Abstract
Introduction
Household air pollution from solid fuel burning kills over 4 million people every year including half a million children from acute lower respiratory infections. Although biologically plausible, it is not clear whether household air pollution is also associated with acute lower respiratory infections in adults. We systematically reviewed the literature on household air pollution and acute lower respiratory infection in adults to identify knowledge gaps and research opportunities.
Methods
Ten bibliographic databases were searched to identify studies of household air pollution and adult acute lower respiratory infection. Data were extracted from eligible studies using standardised forms.
Results
From 4617 titles, 513 abstracts and 72 full-text articles were reviewed. Eight studies met the inclusion criteria of which 2 found a significant adjusted increased risk of acute lower respiratory infection, 2 identified a univariate association whilst 4 found no significant association. Study quality was generally limited. Heterogeneity in methods and findings precluded meta-analysis.
Discussion
A systematic review of the literature found limited evidence for an association between household air pollution and risk of acute lower respiratory infection in adults. Additional research, with carefully defined exposure and outcome measures, is required to complete the risk profile caused by household air pollution in adults.
Registration number
CRD42015028042.
Introduction
Exposure to household air pollution from domestic solid fuel use causes over 4 million deaths per year, and is the most important environmental risk factor for disability adjusted life years worldwide [1]. Household air pollution has been found to increase the risk of childhood acute lower respiratory tract infection (ALRI) by 78% (pooled odds ratio = 1.78 (95% CI 1.45 to 2.18) [2]) and is responsible for half a million deaths in young children every year. Over a third of the world’s population are exposed to high levels of household air pollution, with women and children experiencing the greatest burden [3].
Disease outcomes in adults attributed to household air pollution from solid fuels include chronic lung and cardiovascular disease [1]. Based on the evidence for an association between household air pollution and child ALRI [2], and evidence from ecological and toxicology studies regarding the health effects ambient air pollution in all ages [4], an association between household air pollution and ALRI in adults is biologically plausible but lacks a convincing evidence base. Although the Global Burden of Disease study 2013 incorporated adult ALRI as an outcome for household air pollution exposure, the risk estimate was derived from an integrated exposure-response curve for PM2.5 (particulate matter <2.5μm diameter) from ambient and household air pollution, and tobacco smoke which has limitations [1,5]. It is important to determine whether or not there is an increased risk of ALRI associated with household air pollution in adults since the attributable risk is potentially high and the burden greatest in people living in low and middle income countries (LMICs). ALRI is a common cause of hospitalisation among adults in LMICs with high associated morbidity and mortality [6]. Mortality among adults with pneumonia in sub-Saharan Africa is around 10%, with over 50% of deaths seen in people under 35 [7–9].
To ensure that public health interventions are appropriately directed, the true burden of disease needs to be clarified [10, 11]. We systematically reviewed the literature, including studies of any design, to synthesise the evidence base for the relationship between household air pollution exposure from domestic solid fuel use and adult ALRI worldwide. We describe the current literature, identify knowledge gaps and future research opportunities.
Methods
This systematic review is registered with the Centre for Reviews and Dissemination (Registration number: CRD42015028042). The full protocol is available at http://www.crd.york.ac.uk/prospero.
Eligibility criteria
Eligibility criteria for studies based on participants, exposures, outcomes and study designs were used to identify studies that provided an effect estimate for household air pollution on adult ALRI in the form of a relative risk, odds ratio (OR) or hazard ratio, or data allowing calculation of an effect estimate. English language papers and non-English language papers (including French and Spanish) where a translation was available were included. Translation was not available for Chinese literature.
Participants
All adults were eligible, without geographical restriction. Studies exclusively focussed on children (<18 years) were excluded, but to avoid the exclusion of potentially useful data on adults, studies that included low numbers of under 18-year olds within a primarily adult population were included.
Exposure
Exposure was defined as air pollution from indoor burning of any solid fuels—including wood, charcoal, animal dung, crop residues and coal—for household purposes. We included studies that quantified exposure through direct measurement of specific pollutants, questionnaires regarding exposure history, comparison of groups exposed to types of exposure (e.g. different stove types), or before and after an intervention to reduce exposure. Studies examining outdoor air pollution, occupational exposures, non-fuel combustion sources or non-solid fuels were excluded.
Outcomes
The primary outcome of interest was ALRI including pneumonia, acute bronchitis or bronchiolitis. However we included studies that defined the outcome as “acute” or specified duration of less than 14 days, even if infection was not confirmed, on the assumption that acute respiratory illnesses in the absence of underlying disease would likely be infectious in origin. Studies reporting illnesses lasting longer than 14 days were excluded, to avoid inclusion of chronic diseases. Studies examining exacerbations of chronic conditions such as asthma or COPD were included if an acute infectious exacerbation was defined. Studies that did not distinguish between upper respiratory tract infections and ALRI were excluded.
Study designs
Individually- and cluster-randomised control trials; controlled before-and-after trials; cohort studies; case-control studies and cross-sectional surveys were included.
Search strategy
The databases listed in Box 1 were searched on 17/12/2015 using the search terms and Boolean phrases listed in Box 2. An example search strategy is shown in Box 3. Searches were limited to papers published from 01/01/1960 onwards, and to human studies. Identified papers were imported into EndNote X7.
Box 1. Databases searched
MEDLINE (OVID)
Scopus (including EMBASE)
Web of Science
CINAHL (Ovid)
Global Health (Ovid)
Cochrane Central Register of Controlled Trials (CENTRAL)
Database of Abstracts of Reviews of Effects (DARE)
Latin American and Caribbean literature (LILACS)
SciELO
African Index Medicus
Box 2. Search terms and Boolean phrases used
Outcomes (OR): “respiratory infection*”, “respiratory tract infection*”, pneumonia, “respiratory illness*”
AND
Exposures/Interventions (OR): “household air”, “indoor air”, biomass,*smoke, fuel*, *stove*.
Box 3. Example search strategy for Medline (OVID)
“respiratory infection*”.mp,tw.
“respiratory tract infection*”.mp,tw.
pneumonia.tw,mp.
“respiratory illness”.tw,mp.
1 or 2 or 3 or 4
“household air”.tw,mp.
“indoor air”.tw,mp.
biomass.tw,mp.
smoke,tw,mp.
stove.tw,mp.
fuel.tw,mp.
6 or 7 or 8 or 9 or 10 or 11
5 and 12
Following removal of duplicates, titles of identified papers were reviewed for eligibility by two authors. The first 20% were all reviewed in duplicate by two independent authors to check for agreement. The remaining 80% were reviewed by one author with 10% of these being cross-checked by a second author to ensure ongoing agreement.
Abstracts of the selected titles and then full text of the selected articles were reviewed for eligibility, according to the selection criteria. Abstracts and full text articles were all reviewed in duplicate by two independent authors—HJ reviewed all abstracts and full text articles and the second review was performed by either HS, DH or GM. Where discordant decisions were not resolved by discussion, the decision was made by a third author. If the full text article was not available in English, translation was sought to determine eligibility.
Alternative sources were also searched for eligible studies: www.who.int/trialsearch, www.clinicaltrials.gov and EAGLE (European Association for Grey Literature Exploitation, www.opengrey.eu). Reference lists of all selected papers were reviewed for any potentially eligible titles. For studies where relevant details were not available, authors were invited to supply information if their contact details were available.
Data extraction
Data were extracted from the selected papers using a previously piloted data extraction form, from which a summary table and narrative synthesis were produced. Where effect estimates were not provided, these were calculated from the published data.
Quality assessment
Studies that quantified exposure by direct household or personal measurement of specific pollutants were considered to provide a higher quality of exposure assessment than those that relied on self-reported exposure. Studies using prospective assessment of ALRI by a physician or trained health care worker using predefined criteria or diagnostic investigations were considered to be of higher quality than those reliant of self-reported episodes of ALRI.
Risk of bias assessment
Risk of bias was assessed using the Liverpool Quality Assessment Tool, which reviewed the study methodology in four domains—subject selection, exposure assessment, outcome assessment and adjustment for confounders—and assigned quality ratings (low, moderate or high risk of bias) [12]. We adopted this instead of the Cochrane Collaboration's tool for assessing risk of bias due to the variety of study designs identified. Publication bias was not assessed due to the small number of papers identified.
Data analysis
Methodological heterogeneity in exposure and outcome assessment precluded a meta-analysis, but a narrative summary of the selected papers and a summary of findings table were produced.
Results
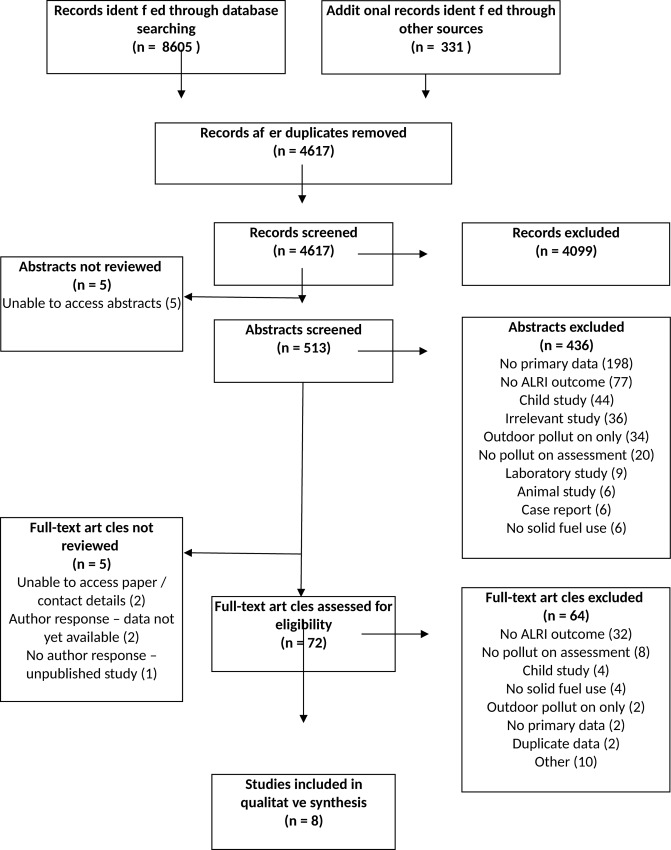
Database searches identified 8605 records and 331 records were identified from the alternative sources set out in the methods. After removal of duplicates 4617 records remained. The number of titles, abstracts and papers reviewed was 4617, 513 and 72 respectively, as summarised in the PRISMA flowchart (Fig 1), with the reasons for exclusion at the abstract and full text stages (see S1 File for PRISMA Checklist). Agreement between authors was 95–96% for the first 20% of titles reviewed, and 95–98% for the 10% overlap of remaining titles. It was not possible to locate 5 selected abstracts [13–17] and 2 selected full text papers—both published in Chinese [18, 19]—for review. Two authors were contacted for further details about their recent or ongoing studies identified at www.clinicaltrials.gov. One study is a prospective cohort study of febrile adults in Tanzania for which recruitment has been completed but the data are not yet available (NCT01947075). The second study is a randomized trial of smoke reduction interventions in Native American populations, but we were unable to obtain further details (NCT02240069). In addition, we are conducting a case-control study of the effects of air pollution exposure and chronic respiratory disease on pneumonia risk in Malawian adults [20].
Fig 1. PRISMA Flow Chart A flow chart depicting the inclusion and exclusion of identified studies.
Ten papers met the criteria for inclusion; data from 2 of these were duplicated in other selected papers [21, 22], and therefore not extracted. The remaining 8 papers included 4 cross-sectional [23–26], 2 cohort [27, 28] and 2 case control studies [29, 30]. The articles covered study locations from 8 countries across 4 continents. The largest study was a retrospective cohort study from China, which reported on the causes of death in 42,422 farmers [28]. The main findings of all included studies are summarised in Table 1, which demonstrates the heterogeneity of study participants, exposure definitions and outcome definitions.
Table 1. Summary of findings table for four studies investigating the effects of household air pollution on adult acute lower respiratory tract infections.
| Study | Sample selection | Sample size | Subject description | Exposure | Outcome | Adjustment for confounders | Effect size |
|---|---|---|---|---|---|---|---|
| COHORT STUDIES | |||||||
| Ezzati, 2001 [27]. Kenya, rural. Conducted 1996–1999. | 55 randomly chosen households. Response rates of household members not stated. | 229 individuals. | 47.6% 5–14 years. 52.4% 15–49 years. Age groups treated as one category, but ALRI more common in the latter. 55% female. | Continuous measurement of PM10 in households for 14–15 hours a day for 200 days. | ALRI (including bronchitis, pneumonia and broncho-pneumonia diagnosed by a nurse who visited all households every 1–2 weeks for 2 years, and examined anybody reported to have respiratory symptoms. | Adjusted for age, sex, smoking, village, and household occupancy. No adjustment for socioeconomic status, but homogenous group of participants. | Adjusted logistic regression, OR (95% CI, p value): Reference category: <200μg/m3 PM10•. 200–500 μg/m3†: 1.65 (0.5–5.45, 0.41). 500–1000 μg/m3†: 1.87 (0.6–5.71, 0.27). 1000–2000μg/m3†: 2.74 {0.93–8.12, 0.07). 2000–4000μg/m3†: 3.28 (1.09–9.85, 0.03). 4000–7000 μg/m3†: 3.21 (1.01–10.24, 0.05). >7000 μg/m3†: 7.10 (2.26–22.32, 0.001). |
| Shen, 2009 [28]. China, rural. Conducted 1992–1996. | Identified from local administrative records. | 44,850 individuals identified. 42,422 followed up to endpoint. | All famers in 4 communes born between 1917 and 1951 and living in Xuanwei on 1/1/1976. 49% female. | Principal use of smoky or smokeless coal, and presence of chimney, assessed by standardised questionnaire. | Death from pneumonia obtained from public records of death certificates, which were completed by physicians. | Adjusted for annual coal use, stove improvement, smoking, years of cooking, education, house size and occupancy, coal mining, COPD and time spent indoors. | Cox proportional hazards model, HR (95%CI, p value). Stove improvement† vs no stove improvement•. Men: 0.49 (0.31–0.78, 0.002). Women: 0.53 (0.32–0.88, 0.014). Smokeless coal† vs smoky coal•. Men: 1.52 (0.98–2.36, 0.060). Women: 1.44 (0.90–2.31, 0.129). |
| CASE CONTROL STUDIES | |||||||
| Loeb, 2009 [29]. Canada, urban. Conducted 2002–2005. | Cases were patients who presented to emergency departments. Not stated if all consecutive patients recruited. Controls recruited contemporaneously using random-digit dialing. Response rates not reported. | 717 cases and 867 controls. | Cases: pneumonia patients, 40% female, age >65 (mean 79). Controls: unmatched healthy community controls, 68% female, age >65 (mean 74). The same geographical restrictions were used for both groups. | Recall of fireplace use in previous 12 months, assessed by structured questionnaire. No quantification of exposure. | Pneumonia, based on appropriate clinical criteria plus radiographic findings, all performed by physicians. | Backward-stepwise logistic regression performed adjusting for multiple variables but fireplace use not included in the final model so only unadjusted results reported. | Unadjusted logistic regression, OR (95%CI, p value): Fireplace use† vs. no fireplace use•. 0.69 (0.54–0.87, 0.002). |
| Figueroa, 2012 [30]. Mexico, urban. Conducted 2000–2007. | Retrospective review of hospital records and social work department records. Implied that all consecutive records included but not implicitly state. | 948 cases and 1305 controls. | Cases: bacterial pneumonia, 42% female, age >18 (mean 55). Controls: otolaryngeal patients, 38% female, age >18 (mean 32). | Past or current exposure to wood smoke in the home, based on retrospective review of secondary source documents. No quantification of exposure. | Bacterial pneumonia, based on retrospective review of hospital records by specialists using a standardised format and clinical definition of pneumonia. | Adjusted for age, gender, occupational exposures, Type 2 Diabetes and household ventilation. No adjustment for socioeconomic status or outdoor exposures. | Logistic regression, OR (95% CI, p value): (unadjusted results calculated from raw data). Current wood smoke exposure† vs No current wood smoke•. Unadjusted: 2.62 (1.78–3.86, <0.0001). Past wood smoke exposure† vs No past wood smoke•. Unadjusted: 2.14 (1.89–2.55, <0.0001). Adjusted: 1.1 (0.9–1.4, 0.5). |
| CROSS SECTIONAL STUDIES | |||||||
| Shrestha, 2005 [23]. Nepal, rural (81%) & urban (19%). Conducted 2003–2004. | Households randomly selected. Household member response rates not stated. | 98 households.168 respondents. | 94% female, mean age 36 years (S.D. 16.7), minimum age not stated. | Use of “unprocessed fuel” (solid bio-fuels) vs “processed fuels” (gas / kerosene), assessed by questionnaire. | ALRI, based on physician examination and review of symptoms but no definition stated. Not stated whether retrospective diagnoses included. | Adjusted for smoking* and age** | Unadjusted OR (95% CI): Unprocessed fuel† vs processed fuel use•. 2.69 (0.76–9.52). Adjusted OR (95% CI): Unprocessed fuel† vs processed fuel use•. *2.77 (0.77–10.00). **2.63 (0.74–9.31). |
| Kilabuko, (2007) [24]. Tanzania, rural. Conducted 2004. | Random sampling of household. No refusal rates for household members stated. | 100 households.390 participants. | No demographics of participants stated, but includes age 5 and over. “Chief cooks” were mainly wives of household heads, so assumed predominantly adult but not necessarily. | “Chief cooks” compared to other household members. No explanation of how chief cooks were chosen or defined. Likely significant overlaps between 2 groups. No quantification of exposure. | ARI, defined as household member reported cough with rapid breathing, assessed by questionnaire. No recall period defined. | No adjustment for confounders. | Unadjusted OR (95% CI, p value) calculated from raw data. Chief cook† vs other household members•. 3.76 (2.19–6.48, <0.0001). |
| Stanković 2011 [25]. Serbia, urban. Conducted 2008. | Individuals recruited from a health centre when attending for health checks. No description of how the sample was selected. Response rates not reported. | 1082 participants. | All female, age 20–40. | Self-reported ‘use of biomass fuels’, assessed by questionnaire. No quantification of exposure. | Self-reported “doctor diagnosed pneumonia’ or ‘doctor diagnosed bronchitis’ in their life time, assessed by questionnaire. | Adjusted for age, education, family history of respiratory illness and outdoor air pollution. Not explicit whether adjusted for environmental tobacco smoke, home dampness or pets. Not adjusted for occupational exposures, comorbidities or socioeconomic factors. | Adjusted logistic regression, OR (95% CI). Biomass use† vs no biomass use•. Bronchitis: 0.91 (0.71–1.15). Pneumonia: 0.99 (0.80–1.22). |
| Taylor, 2012 [26]. Sierra Leone, rural and peri-urban. Conducted 2011. | Participants randomly selected from all eligible individuals in the study area in 16 community strata, using stratified sampling. Response rates not stated. | 520 participants. | All female, age 15–45. | Kitchen location, type of fuel normally used and number of hours spent in the kitchen, assessed by questionnaire. | ARI, defined as self-reported cough followed by rapid breathing, assessed by questionnaire with a 2-week recall period. | Adjusted for age, marital status, kitchen type, smoking, housing type and number of rooms. Not adjusted for other pollution exposures comorbidities or socio-economic factors. | Logistic regression, OR (95% CI, p value): Wood† vs charcoal•. Adjusted: 1.14 (0.71–1.82, 0.580). Kitchen inside the main house† vs separate kitchen•. Adjusted: 0.68 (0.38–1.24, 0.210). Effect of spending 4–6 hours† in kitchen or >7 hours† vs <3 hours•. Unadjusted: 1.31 (0.88–1.94, 0.179) and 2.40 (0.86–6.72, 0.094), respectively. |
PM10 = Particulate Matter < 10μm diameter; ALRI = Acute Lower Respiratory Infection; ARI = Acute Respiratory Infection; COPD = Chronic Obstructive Pulmonary Disease; OR = Odds Ratio; HR = Hazard Ratio; CI = Confidence Interval
The exposure and comparator used in each analysis are notated by † and • respectively
Most studies were based in rural settings and recruited participants from communities. There were 3 exceptions, which were hospital or health-centre based studies, conducted in urban areas of Canada, Mexico and Serbia [25, 29, 30].
Most studies predominantly or exclusively recruited females (accounting for 48–100% of participants within studies). In the study from Tanzania, the cooks were mainly female but the gender and age of the unexposed group were not reported [24].
Except for the study from Canada, which restricted recruitment to individuals over 65 [29], and the study from China [28], in which deaths occurred between 25 and 80 years of age, all studies recruited younger adults. Five studies restricted participants to adults over 18, whilst the 3 remaining studies—from Sierra Leone, Tanzania and Kenya—included younger participants but were conducted in predominantly adult populations.
One study evaluated coal use (Shen et al) and the 7 others evaluated biomass fuel use, predominantly wood. Four studies measured levels of household pollutants directly, but only 1 presented an estimate for the effect of pollutants on ALRI risk. In Kenya, Ezzati et al. measured particulate matter <10μm diameter (PM10) for 14–15 hours per day for 200 days in 55 households, and demonstrated a dose-response relationship between exposure to PM10 and ALRI risk [27]. The authors report that this finding was supported by analysis with a continuous-exposure variable and inverse-quadratic relation, although have not published these results. All studies monitoring air pollution levels measured PM10 rather than PM2.5. This may misrepresent the true harmful exposure, as smaller PM2.5 particles are inhaled deeper in to the lungs than PM10 and are therefore thought to be more pathogenic [31].
The 7 studies that did not provide an effect estimate for measured pollutants all used questionnaire data to classify exposure to household air pollution, based on a variety of self-reported measures including fuel or stove type, quantification of fuel use, ventilation, cooking frequency and location.
Two studies defined the outcome using prospective health care worker diagnosis of ALRI; in Canada, hospitalised cases had symptoms or signs, and radiological changes consistent with pneumonia [29]; in Kenya, the outcome was defined as nurse-diagnosed ALRI following review and physical examination at 1–2 weekly visits over a 2 year period [32]. A third study, from Nepal, included a physician’s review to diagnose ALRI, but no further definition was provided and it is unclear whether retrospective diagnoses were included [23]. The study from China used retrospective review of death certificate records, which had been completed by physicians on the basis of clinical and radiological investigations [32]. Similarly, the study in Mexico used a retrospective review of hospital records by specialists to identify cases [30]. The remaining studies used self-reported symptoms or past diagnoses, with varying recall periods, to define the outcome [24–26].
Six of the 8 studies made some adjustment for potential confounders in their analysis of the effect of household air pollution on ALRI [23, 25, 26, 28, 30, 32], but several studies failed to make adjustments for important potential confounders, such as economic status and smoking.
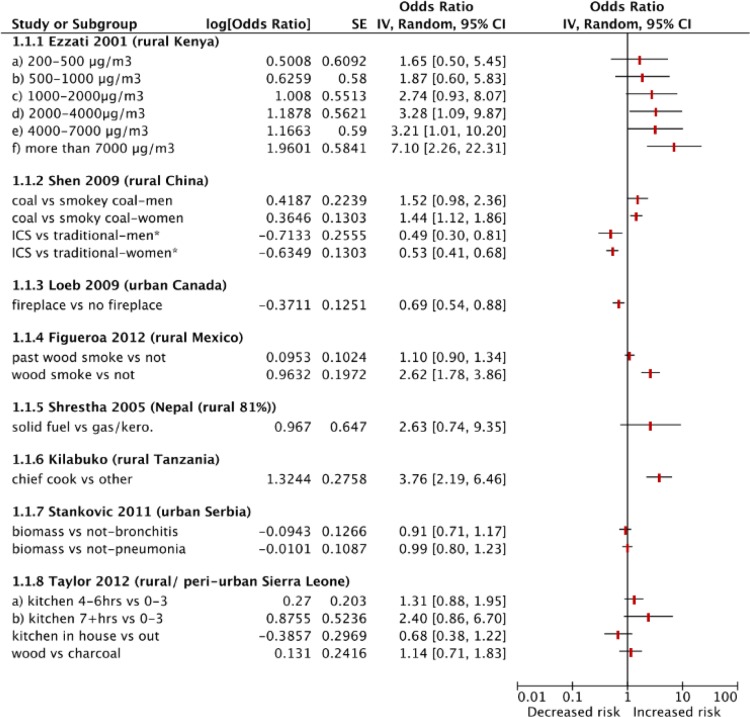
The effect estimates quantified by the studies are shown in Table 1 and in a Forest Plot (Fig 2). There were inconsistent findings across the studies. Whilst the 2 cohort studies have marked differences in study design, they demonstrated statistically significant harmful effects of household air pollution exposure on ALRI after adjustment for confounders. A dose-dependent relationship between PM10 and ALRI was detected in Kenya, (OR = 7.10, (95% CI 2.26 to 22.32) for exposure to >7000μg/m3 PM10 (highest exposure category) vs. 200–500 μg/m3 PM10) [32], and a reduction in risk of pneumonia deaths was observed in participants using an improved coal stove instead of a traditional coal stove (OR = 0.49 (95% CI 0.31 to 0.80) and 0.53 (95% CI 0.32 to 0.88)) in Chinese men and women respectively [28]. The studies from Mexico and Tanzania both reported harmful effects of household air pollution exposure in univariate analysis [24, 30], but these were no longer significant after adjustment for confounders in the Mexican study. The studies from Nepal, Serbia and Sierra Leone did not identify a significant association between household air pollution and risk of ALRI [23, 25, 26]. The study from Canada found that fireplace use was associated with a reduction in the risk of pneumonia, although this was unadjusted [29].
Fig 2. Forest Plot A forest plot showing the Odds Ratio (and lower and upper 95% Confidence Intervals) for different exposures and outcome in the included studies.
Adjusted results are shown where available. Key: ICS: improved cookstove; kero: kerosene; *indicates that the study is assessing for the protective effect of an intervention.
All of the case-control and cross-sectional studies were found to be at high risk of bias in at least 2 out of the 4 domains assessed, leading to an overall assessment of “moderate/high” or “high” risk of bias. Exposure assessments were weak. The cohort studies were of a generally higher standard, although their risk of bias was moderate in at least 2 out of 4 domains.
Discussion
This paper synthesises the current evidence base for the relationship between household air pollution and adult ALRI, identifying 8 eligible studies that have quantified this relationship. Despite a paucity of available studies, the available data provides some evidence for an increased risk of adult ALRI from exposure to household air pollution. The review has highlighted disparities between the findings of the studies, although these are unsurprising given the methodological heterogeneity seen. Methodological limitations were also noted, including poor exposure and outcome classifications, and potential biases including selection and recall biases. Although suggestive of an association, the current evidence is not sufficient to make a direct assessment of the potential impact of household air pollution on adult ALRI.
Methodological differences in exposure classification are a likely source of variation between study findings in this review. Indirect classification of exposure to household air pollution was common, and may have resulted in exposure misclassification. Accurate measurement of exposure to pollutants such as PM2.5 is challenging in the field. Monitoring is becoming more practical, with a variety of devices now available, which will benefit future population studies of health impacts such as ALRI. Exposure, however, needs to be measured at repeated intervals to accurately classify levels for diseases with a long latency period from defined exposures and this remains a challenge.
With regards to major sources of bias and error, the quality of studies identified was varied. Two studies used retrospective records of patients, and none of the prospective studies reported response or refusal rates of participants, so may have been subject to selection bias. Several studies used some form of random sampling but the cross-sectional study from Serbia only recruited women who attended a health centre so the findings are not generalisable to the wider population. The case-control study from Mexico used patients with otolaryngeal disease as controls, but as household air pollution can also be a risk factor for this outcome [3], this may have biased the findings towards the null. Only 1 study directly measured exposure to household pollutants [32]. All other studies relied on questionnaire responses regarding exposures, which are prone to recall bias, with varying recall periods and often poor definitions of exposure. Similarly, several studies relied on potentially biased and inaccurate recall of previous illness; outcome misclassification may have diluted the true effect. Three studies used prospective assessment of patients by a health-care worker for the outcome classification, although the quality of definitions used varied. Two studies made no adjustment for confounders, and other studies failed to adjust for some important confounders such as smoking, and so their findings may be misleading. Four studies were cross-sectional and are therefore unable to establish temporality between exposure and outcome.
Although the literature was previously reviewed as part of the Comparative Risk Assessment conducted for the GBD 2010 [33], this review provides an update, adding 5 studies to the evaluation. This review also provides a narrative summary of the current evidence which was not previously available, and a critical appraisal of methodological limitations. The review included a comprehensive search of published bibliographic databases—including databases from Africa and Latin America—and available grey literature, including contact with known researchers in the field undertaking relevant research. A limitation of this review is that we were unable to include literature published in Chinese. Due to the paucity of evidence, publication bias was not assessed.
Diseases associated with household air pollution disproportionately affect those living in poverty, who rely on solid fuels to meet their energy needs [34]. To consolidate GBD estimates—which currently use modelled data from other sources of air pollution—we set out to quantify the association between household air pollution and adult ALRI. Whilst it is likely that the burden of disease from household air pollution in adults includes ALRI, the published literature does not provide definitive evidence of this. Further research, using robust direct exposure measurement and accurate classification of outcome, is required to improve the evidence base. Exposure to household air pollution is preventable but resources are limited in low-income populations with competing health priorities: with high quality evidence of the true scale of the problem and cost-effectiveness of interventions, resources to reduce the global burden of disease can be effectively allocated.
Supporting Information
PRISMA Checklist.
(DOC)
Acknowledgments
We are grateful to Dr Christine Kelly (University of Liverpool) and Mariana Gallo for their assistance with translating French and Spanish language papers.
Data Availability
All relevant data are within the paper and its cited references.
Funding Statement
This work was supported by the Wellcome Trust (Hannah Jary Wellcome Trust Clinical PhD Fellow, 099929/B/12/Z, https://wellcome.ac.uk) and the UK Medical Research Council and UK Department for International Development (BREATHE-Africa MRC Partnership Grant, MR/L009242/1, http://www.mrc.ac.uk/funding/how-we-fund-research/partnership-grant). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Collaborators GBDRF, Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–323. 10.1016/S0140-6736(15)00128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2008;86(5):390–8C. 10.2471/BLT.07.044529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam KB, et al. Respiratory risks from household air pollution in low and middle income countries. The Lancet Respiratory medicine. 2014;2(10):823–60. 10.1016/S2213-2600(14)70168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S.EPA. 2009 Final Report: Intergrated Science Assessment of Particulate Matter. US Environmental Protection Agency, Washington, DC, EPA/600/R-08/139F, 2009.
- 5.Arnold C. Disease burdens associated with PM2.5 exposure: how a new model provided global estimates. Environmental health perspectives. 2014;122(4):A111 10.1289/ehp.122-A111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis DK, Callaghan M, Phiri K, Chipwete J, Kublin JG, Borgstein E, et al. Prevalence and indicators of HIV and AIDS among adults admitted to medical and surgical wards in Blantyre, Malawi. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97(1):91–6. [DOI] [PubMed] [Google Scholar]
- 7.Scott JA, Hall AJ, Muyodi C, Lowe B, Ross M, Chohan B, et al. Aetiology, outcome, and risk factors for mortality among adults with acute pneumonia in Kenya. Lancet. 2000;355(9211):1225–30. [DOI] [PubMed] [Google Scholar]
- 8.Sow O, Frechet M, Diallo AA, Soumah S, Conde MK, Diot P, et al. Community acquired pneumonia in adults: a study comparing clinical features and outcome in Africa (Republic of Guinea) and Europe (France). Thorax. 1996;51(4):385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yone EW, Balkissou AD, Kengne AP, Kuaban C. Influence of HIV infection on the clinical presentation and outcome of adults with acute community-acquired pneumonia in Yaounde, Cameroon: a retrospective hospital-based study. BMC pulmonary medicine. 2012;12:46 10.1186/1471-2466-12-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortimer K. Chimney stove intervention—ready for scale up? CON. Thorax. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush A. Chimney stove intervention—ready for scale up? PRO. Thorax. 2016;71(5):393–4. 10.1136/thoraxjnl-2015-208241 [DOI] [PubMed] [Google Scholar]
- 12.Puzzolo E, Stanistreet S, Pope D, Bruce NG, Rehfuess EA. Factors Influencing the Large Scale Uptake by Households of Cleaner and More Efficient Household Energy Technologies. A Systematic Review. London:Evidence for Policy and Practice Information and Co-ordinating Centre, University of London, 2013. Available: http://eppi.ioe.ac.uk/cms/Default.aspx?tabid=3426 [accessed 9 November 2016]
- 13.2002 Open Forum at the International Respiratory Congress: the annual convention & exhibition of the American Association for Respiratory Care, October 5–8, 2002, Tampa Bay, Florida U S A. Respiratory Care. 2002;47(9):1032–5. [Google Scholar]
- 14.2005 open forum at the International Respiratory Congress: the annual convention & exhibition of the American Association for Respiratory Care, December 3–6, 2005, San Antonio, Texas, U S A. Respiratory Care. 2005;50(11):1469–553. [Google Scholar]
- 15.ACEP Research Forum, September 26–27, 2005, The Washington Convention Center, Washington, DC. Annals of Emergency Medicine. 2005;46(3):S1–121.
- 16.Benguigui Y. Infecciones respiratorias agudas: fundamentos tecnicos de las estrategias de control Acute respiratory infections: technical fundaments on control strategies. OPS Serie HCT/AIEPI. (8):241–. [Google Scholar]
- 17.Lind T, McDonald JA, Avioli LV. Adult respiratory distress syndrome. Archives of Internal Medicine. 1981;141(13):1749–53. [DOI] [PubMed] [Google Scholar]
- 18.Ma L. Assessing the healthy benefits and cost from household solid fuel intervention in rural Guizhou. Environmental science & technology. [Google Scholar]
- 19.Jin Y, Cheng Y, Wang H, Zhao C. [Effects of air pollution from burning coal on respiratory diseases in adults]. Wei Sheng Yen Chiu/Journal of Hygiene Research. 2001;30(4):241–3, 6. [PubMed] [Google Scholar]
- 20.Jary H, Mallewa J, Nyirenda M, Faragher B, Heyderman R, Peterson I, et al. Study protocol: the effects of air pollution exposure and chronic respiratory disease on pneumonia risk in urban Malawian adults—the Acute Infection of the Respiratory Tract Study (The AIR Study). BMC pulmonary medicine. 2015;15:96 10.1186/s12890-015-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezzati M, Kammen DM. Quantifying the effects of exposure to indoor air pollution from biomass combustion on acute respiratory infections in developing countries. Environmental health perspectives. 2001;109(5):481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezzati M, Kammen DM. Evaluating the health benefits of transitions in household energy technologies in Kenya. Energy Policy. 2002;30(10):815–26. [Google Scholar]
- 23.Shrestha IL, Shrestha SL. Indoor air pollution from biomass fuels and respiratory health of the exposed population in Nepalese households. Int J Occup Environ Health. 2005;11(2):150–60. 10.1179/oeh.2005.11.2.150 [DOI] [PubMed] [Google Scholar]
- 24.Kilabuko JH, Matsuki H, Nakai S. Air quality and acute respiratory illness in biomass fuel using homes in Bagamoyo, Tanzania. International Journal of Environmental Research & Public Health [Electronic Resource]. 2007;4(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanković A, Nikolić M, Arandjelovič M. Effects of indoor air pollution on respiratory symptoms of non-smoking women in Niš, Serbia. Multidisciplinary Respiratory Medicine. 2011;6(6):351–5. 10.1186/2049-6958-6-6-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor ET, Nakai S. Prevalence of Acute Respiratory Infections in Women and Children in Western Sierra Leone due to Smoke from Wood and Charcoal Stoves. International Journal of Environmental Research and Public Health. 2012;9(6):2252–65. 10.3390/ijerph9062252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezzati M, Kammen DM. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: An exposure-response study. Lancet. 2001;358(9282):619–24. [DOI] [PubMed] [Google Scholar]
- 28.Shen M, Chapman RS, Vermeulen R, Tian L, Zheng T, Chen BE, et al. Coal use, stove improvement, and adult pneumonia mortality in Xuanwei, China: a retrospective cohort study. Environmental health perspectives. 2009;117(2):261–6. 10.1289/ehp.11521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeb M, Neupane B, Walter SD, Hanning R, Carusone SC, Lewis D, et al. Environmental risk factors for community-acquired pneumonia hospitalization in older adults. Journal of the American Geriatrics Society. 2009;57(6):1036–40. 10.1111/j.1532-5415.2009.02259.x [DOI] [PubMed] [Google Scholar]
- 30.Figueroa CGS, Plata RF, Briseño DM, de la Garza SR, Pizano AM, Marina FF, et al. Analysis of a routine database to identify risk factors of the host and the environment associated with respiratory diseases. Revista del Instituto Nacional de Enfermedades Respiratorias. 2012;71(1):11–20. [Google Scholar]
- 31.WHO. WHO Factsheet No 313: Air quality and health. http://wwwwhoint/mediacentre/factsheets/fs313/en/ Date last updated: March 2014 Date last accessed: June 23 2016 2011.
- 32.Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: an exposure-response study. Lancet. 2001;358(9282):619–24. [DOI] [PubMed] [Google Scholar]
- 33.Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, et al. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annual review of public health. 2014;35:185–206. 10.1146/annurev-publhealth-032013-182356 [DOI] [PubMed] [Google Scholar]
- 34.Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Pruss-Ustun A, et al. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environmental health perspectives. 2013;121(7):784–90. 10.1289/ehp.1205987 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
Data Availability Statement
All relevant data are within the paper and its cited references.