Abstract
Daclatasvir (Daklinza) for chronic hepatitis C infection
INTRODUCTION
The discovery of the hepatitis C virus (HCV) took place 27 years ago. Initial research was geared toward understanding the HCV life cycle and replication process. The Food and Drug Administration (FDA) approved an injectable formulation of interferon in 1997 as the initial medication active against the virus. Although it was considered a breakthrough in HCV treatment, many adverse events were reported along with a lack of clinically sustained virological response (SVR) that often limited the drug’s role.1
Ongoing efforts sought to develop different means of treating HCV based on the specific genotype of the virus. The addition of pegylated formulations of interferon allowed for less frequent dosing and minimized adverse events. The combination of peginterferon and ribavirin then became the standard of care for patients with chronic HCV infection regardless of genotype. This combination regimen resulted in an SVR of 45% to 50% for HCV genotype 1 (GT1) infection and rates of 70% to 80% for GT2 and GT3. This regimen was also associated with fewer adverse events compared with earlier regimens.2
The approval of two protease inhibitors—boceprevir and telaprevir—was announced as a triple-therapy combination with peginterferon plus ribavirin for GT1 infection. The triple-therapy regimens were short-lived because of their similar response rates to standard-of-care treatment, complicated administration schedules, significant adverse events, and issues with resistance. Subsequently, boceprevir and telaprevir were removed from the U.S. market.3,4
More than 25 years after its initial discovery, HCV remains a global public health concern. According to the Centers for Disease Control and Prevention (CDC), annual U.S. HCV-related deaths in 2013 surpassed the total combined number of deaths from 60 other infectious diseases reported to the CDC, including human immunodeficiency virus (HIV), and CDC surveillance data from 2014 show HCV-related deaths climbed to almost 20,000, an all-time high.5 Unlike hepatitis A and hepatitis B, a vaccine for hepatitis C remains unavailable. As we continue to strive for more preventive treatment options for HCV, we are actively pursuing means to eradicate the spread and incidence of this chronic disease and associated complications.
Today, innovative developments for the treatment of HCV infection have led to the potential eradication of the virus and a cure for infected patients. Direct-acting antiviral agents (DAAs) that target various stages of the HCV life cycle with or without ribavirin are now available. Combining medications that have different targets of action with synergistic antiviral effects will hopefully lessen the burden of resistance to antivirals.
In July 2015, the FDA approved daclatasvir (Daklinza, Bristol-Myers Squibb) for use with sofosbuvir (Sovaldi, Gilead Sciences) as the first 12-week, all-oral treatment option for patients with chronic HCV GT3.6 Daclatasvir, a nonstructural protein 5A (NS5A) inhibitor, when combined with sofosbuvir, an NS5B inhibitor, results in higher SVR rates, fewer adverse events, and limited antiviral resistance.7
INDICATIONS
Daclatasvir is approved for use as combination therapy with sofosbuvir, with or without ribavirin, for the treatment of patients with chronic HCV GT1 or GT3 infection without cirrhosis, with compensated (Child–Pugh A) or decompensated (Child–Pugh B or C) cirrhosis, or post-transplant.8
PHARMACOLOGY
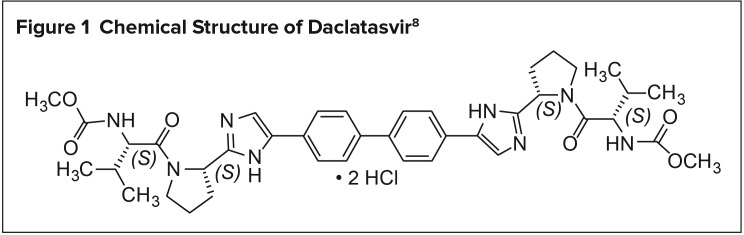
Daclatasvir is chemically described as carbamic acid, N,N′-[[1,1′-biphenyl]-4,4′-diylbis[1H-imidazole-5,2-diyl-(2S)-2,1-pyrrolidinediyl[(1S)-1-(1-methylethyl)-2-oxo-2,1-ethanediyl]]] bis-, C,C′-dimethyl ester, hydrochloride (1:2). Its molecular formula is C40H50N8O6•2HCl (Figure 1), and its molecular weight is 738.88 (free base). Daclatasvir exerts its pharmacological activity by the inhibition of NS5A N-terminus (domain 1), causing structural variations in HCV replication and assembly of HCV virions.8
Figure 1.
Chemical Structure of Daclatasvir8
PHARMACODYNAMICS AND PHARMACOKINETICS
The pharmacokinetic properties of daclatasvir were evaluated in healthy adults and in patients with chronic HCV. Administration of daclatasvir tablets in HCV-infected patients resulted in approximately dose-proportional increases in peak concentration (Cmax), area under the curve (AUC), and minimum concentration up to 60 mg once daily. Daclatasvir is rapidly absorbed with a peak plasma concentration within two hours of administration. Steady state is anticipated after approximately four days of once-daily daclatasvir administration.8
Food may affect the absorption of daclatasvir. The administration of daclatasvir 60 mg after a high-fat, high-calorie meal decreased the Cmax and AUC by 28% and 23%, respectively, compared with fasting conditions. However, administration after a low-fat, low-calorie meal did not result in a significant decrease in absorption.8
Cytochrome P450 (CYP) 3A4 is primarily responsible for the metabolism of daclatasvir, with excretion occurring predominantly in the feces. In healthy subjects, 88% of the dose was excreted in the feces (53% being unchanged drug), and 6.6% of the dose was excreted in the urine (primarily as unchanged drug). There are eight metabolites of daclatasvir, one of which (BMS-805215) has some activity; however, it is 100-fold less potent than the parent drug. The terminal half-life of daclatasvir is 12 to 15 hours.8
DOSAGE AND ADMINISTRATION
Daclatasvir is not recommended as monotherapy and if, for any reason, sofosbuvir is discontinued, discontinuation of daclatasvir is recommended as well. Another important consideration for patients with cirrhosis is the recommendation of testing for NS5A resistance-associated polymorphisms prior to the initiation of therapy.8
The recommended daclatasvir dose is 60 mg once daily in combination with sofosbuvir 400 mg once daily, with or without food, for 12 weeks.8,9 Patients with HCV GT1 infection with decompensated cirrhosis, patients with HCV GT3 infection with compensated or decompensated cirrhosis, and post-transplant patients with HCV GT1 or 3 infection should have ribavirin added to their regimen. The dosage of ribavirin varies in accordance with HCV genotype, patient weight, and patient tolerance and ranges from 600 mg to 1,200 mg daily. If ribavirin is added, administration with food is recommended.8
Because the SVR rate was just 63% in patients with cirrhosis, an extended treatment duration of up to 24 weeks may be appropriate; ribavirin may also be added to the regimen.8
Daclatasvir is available as 30-mg, 60-mg, and 90-mg tablets to allow for dosage adjustments to circumvent drug interactions or to fine-tune daclatasvir concentrations. Patients with renal or hepatic insufficiencies do not require dosage adjustments for daclatasvir.8
RESISTANCE PROFILE
As the standard of care for HCV treatment turns toward DAAs, resistance becomes a major concern due to the selective pressure that favors HCV variants capable of replicating in the presence of DAAs. HCV is predisposed to the development of resistance due to its highly variable genome. Incorrect nucleoside substitutions occur approximately 10–3 to 10–5 times per replication cycle. The virus is estimated to replicate 1012 times per day, resulting in a large number of variant viral genomes or “viral quasispecies.” The dominant wild-type HCV sequence replicates the most efficiently; however, DAA-resistant quasispecies may become the dominant HCV sequence in the presence of DAAs. Resistance to DAAs may be pre-existing or may develop during or after DAA treatment. Resistance to DAAs is also influenced by the HCV genotype, with some HCV genotypes having a higher barrier to resistance than others. Overcoming resistance with the use of more than one DAA that target different parts of the HCV genome has been explored. In vitro studies have demonstrated that HCV variants that are resistant to one DAA remain fully active against other DAAs. Despite this, cross-resistance has been observed with resultant HCV breakthrough.10
Several polymorphisms are associated with daclatasvir resistance based on HCV genotype (Table 1). In general, GT1a has a lower barrier to resistance compared with GT1b. The rate of daclatasvir-resistant polymorphisms did not differ significantly between HCV/HIV coinfected patients compared with HCV monoinfected patients.11
Table 1.
Most Common Polymorphisms Associated With Daclatasvir Resistance11
| Genotype | Substitution |
|---|---|
| 1a | M28T L31V/M Q30E/H/R Y93C/H/N |
| 1b | L31V Y93H |
| 2 | L31M F28S |
| 3 | Y93H |
| 4 | – |
| 5 | L31F |
| 6 | P32L |
CLINCIAL TRIALS
The efficacy of daclatasvir in combination with sofosbuvir and with or without ribavirin was evaluated in three phase 3 clinical trials (Table 2). SVR, the primary endpoint, was defined as HCV RNA below the lower limit of quantification (25 IU/mL) at post-treatment week 12 (SVR12).8
Table 2.
HCV Genotype 1 and 3 Patient Populations From Daclatasvir Clinical Trials8
| Trial | Population | Study Arms and Duration (Number of Patients Treated) |
|---|---|---|
| ALLY-3 | Genotype 3, treatment-näive and treatment-experienced, with or without cirrhosis | Daclatasvir and sofosbuvir for 12 weeks (N = 152) |
| ALLY-2 | Genotypes 1 and 3, treatment-näive and treatment-experienced, with or without cirrhosis, with HCV/HIV-1 coinfection | Daclatasvir and sofosbuvir for 12 weeks (N = 137) |
| ALLY-1 | Genotypes 1 and 3, treatment-näive or treatment-experienced, with or without cirrhosis, including decompensated cirrhosis and post-transplant | Daclatasvir and sofosbuvir plus ribavirin for 12 weeks (N = 103) |
HCV = hepatitis C virus; HIV = human immunodeficiency virus
ALLY-3 Study8,12
ALLY-3 was a phase 3, open-label study that evaluated the safety and efficacy of a 12-week regimen of daclatasvir plus sofosbuvir once daily for the treatment of chronic HCV GT3 in adults 18 years of age or older. Treatment-naïve and treatment-experienced patients with HCV RNA of 10,000 IU/mL or greater at screening were included. Patients with compensated cirrhosis were also eligible for the study.
Results were reported for 152 patients: 101 treatment-naïve patients (66%) and 51 treatment-experienced patients (34%), with an overall SVR12 of 89% (Table 3). SVR12 was 90% in treatment-naïve patients, 86% in treatment-experienced patients, 96% in patients without cirrhosis, 63% in patients with cirrhosis, 93% in patients with F0–F3 fibrosis scores, and 70% in patients with an F4 fibrosis score. One treatment-naïve patient with cirrhosis and an initial platelet count of 83 × 109 cells/L had a detectable HCV RNA of 53 IU/mL at the end of the study.
Table 3.
ALLY-3: SVR12 in Treatment-Naïve and Treatment-Experienced Patients With or Without Cirrhosis With Genotype 3 HCV Treated With Daclatasvir in Combination With Sofosbuvir for 12 Weeks8
| Treatment Outcomes | Total (N = 152) |
|---|---|
|
| |
| SVR12 | |
| All | 89% (135/152) |
| No cirrhosisa | 96% (115/120) |
| With cirrhosis | 63% (20/32) |
|
| |
| Outcomes for patients without SVR12 | |
| On-treatment virological failureb | 0.7% (1/152) |
| Relapsec | 11% (16/151) |
HCV = hepatitis C virus; SVR12 = sustained virological response at post-treatment week 12.
Includes 11 patients with missing or inconclusive cirrhosis status.
One patient had quantifiable HCV RNA at end of treatment.
Relapse rates are calculated with a denominator of patients with HCV RNA not detected at the end of treatment.
Sixteen patients (nine treatment-naïve, seven treatment-experienced) had a post-treatment relapse. Eleven of these patients had cirrhosis at baseline. The daclatasvir-resistant NS5A polymorphism Y93H emerged in nine of the 16. In the other seven relapsed patients, six had the Y93H polymorphism at baseline and one had emergent NS5A-L31I polymorphism. Polymorphisms associated with sofosbuvir were not observed.
ALLY-2 Study8,13
ALLY-2, a phase 3, open-label trial, evaluated the safety and efficacy of daclatasvir plus sofosbuvir for eight to 12 weeks in treatment-naïve patients with controlled HIV infection and for 12 weeks in treatment-experienced patients with controlled HIV infection. A total of 153 HCV GT1–4 patients were enrolled; there were no eligible patients with GT5 or 6. Patients with a creatinine clearance (CrCl) of less than 50 mL/min and patients with uncontrolled diabetes mellitus and/or hypertension were excluded, limiting generalizability of results to these patient populations. Many, but not all, antiretroviral therapy regimens were allowed concomitantly, including: atavanavir/ritonavir, darunavir/ritonavir, lopinavir/ritonavir, efavirenz, nevirapine, rilpivirine, dolutegravir, raltegravir, enfuviritide, maraviroc, abacavir, emtricitabine, lamivudine, tenofovir disoproxil fumarate, and zidovudine.
Daclatasvir was dose-adjusted to 30 mg daily in patients receiving ritonavir-boosted protease inhibitors and to 90 mg in patients receiving efavirenz or nevirapine. Patients without cirrhosis with GT1 HCV/HIV coinfection treated with daclatasvir in combination with sofosbuvir for 12 weeks achieved an SVR12 of 98%, while those with cirrhosis achieved an SVR12 of 91%. Patients with GT3 HCV/HIV coinfection treated for 12 weeks achieved an SVR12 of 100% (Table 4). Study results revealed that eight weeks of therapy resulted in a lower SVR12 compared with 12 weeks of therapy in patients with HCV/HIV coinfection. Control of HIV infection was not negatively impacted by HCV treatment. Overall, it appears that HIV status does not lead to a worse HCV treatment prognosis. Available data on patients with HCV GT2, 4, 5, or 6 infection were insufficient to provide recommendations for those genotypes.
Table 4.
ALLY-2: SVR12 in Patients With Genotype 1 and 3 HCV/HIV Coinfection Treated With Daclatasvir in Combination With Sofosbuvir for 12 Weeks8
| Treatment Outcomes | Total (N = 137) |
|---|---|
|
| |
| SVR12 | |
| Genotype 1 | 97% (123/127) |
| No cirrhosisa | 98% (103/105) |
| With cirrhosis | 91% (20/22) |
| Genotype 3b | 100% (10/10) |
|
| |
| Outcomes for genotype 1 patients without SVR12 | |
| On-treatment virological failurec | 0.8% (1/127) |
| Relapsed | 1.6% (2/126) |
| Missing post-treatment data | 0.8% (1/126) |
HCV = hepatitis C virus; HIV = human immunodeficiency virus; SVR12 = sustained virological response at post-treatment week 12.
Includes five patients with inconclusive cirrhosis status.
One patient with cirrhosis.
One patient had detectable HCV RNA at end of treatment.
Relapse rates are calculated with a denominator of patients with HCV RNA not detected at the end of treatment.
ALLY-1 Study8
ALLY-1 was an open-label trial of daclatasvir, sofosbuvir, and ribavirin that included 113 patients with chronic HCV infection and Child–Pugh A, B, or C cirrhosis (n = 60) or HCV recurrence after liver transplantation (n = 53). Treatment-naïve and treatment-experienced patients with HCV GT1, 2, 3, 4, 5, or 6 infection were eligible to enroll. Prior exposure to NS5A inhibitors was prohibited.
Patients received daclatasvir 60 mg once daily, sofosbuvir 400 mg once daily, and ribavirin for 12 weeks and were monitored for 24 weeks post-treatment. Patients received an initial ribavirin dose of 600mg or less daily with food; the initial and on-treatment dosing of ribavirin was modified based on hemoglobin and CrCl measurements. If tolerated, the ribavirin dose was titrated up to 1,000 mg per day. A high number of reductions in ribavirin dosing occurred in the trial. By week6, approximately half of the patients received 400 mg per day or less of ribavirin. In total, 16patients (15%) completed less than 12weeks and 11 patients (10%) completed less than six weeks of ribavirin therapy. For the cohort of patients with cirrhosis, the median time to discontinuation of ribavirin was 43 days (range, 8–82 days; n = 9). For the post-transplant cohort, the median time to discontinuation of ribavirin was 20 days (range, 3–57 days; n = 7).
The 113 treated patients in ALLY-1 had a median age of 59 years (range, 19–82 years); 67% were male; 96% were white, 4% were black, and 1% were Asian. Most patients (59%) were treatment-experienced, and most (71%) had baseline HCV RNA levels greater than or equal to 800,000 IU/mL. Fifty-eight percent of patients had HCV GT1a, 19% had HCV GT1b, 4% had GT2, 15% had GT3, 4% had GT4, and 1% had GT6, while 77% had the IL28B rs12979860 non-CC genotype. Among the 60 patients in the cirrhosis cohort, 20% were Child–Pugh A, 53% were Child–Pugh B, and 27% were Child–Pugh C, and 35% had a Baseline Model for End-Stage Liver Disease score of 15 or greater. Most (55%) of the 53 patients in the post-transplant cohort had F3 or F4 fibrosis.
SVR12 and outcomes in patients without SVR12 are shown for patients with HCV GT1 by patient population in Table 5. Available data on patients with HCV GT2, 4, 5, or 6 infection were insufficient to provide recommendations. SVR12 rates were comparable regardless of age, gender, IL28B allele status, or baseline HCV RNA level.
Table 5.
ALLY-1: SVR12 in Genotype 1 Patients with Child–Pugh A, B, or C Cirrhosis Or With HCV Genotype 1 Recurrence After Liver Transplantation Treated With Daclatasvir in Combination With Sofosbuvir and Ribavirin for 12 Weeks8
| Treatment Outcomes | Child–Pugh A, B, or C Cirrhosis (n = 45) | Post-Liver Transplant (n = 41) |
|---|---|---|
|
| ||
| SVR12 | ||
| Genotype 1 | 82% (37/45) | 95% (39/41) |
| Genotype 1a | 76% (26/34) | 97% (30/31) |
| Genotype 1b | 100% (11/11) | 90% (9/10) |
| Child–Pugh A | 91% (10/11) | – |
| Child–Pugh B | 92% (22/24) | – |
| Child–Pugh C | 50% (5/10) | – |
|
| ||
| Outcomes for patients without SVR12 | ||
| On-treatment virological failure | 2% (1/45)a | 0 |
| Relapseb | 16% (7/44) | 5% (2/41) |
HCV = hepatitis C virus; SVR12 = sustained virological response at post-treatment week 12.
One subject had detectable HCV RNA at end of treatment.
Relapse rates are calculated with a denominator of patients with HCV RNA not detected at end of treatment.
ADVERSE EVENTS
The most common adverse reactions observed in 10% or more patients with daclatasvir in combination with sofosbuvir were headache and fatigue. The most common adverse reactions observed in 10% or more patients with daclatasvir in combination with sofosbuvir and ribavirin were headache, anemia, fatigue, and nausea.8
CONTRAINDICATIONS
Medications that are strong CYP3A inducers, including phenytoin, carbamazepine, rifampin, and St. John’s wort (Hypericum perforatum), are contra-indicated with daclatasvir. These products lower daclatasvir exposure and decrease virological response.5 Medications that are moderate CYP3A inducers, such as bosentan, dexamethasone, and modafinil, require that the daclatasvir dose be increased to 90 mg. Medications that are considered strong CYP3A inhibitors, such as clarithromycin, itraconazole, and ketoconazole, require a dose adjustment to 30mg. Refer to the full prescribing information for a list of contraindicated drugs and other potential drug–drug interactions.8
WARNINGS AND PRECAUTIONS
Concomitant therapy of amiodarone with daclatasvir and sofosbuvir is not recommended due to post-marketing case reports of serious symptomatic bradycardia, which presents with dizziness, malaise, weakness, shortness of breath, chest pain, and/or confusion. Bradycardia has been observed within hours to two weeks after treatment initiation, with symptoms typically resolving after treatment discontinuation. Risk factors include underlying cardiac comorbidities, concomitant therapy with beta blockers, and/or advanced liver disease. For patients with no alternative treatment options to amiodarone, inpatient cardiac monitoring for the first 48 hours of coadministration and outpatient self-monitoring for the first two weeks of therapy are recommended. 8
SPECIAL POPULATIONS
Geriatric Patients
Clinical trials did not demonstrate any trends in adverse events among the geriatric population compared with younger adults. No dosage adjustment is needed for geriatric patients.8
Pregnancy and Lactation8,14,15
There is an approximate 3% to 10% rate of mother-to-child transmission of HCV. Maternal HCV infection alone is associated with cholestasis of pregnancy, neonatal abstinence syndrome, and neonatal intensive care unit admission. However, after adjusting for sociodemographic variables, large population studies suggest increased rates of preterm birth, low birth weight, premature membrane rupture, gestational diabetes, and congenital anomalies in maternal HCV infection.
Although data in humans are lacking, maternal and embryo-fetal toxicities were observed in animal studies.
Daclatasvir is not recommended for use in pregnant women or women of childbearing potential not on highly effective contraception during therapy and for at least five weeks following completion of daclatasvir treatment.
According to the manufacturer, the decision to breastfeed during therapy should take into account the risk of exposure to the infant and the benefits of treatment to the mother. It is unknown if daclatasvir is present in human breast milk or affects human milk production. In animal studies, daclatasvir was detected in the milk of lactating rats.
COST
Daclatasvir is available in 30-mg, 60-mg, and 90-mg strengths at an average wholesale price (AWP) of $25,200 for a package of 28 tablets.16 Sofosbuvir, a requirement for the combination therapy, is distributed as a 400-mg tablet with an AWP of $33,600 for a package of 28.16 Ribavirin, if indicated, is a generic drug supplied as a 200-mg tablet or capsule that can be acquired for as little as $1.37 per pill AWP (Richmond Pharmaceuticals).16
For the recommended standard treatment of 12 weeks, the patient will need three packages of daclatasvir 60 mg at a total AWP of $75,600 and three packages of sofosbuvir at a total AWP of $100,800 for a total treatment cost of $176,400. The addition of ribavirin will add another $346 to $692 AWP to the total, dependent on the patient’s required dosage.
P&T COMMITTEE CONSIDERATIONS
DDAs have transformed the treatment of HCV infection. These highly tolerable medications significantly improve patient compliance while ultimately moving toward a cure. The major caveat associated with DAAs is the development of resistance.17
Studies demonstrate that shorter-duration therapies are just as effective, if not more effective, in achieving acceptable SVR as the longer regimens used in the past. The trend toward interferon-free treatment provides a more tolerable adverse effect profile, which also improves patient adherence.
The latest HCV guidelines developed by the Infectious Diseases Society of America and the American Association for the Study of Liver Diseases include daclatasvir in their assemblage of recommended regimens for the treatment of the disease. Because the negotiated pricing and cost structure for pharmaceutical products are not transparent in the U.S., it is difficult to estimate the true cost and cost-effectiveness of HCV drugs.18 The primary goal of HCV therapy remains eliminating the virus. The secondary goals are to decrease the progression to liver cirrhosis and the risk of hepatocellular carcinoma. Drugs that meet these goals have the potential to drastically reduce a patient’s future health care expenditures.
Footnotes
Disclosure: The authors report no financial or commercial relationships in regard to this article.
REFERENCES
- 1.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55(9):1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo E. Pegylated interferon and ribavirin treatment for hepatitis C virus infection. Ther Adv Chronic Dis. 2011;2(1):39–45. doi: 10.1177/2040622310384308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HIVandHepatitis.com. Merck plans to discontinue boceprevir for hepatitis C by December 2015. Jan 21, 2015. [Accessed November 8, 2016]. Available at: www.hivandhepatitis.com/hcv-treatment/approved-hcv-drugs/5021-merck-plans-to-discontinue-boceprevir-for-hepatitis-c-by-december-2015.
- 4.HIVandHepatitis.com. Vertex to discontinue sale of telaprevir (Incivek) for hepatitis C. Aug 22, 2014. [Accessed November 8, 2016]. Available at: www.hivand-hepatitis.com/hcv-treatment/approved-hcv-drugs/4808-vertex-to-discontinue-sale-of-telaprevir-incivek-for-hepatitis-c.
- 5.Centers for Disease Control and Prevention. Hepatitis C kills more Americans than any other infectious disease. May 4, 2016. [Accessed November 8, 2016]. Available at: www.cdc.gov/media/releases/2016/p0504-hepc-mortality.html.
- 6.Food and Drug Administration. FDA approves new treatment for chronic hepatitis C genotype 3 infections. [Accessed July 24, 2015]. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm455888.htm.
- 7.Hepatitis Central. Medications to treat hepatitis C—a timeline. [Accessed March 2016]. Available at: www.hepatitiscentral.com/medications-to-treat-hepatitis-c-a-timeline.
- 8.Daklinza (daclatasvir) prescribing information. Princeton, New Jersey: Bristol-Myers Squibb Company; 2016. [Google Scholar]
- 9.Sovaldi (sofosbuvir) prescribing information. Foster City, California: Gilead Sciences; 2015. [Google Scholar]
- 10.Aloia AL, Locarnini S, Beard MR. Antiviral resistance and direct-acting antiviral agents for HCV. Antivir Ther. 2012;17(6Pt B):1147–1162. doi: 10.3851/IMP2426. [DOI] [PubMed] [Google Scholar]
- 11.Plaza Z, Soriano V, Vispo E, et al. Prevalence of natural polymorphisms at the HCV NS5A gene associated with resistance to daclatasvir, an NS5A inhibitor. Antivir Ther. 2012;17(5):921–926. doi: 10.3851/IMP2091. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8):714–725. doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]
- 14.Dunkelberg JC, Berkley EMF, Thiel KW, Leslie KK. Hepatitis B and C in pregnancy: a review and recommendations for care. J Perinatol. 2014;34(12):882–891. doi: 10.1038/jp.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connell LE, Salihu HM, Salemi JL, et al. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 2011;31(8):1163–1170. doi: 10.1111/j.1478-3231.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 16.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; [Accessed October 25, 2016]. [Google Scholar]
- 17.McConachie SM, Wilhelm SM, Kale-Pradhan PB. New direct-acting antivirals in hepatitis C therapy: a review of sofosbuvir, ledipasvir, daclatasvir, simeprevir, paritaprevir, ombitasvir and dasabuvir. Expert Rev Clin Pharmacol. 2016;9(2):287–302. doi: 10.1586/17512433.2016.1129272. [DOI] [PubMed] [Google Scholar]
- 18.American Association for the Study of Liver Diseases-Infectious Diseases Society of America. Overview of cost, reimbursement, and cost-effectiveness considerations for hepatitis C treatment regimens. Jul 6, 2016. [Accessed October 25, 2016]. Available at: www.hcvguidelines.org.