Abstract
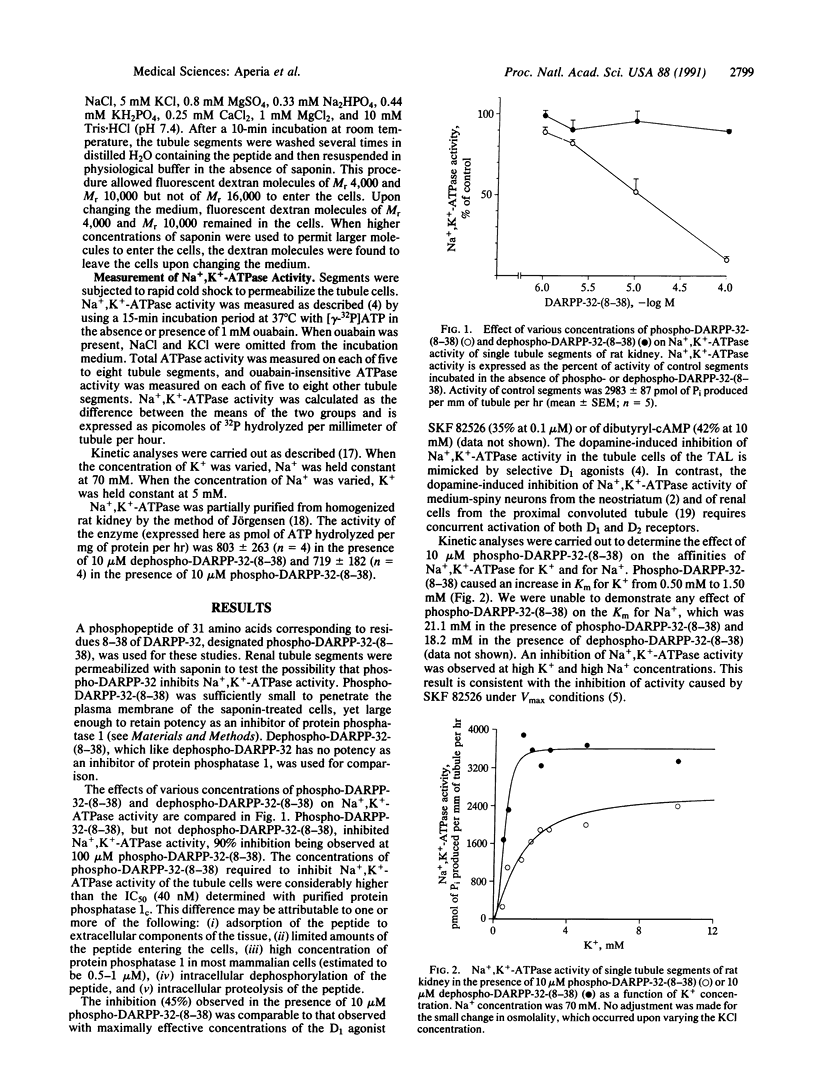
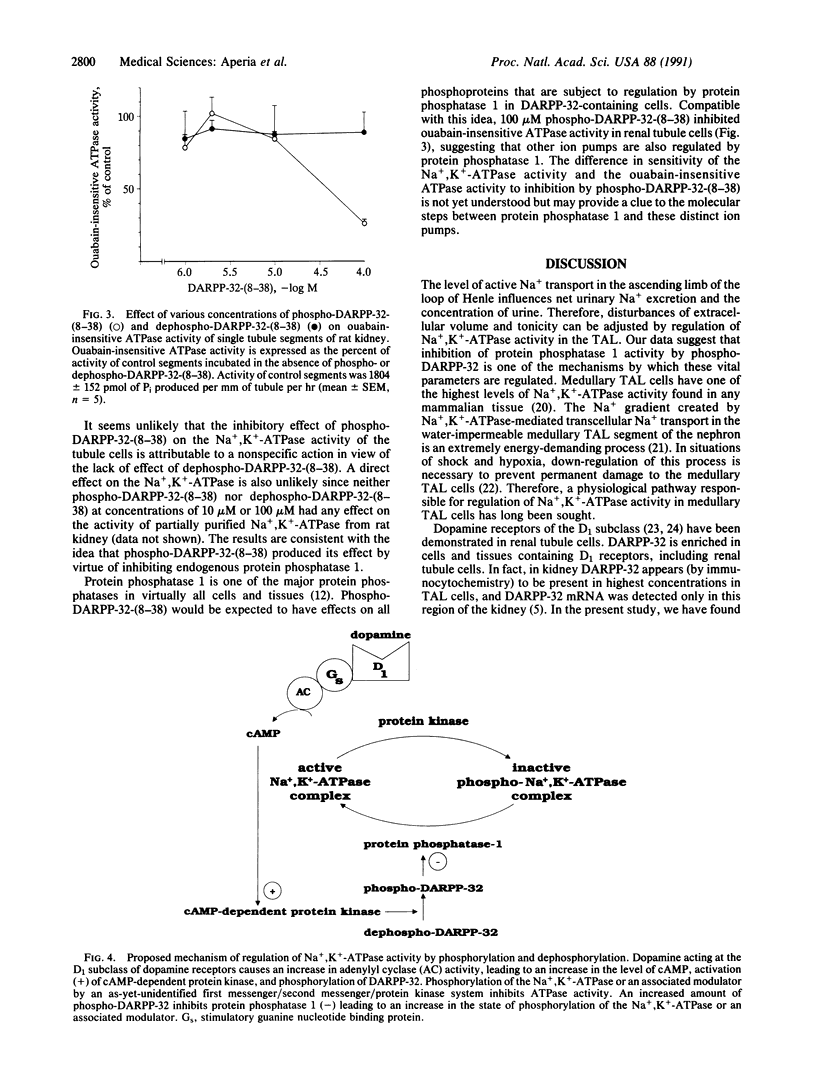
Dopamine inhibits Na+,K(+)-ATPase activity in several renal tubule segments and thereby regulates urinary Na+ excretion. We now show that a phosphopeptide of 31 amino acids, corresponding to residues 8-38 of the protein phosphatase inhibitor DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of Mr 32,000), mimics the inhibitory action of dopamine on Na+,K(+)-ATPase activity in renal tubule cells from the ascending limb of the loop of Henle. The dephosphorylated form of the peptide is ineffective. The results indicate that dopamine acts through a protein phosphorylation pathway to regulate the activity of an ion pump. In addition, the data suggest that inhibition of protein phosphatase 1 by phophorylated DARPP-32 is a component of the mechanism by which dopamine regulates urinary Na+ excretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Bertorello A. M., Hopfield J. F., Aperia A., Greengard P. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990 Sep 27;347(6291):386–388. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- Bertorello A., Hökfelt T., Goldstein M., Aperia A. Proximal tubule Na+-K+-ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol. 1988 Jun;254(6 Pt 2):F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- Epstein F. H. Hypoxia of the renal medulla. Q J Med. 1985 Dec;57(224):807–810. [PubMed] [Google Scholar]
- Felder R. A., Felder C. C., Eisner G. M., Jose P. A. The dopamine receptor in adult and maturing kidney. Am J Physiol. 1989 Sep;257(3 Pt 2):F315–F327. doi: 10.1152/ajprenal.1989.257.3.F315. [DOI] [PubMed] [Google Scholar]
- Halpain S., Girault J. A., Greengard P. Activation of NMDA receptors induces dephosphorylation of DARPP-32 in rat striatal slices. Nature. 1990 Jan 25;343(6256):369–372. doi: 10.1038/343369a0. [DOI] [PubMed] [Google Scholar]
- Hebert S. C., Andreoli T. E. Control of NaCl transport in the thick ascending limb. Am J Physiol. 1984 Jun;246(6 Pt 2):F745–F756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986 May;6(5):1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Greengard P., Tung H. Y., Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984 Aug 9;310(5977):503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., Elliott J. I., Greengard P. Synthetic peptide analogs of DARPP-32 (Mr 32,000 dopamine- and cAMP-regulated phosphoprotein), an inhibitor of protein phosphatase-1. Phosphorylation, dephosphorylation, and inhibitory activity. J Biol Chem. 1990 Nov 25;265(33):20369–20376. [PubMed] [Google Scholar]
- Jørgensen P. L. Structure, function and regulation of Na,K-ATPase in the kidney. Kidney Int. 1986 Jan;29(1):10–20. doi: 10.1038/ki.1986.3. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K. R., Strumwasser F., Nairn A. C., Walter U., Wilson F. D., Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. R. Dopamine and the kidney. Clin Sci (Lond) 1982 May;62(5):439–448. doi: 10.1042/cs0620439. [DOI] [PubMed] [Google Scholar]
- Meister B., Fryckstedt J., Schalling M., Cortés R., Hökfelt T., Aperia A., Hemmings H. C., Jr, Nairn A. C., Ehrlich M., Greengard P. Dopamine- and cAMP-regulated phosphoprotein (DARPP-32) and dopamine DA1 agonist-sensitive Na+,K+-ATPase in renal tubule cells. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8068–8072. doi: 10.1073/pnas.86.20.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet C. C., Greengard P. Distribution of DARPP-32 in the basal ganglia: an electron microscopic study. J Neurocytol. 1990 Feb;19(1):39–52. doi: 10.1007/BF01188438. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Shyjan A. W., Ceña V., Klein D. C., Levenson R. Differential expression and enzymatic properties of the Na+,K(+)-ATPase alpha 3 isoenzyme in rat pineal glands. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1178–1182. doi: 10.1073/pnas.87.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skou J. C. The Na,K-pump. Methods Enzymol. 1988;156:1–25. doi: 10.1016/0076-6879(88)56004-4. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Aswad D. W., Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983 Jan 6;301(5895):69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]



