Abstract
Short on funds and with no therapeutics or vaccines in sight, U.S. health officials are scrambling to prepare for a protracted fight with Zika virus and the mosquitoes that carry it. In this article, the author focuses on the arrival of Zika in the U.S.
INTRODUCTION
In the April 2016 P&T, we described the rapid spread of Zika virus from East Africa, through Southeast Asia, and into South and Central America.1 At that time, the virus had not made significant inroads in the United States. Unfortunately, that is no longer the case. Short on funds and with no therapeutics or vaccines in sight, U.S. health officials are scrambling to prepare for a protracted fight with a tenacious foe. In this article, we focus on the arrival of Zika in the U.S. and on efforts to contain it.
BACKGROUND
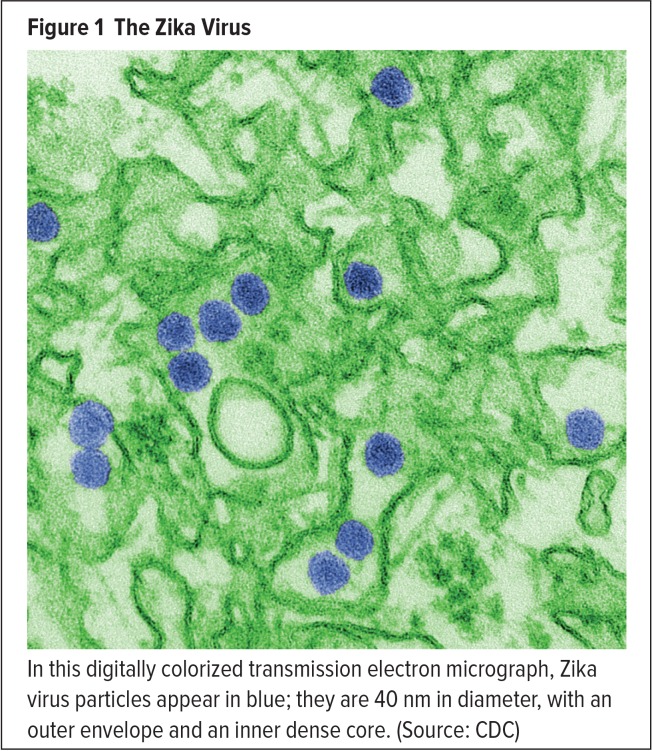
The virus that became known as Zika was first identified in 1947 in rhesus monkeys inhabiting Uganda’s Zika forest.2 The first cases of human infection were reported five years later in Uganda and Tanzania.3 The source of these infections was determined to be a mosquito-borne flavivirus, labeled Zika in reference to its Ugandan origin (Figure 1). The Zika virus is now known to be related to yellow fever virus, dengue virus, and West Nile virus.4,5 It is transmitted to people by mosquitoes of the Aedes species, principally Ae. aegypti (commonly known as the yellow fever mosquito) and Ae. albopictus (the Asian tiger mosquito) (Figures 2 and 3), which thrive in warm climates.4–6
Figure 1.
The Zika Virus
In this digitally colorized transmission electron micrograph, Zika virus particles appear in blue; they are 40 nm in diameter, with an outer envelope and an inner dense core. (Source: CDC)
Figure 2.
An Aedes Aegypti Mosquito
Source: CDC
Figure 3.
An Aedes Albopictus Mosquito
Source: CDC
By the 1980s, mosquitoes had carried the Zika virus across equatorial Asia, from Pakistan to Indonesia.7,8 Continuing its westward migration, the virus passed from Southeast Asia to islands in the South Pacific, where it caused major outbreaks in 2013 and 2014.9–12 On July 15, 2015, Brazil confirmed the circulation of Zika virus in that country—the first report of locally acquired Zika virus infection (ZVI) in the Americas.13 Two days later, Brazilian authorities detected the neurological disorder Guillain-Barré syndrome (GBS) in some adults with ZVI.2,14 Then, in October, Brazilian officials reported an unusual increase in microcephaly among newborns. Fifty-four cases were recorded between August and October 30.15 By the end of 2015, that number would explode to more than 2,900.16
Meanwhile, the virus was moving inexorably north. In November, locally acquired ZVI cases were reported in Colombia, El Salvador, Mexico, Venezuela, and Paraguay.17–21 The following month, the disease had spread to Honduras, Panama, and French Guiana.22–24 On December 31, the Centers for Disease Control and Prevention (CDC) announced the first confirmed case of locally acquired ZVI in Puerto Rico.25 Next stop: the United States.
ZIKA COMES TO AMERICA
Given that Ae. aegypti and Ae. albopictus mosquitoes inhabit most of the mainland U.S., the arrival of Zika virus in this country wasn’t a matter of “if” but of “when.”
Ae. aegypti mosquitoes originated in Africa but have been in the U.S. for centuries, probably reaching the new world on ships used for European exploration and colonization.26 Ae. aegypti occupies urban areas with or without vegetation, and bites, rests, and lays eggs both indoors and outdoors. It prefers taking blood meals from humans and pays less attention to domestic mammals.6
Ae. albopictus mosquitoes, from Asia, were first documented in the U.S. in Texas in 1985 and near Jacksonville, Florida, in 1986. They are believed to have entered the U.S. in shipments of used tires imported from Asia for retreading.27 Ae. albopictus is mostly rural, inhabiting thickets and arboreal vegetation. While it bites humans, it will just as readily feed on domestic and wild mammals.6
In March, Brazilian scientists announced that they were able to infect another mosquito species, Culex quinquefasciatus, with Zika virus in the laboratory, raising concerns that Zika could be carried by a species more prevalent than Ae. aegypti or Ae. albopictus. Culex mosquitoes exist in more-temperate climes, including the southern United States, where they carry the West Nile virus.28 In September, however, scientists at Kansas State University reported that Culex mosquitoes do not appear to transmit Zika virus, which vanishes rather than multiplying in the species.29
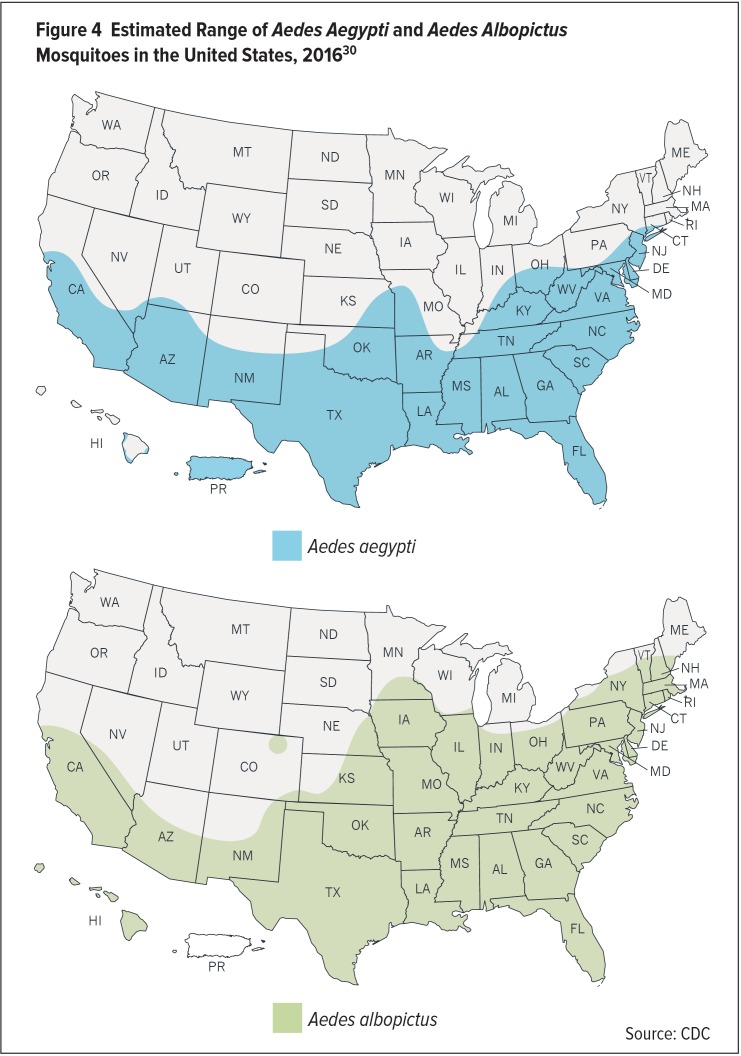
According to the CDC, Ae. aegypti and Ae. albopictus can be found as far north as Michigan, New Hampshire, and Washington state. The mosquitoes are concentrated most heavily, however, in the Southeast and Southwest (Figure 4).30,31 Ae. aegypti mosquitoes were captured at least once in 26 states, whereas Ae. albopictus was found in 40 states. Overall, the mosquitoes were observed in approximately one-third of the 3,141 U.S. counties.31 Only seven states—Washington, Oregon, Idaho, Montana, Wyoming, North Dakota, and South Dakota—are completely free of both species.30
Figure 4.
Estimated Range of Aedes Aegypti and Aedes Albopictus Mosquitoes in the United States, 201630
Source: CDC
In January, researchers at the University of Notre Dame reported the discovery of Ae. aegypti mosquitoes in Washington, D.C. Disturbingly, the team found genetic evidence that the mosquitoes had overwintered for at least four years, meaning they were adapting for persistence in a northern climate.32
At the same time, a resident in Virginia and another in Arkansas tested positive for ZVI. Both had traveled to Zika-affected countries.33,34 Another Zika case occurred in Texas a week later. This one, however, marked the first case of sexual transmission of Zika virus in the U.S. An infected man who had recently returned from Venezuela transmitted the virus to his sexual partner.35 After this, the CDC advised men to abstain from sex or to use condoms correctly after traveling to areas with circulating Zika virus.36
By February 26, 116 residents in 33 states and the District of Columbia had evidence of recent ZVI, according to CDC figures.37
As concern about Zika’s American presence grew, the Department of Homeland Security announced that the U.S. would not screen people entering the country for the virus because most of those infected (an estimated 80%) are asymptomatic.38
It wasn’t long before the American Council on Science and Health was calling Zika “possibly the scariest virus since HIV.” 39 Things took a turn for the worse when Florida health officials noticed Zika cases in the Miami area.
Zika in Florida
With its ubiquitous, almost year-round population of Ae. aegypti and Ae. albopictus mosquitoes and a high volume of travelers from Zika-affected countries, Florida is especially vulnerable to Zika outbreaks.40
In February, Florida Governor Rick Scott declared a public health emergency in four counties with travel-related cases of ZVI and ordered state officials to increase mosquito-control efforts. The counties were Miami–Dade in south Florida, Hillsborough in the Tampa Bay region, Lee in southwest Florida, and Santa Rosa in the Florida Panhandle.41 In late July, Miami–Dade became the U.S. epicenter of Zika activity when local health officials confirmed two cases of disease caused by mosquito bites in a square-mile area of Wynwood, a trendy neighborhood just north of downtown Miami.40,42 They were the first locally acquired cases of ZVI in the continental U.S. The CDC issued a travel advisory warning pregnant women to avoid the area.43
“These cases are not unexpected,” CDC Director Tom Frieden, MD, MPH, told a press conference. “At CDC, we’ve been saying for months, based on experience with chikungunya and dengue, which are viruses spread by the same mosquito that spreads Zika, that individual cases and potentially small clusters of Zika are possible in the U.S. As we have anticipated, Zika is now here.”44
As Dr. Frieden spoke, 1,658 cases of ZVI had been reported to the CDC in the continental U.S. and Hawaii, but none resulted from local mosquito bites. Fifteen cases were believed to be related to sexual transmission, and one case in Pennsylvania was caused by accidental laboratory exposure to the virus.42
In early August, the CDC announced that an additional area of active Zika transmission had been identified in Miami Beach. The Florida Department of Health also found at least four other instances of apparently mosquito-borne Zika in Miami–Dade County.45
The CDC pointed out that detecting the local spread of Zika virus was difficult for several reasons:45
The incubation period for ZVI is up to two weeks.
A high proportion of infected people have no symptoms.
The diagnosis and investigation of cases take weeks.
For these reasons, the CDC said, it was possible that other Miami–Dade neighborhoods could have active Zika transmission that was not yet apparent.44
Anthony Fauci, MD, Director of the National Institute of Allergy and Infectious Diseases (NIAID), warned that Zika could easily continue its march into the continental U.S.46 Not long afterward, a ZVI case was reported more than 200 miles north of Miami in Pinellas County. The case involved a woman with no significant travel history, indicating that the virus was acquired locally.47 A second non–travel-related case of ZVI was discovered in Palm Beach County.48 In mid-August, a Texas resident who had traveled to a Miami area with local Zika transmission tested positive for the virus upon returning home. It was the first time that the virus had spread between states.49
On August 3, officials in Miami–Dade County began the aerial insecticide spraying recommended by the CDC in an effort to kill Aedes mosquitoes in a 10-square-mile area.50 The program used two pesticides—one that kills adult mosquitoes (naled, an organophosphate) and another that eradicates mosquito eggs and larvae. By the end of the month, it was obvious that the plan wasn’t working. The mosquitoes were under control in only 20% of the target area—in zones where both insecticides were used. In the remaining 80% of the target area, where only naled was sprayed, the Aedes mosquitoes held their own.51 On August 29, researchers at the University of Texas Medical Branch in Galveston reported a possible explanation: Adult female mosquitoes can pass the Zika virus to their offspring, making it clear that pesticide programs need to kill both adult mosquitoes and their eggs.52
In late August, Florida health officials announced that they were investigating three more Zika cases that were likely acquired locally in Miami–Dade County, including two cases outside of the known areas of active virus transmission. That brought the state’s total of non–travel-related cases to 46.53 But people began to question the accuracy of information being issued by state agencies. On September 10, the Miami Herald reported that health officials had stopped providing detailed information on epidemiologic investigations into local ZVI; had refused to identify all of the locations where Zika-positive mosquitoes were trapped in Miami Beach; and had under-reported the number of local Zika infections in Florida by excluding anyone who was not a state resident. Arthur Caplan, PhD, Director of Medical Ethics at New York University Langone Medical Center, commented: “It makes no sense—unless you see it through the eyes of the impact on tourism.”54
On September 17, Florida officials tripled the active Zika virus transmission zone in Miami Beach from 1.5 square miles to 4.5 square miles after five new ZVI cases were identified in the area. On the plus side, Governor Scott announced that he expected the Zika zone to be lifted in neighboring Wynwood, where aggressive mosquito control and community outreach measures had proved effective.55
The good news didn’t last. In mid-October, Governor Scott revealed that local transmission of Zika virus was occurring in a new Miami area, where health officials believed two women and three men had been infected.56 Soon the CDC introduced a color-coding system for Miami–Dade County to distinguish between areas of active transmission that present a significant risk of ZVI and areas with a possible risk of ZVI. The system denoted the whole county as a yellow cautionary area except for Miami Beach and the one-square-mile Little River neighborhood of Miami, which were high-risk red zones. The CDC recommended that pregnant women consider postponing travel to the yellow areas and specifically avoid the red areas.57
As of October 19, 2016, Florida remained the only state with locally acquired ZVI, with 137 confirmed cases.58
The Government Response
As the new year began, the White House said its efforts to fight Zika would consist mainly of sharing information about the risks with the public. President Obama, however, acknowledged the need for Zika tests, treatments, and vaccines.59 Soon afterward, he called on Congress to supply $1.9 billion in emergency funding.60
On April 1, more than 300 local, state, and federal government officials; health experts; and nongovernment partners gathered at CDC headquarters in Atlanta to craft a national plan to combat Zika.61,62 The summit’s aims included identifying gaps in readiness and providing technical support to state and local jurisdictions.62 Dr. Frieden suggested that states appoint a Zika coordinator. Other experts urged health officials to establish surveillance networks to monitor the spread of the virus. Many called on Congress to approve the $1.9 billion in funding requested by the White House, much of which would be funneled to local and state agencies that couldn’t afford significant mosquito control.63
In April, federal officials announced that they would transfer $589 million originally intended to protect against Ebola for use in the fight against Zika.64
Finally, on May 16, the Zika Response Appropriations Act–2016 was introduced in the House. The Republican-backed bill provided $622.1 million to federal agencies to fight Zika by “repurposing” leftover Ebola funding and unused Department of Health and Human Services (HHS) administrative funding.65 President Obama immediately threatened to veto the measure, calling the funding “woefully inadequate.”66 Nevertheless, the bill passed the House by a vote of 241 to 184.67 A day later, the Senate, on a vote of 68 to 30, passed its own bill, which called for $1.1 billion in emergency money to fight Zika.68 Heated bickering ensued between House and Senate negotiators before the House grudgingly passed a $1.1 billion Zika bill on June 23 by a vote of 239 to 171—almost entirely along party lines. Democratic leaders bristled at what they called unreasonable spending cuts and policy changes, especially on women’s health, that Republicans had tacked onto the bill.69 On July 14, the measure fell short of the 60 votes needed to advance in the Senate—and both sides went home for a seven-week recess.70
Dr. Frieden said simply: “This is no way to fight an epidemic.” 71
In August, President Obama said he would divert another $81 million from other programs to fund development of a Zika vaccine because Congress had failed to approve funding.72 Meanwhile, Florida Governor Scott complained that the federal government had not delivered all of the support he had requested.48
On August 30, Dr. Frieden said that the CDC would run out of funds to fight Zika by the end of September if nothing was done73—but his warning fell on deaf ears. The Senate returned to Washington on September 6 and again refused to pass a bill that would fund Zika research and prevention. The main source of contention was a Republican effort to prevent Planned Parenthood from receiving money to combat the mosquito-borne disease. The vote was 52 to 46, short of the 60 votes needed to advance the bill.74
In late September, Congress finally allocated $1.1 billion to curb Zika’s spread as part of a larger spending bill designed to keep the federal government running until December 9. Almost $935 million was approved to fight Zika at home: $152 million for NIAID, which is researching vaccines; $394 million for CDC use in Zika-affected areas; and $387 million for the HHS public health emergency fund for activities such as Zika testing and ZVI patient care. Another $175 million was approved for Zika efforts abroad, such as evacuating pregnant Americans from countries where Zika is spreading and helping hard-hit foreign nations.75 Planned Parenthood wasn’t mentioned in the September deal.75
U.S. health officials made it clear that the funding delay hurt U.S. efforts to fight the Zika virus. “Because we’ve had to wait these seven months,” Dr. Frieden said, “we haven’t been able to get a running start on some of the critically important studies to understand more fully the impacts of Zika, to establish better diagnostic tests, [and] to improve our way of controlling mosquitoes.” Vaccine development was also delayed.76
Given how long processing and budgeting can take, public health laboratories didn’t expect a cash infusion until early 2017. Meanwhile, with local health departments shrinking, many don’t have the staff to operate laboratories at the level needed given the volume of samples coming in.77
ZIKA HEALTH EFFECTS AND RISKS
During a Zika press conference on April 11, Anne Schuchat, MD, the CDC’s Principal Deputy Director, remarked that “everything we look at with this virus seems to be a bit scarier than we initially thought.”78 Two days later, the CDC confirmed what many people had suspected: ZVI can cause microcephaly and other serious fetal defects.79 The agency declined, however, to acknowledge a link between Zika and GBS in adults, saying it was still investigating.80
Zika: A Neurotropic Virus
Early in 2016, the World Health Organization (WHO) announced that Zika had been detected in amniotic fluid and that the virus was able to cross the placental barrier and infect the fetus. “We can now conclude,” the agency said, “that Zika virus is neurotropic, preferentially affecting tissues in the brain and brain stem of the developing fetus.”81
In July, a study at the Pasteur Institute in France found that Zika virus could infect and impair neural stem cells in the developing neocortex of mice.82 In the U.S., similar research was conducted at Rockefeller University in New York City, where investigators found that Zika could attack cells in adult mice in the hippocampus, the part of the brain involved in learning and memory—raising disturbing questions about how the virus might affect human adults. The researchers noted that other studies had documented the virus’ ability to cause serious brain and spinal cord infections, including encephalitis, meningitis, and myelitis, in people with ZVI.83 Shortly afterward, scientists in the U.S. and Venezuela described the first case of sensory polyneuropathy associated with acute ZVI.84
In September, American researchers found that Zika can infect cranial neural crest cells (which give rise to bones and cartilage in the skull) and can cause them to secrete signaling molecules that alter their function. In the lab, the increased levels of these molecules were enough to induce the premature differentiation, migration, and death of human neural progenitor cells. This may help explain why infants born to Zika-infected mothers are at risk of microcephaly and disproportionate facial features.85,86
Zika and Pregnancy
There is no evidence to suggest that pregnant women are more susceptible to ZVI or experience more-severe disease compared with women who are not pregnant, according to the CDC.87 Infection during pregnancy, however, can result in congenital microcephaly and other severe brain defects in the fetus.88 ZVI has also been implicated in adverse pregnancy outcomes, including miscarriage and stillbirth.88,89
In April, researchers in Brazil reported placental inflammation and the presence of Zika virus in Hofbauer cells (human placental macrophages) in tissue samples obtained from pregnant women infected with the virus. The findings suggested that a damaged placental barrier may facilitate fetal infection with Zika.90 Yale University investigators later reported similar observations and theorized that Hofbauer cells, because of their migratory characteristics, may help disseminate Zika virus to the fetal brain.91
Because of the potential risks of ZVI during pregnancy, the CDC has stated that its top priority in combating the virus is to protect pregnant women and their fetuses.87 Still, the agency acknowledges that much is not yet known about ZVI in this setting. Uncertainties include:87,92
The incidence of ZVI among pregnant women in areas of Zika virus transmission
The rate of vertical transmission of the virus
The rate with which infected fetuses manifest complications, such as microcephaly or death
How often the Zika virus can pass from a pregnant woman to her fetus during pregnancy or around the time of birth
Other potential health problems that ZVI may cause during pregnancy
Whether pregnant women are more likely to develop Zika symptoms compared with the general population
Whether pregnant women are more likely to develop GBS.
The CDC’s latest guidance recommends that all pregnant women in the U.S. and its territories should be assessed for possible exposure to Zika virus at each prenatal care visit. The agency also recommends that pregnant women not travel to an area with active Zika virus transmission. In addition, pregnant women with a sex partner who has traveled to or lives in an area with active Zika virus transmission should use condoms or other barrier methods to prevent ZVI or should abstain from sex for the duration of the pregnancy.87
In May, the CDC reported that 279 pregnant women were infected with Zika virus in the U.S.93 By October 11, that number had more than tripled to 899.94
Microcephaly and Other Birth Defects
Microcephaly is a rare neurological disorder in which an infant’s head circumference is much smaller than normal for the child’s age and gender.95–97 The deformity is typically caused by genetic abnormalities, the mother’s exposure to toxic substances during pregnancy, or certain viral infections during pregnancy, such as rubella or cytomegalovirus.97 Brazilian officials noticed an apparent link between the birth of microcephalic infants and the presence of Zika virus in pregnant women early in that country’s Zika crisis,98–100 but world health experts were initially hesitant to confirm the connection.101–103
In January, the U.S. experienced its first microcephalic birth. The case involved a Zika-infected woman in Hawaii who had lived in Brazil early in her pregnancy.104 The following month, a researcher at the Yale School of Public Health reported that, in addition to microcephaly, ZVI may cause hydrops fetalis (the abnormal accumulation of fluid in fetal compartments), hydranencephaly (the almost complete loss of brain tissue), and stillbirth.105,106 By April, the data were sufficiently compelling to prompt the CDC to announce that ZVI in pregnant women was definitely a cause of microcephaly and other severe brain abnormalities in fetuses.79
In June, the CDC estimated that the potential risk for micro-cephaly following ZVI ranged from 1% to 13%. The agency based its estimate on statistics from a 2013 Zika outbreak in French Polynesia and on ongoing reports of virus-related birth defects in Brazil’s Bahia state. More importantly, the CDC disclosed nine U.S. cases of ZVI-associated microcephaly. All involved women who contracted ZVI outside the U.S. in areas with active Zika outbreaks or were infected through unprotected sex with an infected partner.107
Meanwhile, progress was being made on the research front. Scientists at the University of Southern California discovered two Zika proteins responsible for microcephaly, an important step toward prevention. The Zika virus contains 10 proteins, but only nonstructural protein 4A (NS4A) and NS4B matter when it comes to microcephaly, according to the research. These proteins stunt brain development and increase autophagy so that the virus can spread. When they hijacked fetal neural stem cells, the size of brain organoids was, on average, halved.108
In another August report, researchers at Harvard Medical School and Beth Israel Deaconess Hospital in Boston described the devastating effects that Zika has on the developing fetal brain. The investigators used radiographs to document brain abnormalities associated with congenital ZVI in 45 cases from Brazil. The most common trait, observed in all subjects, was a visible reduction in brain-tissue volume. All subjects also showed calcification in several regions of the brain and abnormal development of the cortex. Further, the radiologists saw enlarged fluid-filled ventricles in 43 of the babies.109 Finally, in August, the CDC reported on research conducted in Brazil that found sensorineuronal hearing loss in five of 70 (7.1%) infants with ZVI-related microcephaly.110
That same month, officials in Harris County, Texas, reported the first known infant death linked to ZVI in the U.S. The girl had several Zika-related birth defects, including microcephaly. Her mother likely contracted the virus in Latin America, officials said.111
Zika and Guillain-Barré Syndrome
Zika’s neurotropic characteristics and high affinity for brain tissue may also manifest as GBS in infected adults.87,112 GBS is a rare disease of the nervous system that affected an estimated 3,000 to 6,000 people a year in the U.S. before Zika arrived. In GBS patients, damaged nerve cells lead to muscle weakness and sometimes paralysis. In severe cases, weakness or paralysis can affect the muscles that control breathing. Symptoms can last weeks or months, and most people fully recover.87,113 Approximately 30% of those with GBS have residual weakness after three years, while approximately 3% may experience a relapse of muscle weakness and tingling sensations many years after the initial attack.114 Before Zika, GBS was linked to dengue and chikungunya infections, among other causes.115,116
In March, U.S. health officials notified the WHO of America’s first two cases of Zika-related GBS. The first case was an elderly man who had recently traveled to Central America. He developed an acute febrile illness shortly after returning to the U.S. and was hospitalized in January with progressive weakness of the extremities and diminished reflexes. The patient tested positive for ZVI by polymerase chain reaction (PCR). He was about to be discharged when he died suddenly from a ruptured subarachnoid aneurysm.117
The second case was an adult male resident of Haiti who experienced the acute onset of facial weakness, difficulty swallowing, and numbness of the fingers in early January. Days later, he travelled to the U.S. for additional medical care. His cerebrospinal fluid had elevated protein and normal white blood cells. A physical examination showed mild weakness and diminished reflexes. The patient tested positive for ZVI by serology. He improved after intravenous immunoglobulin therapy (the standard treatment for GBS) and was discharged.117
In a surveillance study conducted by the Puerto Rico Department of Health with CDC assistance, 56 suspected cases of GBS with neurological signs were identified between January 1 and July 31, 2016. Thirty-four (61%) of those individuals had evidence of ZVI or flavivirus infection. The subjects’ mean age was 55 years (range, 21–88 years). All were treated with intravenous immunoglobulin G.118
At the end of August, researchers at the Pan American Health Organization compared the rates of GBS before and after Zika arrived in seven countries and announced a strong association between the virus and the illness. In a letter published in the New England Journal of Medicine, Marcos Espinal, MD, PhD, MPH, and his colleagues analyzed the rates of GBS and ZVI in Bahia, Brazil, Colombia, the Dominican Republic, El Salvador, Honduras, Suriname, and Venezuela. They looked at 164,237 confirmed and suspected ZVI cases and 1,474 GBS cases that occurred between April 1, 2015, and March 31, 2016.119 The investigators found a close association between increases in Zika cases and increases in GBS. As Zika infections waned in a country, they found, the incidence of GBS waned as well.119
MODES OF TRANSMISSION
Zika virus is transmitted primarily by the bite of infected Ae. aegypti and Ae. albopictus mosquitoes, which also spread dengue and chikungunya viruses.120 But as more is learned about ZVI, other important modes of transmission have come to light.
Sexual Transmission
In September 2008, an American scientist participating in a mosquito-sampling project in Senegal became ill with ZVI upon his return home to Colorado and infected his wife—the first documented case of sexual transmission of the virus in the U.S. Both the man and his wife noticed signs of hematospermia (red–brown fluid in the man’s ejaculate).121
Five years later, a patient recovering from ZVI on Tahiti Island in French Polynesia sought treatment for bloody sperm. Zika virus was isolated from his semen.122
Next, in February 2016, Texas reported a case of sexually transmitted ZVI. The patient had not recently travelled outside the U.S., but developed symptoms after sexual contact with a traveler. This was the first U.S. Zika case in someone who had not traveled abroad, and the third indication that the virus could be sexually transmitted.123,124
The CDC immediately posted interim guidelines for preventing the sexual transmission of ZVI.123 In its most recent recommendations (updated on September 30), the agency advises that male and female condoms be used from start to finish, every time, during vaginal, anal, and oral sex, and the sharing of sex toys. In addition, dental dams (latex or polyure-thane sheets) may also be used for certain types of oral sex. According to the CDC, testing blood, semen, vaginal fluids, or urine is not recommended to determine how likely a person is to pass Zika virus through sex. Because Zika virus can remain in semen longer than blood, someone might have a negative blood test but a positive semen test.126
Another important discovery took place in February, when a man in England was found to have viable Zika virus in his semen two months after he was infected, suggesting that the virus may linger in semen long after ZVI symptoms fade.127 By that time, the CDC was investigating 14 new reports of possible sexual transmission of Zika virus in the U.S. The agency announced that sexual transmission of the virus might be “more likely” than previously thought.128
In April, the CDC revealed that the case of sexual transmission in Texas involved two men, adding another dimension to the Zika threat: The virus can be spread through unprotected anal sex.129
More surprising news came out of New York City in July: Health officials reported the first documented case of the sexual transmission of Zika virus from a woman to her male sex partner. Until then, all reported cases of sexually transmitted ZVI had been spread from men to their partners.130,131 The following month, an asymptomatic man in Maryland infected his female partner with Zika virus after returning from the Dominican Republic, demonstrating that the virus may be spread from a man even if he has no symptoms of ZVI.132 In August, Yale University investigators found that the virus could persist in the vagina of mice for days after infection—suggesting that Zika may replicate more readily in the female reproductive tract than at other sites of infection.133
Blood Products
In February, the CDC recommended the deferral of individuals from donating blood if they had been to areas with active Zika virus transmission; if they potentially had been exposed to the virus; or if they had confirmed ZVI.134 The agency revised its guidelines in August, advising that all donated blood and blood components in the U.S. should be tested for Zika.135
HHS announced that it was funding two pathogen-reduction technologies to help reduce the risk of Zika virus and other infections from being transmitted through the blood supply. The Biomedical Advanced Research and Development Authority, part of HHS, provided initial funding of $30.8 million to Cerus Corporation and $17.5 million to the U.S. division of Japan’s Terumo Corporation. The Food and Drug Administration (FDA) has approved Cerus’ Intercept technology to reduce pathogens in platelets and plasma. The company is conducting a trial to show that its technology can also reduce pathogens in red blood cells. Terumo is developing its own Mirasol system to confirm that it can reduce the risk of infection through platelets.136
Human Cell and Tissue Products
In March, the FDA issued guidelines aimed at preventing Zika virus transmission via human cells, tissues, and cellular and tissue-based products (HCT/Ps). The agency noted that there was a potential risk that Zika virus could be transmitted by HCT/Ps used as part of a medical, surgical, or reproductive procedure. HCT/Ps include products such as corneas, bone, skin, heart valves, hematopoietic stem/progenitor cells, gestational tissues (such as amniotic membrane), and reproductive tissues (such as semen and oocytes).137
According to the FDA’s guidelines, living donors should be considered ineligible if they were diagnosed with ZVI, were in an area with active Zika virus transmission, or had sex with a male with either of those risk factors within the past six months. Donors of umbilical cord blood, placenta, or other gestational tissues should be considered ineligible if they have had any of these risk factors at any point during their pregnancy. Deceased donors should be considered ineligible if they were diagnosed with ZVI in the past six months.137
A Zika Mystery
Before leaving the subject of Zika virus transmission, mention should be made of a peculiar case that occurred in Utah in June. According to the CDC, an elderly man who died after a bout with Zika may have infected a relative who cared for him during his illness. The caregiver had not traveled to an area with active Zika transmission nor had sex with a person who had recently returned from such a place. Moreover, health officials were not aware of mosquitoes in Utah that are capable of transmitting the virus. Experts outside the CDC said the most likely possibility was that the infected relative came into contact with blood, urine, or other bodily fluids while caring for the man, but the infectious mechanism involved remains a mystery. The relative recovered quickly.138,139
DIAGNOSTIC TESTS
Zika virus may be detected in infected individuals either directly (by identifying virus RNA in tissue or bodily fluids) or indirectly (by testing for virus-specific antibodies).140 The body fluids that can be tested include whole blood, serum, EDTA (ethylenediaminetetraacetic acid) plasma, saliva, and urine.141 The results of serological assays may be confounded by cross-reactivity between virus genus members or by geographical overlap with other pathogens. In those situations, pan-genus and syndromic serum panel tests and antigens are needed and confirmatory techniques are required, such as enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay, and virus-neutralizing testing.141
No commercially available tests for ZVI detection have been approved or cleared by the FDA. However, the agency has permitted applications for emergency use authorizations (EUAs). The Zika IgM [Immunoglobulin M] Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA), developed by the CDC, was the first diagnostic test approved for use in the U.S. for the detection of Zika virus on an emergency basis.142,143 The test detects Zika virus indirectly by identifying Zika-specific IgM in blood or cerebrospinal fluid.144 The assay procedure is complex, however, and the results can be difficult to interpret, making the test unsuitable for rapid Zika diagnosis.140
In February, two Houston hospitals said they had developed the first U.S. hospital-based, rapid RNA test for Zika virus. The test, created by pathologists and clinical laboratory scientists at Texas Children’s Hospital and Houston Methodist Hospital, was customized to each hospital’s diagnostic laboratory and provided results within hours. Assays could be performed on blood, amniotic fluid, urine, or spinal fluid. The hospitals said they were able to distinguish ZVI from dengue, West Nile, or chikungunya infections.145
By early March, the WHO estimated that more than 30 in vitro diagnostic assays for Zika were at various stages of development.146
Meanwhile, the FDA pressed ahead with EUAs, granting the designation to a total of 12 Zika diagnostic tests.144 The CDC followed its IgM assay with a test based on real-time reverse transcriptase polymerase chain reaction (rRT-PCR), using dual-labeled probes to detect viral RNA.140 The test, Trioplex rRT-PCR, was awarded an EUA on March 17. It directly identifies ZVI by detecting Zika RNA in human sera, cerebrospinal fluid, urine, and amniotic fluid.144 Two other RT-PCR assays—Zika Virus RNA Qualitative rRT-PCR (Quest Diagnostics) and RealStar Zika Virus RT-PCR Kit (Altona Diagnostics)—won emergency approval in April and May, respectively.144 These and other Zika assays are listed in Table 1.144
Table 1.
Zika Virus Tests Approved by the FDA for Temporary Emergency Use144
| EUA Date (2016) | Test | Developer/Manufacturer | Description |
|---|---|---|---|
| February 26 | Zika MAC-ELISA | CDC | For detection of Zika virus-specific IgM in human sera or cerebrospinal fluid submitted with patient-matched serum specimen |
| March 17 | Trioplex Real-Time RT-PCR Assay | CDC | For detection and differentiation of RNA from Zika virus, dengue virus, and chikungunya virus in human sera or cerebrospinal fluid collected with patient-matched serum specimen; for detection of Zika virus RNA in urine and amniotic fluid, each collected with patient-matched serum specimen |
| April 28 | Zika Virus RNA Qualitative Real-Time RT-PCR Test | Quest Diagnostics | For detection of Zika virus RNA in human serum specimens |
| May 13 | RealStar Zika Virus RT-PCR Kit | Altona Diagnostics | For detection of Zika virus RNA in serum or urine collected with patient-matched serum specimen |
| June 17 | Aptima Zika Virus Assay | Hologic, Inc. | For detection of Zika virus RNA in human serum and plasma specimens |
| July 19 | Zika Virus Real-Time RT-PCR Test | Viracor-IBT Laboratories | For detection of Zika virus RNA in human serum, plasma, or urine collected with patient-matched serum or plasma specimen |
| July 29 | Versant Zika RNA 1.0 Assay (kPCR) Kit | Siemens Healthcare Diagnostics | For detection of Zika virus RNA in human serum, EDTA plasma, and urine collected with patient-matched serum or plasma specimen |
| August 4 | xMAP MultiFLEX Zika RNA Assay | Luminex Corporation | For detection of Zika virus RNA in human serum, plasma, and urine collected with patient-matched serum or plasma specimen |
| August 17 | ZIKV Detect IgM Capture ELISA | InBios International | For detection of Zika virus IgM antibodies in human serum |
| August 26 | LightMix Zika Real-Time RT-PCR Test | Roche Molecular Systems | For detection of Zika virus RNA in human serum and EDTA plasma; Zika virus RNA generally detectable approximately seven days after onset of symptoms |
| September 23 | Sentosa SA ZIKV RT-PCR Test | Vela Diagnostics | For detection of Zika virus in human serum, EDTA plasma, and urine collected with patient-matched serum or EDTA plasma specimen |
| September 28 | Zika Virus Detection by RT-PCR Test | Arup Laboratories | For detection of Zika virus in human serum, EDTA plasma, and urine collected with patient-matched serum or EDTA plasma specimen |
CDC = Centers for Disease Control and Prevention; EDTA = ethylenediaminetetraacetic acid; ELISA = enzyme-linked immunosorbent assay; EUA = emergency use authorization; FDA = Food and Drug Administration; IgM = immunoglobulin M; kPCR = kappa polymerase chain reaction; MAC-ELISA = IgM antibody capture enzyme-linked immunosorbent assay; RT-PCR = reverse transcriptase polymerase chain reaction; ZIKV = Zika virus.
RT-PCR tests that use urine to detect ZVI have an advantage over other assay systems in that Zika virus RNA is unlikely to be detected in serum after the first week of illness in most patients.147,148 Moreover, data have shown that Zika virus stays at higher levels or lasts longer in urine than in blood.149,150
In the spring, internal strife rocked the CDC when one of its leading Zika experts, Robert Lanciotti, PhD, raised concerns about the agency’s decision to recommend the Trioplex test. Dr. Lanciotti was chief of the CDC lab responsible for developing tests to diagnose viral diseases that are transmitted by mosquitoes, ticks, and fleas. According to his complaint, Trioplex was substantially less effective than an assay he had developed in 2007, called Singleplex, and missed nearly 40% of Zika infections. Dr. Lanciotti also said the agency withheld information about testing differences from state and local public health laboratories.151 In May, the CDC demoted Dr. Lanciotti, but he was reinstated months later after filing a whistleblower retaliation claim.151 His complaint prompted a CDC internal investigation. In its report, the agency said it had made improvements to boost the sensitivity of the Trioplex test. The internal investigation also found that the agency had acted reasonably when it withheld conflicting test data from state public health labs.151
THERAPEUTIC DRUGS
No approved treatments are available for ZVI,152 and the FDA is unaware of any Zika therapies in advanced development.153 In searching for potential Zika therapeutics, scientists have two options: to repurpose existing antiviral compounds or to discover potential Zika inhibitors in compound libraries and develop them for clinical use.154
NIAID used its existing antiviral drug-screening program for other flaviviruses, such as dengue, West Nile, yellow fever, and Japanese encephalitis, to create a test that could examine drug compounds for potential antiviral activity against Zika. By late May, more than 60 potential anti-Zika compounds had been tested; 15 of them were found to have moderate to high activity and are undergoing further evaluation. The agency hopes to develop a broad-spectrum antiviral drug that could treat a variety of flaviviruses, including Zika.155
NIAID is also working to screen a library of approved drugs for potential activity against Zika. In addition, NIAID-supported scientists have developed a rodent model for ZVI to evaluate promising antiviral compounds. Further, NIAID is supporting efforts to develop monoclonal antibodies capable of neutralizing Zika virus.155
In August, American and Chinese scientists collaborated on a drug-repurposing screen of more than 6,000 compounds, including approved drugs and clinical trial candidates. Emricasan, a pan-caspase inhibitor, was identified as the most potent anti–cell-death compound. However, while emricasan showed neuroprotective activity, it did not suppress Zika virus replication. The investigators also found that 10 structurally unrelated inhibitors of cyclin-dependent kinases inhibited Zika replication, as did the FDA-approved anthelmintic drug niclosamide (Nicloside, Bayer Schering Pharma). The investigators suggested that a combination product with the ability to both inhibit Zika replication and protect neural cells from damage might offer the best chance at controlling the virus.156
If viable Zika inhibitors are found, the next step will be to determine whether the concentrations required for clinical efficacy can be achieved in humans.154
Should Zika therapeutics be successfully developed, their use will likely be limited to specific patient subgroups, such as congenitally infected infants and long-term carriers. Testing therapeutic products in the primary clinical target—pregnant women—will require extreme caution. Prophylaxis with small-molecule drugs or passive immunization with monoclonal antibodies may provide a more viable approach.146,154
NIAID researchers exposed another impediment to Zika therapeutics when they reported that the virus may be able to “hide” in organs protected from the immune system, such as the testes, eyes, placenta, and fetal brain. These sites are safeguarded from antibodies to prevent the immune system from attacking vital tissues. But if a virus enters these protected sites, it is much harder to fight them off—a serious challenge for potential Zika treatments.157
VACCINES
Numerous organizations are involved in developing Zika vaccines, but most products are in early preclinical studies, indicating that it will be years before they are eligible for marketing approval.140,158 Dr. Fauci, head of NIAID, remarked that “the earliest we’ll have a vaccine, at best if everything works, will be some time in 2018.”159
According to WHO, females of child-bearing age (including preadolescent and adolescent girls 9 years of age or older) and males of reproductive age (9 years of age or older) comprise the target population for Zika vaccines. In emergency situations, vaccination would be prioritized to women of child-bearing age, as this group is considered at high risk because of the causal relationships between prenatal ZVI and microcephaly, other nervous-system malformations, and pregnancy-related complications.160 Mass vaccination of women of child-bearing age may also help prevent sexual transmission of Zika virus from infected men.161
The research community assumes that Zika vaccines can be developed with the same technologies that have been used to create other flavivirus vaccines (i.e., yellow fever, dengue, Japanese encephalitis, and tick-borne encephalitis).146 NIAID, for example, is working on a DNA-based vaccine that uses a strategy similar to that of an investigational flavivirus vaccine for West Nile virus infection. The West Nile vaccine induced an immune response in a phase 1 study.158 In addition, NIAID is developing a live attenuated investigational Zika vaccine building on a similar approach for dengue virus. The dengue vaccine candidate is being evaluated in a phase 3 trial in Brazil.158 NIAID is also investigating a whole-particle inactivated Zika vaccine based on a similar approach used by the Walter Reed Army Institute of Research to develop vaccines against the Japanese encephalitis and dengue viruses.158
In June, Inovio Pharmaceuticals and GeneOne Life Science, Inc., were the first to initiate a human Zika vaccine trial. The open-label, dose-ranging study is evaluating the safety, tolerability, and immunogenicity of GLS-5700 in 40 healthy adults 18 to 65 years of age. GLS-5700 contains a DNA plasmid encoding for premembrane and envelope proteins of the Zika virus. The vaccine is injected intradermally with a proprietary DNA delivery device, which emits a brief low-voltage electronic pulse that induces cell membranes to open, making them more receptive, theoretically, to accepting the vaccine’s genetic material. Inovio expects to report preliminary results before the end of 2016. The study’s estimated completion date is November 2017.162–164
In July, the National Institutes of Health (NIH) announced that vaccination against a single strain of Zika virus should be sufficient to protect against genetically diverse strains of the virus—heartening news for vaccine developers. Scientists took serum samples from people infected by Zika virus strains circulating in South America and mixed them with multiple strains of the virus in the laboratory to see how well serum antibodies neutralized the virus. The results showed that antibodies elicited after infection with Zika virus strains of Asian lineage were able to inhibit both Asian lineage and African lineage strains. The researchers conducted similar experiments using serum samples from mice and found that sera from mice infected with either Asian or African Zika virus strains were equally effective in neutralizing virus strains from either lineage.165
In early August, the NIH initiated a phase 1 study of NIAID’s DNA-based vaccine. At least 80 healthy volunteers ages 18 to 35 years at three U.S. sites were expected to participate. Like Inovio’s vaccine, the NIAID vaccine includes a plasmid that scientists engineered to contain genes that code for proteins of the Zika virus. When the vaccine is injected into the arm muscle, cells read the genes and make Zika virus proteins, which self-assemble into virus-like particles. The body mounts an immune response to these particles, including neutralizing antibodies and T cells. Initial safety and immunogenicity data are expected by January 2017. If the data are favorable, NIAID plans to initiate a phase 2 study in Zika-endemic countries in early 2017.166
Inovio resurfaced in the vaccine race in late August with its second early-stage trial of GLS-5700. The vaccine was administered to 160 healthy adult volunteers in Puerto Rico, where a Zika emergency had been declared. The study’s primary objective is to evaluate the safety, tolerability, and immunogenicity of GLS-5700 administered with Cellectra-3P, Inovio’s proprietary intradermal DNA vaccine delivery device. The study is also looking at differences in Zika infection rates in participants given either vaccine or placebo.162
Table 2 lists other examples of potential Zika vaccines under investigation in the U.S.146,167–174 But developing a vaccine won’t be easy. Some key hurdles include:1,175,176
Table 2.
| Developer | Technology |
|---|---|
| CaroGen Corp. | VLV-based nanoparticle |
| CDC | VLP expressed by DNA plasmid; live recombinant adenovirus |
| GeoVax Labs, Inc. | MVA-VLP technology elicits antibodies and T cells |
| Hawaii Biotech | Recombinant proteins produced from insect cell line, plus Alhydrogel or proprietary adjuvant |
| Inovio/GeneOne Life Science | DNA (electroporation) |
| Kansas State University | DNA |
| NewLink Genetics Corp. | Purified inactivated virus |
| NIH | Zika-targeted mutation, live attenuated (longer-term); DNA; live VSV recombinant |
| Novavax | E protein (nanoparticles) |
| Pharos Biologicals, Inc. | Nanosphere delivery |
| Protein Sciences Corp. | Recombinant variations of Zika virus E protein |
| Replikins, Ltd. | Synthetic replilink peptides |
| Vaxart, Inc. | Recombinant Zika vaccine in room temperature-stable tablets |
| VaxInnate Corp. | TLR technology; vaccine antigens are genetically fused to bacterial protein flagellin |
| Xenetic Biosciences | Combined Zika/dengue vaccine |
CDC = Centers for Disease Control and Prevention; MVA = modified vacinnia Ankara; NIH = National Institutes of Health; TLR = toll-like receptor; VLP = virus-like particle; VLV = virus-like vesicle; VSV = vesicular stomatitis virus.
Scientists know even less about Zika than they did about the Ebola virus.
There is no simple way to assess the right immune response for Zika; an effective vaccine must strike a delicate balance between provoking a response that is strong enough to stop the virus, but not so strong that it makes the patient ill.
In most cases, ZVI is so mild that individuals may be unaware that they carry the virus, and they are unlikely to see the need for immunization.
To protect the developing fetus, protective immunity must be achieved before the time of peak vulnerability— probably during the first and early second trimesters.
Traditionally, vaccine safety and immunogenicity are firmly established in nonpregnant adults before the vaccination of pregnant women is considered. Pregnant women, however, comprise the primary target for Zika vaccines, making vaccine testing problematic.
Developing vaccines is resource-intensive; vaccines are made from biologic agents that require stringent lot-to-lot consistency and stability.
CONCLUSION
2016 will be remembered as the year that Zika came to America. Is the U.S. technologically and financially prepared to beat the wiliest health foe since the human immunodeficiency virus? Or will we eventually see nearly 27,000 cases of locally acquired infections, as in Puerto Rico,58 or nearly 5,000 cases of Zika-associated microcephaly, as in Brazil?177 Congress’ protracted squabble over Zika funding got things off to a rocky start,68–75 and federal cash infusions aren’t expected to reach public health laboratories until early 2017.77 As this article goes to press, cases of locally acquired ZVI remain restricted to Florida, but Ae. aegypti and Ae. albopictus mosquitoes—the vectors for Zika infection—inhabit roughly half of the continental U.S. No one can predict what the future holds, but it’s safe to say that America, like most of the world, faces a difficult and costly struggle over the coming years.
REFERENCES
- 1.Fellner C. Zika virus: anatomy of a global health crisis. P T. 2016;41:242–253. [PMC free article] [PubMed] [Google Scholar]
- 2.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 3.Smithburn KC. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J Immunol. 1952;69:223–234. [PubMed] [Google Scholar]
- 4.Campos S, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil [letter] Emerg Infect Dis. 2015;21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindhauser MK, Allen T, Frank V, et al. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. E-pub: 9 Feb 2016. doi: http://dx.doi.org/10.2471/BLT.16.171082. [DOI] [PMC free article] [PubMed]
- 6.Centers for Disease Control and Prevention. Aedes aegypti, Aedes albopictus. Jan 30, 2012. [Accessed August 18, 2016]. Available at: www.cdc.gov/dengue/resources/30jan2012/comparisondenguevectors.pdf.
- 7.Darwish MA, Hoogstraal H, Roberts TJ, et al. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg. 1983;77:442–445. doi: 10.1016/0035-9203(83)90106-2. [DOI] [PubMed] [Google Scholar]
- 8.Olson JG, Ksiazek TG, Suhandiman, Triwibowo Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75:389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 9.Duffy MR, Chen T-H, Hancock WT, Powers AM. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 10.Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth A, Mercier A, Lepers C, et al. Concurrent outbreaks of dengue, chikungunya, and Zika virus infections: an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro Surveill. 2014;19:20929. doi: 10.2807/1560-7917.es2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 12.Dupont-Rouzeyrol M, O’Connor O, Calvez E, et al. Co-infection with zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis. 2015;21:381–382. doi: 10.3201/eid2102.141553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Society for Infectious Diseases. Zika virus—Brazil: confirmed. May 15, 2015. [Accessed August 15, 2016]. Available at: www.promedmail.org/direct.php?id=3370768.
- 14.World Health Organization. Zika situation report: neurologic syndrome and congenital anomalies. Feb 5, 2016. [Accessed August 15, 2016]. Available at: http://apps.who.int/iris/bitstream/10665/204348/1/zikasitrep_5Feb2016_eng.pdf.
- 15.Brazilian Ministry of Health. Informative Note No.01/2015: COES microcephaly [in Portuguese] Nov 17, 2015. [Accessed August 15, 2016]. Available at: www.saude.rs.gov.br/upload/1448404998_microcefalia_nota_informativa_17nov2015_MINISTERIO%20DA%20SAUDE.pdf.
- 16.Avian Flu Diary. Brazil: MOH updates microcephalic birth numbers. Dec 29, 2015. [Accessed August 15, 2016]. Available at: http://afludiary.blogspot.com/2015/12/brazil-moh-updates-microcephalic-birth.html.
- 17.European Center for Disease Prevention and Control. Communicable Disease Threats Report. Nov 15–21, 2015. [Accessed August 17, 2016]. Week 47. Available at: http://ecdc.europa.eu/en/publications/Publications/communicable-disease-threats-report-21-nov-2015.pdf.
- 18.World Health Organization. Zika virus infection—El Salvador. Nov 27, 2015. [Accessed February 24, 2016]. Available at: www.who.int/csr/don/27-november-2015-zika-el-salvador/en.
- 19.World Health Organization. Zika virus infection—Mexico. Dec 3, 2015. [Accessed August 17, 2016]. Available at: www.who.int/csr/don/03-december-2015-zika-mexico/en.
- 20.World Health Organization. Zika virus infection—Venezuela. Dec 3, 2015. [Accessed August 17, 2016]. Available at: www.who.int/csr/don/03-december-2015-zika-venezuela/en.
- 21.World Health Organization. Zika virus infection—Paraguay. Dec 3, 2015. [Accessed August 17, 2016]. Available at: www.who.int/csr/don/03-december-2015-zika-paraguay/en.
- 22.World Health Organization. Zika virus infection—Honduras. Dec 21, 2015. [Accessed August 17, 2016]. Available at: www.who.int/csr/don/21-december-2015-zika-honduras/en.
- 23.World Health Organization. Zika virus infection—Panama. Dec 22, 2015. [Accessed August 17, 2016]. Available at: www.who.int/csr/don/22-december-2015-zika-panama/en.
- 24.World Health Organization. Zika virus infection—France—French Guiana and Martinique. Jan 8, 2016. [Accessed August 17, 2016]. Available at: www.who.int/csr/don/8-january-2016-zika-france/en.
- 25.Centers for Disease Control and Prevention. First case of Zika virus reported in Puerto Rico. Dec 31, 2015. [Accessed February 25, 2016]. Available at: www.cdc.gov/media/releases/2015/s1231-zika.html.
- 26.University of Florida, Department of Entomology and Nematology. Aedes aegypti. Apr, 2016. [Accessed August 19, 2016]. Available at: http://entnemdept.ufl.edu/creatures/aquatic/aedes_aegypti.htm.
- 27.University of Florida, Department of Entomology and Nematology. Aedes albopictus. Jul, 2014. [Accessed August 19, 2016]. Available at: http://entnemdept.ufl.edu/creatures/aquatic/asian_tiger.htm.
- 28.Reuters. Research indicates another common mosquito may be able to carry Zika. Mar 3, 2016. [Accessed August 19, 2016]. Available at: www.reuters.com/article/us-health-zika-brazil-idUSKCN0W52AW.
- 29.Kansas State University. Culex mosquitoes do not transmit Zika virus, Kansas State University study finds. Sep 22, 2016. [Accessed October 4, 2016]. Available at: www.k-state.edu/media/newsreleases/2016-09/culex92216.html.
- 30.Centers for Disease Control and Prevention. Zika virus: potential range in US. Aug 2, 2016. [Accessed August 19, 2016]. Available at: www.cdc.gov/zika/vector/range.html.
- 31.Reuters. Official map finds Zika-transmitting mosquitoes in much of U.S. Jun 10, 2016. [Accessed August 19, 2016]. Available at: http://news.trust.org/item/20160610203744-li7a1.
- 32.University of Notre Dame. Mosquitoes capable of carrying Zika virus found in Washington, D.C. Jan 26, 2016. [Accessed August 19, 2016]. Available at: http://news.nd.edu/news/64004-mosquitoes-capable-of-carrying-zika-virus-found-in-washington-dc.
- 33.Virginia Department of Health. Virginia resident tests positive for Zika virus after travel in Zika-affected country. Jan 26, 2016. [Accessed August 19, 2016]. Available at: www.vdh.virginia.gov/news/public-relations-contacts/news-releases/2016-statewide-news-releases/virginia-resident-tests-positive-for-zika-virus-after-travel-in-zika-affected-country.
- 34.Arkansas Times. Dept. of Health: Case of Zika virus confirmed in Arkansas. Jan 26, 2016. [Accessed August 19, 2016]. Available at: www.arktimes.com/ArkansasBlog/archives/2016/01/26/dept-of-health-case-of-zika-virus-confirmed-in-arkansas.
- 35.Reuters. First U.S. Zika virus transmission reported, attributed to sex. Feb 3, 2016. [Accessed August 19, 2016]. Available at: www.reuters.com/article/us-health-zika-idUSKCN0VB145.
- 36.Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:120–121. doi: 10.15585/mmwr.mm6505e1. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong P, Hennessey M, Adams M, et al. Travel-associated Zika virus disease cases among U.S. residents—United States, January 2015–February 2016. MMWR Morb Mortal Wkly Rep. 2016;65:286–289. doi: 10.15585/mmwr.mm6511e1. Available at: www.cdc.gov/mmwr/volumes/65/wr/mm6511e1.htm. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Department of Homeland Security. Zika virus: DHS response plan. Jan 11, 2016. [Accessed September 15, 2016]. Available at: www.dhs.gov/news/2016/02/11/zika-virus-dhs-response-plan.
- 39.American Council on Science and Health. Zika is possibly the scariest virus since HIV. Feb 19, 2016. [Accessed September 15, 2016]. Available at: http://acsh.org/news/2016/02/19/zika-maybe-the-scariest-virus-since-hiv.
- 40.Dapena K, Alcantara C, Franco D. Florida particularly at risk. Miami Herald. [Accessed September 16, 2016]. No date. Available at: www.miamiherald.com/news/health-care/article66790817.html.
- 41.Reuters. Florida governor declares health emergency in four counties over Zika. Feb 3, 2016. [Accessed September 16, 2016]. Available at: www.reuters.com/article/us-health-zika-florida-idUSKCN0VC2S9.
- 42.Centers for Disease Control and Prevention. Florida investigation links four recent Zika cases to local mosquito-borne virus transmission. Jul 29, 2016. [Accessed September 16, 2016]. Available at: www.cdc.gov/media/releases/2016/p0729-florida-zika-cases.html.
- 43.Centers for Disease Control and Prevention. Advice for people living in or traveling to South Florida. Sep 1, 2016. [Accessed September 16, 2016]. Available at: www.cdc.gov/zika/intheus/florida-update.html.
- 44.Centers for Disease Control and Prevention. Transcript for CDC telebriefing: Zika virus update—July 29. Jul 29, 2016. [Accessed September 16, 2016]. Available at: www.cdc.gov/media/releases/2016/t0729-zika-update.html.
- 45.Centers for Disease Control and Prevention. Additional area of active Zika transmission identified in Miami Beach. Aug 19, 2016. [Accessed September 16, 2016]. Available at: www.cdc.gov/media/releases/2016/p0819-zika-miami-beach.html.
- 46.Reuters. Health official warns Zika could spread across U.S. Gulf. Aug 21, 2016. [Accessed September 16, 2016]. Available at: www.reuters.com/article/us-health-zika-miami-idUSKCN10W0SM.
- 47.Reuters. Florida announces Zika case hundreds of miles from Miami. Aug 23, 2016. [Accessed September 16, 2016]. Available at: www.reuters.com/article/us-health-zika-florida-idUSKCN10Y1OO.
- 48.Reuters. Florida governor complains U.S. not doing enough to fight Zika. Aug 24, 2016. [Accessed September 16, 2016]. Available at: www.reuters.com/article/us-health-zika-florida-idUSKCN10Z2SF.
- 49.TIME. Texas traveler gets Zika in Florida. Aug 15, 2016. [Accessed September 16, 2016]. Available at: http://time.com/4453371/texas-florida-zika.
- 50.Reuters. Florida to begin aerial spraying of insecticides to control Zika. Aug 3, 2016. [Accessed September 16, 2016]. Available at: www.reuters.com/article/us-health-zika-insecticide-idUSKCN10E06Q.
- 51.Staletovich J. Air attacks against Zika hit and miss in Miami, CDC reports. Miami Herald. Aug 15, 2016. [Accessed September 16, 2016]. Available at: www.miami-herald.com/news/health-care/article95838697.html.
- 52.Reuters. Adult mosquitoes can pass Zika to their offspring: U.S. study. Aug 29, 2016. [Accessed September 16, 2016]. Available at: www.reuters.com/article/us-health-zika-mosquitoes-idUSKCN1142CD.
- 53.Reuters. Three new cases of local Zika transmission in Florida: officials. Aug 30, 2016. [Accessed September 16, 2016]. Available at: www.reuters.com/article/us-health-zika-florida-idUSKCN11605O.
- 54.Chang D. Florida’s Zika undercount hides extent of virus’ spread, experts say. Miami Herald. Sep 10, 2016. [Accessed September 16, 2016]. Available at: www.miamiherald.com/news/health-care/article100939277.html.
- 55.Reuters. Florida expands Zika zone in Miami Beach after five new cases. Sep 17, 2016. [Accessed September 19, 2016]. Available at: www.reuters.com/article/us-health-zika-miamibeach-idUSKCN11N03K.
- 56.Reuters. Florida declares new area of Zika transmission in Miami. Oct 13, 2016. [Accessed October 14, 2016]. Available at: www.reuters.com/article/us-health-zika-florida-idUSKCN12D2X6.
- 57.Reuters. U.S. health officials create color-coded Zika zones in Florida. Oct 19, 2016. [Accessed October 20, 2016]. Available at: www.reuters.com/article/us-health-zika-florida-idUSKCN12J2R3.
- 58.Centers for Disease Control and Prevention. Zika virus: case counts in the U.S. Oct 6, 2016. [Accessed October 11, 2016]. Available at: www.cdc.gov/zika/geo/united-states.html.
- 59.Reuters. Most U.S. efforts to fight Zika virus to be informational: White House. Jan 27, 2016. [Accessed August 22, 2016]. Available at: www.reuters.com/article/us-health-zika-usa-white-house-idUSKCN0V52FG.
- 60.Associated Press. Obama asking Congress for emergency funding to combat Zika. Feb 8, 2016. [Accessed August 22, 2016]. Available at: http://bigstory.ap.org/article/49c5f168ff214eb2b01ff42730789e44/obama-asking-congress-emergency-funding-combat-zika.
- 61.Reuters. White House and states to craft Zika attack plan at summit. Mar 4, 2016. [Accessed August 23, 2016]. Available at: www.reuters.com/article/us-health-zika-whitehouse-idUSKCN0W60XM.
- 62.Centers for Disease Control and Prevention. National Zika summit focused on coordinated U.S. response. Apr 1, 2016. [Accessed September 21, 2016]. Available at: www.cdc.gov/media/releases/2016/p0401-zika-summit.html.
- 63.Joseph A. At Zika summit, officials stress need for funding and action now to avoid crisis later. STAT. Apr 1, 2016. [Accessed September 21, 2016]. Available at: www.statnews.com/2016/04/01/zika-action-plan-summit.
- 64.McNeil DG., Jr Obama administration to transfer Ebola funds to Zika fight. New York Times. Apr 6, 2016. [Accessed September 21, 2016]. Available at: www.nytimes.com/2016/04/07/health/zika-virus-budget-ebola.html?rref=collection%2Ftimestopic%2FEbola&action=click&contentCollection=timestopics®ion=stream&module=stream_unit&version=latest&contentPlacement=8&pgtype=collection.
- 65.U.S. House of Representatives, Committee on Appropriations. Rogers introduces funding bill to fight the Zika virus. May 16, 2016. [Accessed September 21, 2016]. Available at: http://appropriations.house.gov/news/documents-ingle.aspx?DocumentID=394533.
- 66.Reuters. White House opposes House Zika bill, calls funding inadequate. May 17, 2016. [Accessed September 21, 2016]. Available at: www.reuters.com/article/us-usa-zika-white-house-idUSKCN0Y826Z.
- 67.Reuters. House approves $622 million to combat Zika virus. May 18, 2016. [Accessed September 21, 2016]. Available at: www.reuters.com/article/us-health-zika-house-idUSKCN0YA02O.
- 68.Reuters. Senate approves $1.1 billion to fight Zika virus. May 19, 2016. [Accessed September 21, 2016]. Available at: www.reuters.com/article/us-health-zika-congress-idUSKCN0YA28Z.
- 69.Scott D. House passes Zika bill in early morning vote over Democratic opposition. STAT. Jun 22, 2016. [Accessed September 21, 2016]. Available at: www.statnews.com/2016/06/22/congress-reach-1-1-billion-zika-deal.
- 70.Drabold W. Zika funding bill fails as Congress is unable to reach compromise. TIME. Jul 14, 2016. [Accessed October 4, 2016]. Available at: http://time.com/4406114/zika-funding-bill-fails-as-congress-is-unable-to-reach-compromise.
- 71.McKenna M. CDC director: “This is no way to fight an epidemic.”. National Geographic. Jul 19, 2016. [Accessed October 4, 2016]. Available at: http://news.nationalgeographic.com/2016/07/cdc-warns-zika-epidemic-birth-defects.
- 72.Davis JH. With Congress deadlocked, White House diverts funds to fight Zika. New York Times. Aug 11, 2016. [Accessed October 4, 2016]. Available at: www.nytimes.com/2016/08/12/us/politics/with-congress-deadlocked-white-house-diverts-funds-to-fight-zika.html?_r=0.
- 73.Sun LH. Centers for Disease Control will run out of money to fight Zika in U.S. next month. Washington Post. Aug 30, 2016. [Accessed October 4, 2016]. Available at: www.washingtonpost.com/news/to-your-health/wp/2016/08/30/cdc-will-run-out-of-money-to-fight-zika-in-u-s-next-month.
- 74.Huetteman E, Tavernise S. Senate Democrats block Zika bill over Planned Parenthood provisions. New York Times. Sep 6, 2016. [Accessed October 4, 2016]. Available at: www.nytimes.com/2016/09/07/us/politics/zika-senate-congress.html?_r=0.
- 75.Luthra S. Congress finally approves funding to fight Zika—but what does this mean? Kaiser Health News. Sep 29, 2016. [Accessed October 4, 2016]. Available at: http://khn.org/news/congress-finally-approves-funding-to-fight-zika-but-what-does-this-mean.
- 76.Reuters. Zika funding delay hurt effort to fight virus: U.S. health officials. Oct 3, 2016. [Accessed October 4, 2016]. Available at: www.reuters.com/article/us-health-zika-usa-idUSKCN12327R?utm_medium=referral&utm_source=morefromreuters.
- 77.Luthra S. Got Zika? For pregnant women, lab constraints mean it’s often hard to know. Kaiser Health News. Oct 7, 2016. [Accessed October 7, 2016]. Available at: http://khn.org/news/got-zika-for-pregnant-women-lab-constraints-mean-its-often-hard-to-know.
- 78.Reuters. U.S. officials warn Zika ‘scarier’ than initially thought. Apr 11, 2016. [Accessed October 4, 2016]. Available at: www.reuters.com/article/us-health-zika-whitehouse-idUSKCN0X825A.
- 79.Centers for Disease Control and Prevention. CDC concludes Zika causes microcephaly and other birth defects. Apr 13, 2016. [Accessed October 4, 2016]. Available at: www.cdc.gov/media/releases/2016/s0413-zika-microcephaly.html.
- 80.Centers for Disease Control and Prevention. Zika and Guillain-Barré syndrome. Aug 9, 2016. [Accessed October 4, 2016]. Available at: www.cdc.gov/zika/healtheffects/gbs-qa.html.
- 81.World Health Organization. WHO Director-General addresses media after Zika emergency committee. Mar 8, 2016. [Accessed October 4, 2016]. Available at: www.who.int/mediacentre/news/statements/2016/zika-ec/en.
- 82.Pasteur Institute. Neural stem cells infection by Zika virus in the mouse developing cortex. Jul 16, 2016. [Accessed October 4, 2016]. Available at: https://research.pasteur.fr/en/project/neural-stem-cells-infection-by-zika-virus-in-the-mouse-developing-neocortex.
- 83.Rockefeller University. Zika infection may affect adult brain cells, suggesting risk may not be limited to pregnant women. Aug 18, 2016. [Accessed October 4, 2016]. Available at: http://newswire.rockefeller.edu/2016/08/18/zika-infection-may-affect-adult-brain-cells-suggesting-risk-may-not-be-limited-to-pregnant-women.
- 84.World Federation of Neurology. Zika experts: acute virus infection associated with sensory polyneuropathy. Aug 26, 2016. [Accessed October 4, 2016]. Available at: www.wfneurology.org/zika-experts-acute-virus-infection.
- 85.Reuters. Study finds Zika infects neural cells related to skull formation. Sep 29, 2016. [Accessed October 4, 2016]. Available at: www.reuters.com/article/us-health-zika-skull-idUSKCN11Z2CN.
- 86.Bayless NL, Greenberg RS, Swigut T, et al. Zika virus infection induces cranial neural crest cells to produce cytokines at levels detrimental to neurogenesis. Cell Host Microbe. 2016;20(4):423–428. doi: 10.1016/j.chom.2016.09.006. Epub 2016 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention. Key messages: Zika virus disease. Oct 18, 2016. [Accessed October 20, 2016]. Available at: www.cdc.gov/zika/pdfs/zika-key-messages.pdf.
- 88.Rasmussen SA, Jamieson DJ, Honein MA, et al. Zika virus and birth defects: reviewing the evidence for causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 89.Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127:642–648. doi: 10.1097/AOG.0000000000001378. [DOI] [PubMed] [Google Scholar]
- 90.de Noronha L, Zanluca C, Azevedo MLV, et al. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz. 2016;111:287–293. doi: 10.1590/0074-02760160085. Epub 2016 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jurado KA, Simoni MK, Tang Z, et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016 Aug;18:1. doi: 10.1172/jci.insight.88461. (13). pii: e88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.American Congress of Obstetricians and Gynecologists. Practice advisory on Zika virus. Aug 3, 2016. [Accessed October 5, 2016]. Available at: www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-Interim-Guidance-for-Care-of-Obstetric-Patients-During-a-Zika-Virus-Outbreak.
- 93.Reuters. U.S. reports 279 Zika cases in pregnant women, Obama pushes Congress on funds. May 20, 2016. [Accessed October 5, 2016]. Available at: www.reuters.com/article/us-health-zika-usa-idUSKCN0YB1QN.
- 94.Centers for Disease Control and Prevention. Pregnant women with any laboratory evidence of possible Zika virus infection in the United States and territories, 2016. Oct 6, 2016. [Accessed October 12, 2016]. Available at: www.cdc.gov/zika/geo/pregwomen-uscases.html.
- 95.Opitz JM, Holt MC. Microcephaly: general considerations and aids to nosology. J Craniofac Genet Dev Biol. 1990;10:175–204. [PubMed] [Google Scholar]
- 96.Woods CG. Human microcephaly. Curr Opin Neurobiol. 2004;14:112–117. doi: 10.1016/j.conb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Centers for Disease Control and Prevention. Facts about micro-cephaly. Jul 25, 2016. [Accessed October 5, 2016]. Available at: www.cdc.gov/ncbddd/birth-defects/microcephaly.html.
- 98.Brazilian Ministry of Health. Informative Note No.01/2015: COES microcephaly [in Portuguese] Nov 17, 2015. [Accessed October 5, 2016]. Available at: www.saude.rs.gov.br/upload/1448404998_microcefalia_nota_informativa_17nov2015_MINISTERIO%20DA%20SAUDE.pdf.
- 99.European Center for Disease Prevention and Control. Microcephaly in Brazil potentially linked to the Zika virus epidemic, ECDC assesses the risk. Nov 25, 2015. [Accessed October 5, 2016]. Available at: http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1329&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc.europa.eu%2Fen%2FPages%2Fhome.aspx.
- 100.Pan American Health Organization, World Health Organization. Epidemiological alert: increase of microcephaly in the northeast of Brazil. Nov 17, 2015. [Accessed October 5, 2016]. Available at: www.paho.org/hq/index.php?option=com_docman&task=doc_view&Ite-mid=270&gid=32285=en.
- 101.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 102.Science Daily. New doubts on Zika as cause of micro-cephaly. Jun 24, 2016. [Accessed October 5, 2016]. Available at: www.sciencedaily.com/releases/2016/06/160624150813.htm.
- 103.World Health Organization. WHO Director-General summarizes the outcome of the emergency committee regarding clusters of micro-cephaly and Guillain-Barré syndrome. Feb 1, 2016. [Accessed October 5, 2016]. Available at: www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en.
- 104.Pan American Health Organization, World Health Organization. Epidemiological update: neurological syndrome, congenital anomalies, and Zika virus infection. Jan 17, 2016. [Accessed October 5, 2016]. Available at: www.paho.org/hq/indexphp?option=com_docman&task=doc_view&Itemid=270&gid=32879&lang=en.
- 105.Greenwood M. Zika virus linked to stillbirth, other symptoms in Brazil. Yale News. Feb 25, 2016. [Accessed October 5, 2016]. Available at: http://news.yale.edu/2016/02/25/zika-virus-linked-stillbirth-other-symptoms-brazil.
- 106.Samo M, Sacramento GA, Khouri R, et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly, and fetal demise. PloS Negl Trop Dis. Feb 25, 2016. [Accessed October 5, 2016]. Available at: http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed]
- 107.Reuters. Health agency reports U.S. babies with Zika-related birth defects. Jun 16, 2016. [Accessed October 5, 2016]. Available at: www.reuters.com/article/us-health-zika-usa-idUSKCN0Z22DL.
- 108.Keck School of Medicine of USC. Zika proteins responsible for microcephaly identified. Aug 11, 2016. [Accessed October 5, 2016]. Available at: http://keck.usc.edu/zika-proteins-responsible-for-microcephaly-identified.
- 109.Beth Israel Deaconess Hospital. Special report documents Zika virus’ impact on the fetal brain. Aug 24, 2016. [Accessed October 5, 2016]. Available at: www.bidmc.org/News/PRLandingPage/2016/August/Levine-Zika-RadiologyStudy.aspx.
- 110.Leal MC, Muniz LF, Ferreira TSA, et al. Hearing loss in infants with microcephaly and evidence of congenital Zika virus infection—Brazil, November 2015–May 2016. MMWR Morb Mortal Wkly Rep. 2016;65:917–919. doi: 10.15585/mmwr.mm6534e3. Available at: www.cdc.gov/mmwr/volumes/65/wr/mm6534e3.htm. Accessed October 5, 2016. [DOI] [PubMed] [Google Scholar]
- 111.Phippen JW. The first-known Zika infant death in the U.S. The Atlantic. Aug 9, 2016. [Accessed October 5, 2016]. Available at: www.theatlantic.com/news/archive/2016/08/texas-zika-infant-death/495059.
- 112.Del Carpio-Orantes L. Zika, a neurotropic virus? [in Spanish] Rev Med Inst Mex Seguro Soc. 2016;54:540–543. [PubMed] [Google Scholar]
- 113.Van den Berg B, Walgaard C, Drenthen J, et al. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 114.National Institute of Neurological Disorders and Stroke, National Institutes of Health. NINDS Guillain-Barré information page. Jun 1, 2016. [Accessed October 7, 2016]. Available at: www.ninds.nih.gov/disorders/gbs/gbs.htm.
- 115.Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 116.Wielanek AC, Monredon JD, Amrani ME, et al. Guillain-Barré syndrome complicating a Chikungunya virus infection. Neurology. 2007;69:2105–2107. doi: 10.1212/01.wnl.0000277267.07220.88. [DOI] [PubMed] [Google Scholar]
- 117.World Health Organization. Guillain-Barré syndrome—United States of America. Mar 21, 2016. [Accessed October 7, 2016]. Available at: www.who.int/csr/don/21-march-2016-gbs-usa/en.
- 118.Dirlikov M, Major CG, Mayshack M, et al. Guillain-Barré syndrome during ongoing Zika virus transmission—Puerto Rico, January 1–July 31, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:910–914. doi: 10.15585/mmwr.mm6534e1. Available at: www.cdc.gov/mmwr/volumes/65/wr/mm6534e1.htm. Accessed October 7, 2016. [DOI] [PubMed] [Google Scholar]
- 119.Reuters. Study finds strong link between Zika and Guillain-Barré. Aug 31, 2016. [Accessed October 7, 2016]. Available at: www.reuters.com/article/us-health-zika-guillainbarre-idUSKCN1162X8.
- 120.Centers for Disease Control and Prevention. Zika virus: transmission and risks. Aug 27, 2016. [Accessed October 7, 2016]. Available at: www.cdc.gov/zika/transmission.
- 121.Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable nonvector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Musso D, Roche C, Robin E, et al. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. Feb 2, 2016. [Accessed October 5, 2016]. Available at: www.dallascounty.org/department/hhs/press/documents/PR2-2-16DCHHSReportsFirstCaseofZika-VirusThroughSexualTransmission.pdf.
- 124.Reuters. First U.S. Zika virus transmission reported, attributed to sex. Feb 3, 2016. [Accessed October 5, 2016]. Available at: www.reuters.com/article/us-health-zika-idUSKCN0VB145.
- 125.Centers for Disease Control and Prevention. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:120–121. doi: 10.15585/mmwr.mm6505e1. Available at: www.cdc.gov/mmwr/volumes/65/wr/mm6505e1.htm. Accessed October 5, 2016. [DOI] [PubMed] [Google Scholar]
- 126.Centers for Disease Control and Prevention. Zika and sexual transmission. Sep 30, 2016. [Accessed October 5, 2016]. Available at: www.cdc.gov/zika/transmission/sexual-transmission.html.
- 127.Reuters. Zika may linger in semen long after symptoms fade: doctors’ report. Feb 12, 2016. [Accessed October 5, 2016]. Available at: www.reuters.com/article/us-health-zika-semen-idUSKCN0VL1N5.
- 128.Centers for Disease Control and Prevention. CDC encourages following guidance to prevent sexual transmission of Zika virus. Feb 23, 2016. [Accessed October 5, 2016]. Available at: www.cdc.gov/media/releases/2016/s0223-zika-guidance.html.
- 129.Branswell H. Sexual transmission of Zika seen between gay men for first time. STAT. Apr 14, 2016. [Accessed October 5, 2016]. Available at: www.statnews.com/2016/04/14/zika-men-sexual-transmission/?trendmd-shared=0.
- 130.Centers for Disease Control and Prevention. First female-to-male sexual transmission of Zika virus infection reported in New York City. Jul 15, 2016. [Accessed October 5, 2016]. Available at: www.cdc.gov/media/releases/2016/s0715-zika-female-to-male.html.
- 131.Santora M. Twist in Zika outbreak: New York case shows women can spread it to men. New York Times. Jul 15, 2016. [Accessed October 5, 2016]. Available at: www.nytimes.com/2016/07/16/nyregion/zika-virus-female-to-male-sexual-transmission.html?_r=0.
- 132.Reuters. Zika likely to spread sexually from asymptomatic man to woman: study. Aug 26, 2016. [Accessed October 5, 2016]. Available at: www.reuters.com/article/us-health-zika-research-idUSKCN111224.
- 133.Kashef Z. Zika virus may persist in the vagina days after infection. Yales News. Aug 25, 2016. [Accessed October 5, 2016]. Available at: http://news.yale.edu/2016/08/25/zika-virus-may-persist-vagina-days-after-infection.
- 134.Food and Drug Administration. FDA issues recommendations to reduce the risk for Zika virus blood transmission in the United States. Feb 16, 2016. [Accessed October 5, 2016]. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm486359.htm.
- 135.Food and Drug Administration. FDA advises testing for Zika virus in all donated blood and blood components in the U.S. Aug 26, 2016. [Accessed October 5, 2016]. Available at: www.fda.gov/NewsEvents/Newsroom/Pres-sAnnouncements/ucm518218.htm.
- 136.Reuters. U.S. to help fund technology to eliminate Zika in blood supply. Jun 20, 2016. [Accessed October 5, 2016]. Available at: www.reuters.com/article/us-health-zika-cerus-corp-idUSKCN0Z61Y3.
- 137.Food and Drug Administration. FDA issues recommendations to reduce the risk of Zika virus transmission by human cell and tissue products. Mar 1, 2016. [Accessed October 5, 2016]. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm488612.htm.
- 138.Reuters. Zika mystery widens as Utah caregiver contracts virus. Jul 18, 2016. [Accessed October 5, 2016]. Available at: www.reuters.com/article/us-health-zika-infection-idUSKCN0ZY22S.
- 139.Maron DF. Zika mystery case raises questions about new transmission route. Scientific American. Jul 18, 2016. [Accessed October 5, 2016]. Available at: www.scientificamerican.com/article/zika-mystery-case-raises-questions-about-new-transmission-route.
- 140.Junker M. The Zika virus: an update on the outbreak and the fight against it. GlobalData. Jun, 2016. [Accessed September 12, 2016]. Available at: https://healthcare.glo-baldata.com/resources/white-papers/the-zika-virus-an-update-on-the-outbreak-and-the-fight-against-it.
- 141.Charrel RN, Leparc-Goffart I, Pas S, et al. Background review for diagnostic test development for Zika virus detection. Bull World Health Organ. 2016;94:574D–584D. doi: 10.2471/BLT.16.171207. Available at: www.who.int/bulletin/volumes/94/8/16-171207/en. Accessed October 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Centers for Disease Control and Prevention. New CDC laboratory test for Zika virus authorized for emergency use by FDA. Feb 26, 2016. [Accessed October 5, 2016]. Available at: www.cdc.gov/media/releases/2016/s0226-laboratory-test-for-zika-virus.html.
- 143.Food and Drug Administration. Zika MAC-ELISA authorization letter. Jun 29, 2016. [Accessed October 5, 2016]. Available at: www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM488040.pdf.
- 144.Food and Drug Administration. Emergency use authorizations. Oct 11, 2016. [Accessed October 11, 2016]. Available at: www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm.
- 145.Texas Children’s Hospital. First rapid detection Zika test now available through collaboration with Texas Children’s Hospital and Houston Methodist Hospital. Feb 23, 2016. [Accessed October 7, 2016]. Available at: www.texaschildrens.org/about-us/news/releases/first-rapid-detection-zika-test-now-available-through-collaboration-texas-children%E2%80%99s-hospital-and.
- 146.World Health Organization. Current Zika Product Pipeline. Mar 3, 2016. [Accessed September 12, 2016]. Available at: www.who.int/csr/research-and-development/zika-rd-pipeline.pdf.
- 147.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bingham AM, Cone M, Mock V, et al. Comparison of Zika virus testing in serum, urine, and saliva specimens from travel-associated Zika virus disease cases—Florida, 2016. [Accessed October 7, 2016];MMWR Morb Mortal Wkly Rep. 2016 65(18) doi: 10.15585/mmwr.mm6518e2. Epub May 10, 2016. Available at: www.cdc.gov/mmwr/volumes/65/wr/mm6518e2.htm. [DOI] [PubMed] [Google Scholar]
- 149.de M, Campos R, Cirne-Santos C, et al. Prolonged detection of Zika virus RNA in urine samples during the ongoing Zika virus epidemic in Brazil. J Clin Virol. 2016;77:69–70. doi: 10.1016/j.jcv.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 150.Gourinat AC, O’Connor O, Calvez E, et al. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun LH. CDC whistleblower claims agency has been using wrong Zika test. Washington Post. Sep 27, 2016. [Accessed September 28, 2016]. Available at: www.washingtonpost.com/news/to-your-health/wp/2016/09/27/cdc-whistleblower-claims-agency-has-been-using-wrong-zika-test.
- 152.Centers for Disease Control and Prevention. Zika virus: treatment. Jun 28, 2016. [Accessed October 7, 2016]. Available at: www.cdc.gov/zika/symptoms/treatment.html.
- 153.Food and Drug Administration. Zika virus response updates from FDA. Sep 9, 2016. [Accessed September 22, 2016]. Available at: www.fda.gov/%20Emergen-cyPreparedness/Counterterrorism/MedicalCountermeasures/MCMIssues/ucm485199.htm.
- 154.Shan S, Xie X, Barrett ADT, et al. Zika virus: diagnosis, therapeutics, and vaccine. ACS Infect Dis. 2016;2:170–172. doi: 10.1021/acsinfecdis.6b00030. [DOI] [PubMed] [Google Scholar]
- 155.National Institute of Allergy and Infectious Diseases, National Institutes of Health. Zika virus treatment. May 24, 2016. [Accessed September 22, 2016]. Available at: www.niaid.nih.gov/diseases-conditions/zika-treatment.
- 156.Xu M, Lee EM, Wen Z, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–1107. doi: 10.1038/nm.4184. Epub 2016 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reuters. Corrected—Zika virus may hide in organs protected from the immune system. Feb 16, 2016. [Accessed February 26, 2016]. Available at: www.reuters.com/article/health-zika-immune-idUSL2N15V1C9.
- 158.National Institute of Allergy and Infectious Diseases, National Institutes of Health. Zika virus vaccines. Aug 18, 2016. [Accessed October 11, 2016]. Available at: www.niaid.nih.gov/diseases-conditions/zika-vaccines.
- 159.PR Newswire. Broad-spectrum antiviral drug HSRx 431 tests effective against Zika virus. Sep 6, 2016. [Accessed October 11, 2016]. Available at: www.prnewswire.com/news-releases/broad-spectrum-antiviral-drug-hsrx-431-tests-effective-against-zika-virus-300322646.html.
- 160.World Health Organization. WHO Zika virus vaccine target product profile: vaccine to protect against congenital Zika virus syndrome for use during an emergency. Jul, 2016. [Accessed October 11, 2016]. Available at: www.who.int/csr/research-and-development/who_zika_vaccine_tpp.pdf.
- 161.D’Ortenzio E, Matheron S, Yazdanpanah Y, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. Epub 2016 Apr 13. [DOI] [PubMed] [Google Scholar]
- 162.Inovio Pharmaceuticals. Inovio launches Zika vaccine trial in midst of Puerto Rico epidemic to explore early signals of vaccine efficacy. Aug 29, 2016. [Accessed October 11, 2016]. Available at: http://ir.inovio.com/news/news-releases/news-releases-details/2016/Inovio-Launches-Zika-Vaccine-Trial-in-Midst-of-Puerto-Rico-Epidemic-to-Explore-Early-Signals-of-Vaccine-Efficacy/default.aspx.
- 163.National Institutes of Health. Study of GLS-5700 in healthy volunteers. Aug 29, 2016. [Accessed October 11, 2016]. Available at: https://clinicaltrials.gov/ct2/show/NCT02809443.
- 164.Reuters. Zika vaccine race spurred by crisis and profit potential. Oct 4, 2016. [Accessed October 4, 2016]. Available at: www.reuters.com/article/us-health-zika-vaccines-analysis-idUSKCN12409V.
- 165.National Institute of Allergy and Infectious Diseases, National Institutes of Health. Zika infection is caused by one virus serotype, NIH study finds. Jul 29, 2016. [Accessed October 4, 2016]. Available at: www.niaid.nih.gov/news-events/zika-infection-caused-one-virus-serotype-nih-study-finds.
- 166.National Institutes of Health. NIH begins testing investigational Zika vaccine in humans. Aug 3, 2016. [Accessed October 11, 2016]. Available at: www.nih.gov/news-events/news-releases/nih-begins-testing-investigational-zika-vaccine-humans.
- 167.GeoVax Labs Inc. GeoVax discusses Zika vaccine development at American Society for Virology’s 35th annual meeting. Jun 21, 2016. [Accessed October 11, 2016]. Available at: www.geovax.com/news-events/entry/2016/06/21/geovax-discusses-zika-vaccine-development-at-american-society-for-virology-s-35th-annual-meeting.html.
- 168.Pipeline Review. Xenetic Biosciences announces collaboration with Excivion Ltd., U.K., to develop a novel combined Zika and dengue vaccine. Aug 18, 2016. [Accessed October 11, 2016]. Available at: www.pipelinereview.com/index.php/2016081862101/Vaccines/Xenetic-Biosciences-Announces-Collaboration-with-Excivion-Ltd.-UK-to-Develop-a-Novel-Combined-Zika-and-Dengue-Vaccine.html.
- 169.Protein Sciences Corporation. Vaccines. 2016. [Accessed October 11, 2016]. Available at: www.proteinsciences.com/VAC.htm.
- 170.Vaxart. Vaxart begins preclinical testing of an oral Zika virus vaccine. Mar 3, 2016. [Accessed October 11, 2016]. Available at: www.vaxart.com/NRfiles/VaxartZikaTabletVaccine030316.pdf.
- 171.Pharos Biologicals. Pharos: a completely unique approach to DNA vaccines. 2016. [Accessed October 12, 2016]. Available at: www.pharosbiologicals.com/pharos.html.
- 172.VaxInnate Corporation. Message from the CEO. 2015. [Accessed October 12, 2016]. Available at: http://vaxinnate.com/about-us/message-from-the-ceo.
- 173.CaroGen Corporation. Technology. 2013. [Accessed October 12, 2016]. Available at: www.caro-gencorp.com/technology.html.
- 174.Kansas State University. Kansas State University contributes to potential Zika virus vaccine development. Sep 29, 2016. [Accessed October 12, 2016]. Available at: www.k-state.edu/media/newsreleases/2016-09/zika92916.html.
- 175.Reuters. Scientists’ path to usable Zika vaccine strewn with hurdles. Feb 3, 2016. [Accessed October 4, 2016]. Available at: www.reuters.com/article/us-health-zika-vaccine-idUSKCN0VB21X.
- 176.Marston HD, Lurie N, Borio LL, Fauci AS. Considerations for developing a Zika virus vaccine. N Engl J Med. 2016;375:1209–1212. doi: 10.1056/NEJMp1607762. [DOI] [PubMed] [Google Scholar]
- 177.Reuters. Brazil says Zika-linked microcephaly cases stable at 4,908. Apr 26, 2016. [Accessed October 24, 2016]. Available at: www.reuters.com/article/us-health-zika-brazil-idUSKCN0XN2NP.