differences in PD‐L1 expression and objective response rate (ORR) in 92 patients who received neoadjuvant chemotherapy. Right, relation between PD‐L1 and ORR in 30 patients who had matched biopsy and surgical resection samples. (e,f) Kaplan–Meier survival curves showing that high levels of PD‐L1 were associated with poor disease‐free survival (DFS) and overall survival (OS) in 92 patients with NSCLC. (g,h) Kaplan–Meier survival curves showing that post‐chemotherapy high expression of PD‐L1 was associated with poor DFS and OS in this cohort.
Association between PD‐L1 and chemoresistance of NSCLC patients
To elucidate whether the PD‐L1 expression status in the tumor cells was related to the response to NAC, we then detected and scored PD‐L1 expression in tumor tissues from 92 NSCLC patients with NAC treatment using IHC staining. We divided the patients into two groups according to positive and negative staining based on PD‐L1 expression (Fig. 1c). The IHC results showed that 60% patients were positive for PD‐L1 expression. The relationship between this protein expression and clinicopathologic characteristics is summarized in Table S1. We noticed that positive PD‐L1 expression showed a tight association with advanced stage (P = 0.044) and poor chemotherapy response (P = 0.0006; Table S1). As observed, there was 73.9% PD‐L1 positive staining in SD patients (n = 46), and 45.7% positive staining in PR patients (n = 46) (P = 0.0006; Fig. 1d, left panel, Pearson's χ2‐test). Together, these results underlined that PD‐L1 played an important role in chemoresistance in NSCLC patients with NAC.
Furthermore, we analyzed the association between PD‐L1 expression and the prognosis of these patients with NAC. Figure 1(e,f) shows that PD‐L1 expression in the cancer cells was significantly correlated with a shorter OS and DFS in these patients.
The univariate analysis showed that elevated PD‐L1 expression indicated a higher relapse risk (DFS: HR, 0.481; 95% CI, 0.242–0.948; P = 0.038; OS: HR, 0.473; 95% CI, 0.243–0.899; P = 0.026; Table S2). However, in multivariate analyses, tumor PD‐L1 expression is not an independent prognostic marker (Table S2).
Variation in PD‐L1 before and after NAC for NSCLC tissues
We further studied the changes in PD‐L1 expression pre‐ and post‐NAC, and the corresponding chemotherapy response and prognosis of NSCLC patients. We assessed PD‐L1 expression in 30 patients with matched biopsy tissues and surgical samples. The correlation between PD‐L1 and the clinicopathological characteristics of this cohort is summarized in Table S3. The evaluation of tumor response to NAC revealed that 12 patients had achieved PR, and 18 had achieved SD. According to the expression changes of PD‐L1 before and after treatment, we divided those patients into two groups: group 1, low/high expression before treatment to high expression after treatment (n = 17); and group 2, low/high expression before treatment to low expression after treatment (n = 13). The SD rates of these two groups were 76.4% and 38.4%, respectively. The difference was statistically significant (P = 0.0072; Fig. 1d, right panel). Finally, we evaluated the prognostic value of PD‐L1 status. As shown in Figure 1(g,h), right panel, the low/high to high switch of PD‐L1 status was associated with inferior OS, compared with the other group, in which the change in PD‐L1 status was the reverse (P = 0.021), but was not associated with DFS (P = 0.069). The univariate analysis showed that PD‐L1 status, TNM stage, and NAC response indicated a higher risk for disease relapse. However, in multivariate analyses, PD‐L1 status and TNM stage were independent prognostic markers for NSCLC (Table S4). Our results revealed that the expression of PD‐L1 was not consistent for NSCLC patients before and after NAC. The upregulated and constantly high expression of PD‐L1 had predictive value for the resistance response of NAC.
Correlation between PD‐L1 and CD8+ TILs in NSCLC tissues
As TILs such as the major cytotoxic T cells have been identified to influence outcome and chemotherapy response of patients with cancer,18 we further explored the relationship between TILs in the cancerous microenvironment and PD‐L1 expression in NSCLC tissues after NAC treatment. We used CD8 marker to stain the cytotoxic T cells in 92 NSCLC tissues after NAC and found a higher rate of CD8+ lymphocytes in PR patients (CD8+, 66%; CD8−, 28.2%) compared to SD patients (CD8+, 34%; CD8−, 71.8%), with statistically significantly difference (Fig. 2a,b; P < 0.0001). We also obtained results that PD‐L1 was negatively associated with CD8+ TILs in these NSCLC specimens (Fig. 2c; P < 0.0001).
Figure 2.
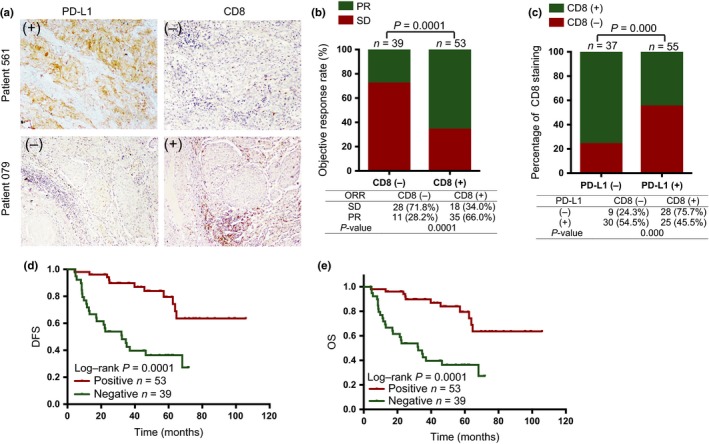
Correlation between programmed cell death ligand 1 (PD‐L1) and CD8 in non‐small‐cell lung carcinoma (NSCLC) tissues. (a) Representative images of PD‐L1 and CD8 in tissues from patients with NSCLC by immunohistochemical staining. Images were taken at ×10 magnification. (b) Low CD8 expression rate was observed in patients with stable disease (SD) and high expression of this protein was seen in cases with partial response (PR). P < 0.0001. (c) PD‐L1 was negatively related to CD8 in these NSCLC tissues. P < 0.0001. (d,e) CD8 expression was associated with improved disease‐free survival (DFS) and overall survival (OS) of NSCLC patients after neo‐adjuvant chemotherapy in the survival curves. ORR, objective response rate.
Next, we analyzed how TIL status influenced prognosis for these NSCLC patients. Consistent with previous reports,24, 25 CD8+ TILs were associated with longer DFS and OS in NSCLC patients who received surgical treatment (Fig. 2d,e).
Cisplatin treatment upregulated PD‐L1 expression in PDX models and NSCLC cell lines
Encouraged by the results mentioned above, we started to investigate the underlying mechanisms. As our standard NAC for lung cancer patients involves cisplatin, we checked the expression level of PD‐L1 under cisplatin treatment in vivo and in vitro. We grafted two human NSCLC tissues in NOD/SCID mice and evidenced PD‐L1 expression in s.c. tumors using FACS and IHC. In both PDX1 and PDX2 systems, we observed that cisplatin treatment inhibited tumor growth (Fig. 3a,b), which confirmed the effect of cisplatin. Cisplatin treatment upregulated PD‐L1 expression in both tumor tissues by FACS (Fig. 3c) and IHC analyses (Fig. 3d).
Figure 3.
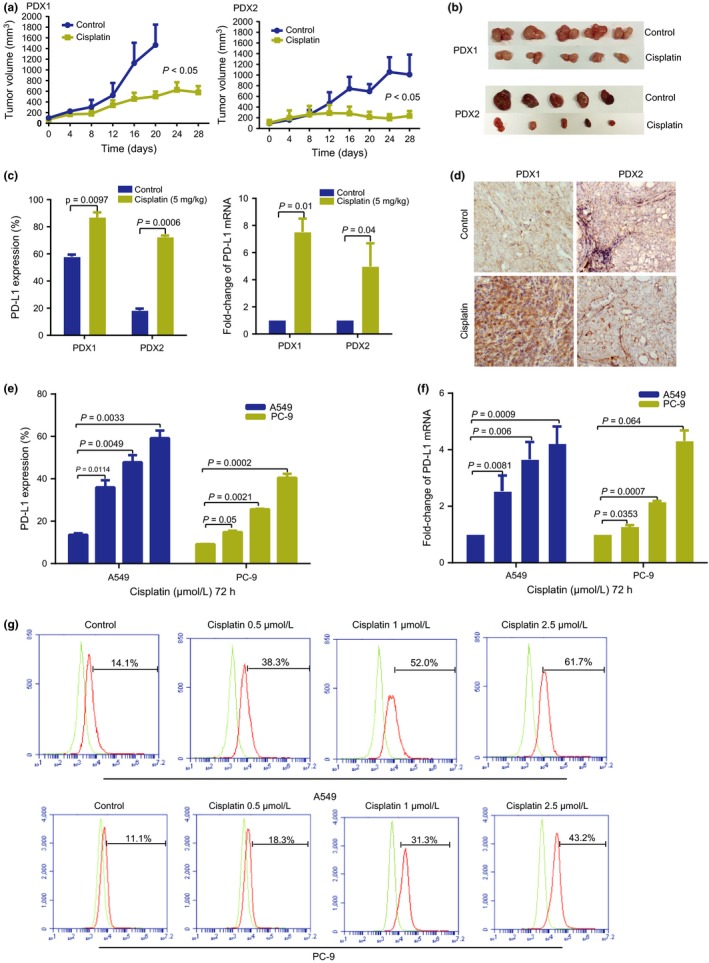
Effect of cisplatin on programmed cell death ligand 1 (PD‐L1) expression in lung cancer cell lines and patient‐derived xenograft (PDX) models. (a) Tumor growth in PDX1 and PDX2 mice after treatment with PBS (control) or cisplatin. P < 0.05. (b) Representative images of tumor sizes in cisplatin‐treated and untreated (control) PDX mice. (c) Protein and RNA levels of PD‐L1 expression in cisplatin‐treated and untreated PDX mice. (d) Representative immunohistochemical images for PD‐L1 expression in cisplatin‐treated and untreated PDX mice. (e,f) PC‐9 and A549 cell lines were cultured with medium alone (control), or cisplatin (0.5, 1, or 2.5 μmol/L) for 72 h and PD‐L1 expression was analyzed by flow cytometry and quantitative PCR. (g) FACS showing PD‐L1 expression in cell lines treated with cisplatin. All experiments were performed in triplicate independently.
We then used various concentrations of cisplatin (0, 0.5, 1, and 2.5 μmol/L) to treat NSCLC cell lines PC‐9 and A549 for 72 h. The levels of PD‐L1 in lung cancer cells were increased when compared with non‐treated cells in a dose‐dependent manner by FACS (Fig. 3e,g) and quantitative PCR detection (Fig. 3f).
Depletion of PD‐L1 inhibited cisplatin resistance in lung cancer cells
To elucidate whether the upregulation of PD‐L1 by cisplatin contributed to the resistance of cancer cells, we depleted PD‐L1 through shRNA in A549 and PC‐9 cells to check the sensitivity changes under cisplatin treatment. We verified that the depletion of PD‐L1 resulted in decreased PD‐L1 expression (Fig. 4a). Moreover, the depletion of PD‐L1 led to more than 50% decrease in IC50 values compared with the control group in A549 and PC‐9 cells (Fig. 4b). These data indicated that PD‐L1 depletion enhanced the sensitivity of lung cancer cells to cisplatin treatment.
Figure 4.
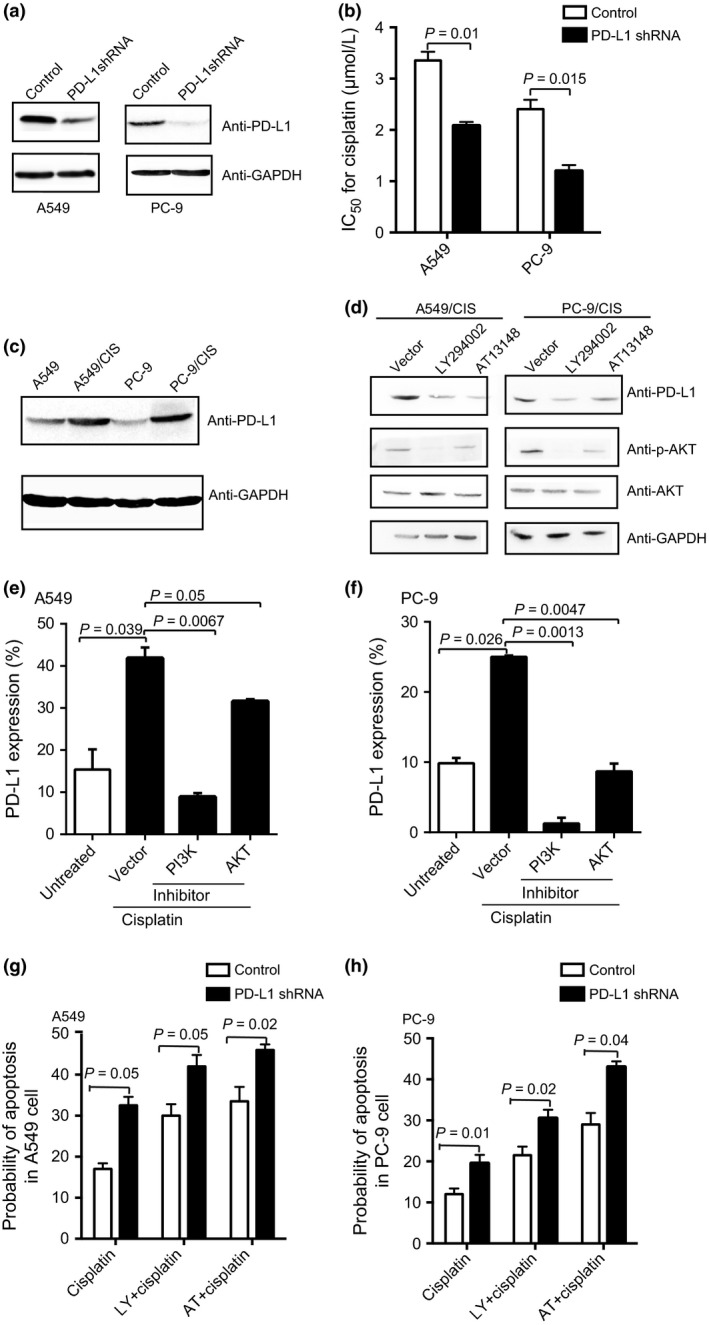
Molecular mechanism of programmed cell death ligand 1 (PD‐L1) expression elevated by cisplatin. (a) Knockdown of PD‐L1 was evaluated by Western blot in A549 and PC‐9 non‐small‐cell lung carcinoma cells. (b) IC 50 values after transient transfection of shRNA. (c) Western blot showing PD‐L1 expression in cisplatin‐resistant cell lines (A549/CIS and PC‐9/CIS) and parental cells. (d) Western blot analysis showing PD‐L1, phosphorylated protein kinase B (p‐AKT) and AKT expression in different protein fractions of PC‐9/CIS and A549/CIS cells treated with phosphatidylinositol 3‐kinase (PI3K)/AKT inhibitors of PI3K (LY294002, 500 nM), and AKT (AT13148, 10 nM) for 72 h. (e,f) A549 and PC‐9 cells were pretreated with inhibitors of signal transduction, for example, 500 nM PI3K inhibitor LY294002 and 10 nM AKT inhibitor AT13148, then cultured with cisplatin before FACS analysis. (g,h) Apoptosis was reduced in A549 and PC‐9 cells with knockdown of PD‐L1 by FACS analysis. PI3K inhibitor (LY294002, 500 nM) or AKT inhibitor (AT13148, 10 nM) resulted in even more apoptosis in cells with suppression of PD‐L1 compared to corresponding control cells. All experiments were performed in triplicate independently.
Next, consistent with the trend from RNA‐Seq results, higher expression of PD‐L1 was observed in cisplatin‐resistant lung cancer cells A549/CIS and PC‐9/CIS compared with the parental cells using Western blot (Fig. 4c).
Inhibition of PI3K/AKT signaling reduced PD‐L1 expression in lung cancer cells
PI3K/AKT signaling has been verified to be associated with chemoresistance.26, 27, 28 To investigate the pathway mediating the upregulation effect of cisplatin on PD‐L1, we blocked PI3K/AKT signaling using the specific inhibitors LY294002 and AT13148 in the resistant sublines. The downregulation of PD‐L1 and the level of phosphorylated AKT were observed (Fig. 4d). Furthermore, these two inhibitors also downregulated PD‐L1 expression in the parental A549 and PC‐9 cell lines treated with cisplatin, as revealed by FACS (Fig. 4e,f). In addition, we obtained induction of apoptosis in cisplatin‐treated A549 and PC‐9 lung cancer cells with knockdown PD‐L1 expression than cells transfected with control shRNA (Fig. 4g,h). With cisplatin treatment, the combination of LY294002/AT13148 and suppression of PD‐L1 resulted in even more cells undergoing apoptosis (Fig. 4g,h). Therefore, the activated PI3K/AKT pathway might, at least in part, be responsible for the upregulation of PD‐L1, which was associated with chemoresistance in lung cancer cells.
Discussion
The PD‐1/PD‐L1 axis plays an important role in immune‐escape, and these pathways are currently attractive therapeutic targets for human cancers, including NSCLC.29 Although many preclinical studies and ongoing clinical trials have focused on the association between PD‐L1 and immune‐escape, investigation into its predictive role in prognosis and chemotherapy response in NSCLC was limited.
Overexpression of PD‐L1 has been correlated with poor prognosis in NSCLC.11 We observed an association between PD‐L1‐positive expression and shorter survival of lung cancer patients, and the positivity of PD‐L1 were significantly associated with NAC response and TNM stage. Identification of potential factors that can assess chemotherapy response will aid in the selection of chemotherapy regimens for lung cancer patients. Increasing numbers of studies have identified that PD‐L1 plays an essential role in chemotherapy of cancers.30, 31 Consistently, higher rates of positivity of this protein were observed for lung cancer patients with chemoresistance. For NSCLC, the treatment response is an independent prognostic factor. We also found that the expression change of PD‐L1 was significantly linked to chemotherapy response, rather than PD‐L1 expression before treatment.
Antitumor immune responses could be induced by blockade of the PD‐1/PD‐L1 pathway in NSCLC.29 The tumor infiltrating lymphocyte, namely TILs, have been reported to be related to improved survival in NSCLC patients with surgical treatment.24, 25 Our results showed that strong CD8+ TILs were significantly associated with increased DFS and OS in the resected specimens of NSCLC patients after NAC. Moreover, high TIL expression rate was mostly detected in chemosensitive samples with significant difference. Previous studies have reported the association between PD‐L1 and cytotoxic CD8+ TILs for lung cancer patients.32, 33 In the present study, we found that the immune suppressor of PD‐L1 expression was negatively correlated to CD8+ TILs in NSCLC samples. Previous reports showed that tumor‐associated PD‐L1 had a function in inducing apoptosis of infiltrating T cells.8, 34 In another study, the authors explained the immunomodulatory effect of anthracyclines on cancer cells, and provided a link between immunoresistance and chemoresistance.19 Considering these results, we hypothesized that PD‐L1 highly expressed in tumor tissues might inhibit TILs, probably leading to suppressed immune reaction and poor outcome for NSCLC patients.
Furthermore, we started to elucidate how a chemotherapy reagent regulates the PD‐L1 status in lung cancer cells. It has been reported that the downregulation of microRNA‐197 enhances PD‐L1 expression and promotes chemoresistance in lung cancer cells, independent of affecting immuno‐inhibitory signals.19 Low doses of fractionated radiotherapy result in increased PD‐L1 in tumor cells in a variety of syngeneic mouse models of melanoma, colorectal, and triple‐negative breast cancers.35 Our results showed that PD‐L1 expression was elevated in vitro and in vivo. Higher expression of this protein was also observed in cisplatin‐resistant sublines. The alteration of PD‐L1 levels may play a role in the chemotherapy response of lung cancer.
In a phase II clinical trial, PD‐L1 blockade combined with chemotherapy has been reported to be safe and effective for stage IIIB/IV NSCLC patients.36 We also checked the effect of PD‐L1 inhibition on drug resistance. Knockdown of PD‐L1 expression increased the sensitive response to cisplatin in lung cancer cells. Recent studies have also shown that the microenvironment induces PD‐L1 expression in myeloma cells, which is associated with immune evasion, drug resistance, and an intrinsic proliferative advantage in multiple myeloma.10 Based on our finding that PD‐L1 was associated with chemoresistance, we further explored the molecular mechanism of the cisplatin effect on PD‐L1. We verified that inhibition of PD‐L1 could induce apoptosis in lung cancer cells. Previous reports indicated that the expression of PD‐L1 is dependent on the upstream part of the PI3K/AKT pathway in melanoma, breast, and prostate carcinoma.37, 38, 39 In addition, the suppression of the PI3K/AKT pathway attenuates laminin‐mediated resistance to imatinib mesylate or chemotherapy and cellular survival in small‐cell lung cancer.40 We also found that PI3K/AKT inhibitors reversed PD‐L1 expression and induced apoptosis of lung cancer cells treated with cisplatin. After cisplatin treatment,more apoptotic cells were observed in PD‐L1 knocked‐down cells combined with PI3K/AKT inhibitors, suggesting that this pathway may target PD‐L1 to overcome drug resistance in NSCLC.
Therefore, PD‐L1 could be a potential indicator for NSCLC patients with chemotherapy response, and targeting PD‐L1 might be a promising strategy for the reversal of chemoresistance of lung cancer.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- Akt
protein kinase B
- CI
confidence interval
- DFS
disease‐free survival
- HR
hazard ratio
- IHC
immunohistochemistry
- NAC
neo‐adjuvant chemotherapy
- NSCLC
non‐small‐cell lung cancer
- OS
overall survival
- PD‐1
programmed cell death 1
- PD‐L1
programmed cell death ligand 1
- PDX
patient‐derived xenograft
- PI3K
phosphatidylinositol 3‐kinase
- PR
partial response
- RNA‐Seq
RNA sequencing
- SD
stable disease
- TIL
tumor‐infiltrating lymphocyte
Supporting information
Table S1. Clinicopathological variables and PD‐L1 expression of the patients treated with neoadjuvant chemotherapy (n = 92).
Table S2. Univariate and multivariate Cox regression analyses for disease free survival (DFS) and overall survival (OS) of patients (n = 92).
Table S3. Clinicopathological variables and PD‐L1 expression shift of the patients treated with neoadjuvant chemotherapy (n = 30).
Table S4. Univariate and multivariate Cox regression analyses for disease free survival (DFS) and overall survival (OS) of patients (n = 30).
Acknowledgments
This work was supported by the Peking University (PKU) 985 Special Fund for Collaborative Research with PKU Hospitals, the Beijing Municipal Administration of Hospitals’ Clinical Medicine Development Special Fund (Grant No. ZYLX201509), the Capital Health Research and Development Special Fund (Grant No. 2014‐2‐1021), and the National High Technology Research and Development Program of China (863 Program) (Grant No. 2014AA020602).
Cancer Sci 107 (2016) 1563–1571
Funding Information
Peking University (PKU) 985 Special Fund for Collaborative Research with PKU Hospitals, the Beijing Municipal Administration of Hospitals‘ Clinical Medicine Development Special Fund, the Capital Health Research and Development Special Fund, the National High Technology Research and Development Program of China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2. Xue C, Hu Z, Jiang W et al National survey of the medical treatment status for non‐small cell lung cancer (NSCLC) in China. Lung Cancer 2012; 77: 371–5. [DOI] [PubMed] [Google Scholar]
- 3. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015; 28: 690–714. [DOI] [PubMed] [Google Scholar]
- 4. Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr Cancer Drug Targets 2015; 15: 452–62. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer JR, Pardoll DM. Immune checkpoint inhibitors: making immunotherapy a reality for the treatment of lung cancer. Cancer Immunol Res 2013; 1: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol 2013; 34: 556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong H, Strome SE, Salomao DR et al Tumor‐associated B7‐H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800. [DOI] [PubMed] [Google Scholar]
- 9. Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD‐1/PD‐L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol 2016; 21: 462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamura H, Ishibashi M, Yamashita T et al Marrow stromal cells induce B7‐H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia 2013; 27: 464–72. [DOI] [PubMed] [Google Scholar]
- 11. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD‐L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28: 682–8. [DOI] [PubMed] [Google Scholar]
- 12. Ohigashi Y, Sho M, Yamada Y et al Clinical significance of programmed death‐1 ligand‐1 and programmed death‐1 ligand‐2 expression in human esophageal cancer. Clin Cancer Res 2005; 11: 2947–53. [DOI] [PubMed] [Google Scholar]
- 13. Thompson RH, Kuntz SM, Leibovich BC et al Tumor B7‐H1 is associated with poor prognosis in renal cell carcinoma patients with long‐term follow‐up. Cancer Res 2006; 66: 3381–5. [DOI] [PubMed] [Google Scholar]
- 14. Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death‐1 ligand‐1 (PD‐L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006; 108: 19–24. [DOI] [PubMed] [Google Scholar]
- 15. Al‐Shibli KI, Donnem T, Al‐Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non‐small cell lung cancer. Clin Cancer Res 2008; 14: 5220–7. [DOI] [PubMed] [Google Scholar]
- 16. Dieu‐Nosjean MC, Antoine M, Danel C et al Long‐term survival for patients with non‐small‐cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26: 4410–7. [DOI] [PubMed] [Google Scholar]
- 17. Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD‐L1: a novel role of pro‐survival signalling in cancer. Ann Oncol 2016; 27: 409–16. [DOI] [PubMed] [Google Scholar]
- 18. Wimberly H, Brown JR, Schalper K et al PD‐L1 expression correlates with tumor‐infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res 2015; 3: 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujita Y, Yagishita S, Hagiwara K et al The clinical relevance of the miR‐197/CKS1B/STAT3‐mediated PD‐L1 network in chemoresistant non‐small‐cell lung cancer. Mol Ther 2015; 23: 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galluzzi L, Senovilla L, Vitale I et al Molecular mechanisms of cisplatin resistance. Oncogene 2012; 31: 1869–83. [DOI] [PubMed] [Google Scholar]
- 21. Poulikakos PI, Rosen N. Mutant BRAF melanomas–dependence and resistance. Cancer Cell 2011; 19: 11–5. [DOI] [PubMed] [Google Scholar]
- 22. Tsaur I, Makarevic J, Juengel E et al Resistance to the mTOR‐inhibitor RAD001 elevates integrin alpha2‐ and beta1‐triggered motility, migration and invasion of prostate cancer cells. Br J Cancer 2012; 107: 847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68. [PubMed] [Google Scholar]
- 24. Horne ZD, Jack R, Gray ZT et al Increased levels of tumor‐infiltrating lymphocytes are associated with improved recurrence‐free survival in stage 1A non‐small‐cell lung cancer. J Surg Res 2011; 171: 1–5. [DOI] [PubMed] [Google Scholar]
- 25. Kilic A, Landreneau RJ, Luketich JD, Pennathur A, Schuchert MJ. Density of tumor‐infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non‐small‐cell lung cancer tumors. J Surg Res 2011; 167: 207–10. [DOI] [PubMed] [Google Scholar]
- 26. Guerreiro AS, Fattet S, Fischer B et al Targeting the PI3K p110alpha isoform inhibits medulloblastoma proliferation, chemoresistance, and migration. Clin Cancer Res 2008; 14: 6761–9. [DOI] [PubMed] [Google Scholar]
- 27. Yuge K, Kikuchi E, Hagiwara M et al Nicotine induces tumor growth and chemoresistance through activation of the PI3K/Akt/mTOR pathway in bladder cancer. Mol Cancer Ther 2015; 14: 2112–20. [DOI] [PubMed] [Google Scholar]
- 28. Zhu Y, Yu J, Wang S, Lu R, Wu J, Jiang B. Overexpression of CD133 enhances chemoresistance to 5‐fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric cancer cells. Oncol Rep 2014; 32: 2437–44. [DOI] [PubMed] [Google Scholar]
- 29. He J, Hu Y, Hu M, Li B. Development of PD‐1/PD‐L1 pathway in tumor immune microenvironment and treatment for non‐small cell lung cancer. Sci Rep 2015; 5: 13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Black M, Barsoum IB, Truesdell P et al Activation of the PD‐1/PD‐L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget 2016; 7: 10557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ishibashi M, Tamura H, Sunakawa M et al Myeloma drug resistance induced by binding of myeloma B7‐H1 (PD‐L1) to PD‐1. Cancer Immunol Res 2016; 4: 779–88. [DOI] [PubMed] [Google Scholar]
- 32. Chen YB, Mu CY, Huang JA. Clinical significance of programmed death‐1 ligand‐1 expression in patients with non‐small cell lung cancer: a 5‐year‐follow‐up study. Tumori 2012; 98: 751–5. [DOI] [PubMed] [Google Scholar]
- 33. Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka‐Akita H, Nishimura M. B7‐H1 expression on non‐small cell lung cancer cells and its relationship with tumor‐infiltrating lymphocytes and their PD‐1 expression. Clin Cancer Res 2004; 10: 5094–100. [DOI] [PubMed] [Google Scholar]
- 34. Gibbons RM, Liu X, Pulko V et al B7‐H1 limits the entry of effector CD8(+) T cells to the memory pool by upregulating Bim. Oncoimmunology 2012; 1: 1061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dovedi SJ, Adlard AL, Lipowska‐Bhalla G et al Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD‐L1 blockade. Cancer Res 2014; 74: 5458–68. [DOI] [PubMed] [Google Scholar]
- 36. Lynch TJ, Bondarenko I, Luft A et al Ipilimumab in combination with paclitaxel and carboplatin as first‐line treatment in stage IIIB/IV non‐small‐cell lung cancer: results from a randomized, double‐blind, multicenter phase II study. J Clin Oncol 2012; 30: 2046–54. [DOI] [PubMed] [Google Scholar]
- 37. Crane CA, Panner A, Murray JC et al PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene 2009; 28: 306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang X, Zhou J, Giobbie‐Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD‐L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 2013; 19: 598–609. [DOI] [PubMed] [Google Scholar]
- 39. Liu L, Mayes PA, Eastman S et al The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD‐1, PD‐L1, and CTLA‐4. Clin Cancer Res 2015; 21: 1639–51. [DOI] [PubMed] [Google Scholar]
- 40. Tsurutani J, West KA, Sayyah J, Gills JJ, Dennis PA. Inhibition of the phosphatidylinositol 3‐kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin‐mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer Res 2005; 65: 8423–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinicopathological variables and PD‐L1 expression of the patients treated with neoadjuvant chemotherapy (n = 92).
Table S2. Univariate and multivariate Cox regression analyses for disease free survival (DFS) and overall survival (OS) of patients (n = 92).
Table S3. Clinicopathological variables and PD‐L1 expression shift of the patients treated with neoadjuvant chemotherapy (n = 30).
Table S4. Univariate and multivariate Cox regression analyses for disease free survival (DFS) and overall survival (OS) of patients (n = 30).


