Abstract
Background
The goal of the fMRI experiment was to explore the involvement of central auditory structures in pathomechanisms of a behaviorally manifested auditory temporary threshold shift in humans.
Material/Methods
The material included 18 healthy volunteers with normal hearing. Subjects in the exposure group were presented with 15 min of binaural acoustic overstimulation of narrowband noise (3 kHz central frequency) at 95 dB(A). The control group was not exposed to noise but instead relaxed in silence. Auditory fMRI was performed in 1 session before and 3 sessions after acoustic overstimulation and involved 3.5–4.5 kHz sweeps.
Results
The outcomes of the study indicate a possible effect of acoustic overstimulation on central processing, with decreased brain responses to auditory stimulation up to 20 min after exposure to noise. The effect can be seen already in the primary auditory cortex. Decreased BOLD signal change can be due to increased excitation thresholds and/or increased spontaneous activity of auditory neurons throughout the auditory system.
Conclusions
The trial shows that fMRI can be a valuable tool in acoustic overstimulation studies but has to be used with caution and considered complimentary to audiological measures. Further methodological improvements are needed to distinguish the effects of TTS and neuronal habituation to repetitive stimulation.
MeSH Keywords: Auditory Cortex; Auditory Fatigue; Auditory Threshold; Habituation, Psychophysiologic; Magnetic Resonance Imaging
Background
Temporary threshold shift (TTS) is a reversible impairment of hearing acuity following noise exposure (or chemical/mechanical manipulation) [1]. It is most frequently manifested with an increase of pure tone audiometric thresholds (PTA) [2–4]. If the measured TTS is above 40–50dB, hearing deterioration can become permanent (permanent threshold shift, PTS) and, therefore, this effect has been studied as a predictor of the ever more prevalent occupational hearing loss [5]. Other authors have indicated TTS as a correlate of tinnitus and hyperacousics, both in animals [3,6] and in humans (see Knipper group’s work for a review [7]). Both animal and scarce human models show that exposure to a sufficient duration and intensity of noise leads to structural and functional changes in the peripheral and central auditory system. Preclinical studies have demonstrated that structural effects involve mechanical damage of hair cells, accompanied by decreased basal dendritic length and spine density [8,9]. Considering functional changes in the peripheral system, temporally decreased distortion product otoacoustic emission levels (DPOAEs), reflecting summed activity of outer hair cells (OHCs), have been reported after acoustic overstimulation. It has been shown that noise preferentially damages OHCs [10,11]. In case of temporal hearing loses, DPOAEs decrease linearly as a function of duration of exposure, with permanent effects developing exponentially [12]. Furthermore, noise exposure leads to enhanced spontaneous neural responses, as measured with single-cell recording, in both the peripheral (the cochlear nucleus, CN), and the central auditory system, in the cochlear nucleus (CN), the inferior colliculus (IC), the medial geniculate body (MGB), and the primary auditory cortex (PAC) [2,13–18]. In addition, numerous findings have been provided concerning the effect of noise exposure on induced central auditory responses in animals. After various durations (hours-days) of high-frequency noise (2–12 kHz) at frequencies exceeding the frequency of noise used for overstimulation, authors showed elevated hearing thresholds [9,16,18], increased [18] or decreased [16] responses in the auditory cortex, and disorganized tonotopy of the primary auditory cortex [9]. Animal studies measuring auditory brainstem responses (ABR) have shown temporary elevated thresholds, as well as decreased amplitudes of wave I and wave IV following suprathreshold auditory stimulation [2,4,19–21]. In one of the very few functional magnetic resonance (fMRI) studies in animals, Lau and colleagues found that a 2-month presentation of 30-kHz broadband noise (65 dB SPL) induced a decrease of BOLD signal amplitude in the contralateral medial geniculate body (MGB), the thalamic part of the central auditory pathway, and in bilateral primary auditory cortices (PAC) after a presentation of sound pulsed at 10 Hz [22]. These results show involvement of these neuronal structures in adaptation to long-term moderate noise exposures and possibly some damage to the peripheral auditory system. The authors reported that the outcomes were accounted for in terms of a compensatory central mechanism of synaptic gain reduction. In scarce human studies, the extent of TTS has been evaluated by comparing auditory thresholds (pure tone audiometry, PTA) before and either immediately or 1–2 min after acoustic overstimulation, with final measurements performed after 30–120 min [23–25]. The largest TTS effect has been reported for continuous sounds at 4 kHz ±½ octave (audiometric notch at 3–6 kHz) and intensity exceeding 70–80 dB SPL [5,26–30]. OAEs have been used to study auditory function after an acute acoustic trauma, as well as chronic noise exposure in humans. This despite some authors demonstrating insufficient sensitivity of the method as regards exposure to loud music [31]. Interestingly, reduction in TTS following noise exposure has been usually manifested with suppressed otoacoustic emissions elicited by stimulation of the contralateral ear, which suggests involvement of the descending olivocochlear bundle in protection and susceptibility to noise [32,33]. In addition, low-level distortion product and transiently-evoked OAEs decreases, especially in high-frequency ranges, have been reported in patients with chronic tinnitus with or without hearing loss [34]. As is the case of PTA and for OAEs level shifts, the most significant effects have been found for high frequencies and in the range from half to an octave above the exposure noise band [35]. The involvement of central auditory structures following acoustic overstimulation has not yet been systematically examined in human. Nevertheless, some evidence has been provided in ABR studies. Kochanek et al. reported a shift of latency (and a decrease in amplitude) of wave V in response to 4 kHz presented at high levels following overstimulation with noise centered at 3 kHz (1/8 band) [36]. The latency effect for quiet suprathreshold stimulation was apparent up to 50 min after overstimulation, although TTS was no longer detectable with pure tone audiometry. This lack of a linear relationship between PTA and ABR measurements has been reported in other similar investigations [28,37–39]. A recent ABR study by Stamper et al. has revealed an unaltered suprathreshold amplitude of wave V and reduced amplitude of wave 1 in participants with higher levels of noise exposure background [28]. Interestingly, Harkrider and Tampas found larger amplitudes of ABR wave V in people with higher noise acceptance scores, which they explained as either enhanced excitation in afferent auditory pathways or limited inhibition in the efferent nerve fibers following differentiation [40]. In line with animal studies, there appears to be interplay between peripheral and central auditory structures following acoustic overstimulation in human. Nevertheless, to the best of our knowledge there have been no studies published investigating the effects of exposure to acoustic overstimulation in the human auditory cortex directly, except for 1 preliminary fMRI trial. Activation in bilateral auditory cortices has shown various temporal dynamics at 4 measurement intervals after noise exposure [41]. The goal of the present study was to further examine the involvement of central auditory structures in pathomechanisms of behaviorally manifested auditory TTS in humans. We hypothesized that post-overstimulation auditory fMRI sessions would reveal decreased BOLD (blood oxygen level-dependent) signal change in the auditory cortex, converging with the reported increased thresholds in TTS studies using PTA and ABR.
Material and Methods
Participants
There were 18 subjects recruited for the study. Ten volunteers, aged 22–39 (M=27.2±5.5) years, were enrolled to the exposure group. Eight subjects (22–41, M=29.0±5.9) participated as the control group. All subjects were included in the study only after a comprehensive assessment by an ENT specialist. All were right-handed, had normal hearing (HL<15 dB for 0.125–8 kHz), and no history of neurological, psychiatric, and/or auditory conditions. Participants signed an informed consent form to take part in the experiment and were paid for participation. They were informed that in certain individuals the TTS effect (and the potentially accompanying tinnitus) might be present for longer than in others. Should the effect remain longer than 24 hours, treatment would be provided (e.g., cardiovascular medications). The study was approved by the Ethics Committee of the Institute of Physiology and Pathology of Hearing and conformed to the Declaration of Helsinki.
Pure tone audiometry
Pure tone audiometry (PTA) measurements in all subjects (N=18) were performed before the fMRI study (24 hours and immediately before), in a sound-proof booth, with the use of a Madsen Itera audiometer (URL:http://www.otometrics.com) and ER3A earphones by Etymotic (URL:http://www.etymotic.com/). PTA (0.125–8 kHz) with 5-dB increments and a presentation rate of 2.5 Hz (descending). In addition, the day before fMRI, auditory thresholds were measured for 4 kHz with 1 dB increments before and after a single binaural exposure to a continuous narrowband noise with a central frequency of 3 kHz (bandwidth at half-maximum=2.5–3.3 kHz), 15 min in duration, at 95 dB(A) (hereafter: acoustic overstimulation). The exact parameters were in accordance with international reference levels of narrowband masking noise (ISO 389–4: 1994). A narrowband noise was selected, among other factors, because a broadband noise would comprise frequency ranges below 2 kHz, which have been shown to induce the acoustic reflex limiting the effect of acoustic overstimulation [25]. The measurement time points were: 1 min post-overstimulation, 2 min post-overstimulation, 10 min post-overstimulation, 20 min post-overstimulation, and 30 min post-overstimulation. Auditory thresholds were determined for 1 pseudo-randomly selected ear (number of left and right ears was equal) [23] and were found to return to normal after noise exposure. At 2 time points after the f/MRI study (immediately after and 24 hours later), all participants had standard tonal audiometry measurements repeated for 0.125–8 kHz (with no acoustic overstimulation). Their hearing acuity was equal to that measured before their participation in the study.
f/MRI
Data acquisition
The f/MRI study was performed at the Bioimaging Research Center of the World Hearing Center of the Institute of Physiology and Pathology of Hearing in Kajetany, Warsaw, Poland, on a 3T Siemens Trio TIM scanner, equipped with a 12-channel matrix head coil. Nordic Neuro Lab (NNL) MRI-compatible electrostatic headphones (URL:www.nordicneurolab.com) were used to deliver the acoustic stimulation. Whole-brain functional imaging was performed with a T2* SingleShot EPI, with the following parameters: 46 axial slices with isotropic resolution 3×3×3 mm, TR=10000 ms (sparse paradigm with 2.5s data acquisition), TE=30ms, FA=90°, FOV=192×192mm, matrix=64×64, pixel bandwidth=2232 Hz/pix, iPAT=2, 60 volumes in a series, TA=10 min 32 s (including 3 non-registered dummy scans required for magnetization stabilization). A 3D high-resolution T1 MPR sequence was used for anatomical reference. The parameters of the sequence were: 208 sagittal slices with isotropic resolution 0.9×0.9×0.9 mm, TR=1900 ms, TE=2.21 ms, TI=900 ms, FA=9, FOV=26×28.8 cm, matrix=290×320, pixel bandwidth= 200 Hz/pix, iPAT=2, TA=5 min. Standard T1 and T2 MR sequences were applied to exclude subjects with brain pathology.
fMRI paradigm
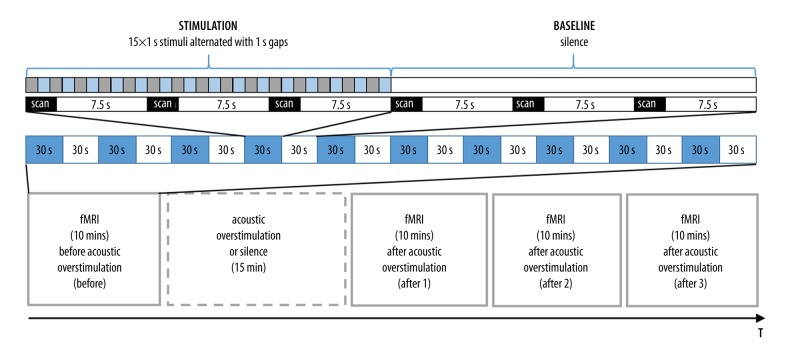
Although sustained increases in spontaneous activity of cortical neurons have been most consistently documented in animal TTS studies, fMRI cannot be used to evaluate this directly [42,43]. Therefore, to test central correlates of TTS, auditory stimulation before and after acoustic stimulation was applied and the outcomes compared. FMRI data were collected in 4×10 min runs: a) prior to acoustic overstimulation/silence (Before), b) starting immediately after overstimulation/silence was completed (at T0; After 1), c) starting 10 mins later (at T0+10 mins; After 2), d) starting next 10 mins later (at T0+20 mins; After 3). A standard box-car paradigm was used with blocks of sound (30 s) intertwined with blocks of silence (30 s). Within 1 sound block, 15×1s 3.5–4.5 kHz sweeps (hereafter: ‘4 kHz’) with a 50 ms rising and falling time were presented at 85 dB(A), alternated with 15×1s silent gaps (Figure 1). Sound parameters were based on the existing findings in animals and humans demonstrating largest TTS effects for frequencies exceeding the frequency of the overstimulation by half an octave [26]. During one sound block, 3 brain volumes were collected. During each time of repetition (TR), which was 10s, a 2.5-s image acquisition was preceded by 7.5 s of scanner silence (sparse data acquisition) (Figure 1). This long TR was selected to limit the influence of the scanner noise on the obtained signal of interest. The acoustic overstimulation involved 15 min of narrowband noise with a central frequency of 3 kHz (the same sound as for audiometric assessments), presented binaurally at 95 dB(A) via NNL electrostatic headphones while in the scanner. The control group had no acoustic overstimulation presented in the MR scanner and instead relaxed in silence. Figure 1 depicts the timeline of the fMRI experiment. During study preparations, an artificial ear simulator by Bruel & Kjaer was connected to a Madsen Itera audiometer by ER3a earphones to estimate the parameters of the noise used for acoustic overstimulation in behavioral audiometric assessments. The same was done for the MRI-compatible NNL headphones. The parameters of the narrowband noise transmitted via the NNL headphones were adjusted, so that was most similar to the noise provided by audiometric earphones. Before each individual fMRI session, NNL headphones were calibrated and, if needed, the amplitude and spectrum of the noise were corrected. Background noise in the scanner room (without scanning) was attenuated with headphones to an approximate level of 50 dB SPL. During fMRI scanning, participants were requested to stay vigilant. Subjects were also kept attentive by the experimenter, who talked to them between functional MR runs.
Figure 1.
Timeline of the fMRI experiment.
fMRI data processing
Initial data analysis and preprocessing were done using SPM12 (Wellcome Department of Imaging Neuroscience, London, UK; URL:www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks Inc., Sherborn, MA). Single-subject preprocessing was performed using standard procedures. Functional scans were realigned to the first image of the time series. Subjects’ moving patterns (translation and rotation) were below 1 mm in all fMRI sessions. Next, images were resampled to 2×2×2 mm. Normalization to a standard brain atlas (SPM MNI space) was performed on segmented T1 volumetric images (voxel size 1×1×1 mm) co-registered with EPI images. Images were smoothed with an 8mm FWHM Gaussian kernel. A general linear model (GLM) and a canonical hemodynamic response function (HRF) were fitted to the data. A time series for each voxel was high-pass filtered (1/128 Hz cut-off) to remove low-frequency noise and signal drifts.
In the whole-brain statistical analysis in SPM, 12 contrasts ‘4 kHz vs. silence’ were calculated for sessions Before and After 1–3 in each participant (first level analysis). Outcomes of these contrasts were compared using ANOVA full factorial analysis (factors: 2 independent groups with unequal variance and 4 dependent sessions with equal variance). Next, group results in each session were compared with one another (interactions between groups and between sessions). Outcome post-hoc SPM t-maps were FWE-corrected at p<0.05. Anatomical brain regions were localized using the AAL atlas implemented in MRICRON (URL:http://www.mccauslandcenter.sc.edu/mricro/mricron/). In an additional analysis, the size of the activated region (no. of voxels) was normalized between sessions, using session Before as a reference (the obtained region size in each session After was divided by the region size obtained in session Before). This was done to present a trend in which the size of the active region was changing.
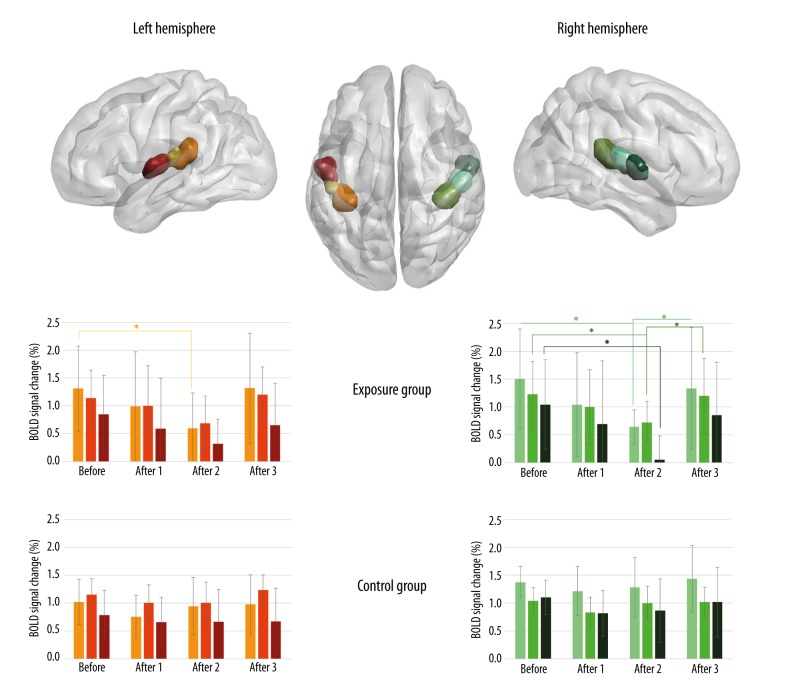
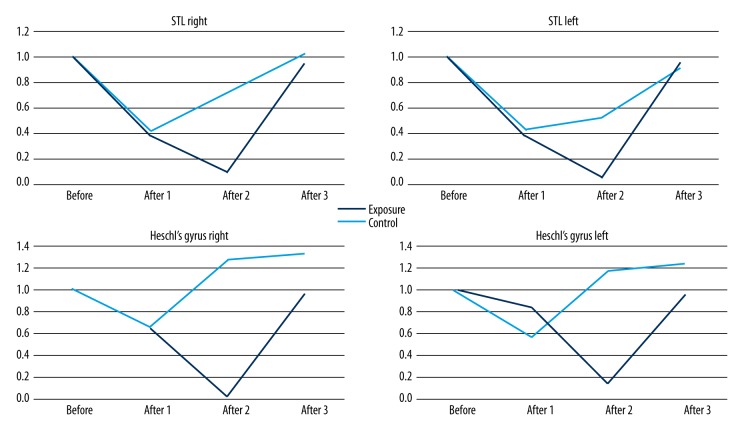
In addition, a region-of-interest (ROI) analysis was performed to test whether there was evidence of significant BOLD signal change in the primary auditory cortex [44]. Anatomical ROIs representing bilateral primary auditory cortex (PAC) (Te 1.0, 1.1, and 1.2) were defined, as provided in the Juelich Histological Atlas (thresholded at 25%) (Figure 2) [45,46]. Next, using the MarsBar toolbox for SPM (URL:http://marsbar.sourceforge.net/), mean BOLD signal change was calculated within ROIs for each subject and each fMRI session, independently. The values were then averaged across subjects in the exposure and the control groups [47].
Figure 2.
Results of the ROI analysis in the exposure group and the control group in bilateral anatomical ROIs in subsequent fMRI sessions, before acoustic overstimulation/period of silence (Before) and after acoustic overstimulation/period of silence (After 1, After 2, After 3) for contrast 4 kHz vs. silence. The mean percentage BOLD signal change is depicted (β values); asterisk * indicates statistical significance (paired t-tests, p<0.05) and bars represent standard deviations.
Results
Audiometric measurements – temporary threshold shift
Average TTS values are presented in Figure 3. The largest TTS was found at 1 min after exposure and decreased gradually down to the measurement time-point 30 min after overstimulation was completed. Differences between auditory thresholds before and after acoustic overstimulation (TTS values) were statistically significant for measurements at 1, 2, 10, 20, and 30 min post-overstimulation (p<0.001). Differences between TTS values at post-overstimulation measurements were statistically significant for contrasts 10 min vs. 2 min and 20 min vs. 10 min.
Figure 3.
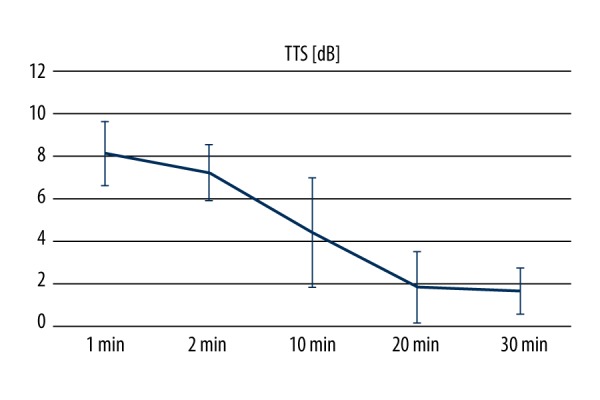
Group average TTS values in subsequent measurement time points; bars indicate standard deviations.
fMRI results
Whole-brain analysis
Exposure group (exposed to acoustic overstimulation)
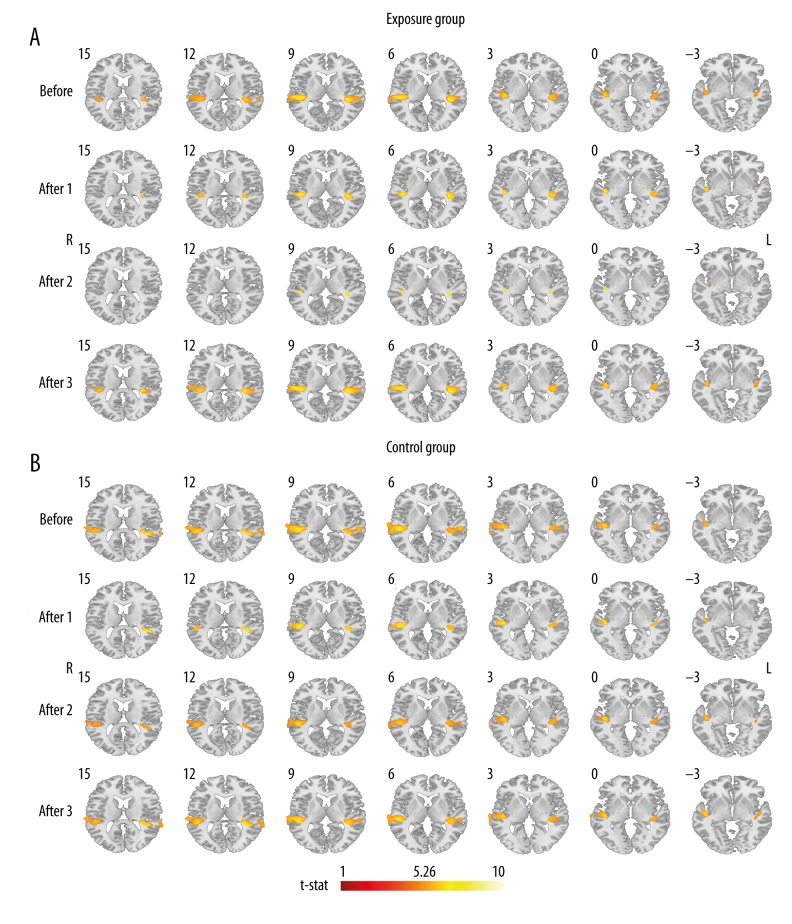
In the exposure group before overstimulation, brain responses to auditory stimulation were identified in bilateral auditory cortices, i.e., superior temporal lobes and Heschl gyri (contrast ‘4 kHz vs. silence’). Cluster sizes in 2 first post-overstimulation sessions in bilateral auditory cortices were of a lesser size, especially in session After 2. In session After 3, the signal recorded in bilateral auditory cortices seemed to recover. The outcomes in the auditory cortex were thresholded at p<0.05 (FWE-corrected) and are presented in Table 1 and Figure 4A. ANOVA t-tests directly comparing 4 fMRI sessions with one another showed significantly more restricted activation in session After 2, when compared to Before (AlphaSim-corrected for multiple comparisons at cluster level p<0.05 after passing an uncorrected threshold at p=0.001). The activation was detected in the right superior temporal lobe (cluster size 159 voxels, peak t-value=4.08) (Figure 5A). There were no other between-session differences revealed. Some of the presented data has been used in a PhD dissertation by Piotr Skarżyński, Assessment of auditory fatigue using fMRI, Medical University of Warsaw, 2012.
Table 1.
Results of the fMRI ANOVA post-hoc analysis in the exposure group and the control group in bilateral superior temporal lobes and Heschl gyri; before acoustic overstimulation/period of silence (Before) and three fMRI sessions after acoustic overstimulation/period of silence (After 1, After 2, After 3) for contrast 4 kHz vs. silence; FWE-corrected (p<0.05), threshold t-stat 5.26.
| Session | Hem. | Main Anatomical region (AAL) | Exposure group | Control group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region size (vox.) | Peak t-value | MNI coord. | Total cluster size (vox.) | Region size (vox.) | Peak t-value | MNI coord. | Total cluster size (vox.) | |||
| Before | R | T1 | 510 | 9.52 | 48–27 7 | 680 | 868 | 9.15 | 48–29 9 | 976 |
| HES | 83 | 8.79 | 42–22 6 | 52 | 7.63 | 42–22 6 | ||||
| L | T1 | 375 | 9.43 | −40–29 7 | 617 | 495 | 8.73 | −46–39 15 | 758 | |
| HES | 77 | 9.11 | −38–29 9 | 45 | 7.91 | −34–31 13 | ||||
| After 1 | R | T1 | 194 | 8.04 | 44–19 1 | 285 | 362 | 7.91 | 48–29 9 | 406 |
| HES | 54 | 7.74 | 42–22 6 | 34 | 6.85 | 44–20 6 | ||||
| L | T1 | 144 | 7.73 | −40–29 7 | 326 | 215 | 7.72 | −48–41 17 | 376 | |
| HES | 65 | 7.63 | −38–29 9 | 26 | 7.08 | −35–32 11 | ||||
| After 2 | R | T1 | 51 | 6.20 | 44–19 1 | 64 | 624 | 10.00 | 46–19 1 | 758 |
| HES | 2 | 5.77 | 42–22 6 | 66 | 7.52 | 42–22 6 | ||||
| L | T1 | 20 | 6.15 | −40–29 5 | 61 | 263 | 7.60 | −40–27 5 | 482 | |
| HES | 11 | 5.96 | −37–29 8 | 53 | 7.25 | −37–24 5 | ||||
| After 3 | R | T1 | 482 | 9.06 | 46–29 9 | 651 | 889 | 9.33 | 48–29 9 | 1003 |
| HES | 80 | 8.11 | 40–27 9 | 69 | 7.34 | 46–22 8 | ||||
| L | T1 | 356 | 8.81 | −42–33 7 | 659 | 452 | 8.29 | −40–37 13 | 779 | |
| HES | 74 | 8.72 | −36–31 11 | 56 | 8.25 | −34–31 13 | ||||
Hem. – brain hemisphere; R – right hemisphere; L – left hemisphere; AAL – Automatic Anatomical Labeling brain atlas; T1 – superior temporal lobe, HES – Heschl gyrus; vox. – number of voxels; MNI – Montreal Neurological Institute brain atlas; coord. – coordinates.
Figure 4.
Results of the fMRI ANOVA post hoc analysis: (A) in the exposure group and (B) in the control group, in bilateral superior temporal lobes and Heschl gyri; before acoustic overstimulation (Before) and 3 fMRI sessions after acoustic overstimulation (After 1, After 2, After 3) for contrast 4 kHz vs. silence; FWE-corrected (p<0.05, threshold t-stat 5.26); L – left hemisphere, R – right hemisphere.
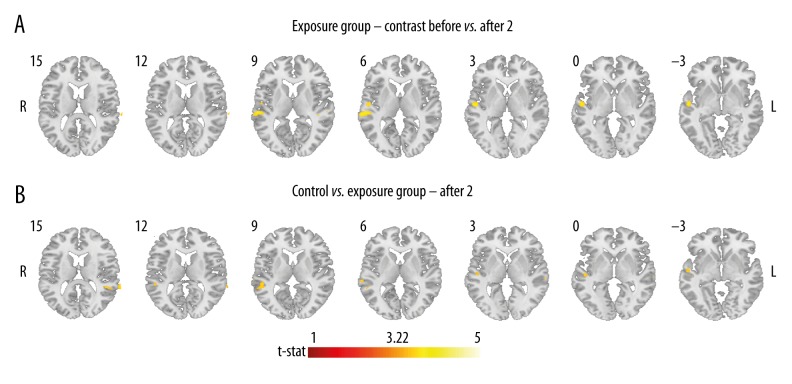
Figure 5.
Results of the fMRI ANOVA post hoc analysis: (A) in the exposure group in bilateral superior temporal lobes and Heschl gyri; contrast between sessions Before > After 2; AlphaSim-corrected (involving cluster-level correction for multiple comparisons at p<0.05 after passing an uncorrected threshold of p=0.001, threshold t-stat 3.22, voxel cluster threshold > 80); (B) contrast between Control group > Exposure group; in session After 2; in bilateral superior temporal lobes and Heschl gyri; uncorrected (p=0.001); L – left hemisphere, R – right hemisphere.
Control group (not exposed to acoustic overstimulation)
The control group revealed auditory brain responses in bilateral superior temporal lobes and Heschl gyri following auditory stimulation before and after the 15-min period of silence. As shown in Table 1 and Figure 4B (contrast ‘4 kHz vs. silence’), activation cluster sizes were more restricted immediately after the period of silence and then seemed to recover to the original state in sessions After 2 and After 3. ANOVA post hoc t-tests comparing the outcomes of 4 fMRI sessions with one another showed no effects with statistical significance (AlphaSim-corrected for multiple comparisons at cluster level p<0.05 after passing an uncorrected threshold at p=0.001).
Figure 6 depicts the active cluster size in bilateral STL and Heschl gyri changing across sessions in the exposure and the control group (outcomes of ANOVA post hoc). Both groups showed the size decreasing in session After 1 (by approximately 40% in Heschl gyri and approximately 60% in STLs, when compared with the reference Before session). In session After 2, in the control group the size of the active regions in auditory cortices starts to recover, whereas in the exposure group it continues to decrease to 10% of the size revealed in session Before. In session After 3, active regions are of comparable size to session Before in both study groups (in the control group the size of the HG cluster was even 20% larger).
Figure 6.
Size of active regions changing across sessions in both study groups in bilateral superior temporal lobes and Heschl gyri; the size of active regions in sessions After 1–After 3 was normalized to session Before.
Between-group comparisons revealed no statistically significant outcomes, with AlphaSim corrected for multiple comparisons at cluster level p<0.05 after passing an uncorrected threshold at p=0.001. Nevertheless, the exposure group had a slightly larger active region in session After 2 in the right superior temporal gyrus (cluster size 62, peak t-value 4.17), and left superior temporal gyrus (cluster size 38, peak t-value 3.79) at p<0.001 unc. As shown in Figure 5B, the cluster on the right was similar to the one differentiating sessions Before and After 2 in the exposure group (Figure 5A).
Region-of-interest analysis
The anatomical region-of-interest (ROI) analysis in the exposure group (exposed to acoustic overstimulation) revealed decreased BOLD signal change (β values) for contrast ‘4 kHz vs. silence’ after acoustic overstimulation in sessions After 1 and After 2 in all selected ROIs in bilateral primary auditory cortices. In the exposure group, as shown with paired t-tests, the decrease in session After 2, compared to session Before, was statistically significant (p<0.05) in all PAC subregions in the right hemisphere and in region Te 1.0 in the left hemisphere. Furthermore, there was a significant increase detected in the right ROI from session After 2 to After 3 in PAC subdivisions Te 1.0 and Te 1.2. No statistically significant changes were demonstrated in control individuals. The produced ROIs and group outcomes are presented in Figure 2.
Discussion
In the present sparse fMRI study, we undertook to examine brain correlates accompanying behaviorally detected TTS by looking at induced central auditory responses at different time intervals following a period of acoustic overstimulation. Two complementary approaches to data analysis provided similar outcomes. Bilateral cortical auditory responses revealed in the exposure group decreased gradually up to 20 min after completion of acoustic overstimulation. The effect was more visible and statistically significant in the right hemisphere and in the time period between the fMRI session before noise exposure and the second session after (Table 1, Figures 2, 4–6). The control group that was not exposed to any acoustic overstimulation while in the scanner also demonstrated responses in bilateral auditory cortices induced with auditory stimulation, whose extent was relatively more constant across all 4 fMRI sessions, apart from a decrease of hemodynamic response, which was not statistically significant in the right hemisphere in ROI analysis just after the 15-minute period of silence (Table 1, Figures 2, 4, 6). In both study groups, the hemodynamic response seemed to have “returned” to the initial state (before the period of acoustic overstimulation/silence) in the third fMRI session (20 to 30 min after acoustic overstimulation/silence) (Table 1, Figures 2, 4, 6).
Habituation due to repetitive auditory stimulation?
Although weak, there was a decrease in the extent of the hemodynamic response and BOLD signal change in both study groups up to 10 min after the period of overstimulation/silence (session After 1). In the exposure group the decrease was in addition present in session After 2. At the first attempt, this outcome can be discussed in reference to habituation (a type of per-stimulus adaptation), which has been demonstrated to exist at different time scales depending on changing environmental requirements and is attributed to different levels of the auditory system, including the auditory cortex [48–50]. Due to this progressive suppression of neuronal responses, the amount of high-probability stimuli being directed to the encoding system is limited, which facilitates abrupt detection of novel events [51,52]. It has been widely demonstrated in behavioral and fMRI research (both in awake and anesthetized states) that after recurrent sensory stimulation the brain would recognize it and habituate towards it [51,53,54] (‘cf. fast learning theory’ with the brain gradually selecting optimal sensory information to represent the features of sound in a form of a specific population of synchronically firing cells in Jäncke’s group work [55]).
In the current study, it cannot be excluded that the same monotonous and non-informative stimulation presented to the participants in fMRI sessions Before and After 1 induced neuronal habituation. The objectively unspectacular effect of habituation could be due to the sparse nature of the fMRI auditory stimuli (sparse fMRI data acquisition). Temporal features of auditory stimulation have been shown to affect the parameters of the hemodynamic response, with more frequently-probed stimuli inducing more profound cortical representations [56]. In the control group, these identical sessions were separated with a period of silence, but in the exposure group the introduced novel noise of different acoustic parameters might have interfered with habituation mechanisms and shifted the main habituation effect to session After 2 [57].
The suggested habituation theory implies that no TTS effect has been detected, and the overstimulation sound only acted as a novelty. This is possible but probably not fully true. The main concern is the recovery of the signal in the auditory cortex seen in the study group with no noise exposure. It seems that due to prolonged exposure to monotonous auditory stimulation, the habituation effect in the control group should be more visible in the more delayed sessions. This should especially be so since time intervals between subsequent post-silence fMRI sessions were all shorter that the 15-min period of silence. Although recovery of the auditory cortex has already been reported in previous studies using repetitive sensory stimulation, it has also been emphasized that the time constant for adaptation build up is faster than for recovery [58,59]. An alternative explanation for the statistically significant drop of the recorded signal in session After 1 in the non-exposure group would be that the participants remaining in the scanner for 15 min in silence simply felt drowsy. Attention has been consistently shown to increase central auditory responses [60,61]. It might well be that otherwise the signal would remain relatively stable across sessions.
Central mechanisms accompanying TTS?
The most visible difference between the two study groups was demonstrated during the fMRI assessment 10 to 20 min after the period of overstimulation/silence (session After 2). In the exposure group there was reduced BOLD signal change in bilateral ROIs in the auditory cortex. Therefore, it cannot be excluded that the applied fMRI procedure managed to reflect some central mechanisms accompanying acoustic overstimulation.
The authors cannot, however, indisputably infer a direct relationship between the behaviorally measured TTS effect and auditory BOLD response changes, particularly when the distinct temporal dynamics of the two observed effects are considered. Although several authors indicated there is lack of a linear relationship between audiometric thresholds and central processing measurements (ABR) following noise exposure, the largest effect has always been found in the immediate ABR measurement [28,36–39]. TTS values in individuals participating in the current experiment were most significant immediately after acoustic overstimulation (i.e., after 1- and 2-min delays, with the psychoacoustic effect fading with time); although the differences between auditory thresholds were significant until 30 min after noise exposure, their absolute values were clinically negligible. It cannot be excluded that cortical responses to suprathreshold auditory stimulation accompanying the detected increase of peripheral auditory thresholds are diminished but with a time lag. Some studies have shown central auditory effects of overstimulation remaining for longer durations than the effects seen with pure tone audiometry [4,28,36].
Furthermore, in terms of timing of the potential TTS-related central correlates, these could have been hampered by the effect novelty exerts on cortical activation. If it is assumed that the temporal threshold shift decreases auditory-induced BOLD signal effects, novelty does the opposite [62]. Therefore, it is possible that by introducing the overstimulation noise before stimulation fMRI session After 1, the latter was perceived as novel, which restricted the effect of the detected TTS and shifted it to session After 2 [57].
The reduced auditory cortical responses following noise exposure could be due to efferent neuroprotective mechanisms steered by the medial olivo-cochlear bundle involving inhibition of outer hair cell electromotility (suppressed gain of the cochlea amplifier) and stiffening of the basilar membrane, which both reduce afferent transduction of severely intensive sounds [63–66]. Moreover, involvement of type I efferent neurons originating in the lateral superior olive has been speculated, which inhibit firing by releasing inhibitory neurotransmitters at synapses with the afferent VIII nerve. Thereby, the effect of the excitatory neurotransmitter produced at the nearby inner hair cells is reduced [67]. Furthermore, temporary ‘deafferentation’ of high-threshold auditory nerve fibers, responding to high sound intensities, might potentially occur in human exposed to acoustic overstimulation, although in the current study no physical damage was induced to the ear structures. As a result, the stimulation reaching the auditory cortex is restricted and it may well be that the sound is received as less intensive [68,69].
Furthermore, in animals, physical deafferentation has been found to cause not only reduced feedback to the cochlea [4,70] but also increased spontaneous activity in central auditory pathways (potentially underlying hyperacusis and tinnitus) [2,13–18,20,39]. With an increased baseline activity of cortical neurons, the fMRI statistical contrast comparing brain responses to auditory simulation with relative silence could reveal a lesser BOLD signal change (in sessions After). Although still not fully understood, a direct relationship of the fMRI signal and spontaneous firing rates of single auditory cells has already been suggested [71–73]. Alternatively, it has been speculated in animal models that acoustic overstimulation results in spontaneous over-excitation of the auditory cortical neurons, which can mask out any further sound stimulation [74]. This mechanism could also be considered as a possible explanation of the findings in the current study, as it would also result in the observed reduced BOLD signal change in response to auditory stimulation. Certainly, it may well be that both described TTS-related mechanisms coexist. Unfortunately, fMRI cannot be used to measure increased baseline activity directly.
In the third fMRI session after acoustic overstimulation, the activation cluster size and the BOLD signal change seem to return to the state before noise exposure. We can only hypothesize that the central effects accompanying the behaviorally detected TTS are only detectable with auditory fMRI up to 20 min after acoustic overstimulation.
Where does the effect occur?
It has been shown in the present study that the allegedly TTS-related central effects might already be detectable at the level of early-stage auditory areas. This result comes as no surprise, since the primary auditory cortex can be considered a buffer between the peripheral auditory system and higher cortical areas. Furthermore, the applied acoustic stimulation involved very simple non-informative sounds normally processed in the primary auditory cortex. This is also the region most sensitive to alternations of sound parameters, such as intensity and loudness [75]. By showing the effect already in the primary auditory cortex, the outcomes of the current fMRI experiment are in line with those reported in TTS animal trials [17,18]. Moreover, the decreased extent of the hemodynamic response in the exposure group was generally more visible in the right primary auditory cortex. This region has been indicated as having greater spectral sensitivity, optimal for global frequency processing, as opposed to the left hemisphere, which specializes in the analysis of fine-structure temporal aspects of sound (such as speech) [75,76]. In terms of the decrease of the BOLD signal change just after the period of silence in the control group, attention has been shown to affect the amount of activation in the primary auditory cortex [77].
Short summary and the hypothesized TTS mechanism
We hypothesize that the central correlate of the temporal threshold shift is in fact a type of temporal habituation, in that excitation thresholds of auditory neurons throughout the whole auditory system become temporarily increased. This effect might be related to or accompanied with increased spontaneous activity of auditory neurons. This 2-part masking mechanism temporarily protects the central nervous system from further overstimulation. The result of noise exposure is thus decreased hemodynamic response to sound presented afterwards, observed already in the primary auditory cortex. The central effect is either intrinsically delayed by several min with respect to the psychoacoustically measurable outcomes, or the effect is impeded in this particular fMRI study by novelty. The TTS-related cortical response reduction is only visible in fMRI up to approximately 20 min after overstimulation is completed.
Methodological remarks
First, it must be emphasized that although preclinical studies provide clear evidence of central short-term plasticity following exposure to acoustic overstimulation, they cannot be easily translated into human neuroscience. In humans, an acoustic trauma cannot involve intentional physical damage to cochlear/neuronal structures and can only be limited to temporary functional changes. The less pronounced effects seen in the current study with the non-invasive technique of fMRI might thus be related to the restricted duration and intensity of the acoustic overstimulation. Moreover, the auditory stimulation had to be presented at high levels, similar to acoustic overstimulation, in order to obtain robust auditory cortical responses [78]. The latter might have reduced the effect of noise applied between fMRI sessions. Furthermore, the fact that fMRI is a non-direct measure of neuronal responses and has limited spatial and temporal resolution can account for the limited extent of the observed brain functional changes [79]. To minimize the effect of the ever-present noise during fMRI scanning, the authors implemented a sparse paradigm, with the loud MR data acquisition comprising only 25% of the whole-brain volume collection [80–83]. At the same time, however, the background noise of the scanner room (at an estimated level of 50dB SPL) could not be counteracted and could have affected the fMRI measurement sensitivity to the subtle central mechanisms accompanying TTS. This influence, although probably equal in both study groups, cannot be disentangled from the outcomes.
Study limitations
As to the limitations of the current experiment, the authors believe that further investigation is required involving more sizable study groups to validate the current findings. To the best of our knowledge, this fMRI trial is the first such study in humans and aimed in particular at testing the viability of this neuroimaging method in the assessment of the central mechanism accompanying TTS. Furthermore, a more detailed analysis of inter-subject variability would be necessary due to the individual susceptibility to acoustic overstimulation using audiological techniques, including acoustic stapedius reflex and auditory brainstem responses [28,40,84]. We believe that ABRs measured with suprathreshold sounds could better correspond to fMRI outcomes in individual subjects, as compared to PTA, which only evaluates the effect of acoustic overstimulation on threshold auditory responses [36]. Vigilance should also be better monitored during similar examinations, such as by use of an eye-tracker while in the scanner, as it has been found to directly affect the extent of auditory neuronal responses [61, 75].
Conclusions
The present study shows that the fMRI technique holds promise for the investigation of central auditory mechanisms accompanying temporary threshold shift following acoustic overstimulation. It is possible that noise exposure can result in reduced BOLD signal change to auditory stimulation presented afterwards. Since it seems reasonable to closely investigate the extent and the dynamics of the TTS sequelae in central auditory structures, further studies should focus on optimization of fMRI procedures that can be applied to provide this information.
Acknowledgments
We wish to thank Doctor Ewa Piątkowska-Janko and Professor Krzysztof Kochanek for support, advice, and useful comments on the study and the manuscript.
Footnotes
Conflict of interest
The authors report no conflict of interest.
Source of support: Departmental sources
References
- 1.Glorig A, Summerfield A, Ward WD. Observations on temporary auditory threshold shift resulting from noise-exposure. Ann Otol Rhinol Laryngol. 1958;67:824–47. doi: 10.1177/000348945806700317. [DOI] [PubMed] [Google Scholar]
- 2.Gröschel M, Müller S, Götze R, et al. The possible impact of noise-induced Ca2+-dependent activity in the central auditory pathway: A manganese-enhanced MRI study. Neuroimage. 2011;57:190–97. doi: 10.1016/j.neuroimage.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Heeringa AN, van Dijk P. The dissimilar time course of temporary threshold shifts and reduction of inhibition in the inferior colliculus following intense sound exposure. Hear Res. 2014;312:38–47. doi: 10.1016/j.heares.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after „temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson RH. Some observations on the nature of the audiometric 4000 hz notch: Data from 3430 veterans. J Am Acad Audiol. 2011;22:23–33. doi: 10.3766/jaaa.22.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Ochi K, Eggermont JJ. Effects of quinine on neural activity in cat primary auditory cortex. Hear Res. 1997;105:105–18. doi: 10.1016/s0378-5955(96)00201-8. [DOI] [PubMed] [Google Scholar]
- 7.Knipper M, Van Dijk P, Nunes I, et al. Advances in the neurobiology of hearing disorders: Recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Gröschel M, Götze R, Ernst A, Basta D. Differential impact of temporary and permanent noise-induced hearing loss on neuronal cell density in the mouse central auditory pathway. J Neurotrauma. 2010;27:1499–507. doi: 10.1089/neu.2009.1246. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Su W, Bao S. Long-term, but not transient, threshold shifts alter the morphology and increase the excitability of cortical pyramidal neurons. J Neurophysiol. 2012;108:1567–74. doi: 10.1152/jn.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trautwein P, Hofstetter P, Wang J, et al. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear Res. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 11.Kemp DT. Cochlear echoes: implications for noise-induced hearing loss. In: Hamernik RP, Henderson D, Salvi R, editors. New perspectives on noise-induced hearing loss. New York: Raven; 1982. pp. 189–207. [Google Scholar]
- 12.Vázquez AE, Luebke AE, Martin GK, Lonsbury-Martin BL. Temporary and permanent noise-induced changes in distortion product otoacoustic emissions in CBA/CaJ mice. Hear Res. 2001;156:31–43. doi: 10.1016/s0378-5955(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 13.Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–92. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- 14.Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164:733–46. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Noreña AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183:137–53. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- 16.Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–74. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 17.Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 18.Sun W, Deng A, Jayaram A, Gibson B. Noise exposure enhances auditory cortex responses related to hyperacusis behavior. Brain Res. 2012;1485:108–16. doi: 10.1016/j.brainres.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez KA, Jeffers PWC, Lall K, et al. Aging after noise exposure: Acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35:7509–20. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–86. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–16. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau C, Zhang JW, McPherson B, et al. Long-term, passive exposure to non-traumatic acoustic noise induces neural adaptation in the adult rat medial geniculate body and auditory cortex. Neuroimage. 2015;107:1–9. doi: 10.1016/j.neuroimage.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 23.Attias J, Bresloff I. Noise induced temporary otoacoustic emission shifts. J Basic Clin Physiol Pharmacol. 1996;7:221–33. doi: 10.1515/jbcpp.1996.7.3.221. [DOI] [PubMed] [Google Scholar]
- 24.Olszewski J, Miłoński J, Sułkowski WJ, et al. Temporary hearing threshold shift measured by otoacoustic emissions in subjects exposed to short-term impulse noise. Int J Occup Med Environ Health. 2005;18:375–79. [PubMed] [Google Scholar]
- 25.Kochanek K. [Auditory system assessment in exposure to noise: Electrophysiological studies]. Medical University of Warsaw; 1986. [in Polish] [Google Scholar]
- 26.Hetu R. Temporary threshold shift and the time pattern of noise exposure. Can Acoust. 1982;10:36–44. [Google Scholar]
- 27.Melnick W. Human temporary threshold shift (TTS) and damage risk. J Acoust Soc Am. 1991;90:147–54. doi: 10.1121/1.401308. [DOI] [PubMed] [Google Scholar]
- 28.Stamper GC, Johnson TA. Auditory function in normal-hearing, noise-exposed human ears. Ear Hear. 2015;36:172–84. doi: 10.1097/AUD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward WD. The role of intermittence in PTS. J Acoust Soc Am. 1991;90:164–69. doi: 10.1121/1.401310. [DOI] [PubMed] [Google Scholar]
- 30.Yamamura K, Itoh F. The effects of variable noise level on man with peak levels below 75 dB(A) Eur J Appl Physiol. 1981;46:69–76. doi: 10.1007/BF00422178. [DOI] [PubMed] [Google Scholar]
- 31.Trzaskowski B, Jędrzejczak WW, Piłka E, et al. Otoacoustic emissions before and after listening to music on a personal player. Med Sci Monit. 2014;20:1426–31. doi: 10.12659/MSM.890747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonsbury-Martin BL, Martin GK, McCoy MJ, Whitehead ML. New approaches to the evaluation of the auditory system and a current analysis of otoacoustic emissions. Otolaryngol Head Neck Surg. 1995;112:50–63. doi: 10.1016/s0194-5998(95)70303-9. [DOI] [PubMed] [Google Scholar]
- 33.Jędrzejczak WW, Blinowska KJ, Konopka W. Time-frequency analysis of transiently evoked otoacoustic emissions of subjects exposed to noise. Hear Res. 2005;205:249–55. doi: 10.1016/j.heares.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Nottet J-B, Moulin A, Brossard N, et al. Otoacoustic emissions and persistent tinnitus after acute acoustic trauma. Laryngoscope. 2006;116:970–75. doi: 10.1097/01.MLG.0000216823.77995.13. [DOI] [PubMed] [Google Scholar]
- 35.Harding GW, Bohne BA. Temporary DPOAE level shifts, ABR threshold shifts and histopathological damage following below-critical-level noise exposures. Hear Res. 2004;196:94–108. doi: 10.1016/j.heares.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Kochanek K, Janczewski G, Abbate C, et al. A comparative study: TTS Vs wave V after exposure to noise. G Ital Med Lav Ergon. 2002;24:138–43. [PubMed] [Google Scholar]
- 37.Janczewski G, Kochanek K, Dawidowicz J, et al. [Evaluation of the effect of auditory fatigue on the human ear based on the measurement of brain stem auditory potentials (ABR). II. Relation of temporary auditory threshold shift and changes in the latency of wave V]. Med Pr. 1988;39:170–74. [in Polish] [PubMed] [Google Scholar]
- 38.Konrad-Martin D, Dille MF, McMillan G, et al. Age-related changes in the auditory brainstem response. J Am Acad Audiol. 2012;23:18–35. doi: 10.3766/jaaa.23.1.3. quiz 74–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaette R, McAlpine D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–57. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harkrider AW, Tampas JW. Differences in responses from the cochleae and central nervous systems of females with low versus high acceptable noise levels. J Am Acad Audiol. 2006;17:667–76. doi: 10.3766/jaaa.17.9.6. [DOI] [PubMed] [Google Scholar]
- 41.Skarżyński PH. Doctoral dissertation. Warszawa: 2012. Badanie zjawiska zmęczenia słuchowego metodą fMRI. [in Polish] [Google Scholar]
- 42.Boyen K, de Kleine E, van Dijk P, Langers DRM. Tinnitus-related dissociation between cortical and subcortical neural activity in humans with mild to moderate sensorineural hearing loss. Hear Res. 2014;312:48–59. doi: 10.1016/j.heares.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82:601–36. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- 44.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–49. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 45.Morosan P, Rademacher J, Schleicher A, et al. Human primary auditory cortex: Cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- 46.Yi H-G, Smiljanic R, Chandrasekaran B. The neural processing of foreign-accented speech and its relationship to listener bias. Front Hum Neurosci. 2014;8:768. doi: 10.3389/fnhum.2014.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poldrack RA. Region of interest analysis for fMRI. So Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez-González D, Malmierca MS. Adaptation in the auditory system: An overview. Front Integr Neurosci. 2014;8:19. doi: 10.3389/fnint.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gourévitch B, Eggermont JJ. Spectro-temporal sound density-dependent long-term adaptation in cat primary auditory cortex. Eur J Neurosci. 2008;27:3310–21. doi: 10.1111/j.1460-9568.2008.06265.x. [DOI] [PubMed] [Google Scholar]
- 50.Rankin CH, Abrams T, Barry RJ, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92:135–38. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grill-Spector K. Selectivity of adaptation in single units: implications for FMRI experiments. Neuron. 2006;49:170–71. doi: 10.1016/j.neuron.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Malmierca MS, Sanchez-Vives MV, Escera C, Bendixen A. Neuronal adaptation, novelty detection and regularity encoding in audition. Front Syst Neurosci. 2014;8:111. doi: 10.3389/fnsys.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson J, Smith AT. fMRI repetition suppression: neuronal adaptation or stimulus expectation? Cereb Cortex. 2012;22:567–76. doi: 10.1093/cercor/bhr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turk-Browne NB, Golomb JD, Chun MM. Complementary attentional components of successful memory encoding. Neuroimage. 2013;66:553–62. doi: 10.1016/j.neuroimage.2012.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jäncke L, Gaab N, Wüstenberg T, Scheich H, Heinze HJ. Short-term functional plasticity in the human auditory cortex: An fMRI study. Brain Res Cogn Brain Res. 2001;12:479–85. doi: 10.1016/s0926-6410(01)00092-1. [DOI] [PubMed] [Google Scholar]
- 56.Harms MP, Melcher JR. Sound repetition rate in the human auditory pathway: Representations in the waveshape and amplitude of fMRI activation. J Neurophysiol. 2002;88:1433–50. doi: 10.1152/jn.2002.88.3.1433. [DOI] [PubMed] [Google Scholar]
- 57.Gati I, Ben-Shakhar G. Novelty and significance in orientation and habituation: a feature-matching approach. J Exp Psychol Gen. 1990;119:251–63. doi: 10.1037//0096-3445.119.3.251. [DOI] [PubMed] [Google Scholar]
- 58.Fang F, Murray SO, Kersten D, He S. Orientation-tuned FMRI adaptation in human visual cortex. J Neurophysiol. 2005;94:4188–95. doi: 10.1152/jn.00378.2005. [DOI] [PubMed] [Google Scholar]
- 59.Lanting CP, Briley PM, Sumner CJ, Krumbholz K. Mechanisms of adaptation in human auditory cortex. J Neurophysiol. 2013;110:973–83. doi: 10.1152/jn.00547.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woods DL, Herron TJ, Cate AD, et al. Functional properties of human auditory cortical fields. Front Syst Neurosci. 2010;4:155. doi: 10.3389/fnsys.2010.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langers DRM, van Dijk P. Mapping the tonotopic organization in human auditory cortex with minimally salient acoustic stimulation. Cereb Cortex. 2012;22:2024–38. doi: 10.1093/cercor/bhr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Froud KE, Wong ACY, Cederholm JME, et al. Type II spiral ganglion afferent neurons drive medial olivocochlear reflex suppression of the cochlear amplifier. Nat Commun. 2015;6:7115. doi: 10.1038/ncomms8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liberman MC, JJG Feedback control of the auditory periphery: anti-masking effects of middle ear muscles vs. olivocochlear efferents. J Commun Disord. 1998;31:471–82. doi: 10.1016/s0021-9924(98)00019-7. quiz 483; 553. [DOI] [PubMed] [Google Scholar]
- 65.Mukerji S, Windsor AM, Lee DJ. Auditory brainstem circuits that mediate the middle ear muscle reflex. Trends Amplif. 2010;14:170–91. doi: 10.1177/1084713810381771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oghalai JS. The cochlear amplifier: Augmentation of the traveling wave within the inner ear. Curr Opin Otolaryngol Amp Head Neck Surg. 2004;12:431–38. doi: 10.1097/01.moo.0000134449.05454.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamill TA, Price LL. The Hearing Sciences. Available from: https://www.pluralpublishing.com/publication_ths2e.htm.
- 68.Charron S, Botte MC. Frequency selectivity in loudness adaptation and auditory fatigue. J Acoust Soc Am. 1988;83:178–87. doi: 10.1121/1.396443. [DOI] [PubMed] [Google Scholar]
- 69.Langers DRM, van Dijk P, Schoenmaker ES, Backes WH. fMRI activation in relation to sound intensity and loudness. Neuroimage. 2007;35:709–18. doi: 10.1016/j.neuroimage.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 70.Housley GD, Morton-Jones R, Vlajkovic SM, et al. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc Natl Acad Sci. 2013;110:7494–99. doi: 10.1073/pnas.1222295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukamel R, Gelbard H, Arieli A, et al. Coupling between neuronal firing, field potentials, and fMRI in human auditory cortex. Science. 2005;309:951–54. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 72.Nir Y, Fisch L, Mukamel R, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17:1275–85. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 73.Yetkin FZ, Roland PS, Mendelsohn DB, Purdy PD. Functional magnetic resonance imaging of activation in subcortical auditory pathway. Laryngoscope. 2004;114:96–101. doi: 10.1097/00005537-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 74.Pienkowski M, Harrison RV. Tone frequency maps and receptive fields in the developing chinchilla auditory cortex. J Neurophysiol. 2005;93:454–66. doi: 10.1152/jn.00569.2004. [DOI] [PubMed] [Google Scholar]
- 75.Woods DL, Stecker GC, Rinne T, et al. Functional maps of human auditory cortex: Effects of acoustic features and attention. PLoS One. 2009;4:e5183. doi: 10.1371/journal.pone.0005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: Music and speech. Trends Cogn Sci. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- 77.Paltoglou AE, Sumner CJ, Hall DA. Examining the role of frequency specificity in the enhancement and suppression of human cortical activity by auditory selective attention. Hear Res. 2009;257:106–18. doi: 10.1016/j.heares.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Langers DR, van Dijk P. Mapping the tonotopic organization in human auditory cortex with minimally salient acoustic stimulation. Cereb Cortex. 2012;22(9):2024–38. doi: 10.1093/cercor/bhr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Penny W, Friston K, Ashburner J, et al., editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. 1st Edition. Available from: http://store.elsevier.com/Statistical-Parametric-Mapping-The-Analysis-of-Functional-Brain-Images/isbn-9780123725608/
- 80.Hall DA, Haggard MP, Akeroyd MA, et al. :“Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp. 1999;7:213–23. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Langers DRM, Krumbholz K, Bowtell RW, Hall DA. Neuroimaging paradigms for tonotopic mapping (I): The influence of sound stimulus type. Neuroimage. 2014;100:650–62. doi: 10.1016/j.neuroimage.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim EYL, Tang IP, Peyman M, et al. 3 Tesla magnetic resonance imaging noise in standard head and neck sequence does not cause temporary threshold shift in high frequency. Eur Arch Otorhinolaryngol. 2015;272(11):3109–13. doi: 10.1007/s00405-014-3232-y. [DOI] [PubMed] [Google Scholar]
- 83.Moelker A, Pattynama PMT. Acoustic noise concerns in functional magnetic resonance imaging. Hum Brain Mapp. 2003;20:123–41. doi: 10.1002/hbm.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Konings A, Van Laer L, Van Camp G. Genetic studies on noise-induced hearing loss: A review. Ear Hear. 2009;30:151–59. doi: 10.1097/AUD.0b013e3181987080. [DOI] [PubMed] [Google Scholar]