Abstract
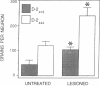
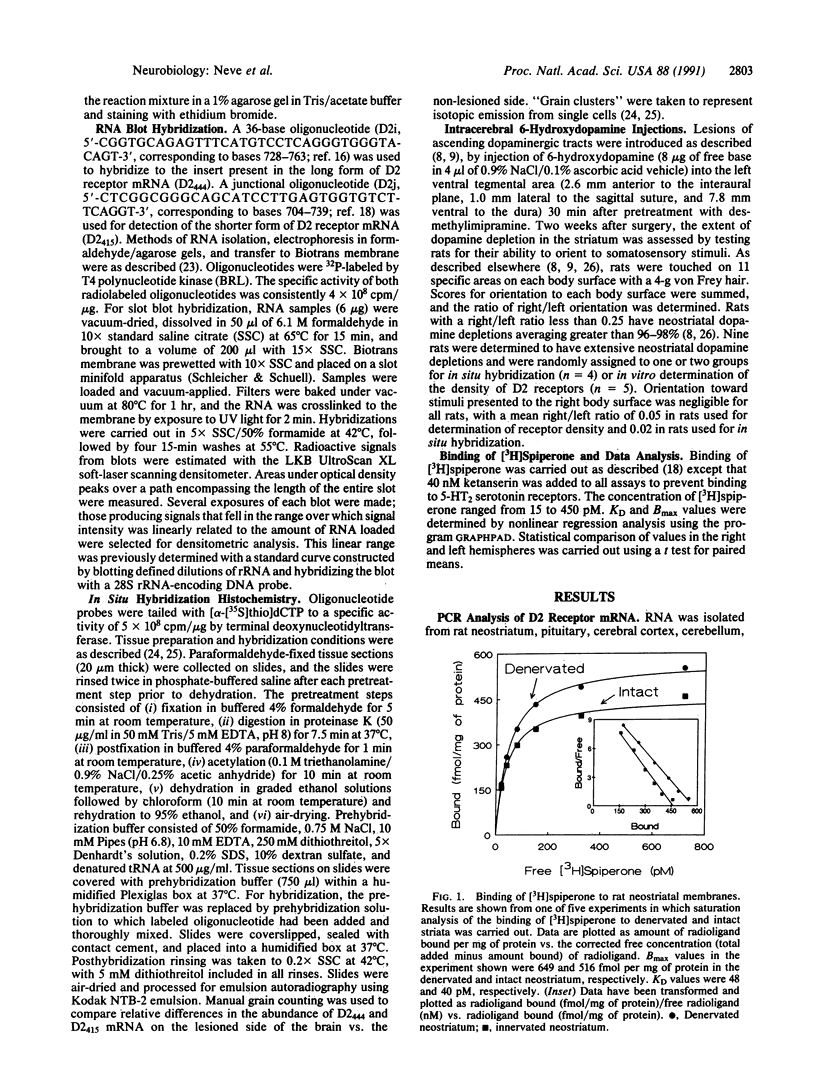
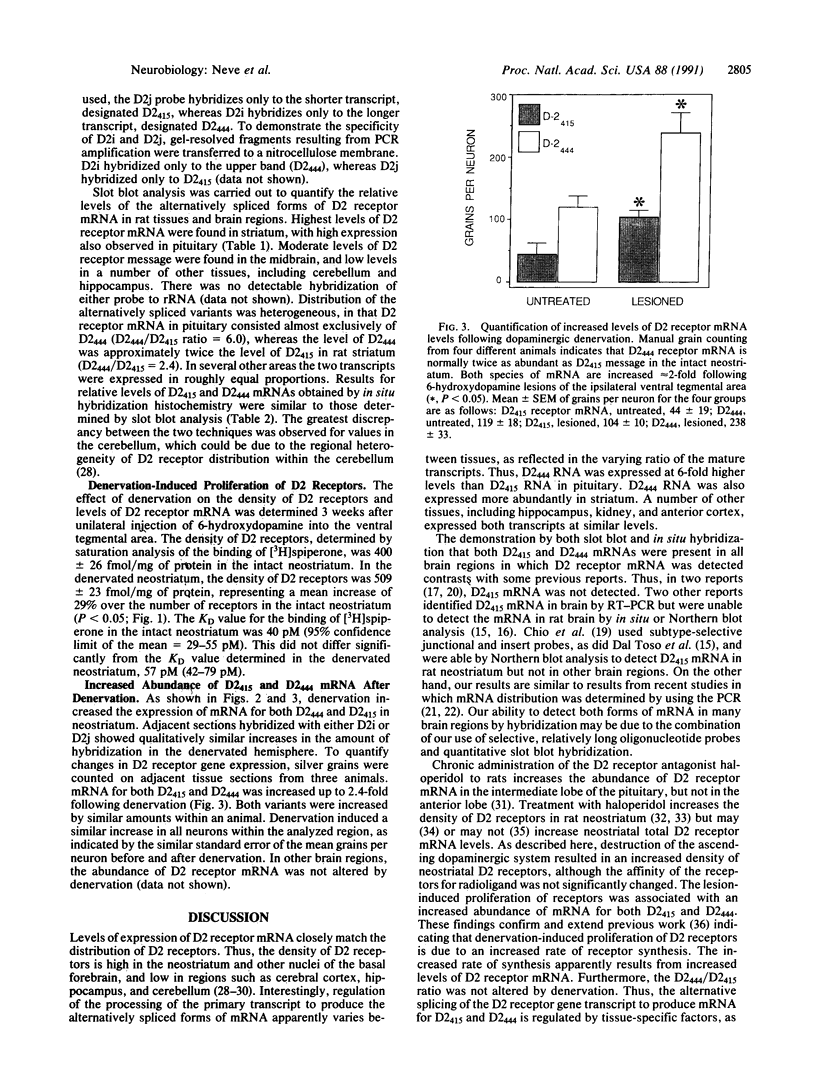
The existence of two molecular forms of D2 dopamine receptors suggests that differences in the distribution or regulation of the two forms could be exploited for the pharmacological treatment of disease. Using probes selective for each alternatively spliced variant of D2 receptor mRNA, we determined that both variants were widely distributed in rat brain and pituitary but that the ratio of the forms varied among regions. mRNA for the 444-amino acid-long variant, D2(444), was the most abundant form in pituitary and neostriatum. Intermediate levels of both D2(444) mRNA and the short form, D2(415), were detected in midbrain, and low levels of D2(444) and D2(415) mRNAs were detected in all other regions examined, including hippocampus, cerebellum, and cortex. The D2(444)/D2(415) ratio was generally lower in the regions of low expression than in pituitary and neostriatum. Dopamine-depleting lesions increased the density of D2 receptors in the denervated neostriatum by 29% without altering the affinity of the receptors for [3H]spiperone. The proliferation of receptors appeared to be due to a lesion-induced increase of up to 120% in the abundance of both variants of mRNA in the neostriatum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Neve K. A., Bunzow J. R., Civelli O. Coupling of a cloned rat dopamine-D2 receptor to inhibition of adenylyl cyclase and prolactin secretion. J Biol Chem. 1990 Feb 5;265(4):2098–2104. [PubMed] [Google Scholar]
- Autelitano D. J., Snyder L., Sealfon S. C., Roberts J. L. Dopamine D2-receptor messenger RNA is differentially regulated by dopaminergic agents in rat anterior and neurointermediate pituitary. Mol Cell Endocrinol. 1989 Nov;67(1):101–105. doi: 10.1016/0303-7207(89)90235-9. [DOI] [PubMed] [Google Scholar]
- Bouthenet M. L., Martres M. P., Sales N., Schwartz J. C. A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience. 1987 Jan;20(1):117–155. doi: 10.1016/0306-4522(87)90008-x. [DOI] [PubMed] [Google Scholar]
- Boyson S. J., McGonigle P., Molinoff P. B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986 Nov;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Burt D. R., Creese I., Snyder S. H. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977 Apr 15;196(4287):326–328. doi: 10.1126/science.847477. [DOI] [PubMed] [Google Scholar]
- Chio C. L., Hess G. F., Graham R. S., Huff R. M. A second molecular form of D2 dopamine receptor in rat and bovine caudate nucleus. Nature. 1990 Jan 18;343(6255):266–269. doi: 10.1038/343266a0. [DOI] [PubMed] [Google Scholar]
- Creese I., Burt D. R., Snyder S. H. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977 Aug 5;197(4303):596–598. doi: 10.1126/science.877576. [DOI] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Sokoloff P., Martres M. P., Riou J. F., Emorine L. J., Schwartz J. C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989 Dec 21;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Higgins G. A., Koh S., Chen K. S., Gage F. H. NGF induction of NGF receptor gene expression and cholinergic neuronal hypertrophy within the basal forebrain of the adult rat. Neuron. 1989 Aug;3(2):247–256. doi: 10.1016/0896-6273(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Higgins G. A., Lewis D. A., Bahmanyar S., Goldgaber D., Gajdusek D. C., Young W. G., Morrison J. H., Wilson M. C. Differential regulation of amyloid-beta-protein mRNA expression within hippocampal neuronal subpopulations in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1297–1301. doi: 10.1073/pnas.85.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. The mechanisms of action of L-dopa in Parkinson's disease. Life Sci. 1974 Oct 1;15(7):1249–1259. doi: 10.1016/0024-3205(74)90306-3. [DOI] [PubMed] [Google Scholar]
- Janowsky A., Berger P., Vocci F., Labarca R., Skolnick P., Paul S. M. Characterization of sodium-dependent [3H]GBR-12935 binding in brain: a radioligand for selective labelling of the dopamine transport complex. J Neurochem. 1986 Apr;46(4):1272–1276. doi: 10.1111/j.1471-4159.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Janowsky A., Vocci F., Berger P., Angel I., Zelnik N., Kleinman J. E., Skolnick P., Paul S. M. [3H]GBR-12935 binding to the dopamine transporter is decreased in the caudate nucleus in Parkinson's disease. J Neurochem. 1987 Aug;49(2):617–621. doi: 10.1111/j.1471-4159.1987.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Le Moine C., Normand E., Guitteny A. F., Fouque B., Teoule R., Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc Natl Acad Sci U S A. 1990 Jan;87(1):230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Seeman P., Rajput A., Farley I. J., Hornykiewicz O. Receptor basis for dopaminergic supersensitivity in Parkinson's disease. Nature. 1978 May 4;273(5657):59–61. doi: 10.1038/273059a0. [DOI] [PubMed] [Google Scholar]
- Lloyd K. G. CNS compensation to dopamine neuron loss in Parkinson's disease. Adv Exp Med Biol. 1977;90:255–266. doi: 10.1007/978-1-4684-2511-6_16. [DOI] [PubMed] [Google Scholar]
- Marshall J. F. Somatosensory inattention after dopamine-depleting intracerebral 6-OHDA injections: spontaneous recovery and pharmacological control. Brain Res. 1979 Nov 16;177(2):311–324. doi: 10.1016/0006-8993(79)90782-0. [DOI] [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Muller P., Seeman P. Brain neurotransmitter receptors after long-term haloperidol: dopamine, acetylcholine, serotonin, alpha-noradrenergic and naloxone receptors. Life Sci. 1977 Dec 15;21(12):1751–1758. doi: 10.1016/0024-3205(77)90155-2. [DOI] [PubMed] [Google Scholar]
- Murrin L. C., Gale K., Kuhar M. J. Autoradiographic localization of neuroleptic and dopamine receptors in the caudate-putamen and substantia nigra: effects of lesions. Eur J Pharmacol. 1979 Dec 7;60(2-3):229–235. doi: 10.1016/0014-2999(79)90222-x. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Altar C. A., Wong C. A., Marshall J. F. Quantitative analysis of [3H]spiroperidol binding to rat forebrain sections: plasticity of neostriatal dopamine receptors after nigrostriatal injury. Brain Res. 1984 Jun 4;302(1):9–18. doi: 10.1016/0006-8993(84)91280-0. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Henningsen R. A., Kinzie J. M., De Paulis T., Schmidt D. E., Kessler R. M., Janowsky A. Sodium-dependent isomerization of dopamine D-2 receptors characterized using [125I]epidepride, a high-affinity substituted benzamide ligand. J Pharmacol Exp Ther. 1990 Mar;252(3):1108–1116. [PubMed] [Google Scholar]
- Neve K. A., Kozlowski M. R., Marshall J. F. Plasticity of neostriatal dopamine receptors after nigrostriatal injury: relationship to recovery of sensorimotor functions and behavioral supersensitivity. Brain Res. 1982 Jul 22;244(1):33–44. doi: 10.1016/0006-8993(82)90901-5. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Loeschen S., Marshall J. F. Denervation accelerates the reappearance of neostriatal D-2 receptors after irreversible receptor blockade. Brain Res. 1985 Mar 11;329(1-2):225–231. doi: 10.1016/0006-8993(85)90528-1. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Harris P., Kosik K. S., Kurnit D. M., Donlon T. A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986 Dec;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- O'Malley K. L., Mack K. J., Gandelman K. Y., Todd R. D. Organization and expression of the rat D2A receptor gene: identification of alternative transcripts and a variant donor splice site. Biochemistry. 1990 Feb 13;29(6):1367–1371. doi: 10.1021/bi00458a003. [DOI] [PubMed] [Google Scholar]
- Rao D. D., McKelvy J., Kebabian J., MacKenzie R. G. Two forms of the rat D2 dopamine receptor as revealed by the polymerase chain reaction. FEBS Lett. 1990 Apr 9;263(1):18–22. doi: 10.1016/0014-5793(90)80695-f. [DOI] [PubMed] [Google Scholar]
- Reisine T. D., Nagy J. I., Fibiger H. C., Yamamura H. I. Localization of dopamine receptors in rat brain. Brain Res. 1979 Jun 15;169(1):209–214. doi: 10.1016/0006-8993(79)90391-3. [DOI] [PubMed] [Google Scholar]
- Selbie L. A., Hayes G., Shine J. The major dopamine D2 receptor: molecular analysis of the human D2A subtype. DNA. 1989 Nov;8(9):683–689. doi: 10.1089/dna.1.1989.8.683. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Waddington J. L., Cross A. J., Longden A., Owen F., Poulter M. Apomorphine-induced rotation in the unilateral 6-OHDA-lesioned rat: relationship to changes in striatal adenylate cyclase activity and 3H-spiperone binding. Neuropharmacology. 1979 Jul;18(7):643–645. doi: 10.1016/0028-3908(79)90119-9. [DOI] [PubMed] [Google Scholar]
- van Tol H. H., Riva M., Civelli O., Creese I. Lack of effect of chronic dopamine receptor blockade on D2 dopamine receptor mRNA level. Neurosci Lett. 1990 Apr 6;111(3):303–308. doi: 10.1016/0304-3940(90)90279-i. [DOI] [PubMed] [Google Scholar]