Abstract
Objective
We set out to determine what proportion of the mortality decline from 1997 to 2007 in coronary heart disease (CHD) in the Netherlands could be attributed to advances in medical treatment and to improvements in population-wide cardiovascular risk factors.
Methods
We used the IMPACT-SEC model. Nationwide information was obtained on changes between 1997 and 2007 in the use of 42 treatments and in cardiovascular risk factor levels in adults, aged 25 or over. The primary outcome was the number of CHD deaths prevented or postponed.
Results
The age-standardized CHD mortality fell by 48% from 269 to 141 per 100.000, with remarkably similar relative declines across socioeconomic groups. This resulted in 11,200 fewer CHD deaths in 2007 than expected. The model was able to explain 72% of the mortality decline. Approximately 37% (95% CI: 10%-80%) of the decline was attributable to changes in acute phase and secondary prevention treatments: the largest contributions came from treating patients in the community with heart failure (11%) or chronic angina (9%). Approximately 36% (24%-67%) was attributable to decreases in risk factors: blood pressure (30%), total cholesterol levels (10%), smoking (5%) and physical inactivity (1%). Ten% more deaths could have been prevented if body mass index and diabetes would not have increased. Overall, these findings did not vary across socioeconomic groups, although within socioeconomic groups the contribution of risk factors differed.
Conclusion
CHD mortality has recently halved in The Netherlands. Equally large contributions have come from the increased use of acute and secondary prevention treatments and from improvements in population risk factors (including primary prevention treatments). Increases in obesity and diabetes represent a major challenge for future prevention policies.
Introduction
Coronary heart disease (CHD) remains the leading cause of death worldwide and is a major contributor to chronic disease morbidity.[1] Since the 1970s, CHD mortality has fallen dramatically in Western societies. The IMPACT model has been developed to estimate the contribution of changes in uptakes of evidence-based treatments and nationwide changes in cardiovascular risk factors to the changes in CHD mortality. The model has been applied in more than 20 countries.[2–18] Results vary by country, with the contribution from treatments ranging from 25–50% and risk factor changes explaining 50–75%.[2–18] These differences between countries can mostly be explained by the precise time period chosen, and the scale of change in major CHD risk factors.
Analysis from England and then Scotland using an extended IMPACT-SEC model showed that relative inequalities between the most affluent and most deprived groups have actually widened.[4,11] CHD burden tends to differ by socioeconomic circumstances (SEC), with incidence and mortality generally being lower in more affluent groups. Socioeconomic inequalities also exist in the Netherlands, although of smaller magnitude compared to the UK. Nevertheless, the healthy life expectancy without physical limitations is 14 years lower for men with low education compared to men with a high education; for women the difference is 15 years.[19] Furthermore, we have shown previously that socioeconomic inequalities in incidence of acute myocardial infarction (AMI) persisted between 1997 and 2007.[20] Exploration of socioeconomic differences thus remains in the Netherlands.
We therefore aimed to determine what proportion of the recent decline (from 1997 to 2007) in CHD mortality could be attributed to advancements in medical treatment and to nationwide time trends in CHD risk factors, particularly in socioeconomic subgroups of the population.
Methods
The Dutch population aged 25 years and over between 1997 and 2007 was evaluated using the IMPACT-SEC model.[4,11] This model integrates nationwide data at two time points to explain an observed change in mortality. The IMPACT model was developed to (1) model CHD mortality trends and incorporates time trends in uptake of evidence-based acute phase and secondary prevention treatments, in addition to time trends in major CHD risk factors and (2) estimate the relative change in CHD mortality associated with each of these items. The IMPACT model is validated and applied in several countries.[2–18]
Data sources
Data used are described in detail in the S1 Appendix. In short, data on the age, sex and socioeconomic distribution of the Dutch population and on specific CHD death counts (International Classification of Diseases version 10 code I20-I25) were obtained from Statistics Netherlands. Dutch inhabitants were divided in three socioeconomic groups: lowest (20% most deprived persons within age-sex-stratum), medium (60% of age-sex-stratum) and highest (20% most affluent persons within age-sex-stratum). Socioeconomic circumstances were based on an area-level socioeconomic indicator constructed by the Netherlands Institute for Social Research in 2002–2006.[21] The national Dutch hospital discharge register was used to determine the number of eligible patients and their 1-year mortality rate. Information on drug use came from the PHARMO Database Network, linking pharmacy and hospitalization records of over 2.3 million subjects.[22–24] Data on drug use in the community came from the general practitioners (GP) register HNU (Huisartsen Netwerk Utrecht). Data of major cardiovascular risk factors (blood pressure, cholesterol, body mass index (BMI), diabetes, smoking, physical inactivity) came from the Doetinchem/MORGEN (RIVM) cohort for those aged up to 65 years and the LASA (Longitudinal Aging Study Amsterdam) cohort for those aged 65 years and over.[25,26] Smoking information came from annual nationwide surveys on smoking habits (STIVORO) and diabetes information from the GP register.
The primary output was the number of Deaths Prevented or Postponed (DPP) in 2007 due to lower CHD mortality rates. The DPP was calculated as the difference between the observed 2007 CHD deaths and the expected CHD deaths in 2007, had 1997 mortality rates remained constant. Change in population size and age distribution was considered using indirect standardization to the Dutch population of 2007. The expected number of CHD deaths was calculated by multiplying age-sex-socioeconomic group-specific mortality rates in 1997 by the population size for each 10-year age-sex-socioeconomic stratum in 2007.
Deaths prevented or postponed: treatment uptake
For example, in 2007, 1,143 men aged 65 to 74 years in the most deprived group were hospitalized with an acute myocardial infarction (AMI), of whom, 81% received aspirin. Aspirin reduces one year mortality rate by 15% based on recent meta-analyses.[27] The one year mortality rate in these men was 8% in 1997. The approximate number of DPP attributable to aspirin use in AMI for 65–74 year old men in the most deprived group was calculated as:
As all treatments were in use in 1997, the net benefit of an intervention in 2007 was calculated as:
Simply assuming that the efficacy of multiple treatments was additive would overestimate the treatment effect. The Mant and Hicks method was used instead to estimate one-year mortality reduction by polypharmacy.[28] This approach estimates the cumulative relative benefit:
The acute-phase and secondary prevention treatment component of the model comprised seven mutually exclusive CHD subgroups: patients hospitalized for an AMI, unstable angina, or heart failure associated with CHD and patients in the community who were AMI survivors, or who were patients with stable coronary artery disease (with and without percutaneous or surgical revascularization), or who were patients with CHD-related heart failure. Within each of these groups, 42 medical and surgical therapies were evaluated (listed in Table 1).
Table 1. Deaths Prevented or Postponed (DPPs) due to change in acute-phase and secondary prevention treatments for CHD between 1997 and 2007.
| Treatment uptake | ||||||
|---|---|---|---|---|---|---|
| Nr. of patients | 1997 (%) | 2007 (%) | RRR (%) | 1-year mortality | DPPs, Mean (%) (Range) | |
| AMI | 18,002 | 21.0% | 668 (6.0) (0.3, 12.3) | |||
| Thrombolysis | 55.0 | 2.0 | 0.24 | 0 | ||
| Antiplatelets | 87.3 | 91.0 | 0.23 | 29 (0.3) (0.1, 1.1) | ||
| B-Blocker | 75.9 | 89.5 | 0.04 | 18 (0.2) (-0.2, 1.3) | ||
| ACE inhibitor or ARB | 29.9 | 57.4 | 0.07 | 45 (0.4) (0.1, 1.1) | ||
| Clopidogrel | 0.9 | 77.7 | 0.03 | 66 (0.6) (0.1, 2.0) | ||
| Primary PCI (within 14 days) | 8.0 | 39.5 | 0.30 | 254 (2.3) (0.2, 6.5) | ||
| Primary CABG (within 6 wks) | 3.8 | 4.5 | 0.39 | 12 (0.1) (0.0, 0.2) | ||
| CPR in the community | 2.3 | 4.3 | 0.79 | 244 (2.2) (1.8, 2.6) | ||
| Unstable angina | 29,000 | 7.2% | 255 (2.3) (0.4, 6.3) | |||
| Heparin | 49.6 | 55.0 | 0.33 | 63 (0.6) (0.2, 1.2) | ||
| Antiplatelets | 76.1 | 77.1 | 0.15 | 18 (0.2) (0.1, 0.4) | ||
| IIB/IIIA | 0.0 | 0.6 | 0.09 | 0.4 (0.0) (0.00, 0.01) | ||
| ACE inhibitor or ARB | 17.3 | 46.1 | 0.07 | 40 (0.4) (0.1, 1.0) | ||
| B-Blocker | 66.8 | 83.4 | 0.04 | 12 (0.1) (-0.1, 0.8) | ||
| Clopidogrel | 0.1 | 60.4 | 0.07 | 74 (0.7) (0.1, 1.8) | ||
| CABG surgery (within 6 wks) | 9.4 | 6.8 | 0.39 | 0 | ||
| PCI (within 14 days) | 5.8 | 14.3 | 0.32 | 47 (0.4) (0.0, 1.2) | ||
| Secondary prevention post MI | 110,770 | 3.9% | 228 (2.0) (0.6, 4.8) | |||
| Antiplatelets | 52.1 | 52.8 | 0,15 | 7 (0.1) (0.0, 0.1) | ||
| B-Blocker | 40.1 | 46.6 | 0.23 | 61 (0.5) (0.2, 1.3) | ||
| ACE inhibitor or ARB | 21.8 | 38.0 | 0.20 | 62 (0.6) (0.2, 1.3) | ||
| Statin | 33.0 | 47.0 | 0.24 | 85 (0.8) (0.2, 1.9) | ||
| Acenocoumarol | 10.9 | 10.7 | 0.22 | 12 (0.1) (0.0, 0.3) | ||
| Rehabilitation | 28.5a | 28.5a | 0.26 | 0 (0) (0, 0) | ||
| Secondary prevention post revascularization | 82,467 | 5.2% | 228 (2.0) (0.6, 4.4) | |||
| Antiplatelets | 51.8 | 52.7 | 0.15 | 6 (0.1) (0.0, 0.1) | ||
| B-Blocker | 40.4 | 46.8 | 0.23 | 40 (0.4) (0.1, 0.8) | ||
| ACE inhibitor or ARB | 21.8 | 38.3 | 0.20 | 77 (0.7) (0.2, 1.5) | ||
| Statin | 33.5 | 47.6 | 0.22 | 101 (0.9) (0.2, 1.8) | ||
| Acenocoumarol | 11.4 | 10.8 | 0.22 | 3 (0) (0.0, 0.1) | ||
| Rehabilitation | 28.5a | 28.5a | 0.26 | 0 (0) (0, 0) | ||
| Chronic stable CAD | 277,170 | 4.0% | 1,017 (9.1) (2.6, 18.6) | |||
| Antiplatelets | 40.4 | 64.8 | 0.15 | 294 (2.6) (1.0, 5.7) | ||
| Statins | 15.1 | 50.1 | 0.23 | 420 (3.8) (0.8, 7.3) | ||
| ACE inhibitor or ARB | 16.0 | 37.9 | 0.17 | 303 (2.7) (0.8, 5.5) | ||
| CABG surgery (last 5 yrs) | 12.1 | 8.7 | 0.39 | 0 | ||
| Heart failure in the hospital | 13,320 | 42.1% | 479 (4.3) (1.6, 9.2) | |||
| ACE inhibitor or ARB | 62.4 | 71.7 | 0.20 | 33 (0.3) (0.1, 0.7) | ||
| B-Blocker | 22.5 | 69.2 | 0.35 | 388 (3.5) (1.3, 7.3) | ||
| Spironolactone | 44.9 | 49.6 | 0.30 | 39 (0.4) (0.1, 0.8) | ||
| Antiplatelets | 45.1 | 51.0 | 0.15 | 19 (0.2) (0.1, 0.4) | ||
| Heart failure in the community | 46,435 | 18.3% | 1,258 (11.2) (4.0, 24.7) | |||
| ACE inhibitor/ARB | 34.4 | 59.1 | 0.20 | 143 (1.3) (0.4, 2.9) | ||
| B-Blocker | 19.7 | 51.9 | 0.35 | 566 (5.1) (1.9, 10.7) | ||
| Spironolactone | 4.5 | 22.5 | 0.31 | 263 (2.3) (0.7, 5.5) | ||
| Antiplatelets | 31.2 | 69.1 | 0.15 | 287 (2.6) (1.0, 5.6) | ||
| Total treatments | 4,134 (36.9) (10.1, 80.2) | |||||
Abbreviations: ACE, angiotensin-converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CPR, cardiopulmonary resuscitation; PCI, percutaneous coronary intervention (with or without stenting); RRR, relative risk reduction
aNo change assumed in uptake between 1997 and 2007.
Deaths prevented or postponed: risk factor changes
Two approaches were used to estimate the number of DPPs due to changes in risk factors. The regression coefficient approach was used for risk factors expressed in continuous data: systolic blood pressure (SBP), total cholesterol level, and BMI. Three variables were used for this approach: (1) the expected number of CHD deaths in 2007, (2) multiplied by the absolute change in risk factor prevalence, (3) multiplied by a regression coefficient that quantified the change in CHD mortality expected for the change in risk factor level. For example, in 2007, there were 161 expected CHD deaths among 202,031 women aged 55 to 64 years in the most deprived group. The mean systolic blood pressure in this group decreased between 1997 and 2007 by 13.3 mm Hg. The relation of blood pressure treatment with CHD mortality estimated age- and sex-specific reduction in CHD mortality to be 50% for every 20-mmHg reduction in SBP, yielding a natural logarithmic coefficient of –0.035.[29] The number of DPP as a result of SBP change was:
The second approach used was the population-attributable risk fraction (PARF). This approach was used to for dichotomous risk factors:
where P is the risk factor prevalence and RR is the relative risk for CHD mortality associated with the presence of that risk factor. DPP were estimated as the expected CHD deaths in 2007 multiplied by the difference in PARF between 1997 and 2007. For example, diabetes prevalence among men aged 65 to 74 years in the most deprived group was 11% in 1997 and 28% in 2007. Assuming a relative risk of 1.86 constant over time,[30] the PARF was calculated as 0.087 in 1997 and 0.194 in 2007. The number of deaths attributable to the increase in diabetes prevalence from 1997 to 2007 was:
Sensitivity analyses
For each model parameter, minimum and maximum plausible values were assigned using the 95% confidence intervals (from the source documentation); if these were unavailable, these limits were defined as 20% above and below the best estimate. The minimum and maximum plausible values were introduced into the model, generating minimum and maximum estimates for DPP. This represents a conservative estimation of uncertainty as has been applied before.[10,18]
Results
Overall findings
Between 1997 and 2007, the age-standardized CHD mortality rate in adults aged 25 years and over fell from 269 to 141 per 100,000 population; a decline of 48% or 6.3% per year (Table 2). In 2007, there were 11,855 CHD deaths, 57% of these were in men. Nationally, there were 11,200 fewer CHD deaths in 2007 than in 1997 mortality rates had persisted, representing the “total” deaths prevented or postponed. A socioeconomic gradient in death rates was observed, with the lowest death rates in the most affluent group. The rate of decline did not differ significantly between socioeconomic groups. Thus, relative socioeconomic inequalities remained stable between 1997 and 2007 (Table 2).
Table 2. CHD mortality rates 1997 and 2007 by sex and socioeconomic group.
| Year | National | Most affluent group | Middle group | Most deprived group | |
|---|---|---|---|---|---|
| (100%) | (20%) | (60%) | (20%) | ||
| Men | |||||
| Population ≥25 years | 1997 | 5,236,772 | 890,568 | 3,237,785 | 1,108,419 |
| 2007 | 5,572,741 | 1,114,194 | 3,344,676 | 1,113,871 | |
| Observed CHD deaths | 1997 | 11,046 | 1,644 | 6,565 | 2,837 |
| 2007 | 6,743 | 1,178 | 4,014 | 1,551 | |
| Age-standardised rates(per 100,000)a | 1997 | 362 | 316 | 362 | 396 |
| 2007 | 188 | 167 | 187 | 210 | |
| Annual % fallb | 6.3 | 6.2 | 6.4 | 6.1 | |
| Expected deathsc | 2007 | 13,631 | 2,342 | 2,744 | 3,059 |
| Target DPPsd | 2007 | 6,888 | 1,164 | 1,405 | 1,508 |
| % of expected deaths prevented | 2007 | 50.5 | 49.7 | 51.2 | 49.3 |
| Women | |||||
| Population ≥25 years | 1997 | 5,511,880 | 936,584 | 3,391,221 | 1,184,075 |
| 2007 | 5,856,439 | 1,171,650 | 3,513,791 | 1,170,998 | |
| Observed CHD deaths | 1997 | 8,276 | 1,327 | 4,775 | 2,174 |
| 2007 | 5,112 | 889 | 2,998 | 1,225 | |
| Age-standardised rates(per 100,000)a | 1997 | 177 | 151 | 175 | 201 |
| 2007 | 95 | 82 | 93 | 114 | |
| Annual % fallb | 6.1 | 5.9 | 6.2 | 5.6 | |
| Expected deathsc | 2007 | 9,423 | 1,631 | 1,879 | 2,157 |
| Target DPPsd | 2007 | 4,311 | 742 | 879 | 932 |
| % of expected deaths prevented | 2007 | 45.8 | 45.5 | 46.8 | 43.2 |
| Total | |||||
| Population ≥25 years | 1997 | 10,748,652 | 1,827,152 | 6,629,006 | 2,292,494 |
| 2007 | 11,429,180 | 2,285,844 | 6,858,467 | 2,284,869 | |
| Observed CHD deaths | 1997 | 19,322 | 2,971 | 11,340 | 5,011 |
| 2007 | 11,855 | 2,067 | 7,012 | 2,776 | |
| Age-standardised rates(per 100,000)a | 1997 | 269 | 234 | 268 | 299 |
| 2007 | 141 | 125 | 140 | 162 | |
| Annual % fallb | 6.3 | 6.1 | 6.3 | 5.9 | |
| Expected deathsc | 2007 | 23,055 | 3,972 | 4,622 | 5,216 |
| Target DPPsd | 2007 | 11,200 | 1,905 | 2,285 | 2,440 |
| % of expected deaths prevented | 2007 | 48.6 | 48.0 | 49.4 | 46.8 |
a Rates in this table are standardised to the European Standard Population (version 2013) aged 25+ years.
b Annual % fall = (1-(observed 2007 rate/observed 1997 rate)^(1/10)).
c Expected deaths = CHD deaths expected in 2007 based on 2007 population had 1997 CHD rates remained.
d DPPs, deaths prevented or postponed. DPPs = expected – observed deaths in 2007.
As a result of changes in the acute phase and secondary prevention treatment of CHD patients, some 4,134 fewer CHD deaths occurred (Table 3 and Table 1). This accounted for 37% (95% CI: 10% to 80%) of the fall in total CHD mortality in the Netherlands (Table 3). The proportion of changes in uptake of medical and surgical treatment attributable to the fall in CHD deaths was the same across socioeconomic groups. Population level changes in the prevalence of risk factors accounted for 3,979 fewer deaths or 36% (95% CI: 24% to 67%) of the fall (Table 4), of which 25% was related to non-pharmacological changes (in diet and lifestyle) and 11% to changes in the use of blood pressure and cholesterol lowering drugs for primary prevention. Substantial differences existed in the contribution of risk factor changes by sex and socioeconomic circumstances (ranging from 7% in the most affluent women to 57% in the most affluent men, Table 3). However, no consistent gradient or pattern was observed. The model explained 72% of the overall fall in CHD mortality (a shortfall of 3,087 deaths, Table 3). The proportion that could not be explained by changes in treatments and risk factors was larger in the most deprived group (35%) compared to the middle (24%) and most affluent group (29%, Table 3).
Table 3. Proportion of CHD deaths prevented or postponed by socioeconomic group, due to change in treatments and risk factors between 1997 and 2007.
| National | Most affluent group | Middle group | Most deprived group | |
|---|---|---|---|---|
| (100%) | (20%) | (60%) | ||
| Treatments | ||||
| Acute myocardial infarction | 6.0% | 6.7% | 6.0% | 5.4% |
| Unstable angina pectoris | 2.3% | 2.1% | 2.3% | 2.5% |
| Secondary prevention post AMI | 2.0% | 2.4% | 1.8% | 2.4% |
| Secondary prevention post revascularization | 2.0% | 2.4% | 1.8% | 2.5% |
| Chronic stable CAD | 9.1% | 9.1% | 9.0% | 9.4% |
| Heart failure in the hospital | 4.3% | 1.5% | 4.7% | 5.4% |
| Heart failure in the community | 11.2% | 9.2% | 13.0% | 7.7% |
| Total treatments | 36.9% | 33.3% | 38.6% | 35.3% |
| Total treatments – men | 32.4% | 29.4% | 33.3% | 32.7% |
| Total treatments – women | 44.1% | 38.8% | 47.1% | 39.4% |
| Risk factors | ||||
| Smoking | 4.5% | 4.0% | 4.3% | 5.7% |
| Diabetes | -9.0% | 4.5% | -10.0% | -16.5% |
| Physical inactivity | 1.3% | 2.9% | 1.6% | -0.8% |
| Systolic blood pressure, mmHg | 29.5% | 18.4% | 31.9% | 31.5% |
| -due to changes in the uptake of blood pressure lowering drugs | 3.8% | 4.3% | 3.8% | 3.4% |
| Total cholesterol, mmol/l | 10.4% | 9.3% | 10.4% | 11.2% |
| -due to changes in the uptake of cholesterol lowering drugs | 7.0% | 7.8% | 6.9% | 6.7% |
| Body Mass Index, m/kg2 | -1.2% | -1.6% | -1,0% | -1.5% |
| Total risk factors | 35.5% | 37.5% | 37.1% | 29.5% |
| Total risk factors –men | 37.5% | 57.0% | 34.6% | 30.7% |
| Total risk factors –women | 32.4% | 7.0% | 41.3% | 27.6% |
| Total treatments + risk factors | ||||
| %DPPs explained by model | 72.4% | 70.9% | 75.7% | 64.8% |
| %DPPs explained by model – men | 69.9% | 86.3% | 67.9% | 63.4% |
| %DPPs explained by model – women | 76.6% | 45.8% | 88.4% | 67.0% |
| %DPPs not explained by model | 27.6% | 29.1% | 24.3% | 35.2% |
| DPP Counts | ||||
| DPPs explained by the model | ||||
| - Due to treatment uptake | 4,134 (37%) | 635 (33%) | 2,639 (39%) | 861 (35%) |
| - Due to risk factor change | 3,979 (36%) | 715 (38%) | 2,544 (37%) | 721 (30%) |
| DPPs unexplained by model | 3,087 (28%) | 555 (29%) | 1,671 (24%) | 858 (35%) |
| Target DPPs | 11,200 (100%) | 1,905 (100%) | 6,854 (100%) | 2,440 (100%) |
CAD, coronary artery disease. AMI, acute myocardial infarction. DPP, death prevented or postponed.
Table 4. Deaths Prevented or Postponed (DPPs) due to changes in risk factors for coronary heart disease including the effect of changes in primary prevention treatments between 1997 and 2007.
| Risk factor level | Risk factor level | Absolute change in risk factorsa | Deaths Prevented or Postponed, | |
|---|---|---|---|---|
| 1997 | 2007 | Mean (%) (range) | ||
| Smoking prevalence | 32.5% | 27.2% | -5.3% | 507 (4.5) (4.3, 6.5) |
| Diabetes prevalence | 5.5% | 8.1% | 2.6% | -1,003 (-9.0) (-8.3, -12.5) |
| Physical inactivity | 60.2% | 54.9% | -5.3% | 144 (1.3) (1.2, 1.7) |
| SBP, mmHg | 132.2 | 129.4 | -2.8 | 3,304 (29.5) (23.5, 45.8) |
| Treatment uptake | Treatment uptake | |||
| -due to changes in the uptake of blood pressure lowering drugsa | 9.4% | 13.7% | +4.3% | 422 (3.8) (1.8, 6.8) |
| Total cholesterol, mmol/l | 5.6 | 5.4 | -0.2 | 1,161 (10.4) (7.9, 17.1) |
| Treatment uptake | Treatment uptake | |||
| -due to changes in the uptake of cholesterol lowering drugsa | 0.3% | 6.6% | +6.3% | 787 (7.0) (1.7, 17.6) |
| Body mass index, kg/m2 | 25.9 | 26.5 | 0.6 | -134 (-1.2) (-0.8, -2.1) |
| Total risk factors | 3,979 (35.5) (23.8, 67.0) | |||
SBP, systolic blood pressure.
a Eligible persons (n = 9,747,083) for primary prevention treatment were defined as all persons who did not have a cardiovascular-related hospital admission during the 5 years and 9 months prior to October 1 in the index year, and did not use nitrates, digitalis glycosides or antithrombotic drugs in the index year.[32]
Changes in acute phase and secondary prevention treatments
The largest contribution to CHD deaths prevented or postponed by increased uptake of treatments came from patients in the community with heart failure (1,258 DPPs, 11%) and chronic stable coronary artery disease (CAD, 1,017 DPPs, 9%), followed by the acute phase treatment of patients with an AMI (668 DPPs, 6%) and hospital treatment of heart failure (470 DPPs, 4%, Table 1). Uptake rates of beta-blockers in heart failure patients in the community more than doubled between 1997 and 2007, representing 5% of the total DPPs. Uptake rates of statins in chronic stable CAD patients increased more than three-fold, representing 4% of the total DPPs. Between 1997 and 2007 small improvements were observed in secondary prevention therapy after a hospital admission for an AMI or revascularization procedure. In general, the improvements due to changes in treatment uptakes were evenly distributed across socioeconomic groups.
Risk factor changes
The largest contribution from population level changes in risk factors came from the 3 mmHg fall in SBP. This SBP fall generated 3,304 fewer CHD deaths, representing 30% of the CHD mortality decrease (Table 4). Between 1997 and 2007, the uptake of blood pressure lowering drugs for primary prevention therapy increased from 7% to 12% in men and from 11% to 16% in women (Table 4). The pharmacological contribution was fairly small (4% of total DPPs) compared to the non-pharmacological contribution from changes in SBP. The most affluent group showed about one-third smaller total contributions from total SBP changes (19%) compared to the middle and most deprived group (32%). In contrast, the pharmacological benefits were about one-fourth larger in the most affluent (4%) compared to the most deprived (3%) group.
The second largest contribution from risk factor changes was the 0.2 mmol/l fall in cholesterol levels, generating 1,161 (10%) fewer CHD deaths. About two-third of the fall in total cholesterol was related to drug use. The uptake of cholesterol lowering drugs for primary prevention increased between 1997 and 2007 from 0.3% to 7% in men and from 0.2% to 6% in women. In the most affluent SEC group, the largest proportion of DPPs due to changes in cholesterol were related to an increased use of drugs (7.8% pharmacological from 9.3% total, Table 3). Trends in risk factors by SEC group, age and sex are presented in S1 Appendix Table K and L.
Favorable changes in the behavioral risk factors made a modest contribution to DPPs. From 1997 to 2007, smoking prevalence dropped by about 5%, leading to 500 fewer deaths (5% DPPs, Table 4). Socioeconomic gradients were observed in the proportion of DPPs due to changes in smoking and physical inactivity. The most deprived group was the only socioeconomic group that showed increases in physical inactivity, leading to 20 additional deaths (-1% DPPs). The most deprived group, however, did show the largest improvement (-6%) in smoking rates (from 35% to 29%) compared to -4% (from 0% to 26%) in the most affluent group (Table 1, S1 Appendix Table K).
Adverse risk factor trends
The mortality gains due to positive trends in SBP, cholesterol, smoking and physical inactivity were negated by increases in BMI and diabetes (together contributing 1,137 additional deaths, equivalent to a 10% increase in CHD mortality). The most affluent group was the only SEC group that showed a decline in diabetes (from 6% to 5%, S1 Appendix Table K).
Discussion
Between 1997 and 2007, Dutch CHD mortality rates fell by an impressive 48%, resulting in 11,200 fewer CHD deaths in 2007. At least 37% of this decline could be attributed to the increased use of acute phase and secondary prevention treatments. Similarly at least 36% could be attributed to changes in population levels of several cardiovascular risk factors (predominantly non-pharmacological). Our results suggest that in the most affluent part of the population the contribution to the decline in CHD mortality was driven by a different set of risk factors changes than in the most deprived part of the population. Approximately 10% more deaths could have been prevented if body mass index and diabetes would not have increased.
Comparison overall results with other studies
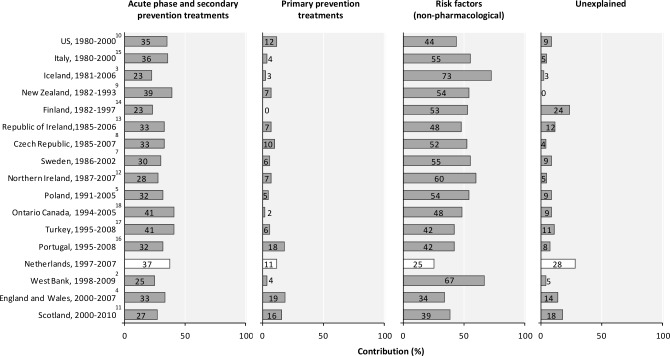
Several studies reported country specific results from the IMPACT model.[2–18] With respect to the contribution of changes in uptake in medical treatment in the acute phase and in secondary prevention, estimates ranged from 23 to 41%, with our estimate (37%) thus being towards the upper end of the distribution (Fig 1). In all IMPACT studies with a starting year before 1995 non-pharmacological changes in population risk factors explained changes in mortality to a larger extent than in more recently started studies. Our contribution of 25% is the lowest observed. It is to partly reflect our relatively low (3%) non-pharmacological contribution from changes in population cholesterol levels in our study, although the intake of trans fatty acids declined from 4,5% in 1987/1988 to 0.5% in 2007/2010 in the Netherlands.[31] Changes in diet could possibly have had a beneficial effect on CVD mortality beyond the effects of declining cholesterol levels. Some 28% of the mortality fall was not explained by our model. This relatively high percentage may reflect measurement imprecision, lag effects and changes in other, unmeasured risk factor. Although all IMPACT studies have used a broadly similar methodology, the assumptions underlying specific data have inevitably differed between some studies, making direct comparisons imprecise.
Fig 1. Percentage of the decrease in deaths from coronary heart disease attributed to changes in acute phase and secondary prevention treatments, primary prevention treatments and non-pharmacological risk factor changes in our study population and in other populations.
Studies are ranked by starting year.
Comparison IMPACT-SEC results with other studies
This is the first IMPACT-SEC study outside the United Kingdom to address socioeconomic differentials concealed within the overall decline in CHD mortality.[4,11] CHD mortality falls were remarkably similar across socioeconomic groups in the Netherlands. This represents a striking contrast with England and Scotland where relative socioeconomic inequalities widened over time.[4,11] Furthermore, in the Netherlands, we also found that the total contribution from risk factor changes was also similar across socioeconomic groups in the Netherlands (although the individual contributions of risk factors differed). The deprived groups benefited from greater falls in smoking rates, blood pressure and cholesterol. However, the most affluent socioeconomic group seemed to show slightly larger benefits from the increased use of blood pressure and cholesterol lowering drugs for primary prevention therapy as compared to the most deprived socioeconomic group. In addition, a more modest change in diabetes prevalence and decline in physical inactivity was observed in the affluent group. This might perhaps reflect more supportive environments, easier access to health care, or greater responsiveness to the general practitioner primary prevention interventions. This insight is very similar to the findings in the UK.[4,11] The underlying contributory factors remain unclear and therefore represent an important area for future investigation.
Potential implication
Our data highlight several areas meriting greater attention and prevention efforts. For example, the low uptake levels of many secondary preventive medications. Primary prevention interventions should clearly complement secondary prevention efforts, and reflect cost benefit estimates.
Limitations
The quality of any modelling study is largely determined by the adequacy of the available data to reflect what is going on in a country. Like others we made several assumptions. All were transparent and documented in the S1 Appendix. The use of record linkage represented a substantial methodological improvement compared with some previous IMPACT studies because it allowed accurate accounting for potential overlaps between patient groups. Hospital, population, pharmacy and GP registers were fairly representative for the entire population and large enough for reasonably accurate estimates. Trends in population based risk factor levels were based on individual cohort data, not necessarily precisely representing the entire Dutch population.[25,26] Though not perfect, these remain the best available data for the Netherlands.
Socioeconomic groups were based on an area-level indicator by postal code.[32] Although neighborhood socioeconomic circumstances correlate well with individual socioeconomic position,[33,34] some misclassification may have occurred which could reduce the possibility to observe differences.
Conclusions
CHD mortality in The Netherlands has dramatically declined since 1997. Equally large contributions have come from the increased use of acute phase and secondary prevention treatments, and from improvements in population risk factors (including primary prevention treatments). These positive trends have been negated by substantial increases in obesity and diabetes, which represent a major challenge for future, more effective prevention policies.
Supporting Information
(PDF)
Acknowledgments
We adapted the English IMPACT-SEC to create the Dutch IMPACT-SEC model. The English IMPACT-SEC project was conducted by Madhavi Bajekal (MB) and Shaun Scholes (SS). Hande Love set up the worksheet template; SS populated the model and was its custodian. MB ensured the integrity of in/outputs and provided SEC-related methodological solutions. Martin O’Flaherty & Nat Hawkins provided support, clinical expertise and generated the therapeutic input. The UCL team was led by Rosalind Raine, and Simon Capewell co-ordinated the overall project.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
CK and IV are supported by a grant from the Dutch Heart Foundation (grant 2008T111, http://www.hartstichting.nl). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. The top 10 causes of death, Factsheet no. 310, updated May 2014 [Available: http://www.who.int/mediacentre/factsheets/fs310/en/index.html]
- 2.Abu-Rmeileh NM, Shoaibi A, O'Flaherty M, Capewell S, Husseini A. Analysing falls in coronary heart disease mortality in the West Bank between 1998 and 2009. BMJ Open 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspelund T, Gudnason V, Magnusdottir BT, Andersen K, Sigurdsson G, Thorsson B, et al. Analysing the large decline in coronary heart disease mortality in the Icelandic population aged 25–74 between the years 1981 and 2006. PLoS One 2010;5:13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajekal M, Scholes S, Love H, Hawkins N, O'Flaherty M, Raine R, et al. Analysing recent socioeconomic trends in coronary heart disease mortality in England, 2000–2007: a population modelling study. PLoS Med 2012;9:e1001237 10.1371/journal.pmed.1001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandosz P, O'Flaherty M, Drygas W, Rutkowski M, Koziarek J, Wyrzykowski B, et al. Decline in mortality from coronary heart disease in Poland after socioeconomic transformation: modelling study. BMJ 2012;344:d8136 10.1136/bmj.d8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett K, Kabir Z, Unal B, Shelley E, Critchley J, Perry I, et al. Explaining the recent decrease in coronary heart disease mortality rates in Ireland, 1985–2000. J Epidemiol Community Health 2006;60:322–327. 10.1136/jech.2005.038638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorck L, Rosengren A, Bennett K, Lappas G, Capewell S. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. Eur Heart J 2009;30:1046–1056. 10.1093/eurheartj/ehn554 [DOI] [PubMed] [Google Scholar]
- 8.Bruthans J, Cifkova R, Lanska V, O'Flaherty M, Critchley JA, Holub J, et al. Explaining the decline in coronary heart disease mortality in the Czech Republic between 1985 and 2007. Eur J Prev Cardiol 2012;21:829–839. 10.1177/2047487312469476 [DOI] [PubMed] [Google Scholar]
- 9.Capewell S, Beaglehole R, Seddon M, McMurray J. Explanation for the decline in coronary heart disease mortality rates in Auckland, New Zealand, between 1982 and 1993. Circulation 2000;102:1511–1516. [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 2007;356:2388–2398. 10.1056/NEJMsa053935 [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss JW, Davies CA, Dundas R, Hawkins N, Jhund PS, Scholes S, et al. Explaining trends in Scottish coronary heart disease mortality between 2000 and 2010 using IMPACT-SEC model: retrospective analysis using routine data. BMJ 2014;348:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes J, Kee F, O'Flaherty M, Critchley J, Cupples M, Capewell S, et al. Modelling coronary heart disease mortality in Northern Ireland between 1987 and 2007: broader lessons for prevention. Eur J Prev Cardiol 2013;20:310–321. 10.1177/2047487312441725 [DOI] [PubMed] [Google Scholar]
- 13.Kabir Z, Perry IJ, Critchley J, O'Flaherty M, Capewell S, Bennett K. Modelling Coronary Heart Disease Mortality declines in the Republic of Ireland, 1985–2006. Int J Cardiol 2013;168:2462–2467. 10.1016/j.ijcard.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Laatikainen T, Critchley J, Vartiainen E, Salomaa V, Ketonen M, Capewell S. Explaining the decline in coronary heart disease mortality in Finland between 1982 and 1997. Am J Epidemiol 2005;162:764–773. 10.1093/aje/kwi274 [DOI] [PubMed] [Google Scholar]
- 15.Palmieri L, Bennett K, Giampaoli S, Capewell S. Explaining the decrease in coronary heart disease mortality in Italy between 1980 and 2000. Am J Public Health 2010;100:684–692. 10.2105/AJPH.2008.147173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira M, Azevedo A, Lunet N, Carreira H, O'Flaherty M, Capewell S, et al. Explaining the decline in coronary heart disease mortality in Portugal between 1995 and 2008. Circ Cardiovasc Qual Outcomes 2013;6:634–642. 10.1161/CIRCOUTCOMES.113.000264 [DOI] [PubMed] [Google Scholar]
- 17.Unal B, Sozmen K, Arik H, Gerceklioglu G, Altun DU, Simsek H, et al. Explaining the decline in coronary heart disease mortality in Turkey between 1995 and 2008. BMC Public Health 2013;13:1135 10.1186/1471-2458-13-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wijeysundera HC, Machado M, Farahati F, Wang X, Witteman W, an der Velde G, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005. JAMA 2010;303:1841–1847. 10.1001/jama.2010.580 [DOI] [PubMed] [Google Scholar]
- 19.Bruggink JW. Ontwikkelingen in (gezonde) leevnsverwachtin naar opleidingsniveau [in Dutch]. Bevolkingstrends 4e kwartaal 2009.
- 20.Koopman C, Bots ML, van Oeffelen AA, van Dis I, Verschuren WM, Engelfriet PM, et al. Population trends and inequalities in incidence and short-term outcome of acute myocardial infarction between 1998 and 2007. Int J Cardiol 2012;168:993–8. 10.1016/j.ijcard.2012.10.036 [DOI] [PubMed] [Google Scholar]
- 21.Knol FA. From high to low; from low to high: the development of social status of neighbourhoods between 1971 and 1995 The Hague: Sociaal Cultureel Planbureau; 1998. [in Dutch]. [Google Scholar]
- 22.Herings R, Pedersen L. Pharmacy-based Medical Record Linkage Systems In: Strom B, Kimmel S, Hennessy S, editors. Pharmacoepidemiology. 5 ed: John Wiley & Sons, Ltd; 2012. p270-86. [Google Scholar]
- 23.van Herk-Sukel MP, van de Poll-Franse LV, Lemmens VE, Vreugdenhil G, Pruijt JF, Coebergh JW, et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer 2010;46:395–404. 10.1016/j.ejca.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 24.Koopman C, Vaartjes I, Heintjes EM, Spiering W, van Dis I, Herings RM, et al. Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998–2010. Eur Heart J 2013;34:p 3198–3205. 10.1093/eurheartj/eht368 [DOI] [PubMed] [Google Scholar]
- 25.Huisman M, Poppelaars J, van der HM, Beekman AT, Brug J, van Tilburg TG, et al. Cohort profile: the Longitudinal Aging Study Amsterdam. Int J Epidemiol 2011;40:868–876. 10.1093/ije/dyq219 [DOI] [PubMed] [Google Scholar]
- 26.Verschuren WM, Blokstra A, Picavet HS, Smit HA. Cohort profile: the Doetinchem Cohort Study. Int J Epidemiol 2008;37:1236–1241. 10.1093/ije/dym292 [DOI] [PubMed] [Google Scholar]
- 27.Nichol G, Stiell IG, Hebert P, Wells GA, Vandemheen K, Laupacis A. What is the quality of life for survivors of cardiac arrest? A prospective study. Acad Emerg Med 1999;6:95–102. [DOI] [PubMed] [Google Scholar]
- 28.Mant J, Hicks N. Detecting differences in quality of care: the sensitivity of measures of process and outcome in treating acute myocardial infarction. BMJ 1995;311:793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 31.Voedingscentrum. Zo eet Nederland. Resultaten van de voedselconsumptiepeiling 1997–1998. Den Haag: 1998. [in Dutch]
- 32.Knol FA. From high to low; from low to high: the development of social status of neighbourhoods between 1971 and 1995 The Hague: Sociaal Cultureel Planbureau; 1998. [in Dutch]. [Google Scholar]
- 33.Stjarne MK, Fritzell J, De Leon AP, Hallqvist J. Neighborhood socioeconomic context, individual income and myocardial infarction. Epidemiology 2006;17:14–23. [DOI] [PubMed] [Google Scholar]
- 34.Sundquist K, Malmstrom M, Johansson SE. Neighbourhood deprivation and incidence of coronary heart disease: a multilevel study of 2.6 million women and men in Sweden. J Epidemiol Community Health 2004;58:71–77. 10.1136/jech.58.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.