Abstract
Schizophrenia is characterized by deficits of context processing, thought to be related to dorsolateral prefrontal cortex (DLPFC) impairment. Despite emerging evidence suggesting a crucial role of the DLPFC in integrating reward and goal information, we do not know whether individuals with schizophrenia can represent and integrate reward-related context information to modulate cognitive control. To address this question, thirty-six individuals with schizophrenia (n=29) or schizoaffective disorder (n=7) and twenty-seven healthy controls performed a variant of response conflict task (Padmala and Pessoa, 2011) during fMRI scanning, in both baseline and reward conditions, with monetary incentives on some reward trials. We used a mixed state-item design that allowed us to examine both sustained and transient reward effects on cognitive control. Different from predictions about impaired DLPFC function in schizophrenia, we found an intact pattern of increased sustained DLPFC activity during reward vs. baseline blocks in individuals with schizophrenia at a group level but blunted sustained activations in the putamen. Contrary to our predictions, individuals with schizophrenia showed blunted cue-related activations in several regions of the basal ganglia responding to reward-predicting cues. Importantly, as predicted, individual differences in anhedonia/amotivation symptoms severity were significantly associated with reduced sustained DLPFC activation in the same region that showed overall increased activity as a function of reward. These results suggest that individual differences in motivational impairments in schizophrenia may be related to dysfunction of the DLPFC and striatum in motivationally salient situations.
Keywords: negative symptoms, reward, dorsolateral prefrontal cortex, schizophrenia, mixed fMRI design
General Scientific Summary
This study found that individual differences in anhedonia and/or amotivation symptoms in schizophrenia were related to sustained brain activity in the dorsolateral prefrontal cortex during reward context. These findings suggest one of the potential neural mechanisms that may lead to motivational impairments in individuals with schizophrenia.
Introduction
Amotivation, a “negative” symptom of schizophrenia, is often observed before the onset of psychosis and persists even after successful treatment with antipsychotics that reduce positive symptoms (Ucok & Ergul, 2014). Despite the crucial impact of amotivation on functional outcomes in schizophrenia (Best, Grossman, Oyewumi, & Bowie, 2014; Robertson et al., 2014; Ventura et al., 2014), the psychological and neural mechanisms contributing to motivational impairments in schizophrenia are still not clear. To date, separate lines of research have shown that individuals with schizophrenia display deficits in cognitive control (e.g., Cohen, Barch, Carter, & Servan-Schreiber, 1999) and both intact and impaired aspects of reward processing (Gold, Waltz, Prentice, Morris, & Heerey, 2008). Deficits in cognitive control are thought to reflect, at least in part, impairments in the function of the dorsolateral prefrontal cortex (DLPFC), hypothesized to have dysregulated input from the midbrain dopamine system in schizophrenia (Cohen & Servan-Schreiber, 1992). In terms of reward processing, a growing body of literature suggests that individuals with schizophrenia have intact “in-the-moment” responses to reward (Cohen & Minor, 2010; Dowd & Barch, 2012; Gard, Kring, Gard, Horan, & Green, 2007; Kring, Kerr, Smith, & Neale, 1993), but impaired ability to use rewards to guide learning and future behavior (Gold et al., 2008; Heerey, Bell-Warren, & Gold, 2008; Heerey & Gold, 2007; Kring & Barch, 2014; Morris, Quail, Griffiths, Green, & Balleine, 2015). Importantly, recent evidence suggests that the DLPFC plays a crucial role in integrating cognitive and reward-related information (Dixon & Christoff, 2014; Jimura, Locke, & Braver, 2010; Sakagami & Watanabe, 2007; Watanabe & Sakagami, 2007) to guide behavior. However, little is known about whether individuals with schizophrenia can use reward-related information to modulate cognitive control function, and whether impairments in the ability to integrate incentive information with cognitive control might contribute to motivational impairments in this illness. To address these questions, the current study examined the effect of rewards on cognitive control in schizophrenia using a mixed state-item fMRI design. We also examined the relationship of amotivation severity to the ability to use rewards to modulate cognitive control.
Dorsolateral Prefrontal Cortex Function and Reward Processing
Emerging evidence from primate neurophysiological studies and human neuroimaging studies suggests that the lateral prefrontal cortex is involved in encoding reward-related information to enhance cognitive control functions (Kouneiher, Charron, & Koechlin, 2009; Krawczyk, Gazzaley, & D’Esposito, 2007; Szatkowska, Szymanska, Marchewka, Soluch, & Rymarczyk, 2011), potentially via internal representations of reward value (see Botvinick & Braver, 2015; Dixon & Christoff, 2014 for recent reviews). On a variety of cognitive control paradigms, individuals often perform better and faster when provided with cues that predict rewards for successful performance, referred to as the reward cue effects (Bahlmann, Aarts, & D’Esposito, 2015; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Krawczyk et al., 2007; Locke & Braver, 2008). Neural responses have been shown to be increased in several regions of the lateral PFC, especially DLPFC and other reward-related subcortical regions, including the nucleus accumbens and caudate, when responding to such reward-predicting cues.
In addition to the effects of incentive cues, recent work also suggests that contextual information about incentives can modulate cognitive control performance (Chiew & Braver, 2013; Jimura et al., 2010; Locke & Braver, 2008). For example, in Locke and Braver (2008), healthy individuals performed an AX variant of the Continuous Performance Test (AX-CPT; Beck, Bransome, Mirsky, Rosvold, & Sarason, 1956), which is known to measure cognitive control processes, in particular, the ability to utilize and maintain contextual cue information (Braver, Barch, & Cohen, 1999) under different blocked-wised motivational contexts in a scanner. To be specific, a series of letters one at a time was presented to participants, asking them to make a target response on the letter X only when it follows the letter A. Thus, cues either an A or non-A letters such as B, serve as the context that participants should keep in mind by representing and sustaining to make subsequent target responses. The crucial point of this task is that the high proportion of the AX trials (70%) enables one to differentiate intact or impaired context processing ability. That is, people with intact context processing ability tend to show more errors on AY trials relative to BX trials due to a high expectation of an X probe following an A cue. On the other hand, people with impaired context processing are more likely to make more errors on BX trials than AY trials (MacDonald, Carter, et al., 2005; Servan-Schreiber, Cohen, & Steingard, 1996), as they are less able to use context information provided by the B (i.e., non-A) cue to inhibit a tendency to respond to the X. Locke and Braver (2008) found that healthy individuals showed faster and better behavioral performances on the AX-CPT on trials with no incentive cues when those trials occurred during blocks that contained incentive cues on some trials versus blocks that contained no incentive information. This effect, referred to as the reward context effect, was hypothesized to reflect the use of proactive control to represent information about potential rewards in order to facilitate the processing of the upcoming target-related information. This reward context effect has been associated with increased sustained activation in the lateral prefrontal cortex (Jimura et al., 2010; Locke & Braver, 2008).
Dysfunctional Dorsolateral Prefrontal Cortex and Reward Processing in Schizophrenia
There is a resurgence of research interest in psychological and neural mechanisms of dysfunctional reward processing in schizophrenia, as motivational deficits, often categorized under negative symptoms, are more closely related to patients’ functioning outcome than cognitive deficits (see Kring & Barch, 2014; Reddy, Horan, & Green, 2015; Salamone, Koychev, Correa, & McGuire, 2015 for recent reviews). Based on recent affective neuroscience research, Barch and colleagues (Barch & Dowd, 2010; Barch, Pagliaccio, & Luking, 2015; Kring & Barch, 2014) proposed six major components influencing the ability to translate reward information into goal-directed behavioral responses. One of these components is an important ability for goal-directed behavior, which is to integrate reward values in order to generate action plans, thought to be supported by DLPFC function. Here, we will focus on this component as it may be potentially related to the anhedonic phenotype. The “anhedonic phenotype” collectively refers to the constructs of anhedonia and/or amotivation as distinct factors from diminished expression, comprising blunted affect and alogia (Barch & Dowd, 2010; Barch et al., 2015; Kring & Barch, 2014; Strauss, Hong, et al., 2012). Although there is a body of literature on impaired cognitive control function, thought to be due to abnormal DPLFC function in schizophrenia (e.g., Cohen et al.,1999), more information is needed on the ability of individuals with schizophrenia to integrate reward information with cognitive control, the neural mechanisms that support such integration, and the ways in which these may relate to anhedonia/amotivation symptoms in schizophrenia.
One of promising experimental paradigms to examine the construct of anhedonia/amotivation is to examine the effect of reward on cognitive control. For the most part, reward processing and cognitive control in schizophrenia have been examined in independent studies. Mixed findings exist in the reward processing literature in schizophrenia depending on task demands. On the one hand, individuals with schizophrenia show relatively intact reward-related behavioral and neural responses to the in-the-moment presentation of rewards, which presumably does not require the internal representation of reward value regardless of antipsychotic medication status (Dowd & Barch, 2012; Kirsch, Ronshausen, Mier, & Gallhofer, 2007; Waltz et al., 2010). For example, individuals with schizophrenia showed intact brain responses at the receipt of the expected monetary incentives in the ventral striatum (Dowd & Barch, 2012). However, when rewards were unexpectedly delivered, there is some evidence showing reduced responses in the lateral prefrontal cortex in schizophrenia (Schlagenhauf et al., 2009; Walter et al., 2010; Waltz et al., 2009). Such results might suggest that individuals with schizophrenia would be able to use explicit reward cue information to modulate cognitive control, suggesting intact reward cue effects on cognitive control, even if their overall cognitive control level might be impaired (reviewed in Kring & Barch, 2014). Recent behavioral work from our laboratory on a response conflict paradigm is consistent with this hypothesis, showing intact reward cue effects on reaction times in schizophrenia (Mann, Footer, Chung, Driscoll, & Barch, 2013). Further, such findings might suggest that individuals with schizophrenia should show intact reward-cue related responses in striatal regions associated with the process of reward information.
In contrast, some studies have found that individuals with schizophrenia show reduced neural responses to reward-predicting cues in the ventral striatum (Juckel et al., 2006; Kapur, 2003; Kirsch et al., 2007; Robbins, 1976; Schlagenhauf et al., 2008) and there is a growing body of literature suggesting that individuals with schizophrenia have difficulties maintaining reward or pleasure-related information over time (Gold et al., 2008; Heerey, Matveeva, & Gold, 2011; Kring & Caponigro, 2010; Morris et al., 2015; Morris et al., 2012; Schlagenhauf et al., 2014). Such results might suggest that individuals with schizophrenia should show impaired effects of incentives on cognitive control, especially incentive context effects that might require the maintenance of internal representations about reward information. Consistent with this hypothesis, our recent behavioral work showed reduced incentive context effects on reaction times in a response-conflict paradigm among individuals with schizophrenia (Mann et al., 2013). As of yet, there is no data on the pattern of neural responses to incentive cues or incentive context among individuals with schizophrenia. However, these behavioral results and the literature on altered prefrontal cortex function in schizophrenia might suggest that individuals with schizophrenia should show reductions in the type of sustained DLPFC activation that has been thought to support reward context effects.
Importantly, it is possible that both behavioral and neural responses to incentives cues and incentive context might be influenced by individual differences in negative symptom severity. A number of studies have shown relationships between negative symptom severity in schizophrenia and neural activity during reward anticipation (de Leeuw, Kahn, & Vink, 2015; Gold et al., 2012; Juckel et al., 2006; Schlagenhauf et al., 2008; Simon et al., 2010). For example, Waltz and colleagues (2010) found that patients with more severe negative symptoms showed reduced neural responses in the lateral PFC during obtained gain vs. neutral cues. Dowd and Barch (2012) found that patients with greater anhedonia severity tended to show reduced activations in the ventromedial prefrontal cortex during anticipation of reward. These reduced activations may reflect patients’ reduced internal representation of reward value. These findings provide evidence that patients’ negative symptoms, in particular, motivational deficits, may be related to impaired internal representations of reward value, potentially related to supported dorsolateral prefrontal cortex function. Taken together, these recent works suggest the possibility that abnormalities of motivational behavior in schizophrenia may be related to difficulty integrating reward-related information in the motivational context rather than reward-related experiences per se (Gold et al., 2012; Gold et al., 2008; Strauss et al., 2011).
Hypotheses and Predictions
This study aimed to examine both reward context and cue effects on cognitive control in schizophrenia using a modified response conflict task paradigm (Padmala & Pessoa, 2011) that incorporated a mixed state-item fMRI design. The job of participants was to categorize images as either houses or buildings with either congruent or incongruent overlaid words. First, participants performed no-reward baseline conditions, followed by reward conditions with monetary incentives on some trials (reward cue trials – REW-CUE), but not other trials (reward context trials – RCTX-CUE). The use of mixed state-item fMRI design allowed us to examine both sustained brain activity during the baseline condition (BASE) and during each task condition (i.e., baseline condition (BASE), reward condition (REWARD), as well as cue effects by comparing neural activity during cues occurring during the baseline condition (BASE-CUE), and cues during reward condition that either indicated that they could win a reward (REW-CUE) or reward context trials on which they could not get a reward (RCTX-CUE). Based on the literature reviewed above, we predicted that individuals with schizophrenia would show reduced incentive context effects in behavior and reduced sustained neural activity in the DLPFC during reward versus baseline blocks compared to the healthy controls (HC). In contrast, we predicted that they would show intact reward cue effects in behavior and that they would show intact transient neural activity in the ventral striatum in response to incentive cues. Further, we predicted that anhedonia and/or amotivaion in schizophrenia may reflect, at least in part, patients’ difficulty of translating reward value into goal representation towards goal-directed responses. In this context, we expected that individual differences in anhedonia and/or amotivation symptoms in schizophrenia would be negatively correlated with either or both sustained brain activations in the dorsolateral prefrontal cortex during reward contexts and transient cue-related activity in the ventral striatum during reward anticipations.
Method
Participants and Recruitment Information
Participants consisted of 27 HC and 36 outpatients with DSM-IV-TR schizophrenia (n = 29) or schizoaffective disorder (n =7), taking stable antipsychotic medication doses for at least two weeks before participating in the current study (see Supplement Material for details about recruitment, inclusion and exclusion criteria). All participants were recruited through the Conte Center for the Neuroscience of Mental Disorders at Washington University in St. Louis. A subset of data analyses from the HC (n=27) only was reported in a prior publication (Chung and Barch, 2015).
Clinical symptoms were rated using the Scales for the Assessment of Negative Symptoms (SANS; Andreasen, 1983b), the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1983a) by a trained master’s level clinician. Importantly, we included the Brief Negative Symptom Scale (BNSS; Kirkpatrick et al., 2011) to investigate the relationship between individual differences in anhedonia and/or amotivation and neural responses (see Supplement Methods for details about the BNSS). Written informed consent was obtained from all participants and all procedures were approved by the Washington University.
Task Stimuli and Paradigm
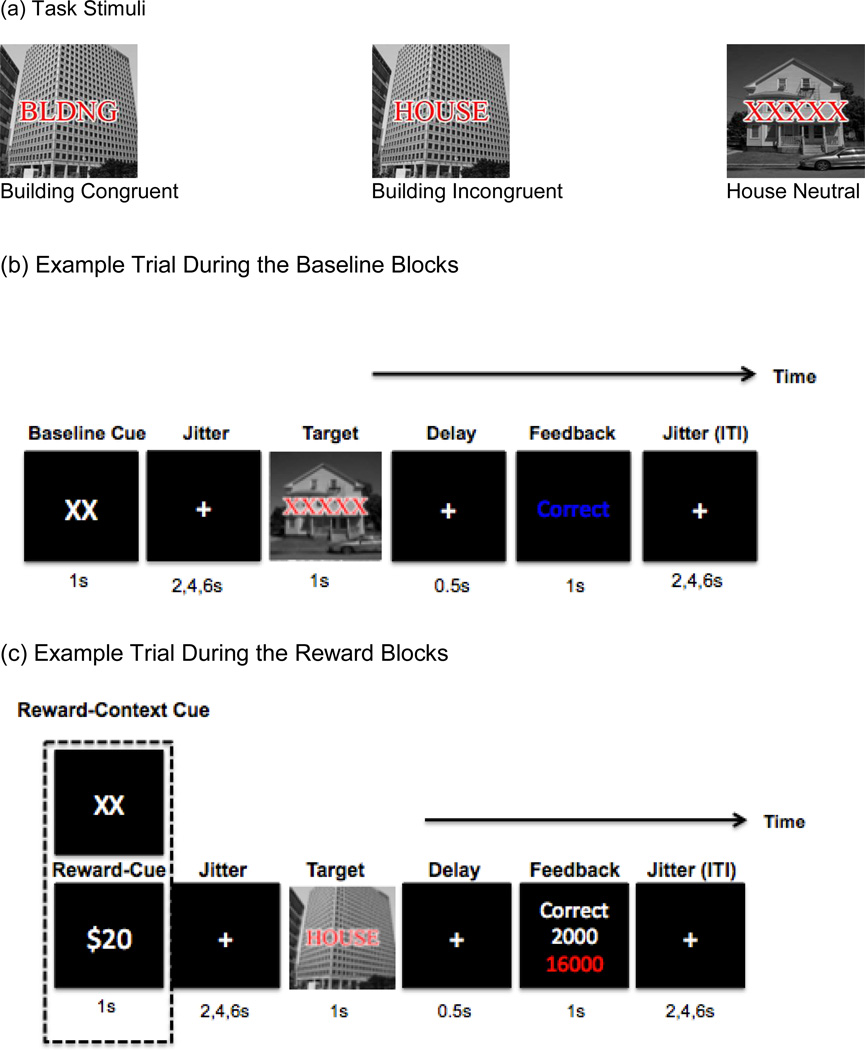
During the task, images-plus-words were presented to participants one at a time. The job of participants was to categorize each image as either a house or building regardless of the overlaid letters by pressing a “1” for a house image or “2” for a building image. The overlaid letters were used to create neutral, incongruent or congruent trials. For example, if a building picture was presented with a matching word, “BLDNG”, this is an example of congruent trial. However, if a building image was presented with non-matching word such as “HOUSE”, this is an example of incongruent trials. For neutral trials, “XXXXX” letters were presented with an image (see Figure 1 (a) for task stimuli).
Figure 1.
Task Stimuli and Paradigm
Note. During reward blocks, Reward-cue (REW, “$20”) and Reward-Context cue (RCTX, “XX”) were intermixed with equal number of trials across trial-type
We modified the cued response conflict processing developed by Padmala and Pessoa (2011) for the use of a mixed-state item design, as described in more details below (see Supplement materials). During the task, images-plus-words were presented to participants one at a time. The job of participants was to categorize each image as either a house or building regardless of the overlaid letters.
Each participant first performed two baseline runs without any knowledge of future rewards where each trial started with a “XX” cue with instruction saying these cues were irrelevant to task (BASE condition, with BASE-CUE trials). Then they completed four reward runs (REWARD condition) with instruction about possibility of obtaining incentive money on some trials (REW-CUE) but not other (RCTX-CUE). Reward runs includes 54 trials per each trial-type, resulting in a total of 162 trials where trials were preceded by a “$20”, REW-CUE indicating that a fast and correct response would be rewarded by 2000 points or by a “XX”, RCTX-CUE, indicating zero points would be possible on the trial. The RT threshold to determine “fast” response was set individually for each subject based on the median RT from the second baseline run (see Figure 1).
As presented in Figure 1 (b), each participant first performed two baseline runs without any knowledge of future rewards. In these runs, each trial started with a “XX” cue with instruction saying these cues were irrelevant to task (BASE condition, with BASE-CUE trials). Then, a jittered fixation period occurred, ranging from 2 to 6s before the onset of the stimuli, designed to allow estimates of event-related responses to the cues. The target stimulus was then presented for 1s, during which time participants responded. Then, visual feedback indicating accuracy of performance was presented to participants. Finally, an intertrial interval (ITI) that varied between 2,4, and 6s occurred. After the two baseline runs, participants completed four reward runs (REWARD condition) with instructions about the possibility of obtaining money on some trials (i.e., “$20”, REW-CUE) but not other trials (i.e., “XX”, RCTX-CUE) for correct and fast responses (see Figure 1(c) for reward blocks). The RT threshold to determine a “fast” response was set individually for each subject based on the median RT from the second baseline run. During reward runs, half of the trials were preceded by a “$20” reward cue (REW-CUE) indicating that a fast and correct response would be rewarded by 2000 points or by a “XX” (RCTX-CUE), indicating zero points would be possible on the trial. Equal numbers of congruent, incongruent, and neutral trials were presented for a total of 108 trials. After the target stimulus, participants received visual feedback indicating the number of reward points that they had earned on that trial as well as their cumulative earnings in points. At the end of experiment, the accumulated reward points were converted to real money. All participants received a maximum of $20 based on their accumulated points in addition to base money for completing the experiment ($25/h).
All BOLD scanning runs of this response conflict task were performed using a mixed block and event-related design, based on the recommendations of Petersen and Dubis (2012). Each run consisted of three blocks of 27 trials (9 trials per block), alternating with three fixation blocks (30 seconds per each) to examine how long sustained effect lasted during each task block. In both baseline and reward sections, each run was separated by pauses for rest. During task blocks, the inter-trial interval of 2 to 6 seconds was temporally jittered to ensure robust deconvolution of even-related fMRI responses.
Behavioral Data Analysis
Repeated measures ANOVAs were conducted on correct trials for both median reaction times (RT) and error data with within-subject factors of a Reward (BASE-CUE, REW-CUE, RCTX-CUE) and Trial type (congruent, incongruent, and neutral trials) and Diagnostic Group (HC, schizophrenia) as a between-subject factor. Significant interactions were followed by post-hoc contrasts to determine the source of the interactions. For analyses of reward context effects, we calculated contrasts on RT data by subtracting the RT in RCTX-CUE trials cued by “XX” in the reward condition from RT in the BASE-CUE trials cued by the same cue, “XX” in the baseline condition across all three trial types. For analyses of reward cue effects, contrasts on RT data were estimated by subtracting the RT in REW-CUE trials (“$20”) from RT in the RCTX-CUE trials (“XX”) from the within the same reward blocks, collapsing across all three trials-type.
fMRI Data Analysis
Additional details on fMRI acquisition and image analysis are presented in Supplemental Methods and Materials. A voxel-wise general linear model (GLM) approach was used, which incorporated regressors for linear trend and baseline shifts. Using the mixed design, sustained and transient effects associated with reward enhancement on cognitive control can be simultaneously but independently coded within the same GLM, enabling dissociation of these two effects (Friston et al., 1995). Baseline and reward task blocks were modeled by box-car functions lasting the length of each task block using an assumption of a fixed-shape response of long duration (Fischl et al., 2002). The event-related transient effects were analyzed separately for each trial-type by estimating the values for eight time point regressors (starting at trial onset) within the hemodynamic response epoch, estimated to be 16 seconds (TR: 2 seconds, 8 scanning frames) using unassumed hemodynamic response shapes. Specifically, for event-related effects, three regressors for each reward-related cue-type (BASE-CUE, REW-CUE, RCTX-CUE) during cue phase, another set of three regressors for target-related trials (congruent, incongruent, neutral trials) during target phase were separately coded with start and done cues.
DLPFC and BG A Priori ROI Mask Analyses
To conduct hypothesis-driven regions of interest (ROI) analyses, we defined as a priori ROI regions within the DLPFC and the basal ganglia (BG). Exploratory whole brain analyses are presented in the Supplement Materials. The anatomical DLPFC mask regions (Brodmann’s areas 9 and 46) were defined on an atlas-representative image using the boundaries described by Rajkowska and Goldman-Rakic (1995) and includes the bilateral middle and superior frontal gyri. The BG mask regions were derived from Wang et al. (2008) and generated by combining the caudate, nucleus accumbens, putamen, and globus pallidus. All statistical activation maps from each mask were appropriately corrected for multiple comparisons using combined p-value and cluster thresholds determined using Monte Carlo simulations; an approach equivalent to that employed by the AlphaSim program in the AFNI software package. For DLPFC ROI mask regions, z-value of 2.05 and a contiguous 13 voxels and for the BG ROI mask region, z -value of 2.05 and a contiguous 14 voxels were used. After the identification of group differences, for significant clusters, we extracted an average value of BOLD responses and imported them into SPSS for further post-hoc analyses to parse the source of significant effects. After the identification of group differences, for significant clusters, we extracted an average value of BOLD responses and imported them into SPSS for further post-hoc analyses to parse the source of significant effects. These analyses included independent and paired t-test as appropriate (see Supplemental Results). For these post-hoc analyses of cue-related activity, given our use of unassumed GLMs, we focused on regions showing interactions with time points and the mean percent signal change across each region was extracted for each of the eight estimated time points to visualize general pattern of activity. Among all time points frames, we focused on the average of time point 3 and 4, as theses time points encompassed 5–8 seconds after stimulus onset, which would capture the initial peak in a stereotyped hemodynamic responses unconfounded by sustained activity.
Individual Difference Analyses
We conducted voxel-wise Pearson product-moment correlations between individual difference measures and BOLD contrasts in the ROIs that showed sustained reward and transient cue effects within each DLPFC and BG mask. In these analyses, REWARD - BASE conditions and the “$20” versus “XX” within reward blocks contrasts were correlated with individual difference scores described above using both the same small-volume correction mentioned above and Bonferroni correction for multiple testing.
Post-Scan Questionnaire of Analysis
Before the fMRI session, participants had two separate practice sessions in the scanner to ensure that they were familiar with what they were supposed to do in the baseline run and then before the reward task runs. After the fMRI session, a subset of participants [schizophrenia: n=7, HC: n=10] completed a post-scan questionnaire asking about self-reported motivation and difficulty levels during task.
Results
Participants Characteristics
The two groups were similar in terms of most demographic variables except slightly higher participants’ education in the HC relative to the schizophrenia (see Table 1), a typical finding in schizophrenia research.
Table 1.
Participants Characteristics
| Variables | HC (N =27) | SCZ (N =36) | Group Comparison |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 35.56 (8.61) | 38.96 (8.47) | F (1, 62) = 2.43, p =.12 |
| Gender (% male) | 55.6 | 69.4 | χ2 (1) = 1.28, p = .25 |
| Race (% Caucasian) | 29.6% | 41.7 | χ2 (1) = 3.33, p = .18 |
| Smoking status (%Smokers) | 37.0% | 68.8% | χ2 (1) = 3.58, p= .06 |
| Handedness (% right) | 92.6% | 80.6% | χ2 (2) = 1.48, p= .47 |
| Highest Parental Education (years) | 14.11 (1.73) | 13.80 (3.62) | F (1, 61) = .16, p = .68 |
| Education (years) | 14.51 (1.86) | 13.13 (2.60) | F (1, 62) = 5.44, p= .02 |
| Clinical Measures | |||
| Diagnosis Subtype (N) | |||
| Schizoaffective (7) | |||
| Schizophrenia (29) | |||
| SAPS: Positivea | - | 4.83 (4.31) | |
| SANS: Negativeb | - | 8.77 (3.33) | |
| BDI | 2.14 (3.55) | 8.47 (8.99) | t (61) = −3.44, p =.001 |
| BNSS, consummatory | - | 3.83 (2.83) | |
| BNSS, anticipatory | - | 1.41 (1.42) | |
| BNSS, total | - | 19.13 (11.16) | |
| Antipsychotic Medications | |||
| Typical antipsychotics (first generation antipsychotics) (%) | - | 11.1% | |
| Atypical antipsychotics (second generation) (%) | - | 69.4% | |
| Both typical and atypical antipsychotics (%) | - | 8.3% | |
| Other Medications | |||
| Antidepressant | - | 44% | |
| Mood Stabilizer | - | 16% | |
| Anticholinergic | - | 25% | |
Note. HC = Healthy Controls, BDI = Beck Depression Inventory (Beck, Steer, & Garbin, 1988), BNSS= The Brief Negative Symptom Scale (Andreasen, 1983b), SCZ = schizophrenia, SAPS= The Scales for the Assessment of Positive Symptoms (Andreasen, 1983b), SANS = The Scales for the Assessment of Negative Symptoms (Andreasen, 1983b).
Sum of the global scores for hallucinations and delusions of the SAPS (Andreasen, Arndt, Alliger, Miller, & Flaum, 1995)
Sum of the global scores for alogia, anhedonia, avolition, affective flattening and attentional impairments (Andreasen et al., 1995)
Post-scan Questionnaire (see Table S1)
Self-reported Motivation: A repeated-measures of ANOVA on self-reported motivation was conducted to make sure that motivational states were manipulated by monetary incentives. There was a significant main effect of Motivation [F (2,30) = 4.78, p= .01, η2p= .24]. We did not find a significant main effect of Group [F (1,15) = .68, p = .42, η2p= .04] or a significant Motivation (RCTX-CUE, REW-CUE, BASE-CUE) x Group interaction [F (2,30) = .04, p = .95, η2p= .003]. Three post-hoc paired t-tests (REW-CUE - BASE-CUE, RCTX-CUE - BASE-CUE, REW-CUE - RCTX-CUE) were conducted to follow up a main effect of Motivation. Both groups reported higher motivation on REW-CUE (“$20”) than BASE-CUE (“XX”) [paired t-test (16)=2.74, p=0.01] and on REW-CUE (“$20”) relative to RCTX (“XX”) within reward blocks [paired t-test (16)=2.63, p= .018]. However, there was no difference in self-reported motivation between RCTX-CUE and BASE-CUE [paired t-test (16) = 0.23, p= .87].
Self-reported Task Difficulty: Another repeated-measures of ANOVA on difficulty was conducted. There was a significant main effect of Difficulty [F (1,15) = 5.38, p= .03, η2p= .26]. We did not find a significant main effect of Group [F (1,15) = .008, p = .92, η2p= .001] or a significant Difficulty (reward, baseline blocks) x Group interaction [F (1,15) = 2.25, p = .15, η2p= .13]. Post-hoc paired t-test on difficulty between reward and baseline blocks indicates that both groups felt somewhat higher level of difficulty on reward than baseline blocks [paired t-test (16)=2.52, p= .02].
Behavioral Data
Repeated measures ANOVAs on accuracy and reaction times were conducted as described above. The error data revealed only a significant main effect of Trial type [F (2,122) = 16.98, p = .00, η2p= .21], reflecting more errors on incongruent compared to congruent trials [F (1,61) = 27.92, p = .000, η2p= .31] and neutral compared to congruent trials at a trend level [F (1,61) = 3.48, p = .06, η2p= .05] but not other interaction effects (all ps > .16). Thus, main behavioral analyses were focused on RT data (See Supplement results).
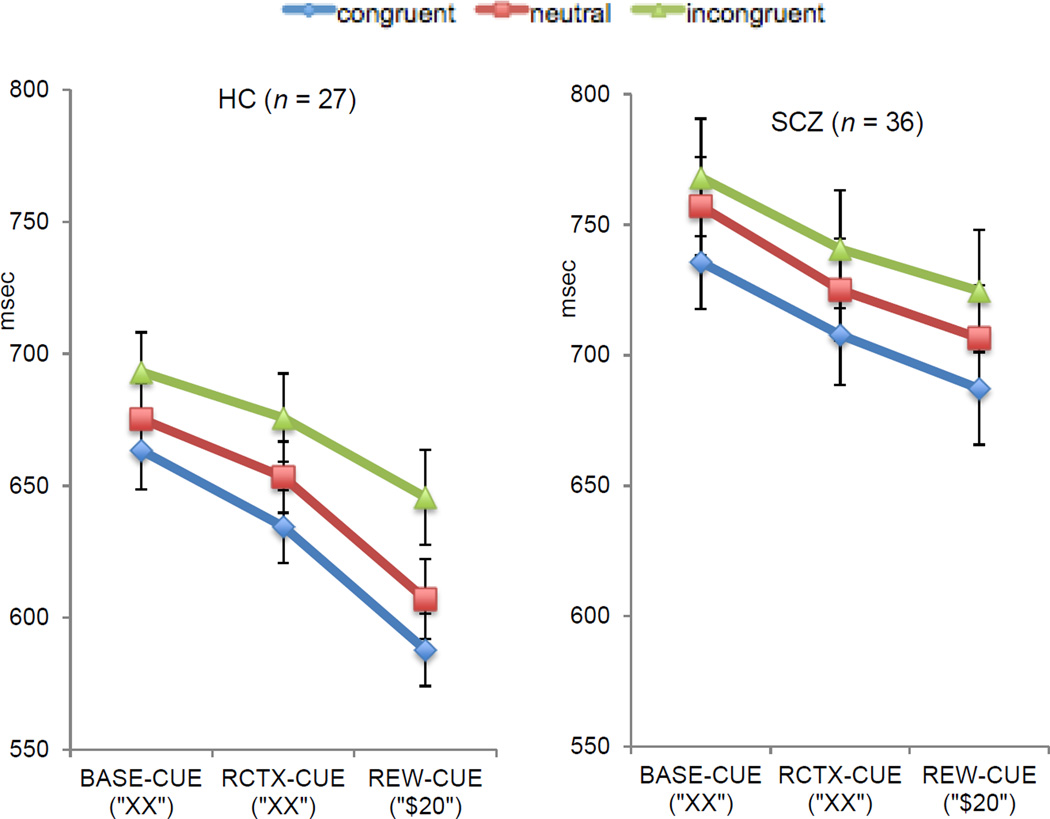
As presented in Figure 2, the repeated measure ANOVA for RTs revealed significant main effects of Group [F (1,61) = 10.38, p = .002, η2p= .14], Reward [F (2,122) = 34.12, p < .001, η2p= .35], and Trial type [F (2,122) = 32.01, p < .001, η2p= .34]. Planned contrasts to determine the source of the main effect of Trial type indicated slower responses on incongruent compared to congruent trials [F (1,61)=51.93, p < .001, η2p= .46] and slower RTs on neutral compared with congruent trials [F (1,61)=18.66, p < .001, η2p= .23]. Planned contrasts to determine the source of the main effect of Reward reflected faster performance on RCTX-CUE compared to BASE-CUE trials [F(1,61) = =12.93, p = .001, η2p= .17], REW-CUE compared to BASE-CUE [F (1,61) = 60.98, p < .001, η2p= .50] as well as REW-CUE compared to RCTX-CUE [F (1,61) =26.32, p < .001, η2p= .30]. As shown in Figure 2, the main effect of Group reflected overall slower RTs in schizophrenia group compared with the HC. We did not find a significant Reward by Group interaction (p = .22), Trial type by Group or Reward x Trial type or Group by Reward x Trial type interactions (all ps > .44).
Figure 2.
Behavioral Data: Median Correct Reaction Times
Note. BASE-CUE = Baseline cue trials, HC = healthy controls, REW-CUE= Reward cue, RCTX-CUE = Reward-Context cue, SCZ = Schizophrenia.
Behavioral Indices of Reward Context and Cue Effects
There were no significant group differences regarding behavioral indices of reward context and cue effects: individuals with schizophrenia showed similar reward context [t (61) = −0.44, p = .65] and cue effects compared with the HC [t (61) = 1.95, p = .06].
Behavior-Negative Symptoms Relationships
There was no significant association between individual differences in negative symptoms and behavioral indices of reward context and cue effects on RT data (all ps > .09).
Neuroimaging Data
Sustained Context-dependent Reward Effects
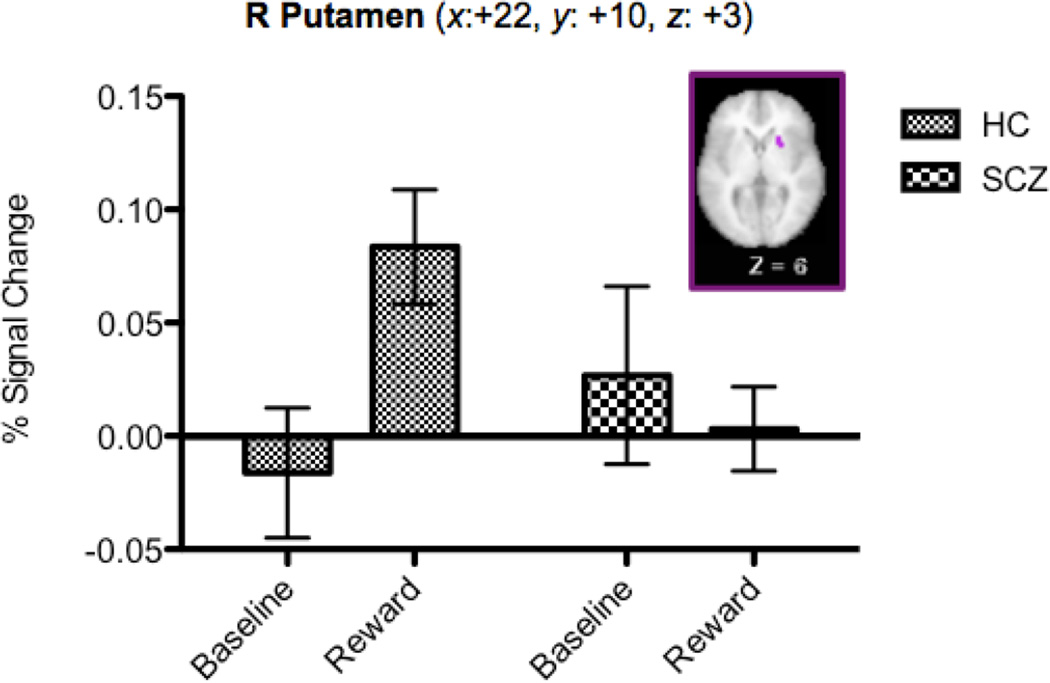
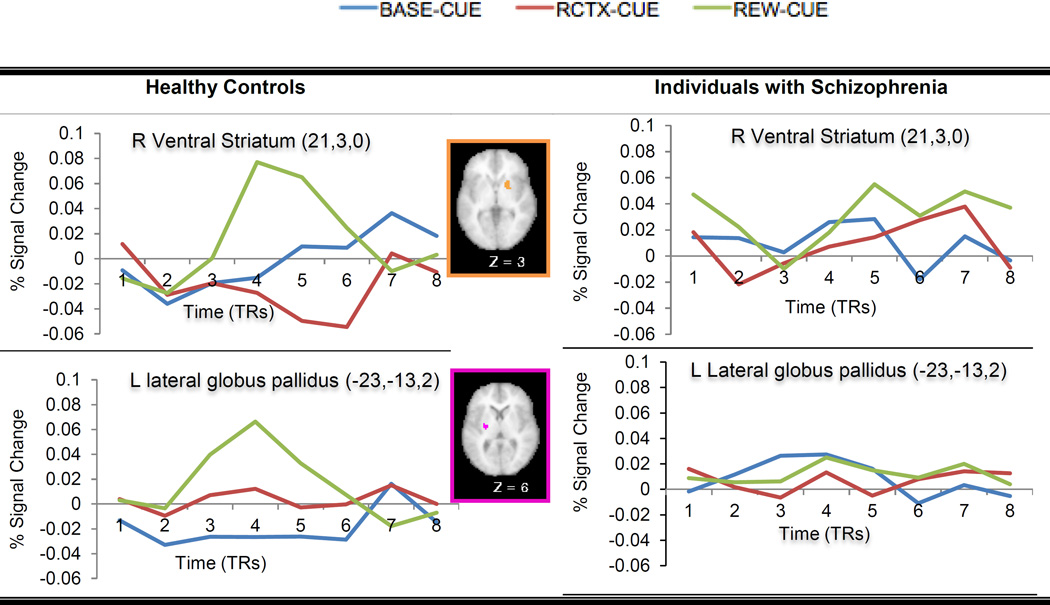
The voxel-wise repeated measures ANOVAs within the DLPFC and BG masks with Reward Condition (BASE, REWARD) as a within-subject factor and Group (HC, schizophrenia) as a between-subject factor revealed significant main effects of Reward Condition and Group, as well as of Group x Reward Condition (see Table 2). In terms of the main effect of Reward Condition, consistent with prior research, we found bilateral regions of DLPFC that showed increased sustained activity in the reward compared to the baseline blocks. In terms of the main effect of Group, we found regions in the PFC and the putamen that showed increased activity in the schizophrenia versus the HC, but bilateral regions in the lateral globus pallidus that showed decreased activity in the schizophrenia compared to the HC. Most importantly, we found a region in the putamen that showed a Group X Reward Condition interaction (see Figure 3), with HC, but not schizophrenia, showing greater activity in the reward blocks versus the baseline blocks.
Table 2.
Sustained Context-dependent Activity
| Effect | BA | Region of Activation | Cluster size (voxels) |
Talairach Coordinates |
Z | Activation Patterna |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Reward Condition |
9 | Middle Frontal Gyrus | 267 | 42 | 16 | 29 | 3.97 | REWARD > BASE |
| 9 | Middle Frontal Gyrus | 81 | −42 | 10 | 30 | 3.34 | REWARD> BASE | |
| Group | 9 | Middle Frontal Gyrus | 44 | 41 | 19 | 27 | 2.61 | SCZ > HC |
| Putamen | 25 | 14 | 10 | −3 | 2.82 | SCZ > HC | ||
| Lateral Globus Pallidus | 24 | −22 | −6 | −2 | 2.99 | HC > SCZ | ||
| Lateral Globus Pallidus | 15 | 23 | −8 | 2 | 2.78 | HC > SCZ | ||
| Reward x Group |
Putamen | 24 | 22 | 10 | 3 | 2.66 | SCZ: BASE = REWARD HC: REWARD > BASE |
|
Note. BASE= Baseline conditions; REWARD = Reward conditions, HC = healthy controls, REWARD= Reward conditions, SCZ = schizophrenia. Z values represent mean activation across the region.
Post-hoc paired t-tests or independent t--tests were conducted (all p < .05).
Figure 3.
Regions Displaying Sustained Reward x Group Interaction
Note. Error bars represent ± SEM
Relation between Sustained Activation and Amotivation in Schizophrenia
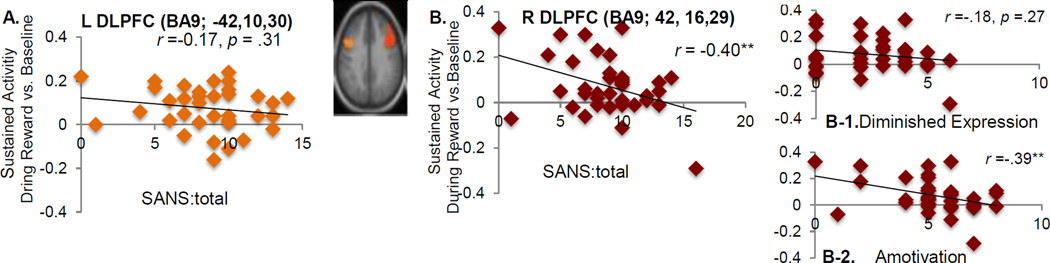
Patients with greater negative symptoms scores as measured by a total score of the SANS showed more reduced sustained activity in the right DLPFC in the REWARD Condition (Figure 4B) but no such significant association was found in the left DLPFC (Figure 4 A). Existing research suggests that the SANS includes two independent factors, which includes diminished expression (i.e., the sum of affect flattening and alogia), and amotivation (i.e., the sum of anhedonia, avolition, and asociality) (e.g., Blanchard & Cohen, 2006; Strauss, Hong, et al., 2012). Thus, we examined whether these relationships to the right DLPFC activity differed across the two subscales. Such significant association in the right DLPFC was driven by the association between individual differences in amotivation and sustained activity during the REWARD condition in the right DLPFC (r = .39 p= 0.01), an effect that passed Bonferroni correction.
Figure 4.
Relationship between Sustained DLPFC Activation and Amotivation
Note. DLPFC= dorsolateral prefrontal cortex, L = left, R= right, SANS = The Scales for the Assessment of Negative Symptoms (Andreasen, 1983b). ** p < 0.02.
Exploratory Whole Brain Analysis
Sustained Interaction of Reward and Group
A significant Group x Reward interaction was also found in the right orbitofrontal cortex (OFC) and right claustrum in the whole-brain analysis (see Table S4 for exact coordinates of each region and the pattern shown in each region). To further identify the source of these Reward x Group interactions effects, we conducted post-hoc paired t-tests to compare sustained activity during reward vs. baseline blocks for each group. As presented in Figure S6, the HC showed greater activity during reward vs. baseline blocks in both regions displaying a Reward x Group interaction [the right OFC: paired t-test (26)=3.52, p =.002), the right claustrum: paired t-test (26)= 3.51, p =.002]. In contrast, individuals with schizophrenia showed a weak effect in the opposite direction, with somewhat greater activity in baseline relative to reward blocks in the right OFC [paired t-test (35)= −2.57, p = .01] and the right claustrum at a trend level [paired t -test (35)= −2.02, p =.05].
Sustained Brain Activity and Negative Symptom Relation
There was no significant association between the sustained activity in the OFC activity during reward vs. baseline conditions and symptom severity as measured by a total score of the SANS (r = −.15, p =.35). For the right claustrum, the sustained activity contrast during reward vs. baseline was associated with the total score of the SANS (r = −.33, p =.04) but did not pass the Bonferroni correction.
Transient Cue-Related Reward Effects
The voxel-wise repeated measures ANOVAs with Cue Type (BASE-CUE, REW-CUE, RCTX-CUE) and Time points (frames 1–8 in a trial) as within-subject factors and Group (HC, schizophrenia) as a between-subject factor revealed several regions showing either significant Cue Type x Time points or Cue Type x Group x Time points interactions (Table 3). All of the regions showing Cue Type x Time points interactions, including bilateral DLPFC, showed greater activity on REW-CUE as compared to both RCTX-CUE and BASE-CUE trials. There were two regions in the BG – putamen and lateral globus pallidus that showed further interactions with Group. In both the ventral striatum and lateral globus pallidus, HC showed increased trial-by-trial activity on REW-CUE trials relative to RCTX-CUE and BASE-CUE trials while individuals with schizophrenia did not show significant differences in the trial-by-trial activity as a function of Cue Type (Figure 5).
Table 3.
Results from the Cue-related Activity Analyses
| BA | Region of Activation | Cluster size (voxels) |
Talairach Coordinates |
Z | Activation Patternsa |
|||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Reward Cue Type X Time Point Interaction | ||||||||
| Putamen | 30 | −19 | 3 | −1 | 3.24 | REW-CUE >BASE-CUE+ =RCTX-CUE | ||
| Medial Globus Pallidus | 16 | −14 | −8 | 0 | 3.33 | REW-CUE > RCTX-CUE= BASE-CUE | ||
| Caudate Body | 85 | 12 | −3 | 14 | 3.81 | REW-CUE> RCTX-CUE = BASE-CUE | ||
| Caudate Body | 30 | −14 | −10 | 19 | 3.22 | REW-CUE > RCTX-CUE = BASE-CUE | ||
| 9 | Middle Frontal Gyrus | 253 | −38 | 20 | 29 | 4.27 | REW-CUE > RCTX-CUE = BASE-CUE | |
| 9 | Middle Frontal Gyrus | 385 | 37 | 23 | 29 | 4.49 | REW-CUE > RCTX-CUE = BASE-CUE | |
| Reward Cue Type x Group x Time Point Interaction | ||||||||
| HC | SCZ | |||||||
| Putamen | 37 | 21 | 3 | 0 | 3.13 | REW-CUE > RCTX-CUE = BASE-CUE |
REW-CUE= BASE-CUE=RCTX-CUE |
|
| Lateral Globus Pallidus | 22 | −23 | −13 | 2 | 2.82 | REW-CUE > RCTX-CUE = BASE-CUE |
REW-CUE = BASE-CUE=RCTX-CUE |
|
Note. BASE-CUE=Baseline cue, HC= Healthy Controls, REW-CUE = Reward cue, RCTX-CUE= Reward-context cue, SCZ= Schizophrenia. Bold indicates deactivated regions.
Post-hoc paired t-tests were conducted to examine the relationship between neural activity on reward trials and other cue-type activity on each region (p <0.05).
p-value was 0.05
Figure 5.
Time Courses for Regions showing Reward Cue Type x Group x Time Point Interactions During Cue Phase
Note. R= Right, L= Left
Transient Target- Related Effects
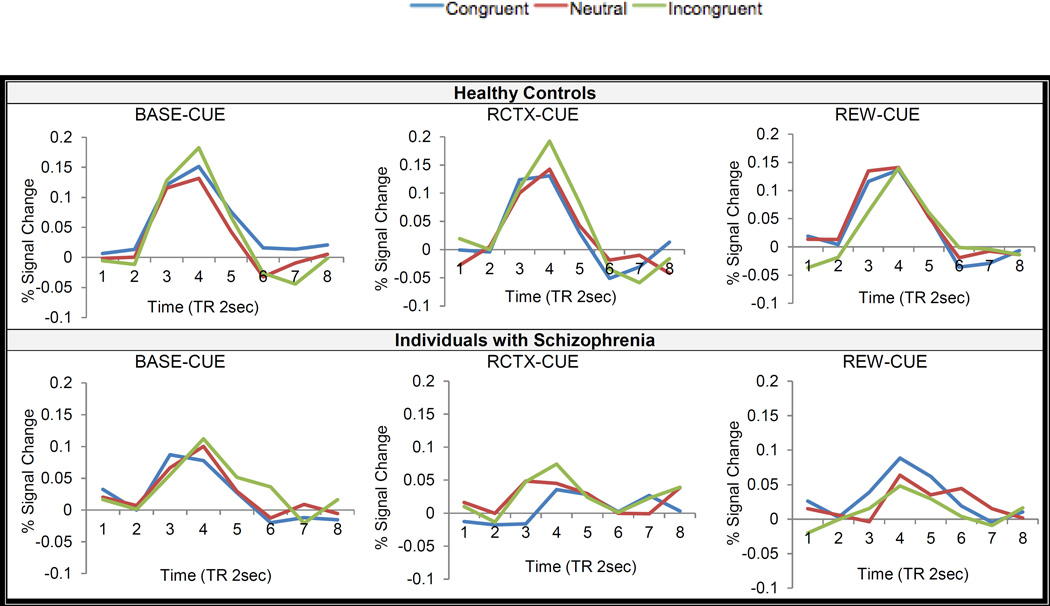
The voxel-wise repeated measures ANOVAs with Cue Type (BASE-CUE, REW-CUE, RCTX-CUE), Trial Type (Congruent, Neutral, Incongruent) and Time points as within-subject factors and Group as a between-subject factor were conducted to examine target-related activity as a function of rewards. Here, we focused on Cue Type x Group x Time points and Cue Type x Trial type x Group x Time points interaction effects as our main interests were to test whether individuals with schizophrenia could use and integrate reward-related context information to modulate their cognitive performance (see Supplement Results for the other interaction effects).
Several regions in the bilateral DLPFC, putamen and medial globus pallidus displayed Cue Type x Group x Time points interaction (see Table 4). In the putamen and medial globus pallidus, the HC did not show a different degree of target-related activity as a function of reward, while individuals with schizophrenia showed greater target-related activity on BASE-CUE than on RCTX-CUE trials at a trend level (p =.05). In the right DLPFC (x:25, y:37, z: 29), the HC showed reduced target-related activity during RCTX/REW-CUE trials vs. BASE-CUE while individuals with schizophrenia showed no different activity as a function of Cue Type in the same right DLPFC (see Figure S2 for time course).
Table 4.
Reward x Group Interaction During Target Phase
| Analysis | BA | Region of Activation | Cluster size (voxels) |
Talairach Coordinates |
Z | Activation Pattern | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | HC | SCZ | |||||||
| Basal Ganglia |
Medial Globus Pallidus | 25 | −14 | 0 | −5 | 3.31 | RCTX-CUE =REW-CUE =BASE-CUEa |
RCTX-CUE= REW-CUE = BASE-CUE |
|||
| TP3 | TP4 | TP3 | TP4 | ||||||||
| RCTX-CUE= REW- CUE = BASE-CUE |
RCTX-CUE> REW- CUE= BASE-CUE |
RCTX-CUE= REW-CUE = BASE-CUE |
|||||||||
| Putamen | 15 | 21 | 6 | 1 | 2.64 | RCTX-CUE=REW-CUE=BASE-CUE | BASE-CUE > RCTX-CUE+= REW-CUE |
||||
| DLPFC | 9 | Superior Frontal Gyrus | 16 | 25 | 37 | 29 | 2.62 | BASE-CUE >RCTX-CUE+=REW-CUE | RCTX-CUE = REW-CUE = BASE-CUE |
||
| 9 | Middle Frontal Gyrus | 51 | −34 | 23 | 33 | 3.09 | RCTX-CUE=REW-CUE=BASE-CUE | BASE-CUE> REW-CUE = RCTX-CUE |
|||
| 9 | Middle Frontal Gyrus | 20 | 43 | 26 | 30 | 2.54 | RCTX-CUE=REW-CUE=BASE-CUE | RCTX-CUE=REW-CUE = BASE-CUE |
|||
| TP3 | TP4 | TP3 | TP4 | ||||||||
| BASE-CUE>REW- CUE++=RCTX-CUE |
RCTX-CUE=REW- CUE= BASE-CUE |
RCTX-CUE=REW-CUE = BASE-CUE |
|||||||||
Note. BASE-CUE=Baseline Cue, REW-CUE = Reward cue, RCTX-CUE= Reward-context cue, TP = Time Point.
p = .05,
p =0.08.
When neural activities at the average of time point 3–4 did not differ as a function of trial-type, another paired t-tests at each time point 3 and 4 for each group were separately conducted to identify the source of significant effects
The only DLPFC a priori mask region revealed Cue Type x Trial type x Group x Time points interaction. As a follow up, we first determined whether the Cue Type x Trial type x time points interaction was significant within each group, separately. This three-way interaction was significant in the schizophrenia at a trend level [F (4, 104)= 2.18, p=.07, η2p =.05], but not the HC [F(4, 104)= .78, p=.53, η2p =.02]. Then we compared the magnitude of difference (incongruent - congruent trials) to targets during REW-CUE to RCTX-CUE in the schizophrenia and found a significantly smaller interference effect on REW-CUE trials compared to RCTX-CUE trials in the schizophrenia [paired t-test (35) = 3.17, p = .003] (see Figure 6).
Figure 6.
Time courses for the Right DLPFC Region Displaying a Reward x Trial type x Group Interaction During Target Phase
Note. BASE-CUE= Baseline cue, RCTX-CUE=Reward-Context cue, REW-CUE = Reward Cue
Relation between Transient Activation and Amotivation in Schizophrenia
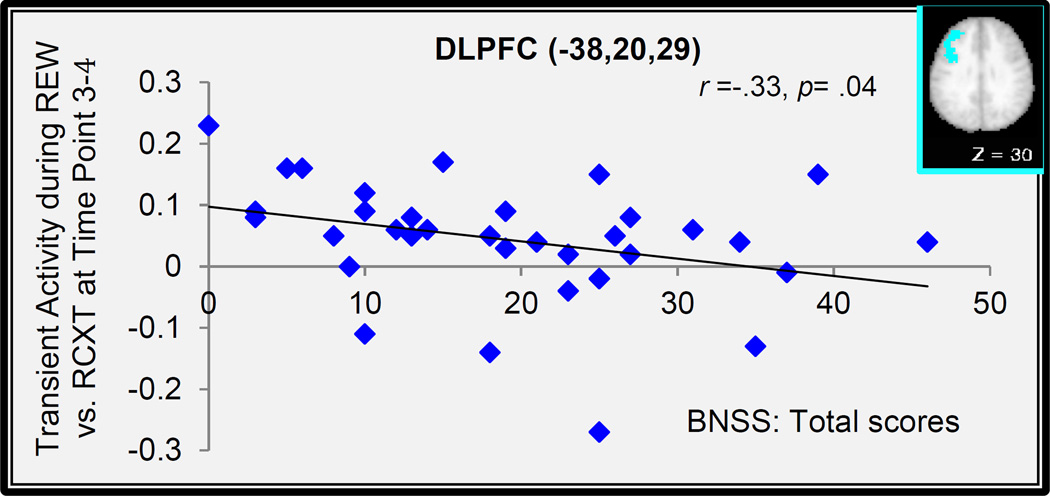
Another set of Pearson Product-Moment correlation analyses were also conducted to examine the relationship between transient cue-related reward context and/or cue effects and individual difference in amotivated symptom in the ventral striatum and lateral globus pallidus regions displaying Cue Type x Group x Time point interactions as well as in the DLPFC region displaying Cue Type x Time points interactions. We found that the transient cue effect during REW-CUE vs. RCTX-CUE trials in the left DLPFC was significantly correlated with individual difference in the BNSS, though this correlation did not pass Bonferroni correction (see Figure 7).
Figure 7.
Relationship between Cue-Related DLPFC Activation and Negative Symptoms
Note. BNSS: Brief Negative Symptom Scale, DLPFC = Dorsolateral Prefrontal Cortex.
Potential Effects of Medications or Depression on Behavior and Neural Data
We also examined whether the current results were affected either by antipsychotic medications or depressive symptoms. First, we estimated olanzapine equivalents for each individual based on Gardner et al. (2010)’s recommendations. Pearson’s Product-Moment correlations with olanzapine equivalent doses in schizophrenia (n =36) revealed no significant associations with either reward context effects (r = −0.03, p = .82) or reward cue effects (r =−0.09, p =.58) in behavior. Similarly, neither sustained nor transient neural activations as a function of rewards were associated with olanzapine equivalent doses. Furthermore, we examined the effect of antipsychotic type on activity in the regions showing significant group effects in the behavior and neural data by conducting one-way ANOVAs comparing individuals with schizophrenia taking atypical versus typical versus both medications. For sustained activity during reward vs. baseline blocks in the putamen and the DLPFC, there were no significant differences among the three groups [F (3,35) = 1.25, p= .30; F(3,35) =0.29, p=.82, respectively]. Similarly, regarding transient cue-related activity as a function of rewards, there were no significant differences among three groups in the right putamen [F(3,35) = 0.54, p= .65] or the left lateral globus pallidus [F (3,35) = 1.61, p= .20], Regarding the behavioral findings, one-way ANOVAs revealed that there were no significant differences among the three groups for behavioral reward context effects [F(3,35) = 1.98, p= .13] or for behavioral reward cue effects [F(3,35) = 1.26, p= .30]. We also did not find any significant relationships between depressive symptoms, as measured by the BDI (Beck, Steer, & Garbin, 1988), and any of the behavioral or neural measures (all ps ≥ .05) (see Tables 5 and 6).
Table 5.
No Significant Associations Between Medications and Reward Cue and Context Effects
| Group | ||
|---|---|---|
| Healthy Controls (n=27) | Individuals with Schizophrenia (n=36) |
|
| BDI1 | ||
| Reward Cue Effects | −.18 (0.35) | .06(0.70) |
| Reward Contexts Effect | −.18 (0.37) | .19 (0.26) |
| Olanzapine Equivalent Doses | ||
| Reward Cue Effects | - | −.09 (0.58) |
| Reward Context Effects | - | −.03 (0.82) |
Note. Numbers in parentheses represents p-value.
BDI= Beck Depression Inventory (Beck, 1988)
Table 6.
No significant associations between medications and neural activations
| Effect | Group | |||
|---|---|---|---|---|
| Region of Activation (Talairach coordinates) |
Olanzapine Equivalent Doses |
BDI1 in SCZ (n=36) |
BDI in HC (n=26) |
|
| Sustained Reward | DLPFC (x:42, y:16, z:29) |
−0.08 (.63) | −.33 (.05) | .24 (.22) |
| Sustained Reward X Group |
Putamen (x:22, y:10, z:3) |
−0.002 (.98) | −.31 (.06) | −.14 (.46) |
| Transient2 Reward Cue x Group x Time point |
Putamen (x: 21,y:3,z:0) |
−.09 (.57) | −.15 (.37) | −.18 (.35) |
| Lateral Globus Pallidus (x:-23, y::-13, z:2) |
.23 (.16) | .13 (.42) | −.30 (.12) | |
Note.
BDI= Beck Depression Inventory (Beck, 1988)
Numbers in parentheses represent p-value.
Pearson correlations were conducted using neural contrasts during Reward-Cue (“$20”) – Reward-Context cue (“$XX”) within reward conditions
Discussion
The goal of this study was to examine the effect of rewards on cognitive control at both behavioral and neural levels using a mixed state-item fMRI design with a special focus on the role of the DLPFC and basal ganglia system. The present results suggest dysfunctional cortico-striatal system in schizophrenia during processing reward-related context information, as evidenced by blunted sustained activations in the putamen and transient activations in the ventral striatum as a function of rewards. Importantly, patients with more severe anhedonia and/or amotivation symptoms tended to show more reduced sustained neural activations in the DLPFC as a function of rewards. These results are consistent with previous studies using various reward-processing task paradigms that might reflect deficits in integrating and transferring internal representation of reward value into action execution to guide goal-directed behavior presumably due to dysfunctional cortico-striatal system (Barch & Dowd, 2010; Gold et al., 2008; Morris et al., 2015; Waltz et al., 2009).
Behavioral Reward Context and Cue Effects
Different from our previous study (Mann et al., 2013) showing a reduced reward context effect in schizophrenia but an intact reward cue effect, we found that individuals with schizophrenia showed no significant differences from the HC in either reward cue or reward context effects. The discrepancy between two studies may be due to methodological differences. The clearest difference in the current study from Mann et al. (2013) is a modification of the task paradigm by adopting a mixed state-item fMRI design. Thus, the current study included a jittered period between the presentation of cues and target phase while our prior behavioral study did not include such a jittered period; the target phase was followed right after the presentation of each cue. Thus, it is possible that due to such jittering after cue presentation, participants had some time to prepare for upcoming stimuli, which in turn may have resulted in relatively intact pattern of reward context effects for the individuals with schizophrenia in current study. Also, different from Padmala and Pessoa (2011), we did not find cognitive control modulation as a function of rewards. Rather, we found a general enhancement of reward on cognitive performance as evidenced by faster responses during reward relative to baseline contexts.
Sustained Context-dependent Activity and Relation to Amotivation in Schizophrenia
Emerging evidence suggests that the maintenance of reward value during the entire task supported by the DLPFC system (Braver et al., 2014; Jimura et al., 2010; Kring & Barch, 2014) is crucial for the enhancement of cognitive control function. That is, keeping goals active in a sustained manner via proactive control in the DLPFC may facilitate preparatory processing and in turn, lead to enhanced cognitive performance (Chiew & Braver, 2013; Jimura et al., 2010; Locke & Braver, 2008). Considering patients’ context processing deficits in non-incentive paradigms, thought to be due to a disturbance in DLPFC function (e.g., Barch et al., 2001), we expected to find reduced sustained activations during reward context in the DLPFC in schizophrenia compared with HC. However, contrary to the prediction, but paralleling the present behavioral findings, individuals with schizophrenia showed an intact pattern of greater sustained activation in the bilateral DLPFC during reward vs. baseline contexts, as did the HC. Interestingly, a diagnostic group difference was identified in the putamen where individuals with schizophrenia showed blunted sustained activation during the reward condition.
The basal ganglia complex is a major component of the neural circuitry that is involved in reward processing in addition to the lateral prefrontal cortex (reviewed in Delgado, 2007). It should be noted that the dorsal striatum receives major input from cortical structures to execute motivated, goal-directed behaviors (Haber, 2011). The dorsal striatum is thought to be one of the motivation-sensitive regions of which neural activity is modulated by reward-related contextual information (Delgado, Locke, Stenger, & Fiez, 2003; Delgado, Stenger, & Fiez, 2004). However, we did not see evidence for behavioral differences at the group level in the reward context effects. It is possible that these would be more apparent in a more challenging task, but that is a speculation that awaits empirical testing.
Consistent with our hypothesis about sustained activation, patients with greater anhedonia/amotivation showed more reduced sustained activity during reward vs. baseline blocks in the right DLPFC. Of note, this is the region where as a group, individuals with schizophrenia showed a similar pattern to the HC of greater sustained activity during the reward vs. the baseline condition. Thus, although not all individuals with schizophrenia may show impairments in sustained DLPFC activity, those individuals with more severe anhedonia/amotivation may reflect have difficulties in the ability to represent and sustain reward value during their cognitive control function potentially due to abnormal DLPFC-medicated context processing. However, we did not find parallel relationships between individual differences in anhedonia/amotivation and the behavioral indices of reward effects. Again, this may suggest that the behavioral indices were not as sensitive as the neural indices.
Transient Cue-related Activity and Relation to Amotivation in Schizophrenia
Contrary to the traditional notion that motivational impairments in schizophrenia may come from their inability to experience pleasure (Meehl, 1989), accumulated evidence from behavioral and neuroimaging studies suggests that individuals with schizophrenia have an intact ability to experience positive events as positive in the moment. Rather, it has been argued that amotivation in schizophrenia may come from a different phase of reward processing. For example, regardless of antipsychotic medication status, several studies found less ventral striatal activation during the presentation of reward-predicting cues in individuals with schizophrenia relative to the HC (Esslinger et al., 2012; Juckel et al., 2006). In line with these findings, in the current study, individuals with schizophrenia showed blunted ventral striatal activations during reward-predicting cues.
Importantly, we predicted that individuals with schizophrenia who had greater anhedonia/amotivation would show less transient cue-related activation in the ventral striatum as a function of reward. Different from the prediction, we did not find any significant correlation between individual difference scores in anhedonia/amotivation as measured by the BNSS and neural activation in the ventral striatum. However, we did find such association in the DLPFC during REW-CUE (“$20”) vs. RCTX-CUE (“XX”). However, this correlation did not pass Bonferroni correction. As such, these associations should be considered provisional and further research is needed to replicate this relevance of anhedonia/amotivation with the DLPFC function, especially by dissociating “liking” and “wanting” (Berridge, 1996, 2007, 2012).
Implications of Cognitive Control, Motivation and Dorsolateral Prefrontal Cortex Function in Schizophrenia
As noted in the Introduction, emerging evidence points to relatively intact in-the-moment pleasurable experiences, but converges on impairments of predicting reward and translating reward value into action execution in schizophrenia, processes thought to be supported by the DLPFC function (reviewed in Barch et al., 2015). The recent focus on studying reward processing paradigms in schizophrenia has come from the hope for a better understanding of the pathophysiology of negative symptoms, in particular, motivational impairments (see Barch & Dowd, 2010; Strauss, Waltz, & Gold, 2014 for recent reviews). In this context, we have used a cognitive control paradigm (i.e., a variant of response conflict task) in reward context to infer to what extent individuals with schizophrenia would be able to use cognitive control to help them engage in real-world functioning, such as work or school activities, to achieve positive outcome. Through a mixed-state item fMRI design, we experimentally measured a presumed proxy of patients’ ability to process in-the-moment pleasurable experience (i.e., transient reward-cue effects) as well as a proxy of patients’ ability to sustain and use reward values during goal-directed behavior, such as working or school activities (i.e., sustained reward context effects).
Consistent with our predictions, the current findings suggest that motivational impairments in schizophrenia are related to dysfunctional DLPFC activity during cognitive control. Along with a body of literature showing cognitive control deficits in schizophrenia (e.g., Barch & Ceaser, 2012; Cohen et al. 1999), we propose that motivational impairments in schizophrenia might reflect patients’ inefficient use of proactive control in potentially rewarding situations. Given our results, one potentially promising pathway for developing negative symptom treatments is to enhance DLPFC-mediated cognitive control function in rewarding situations in order to improve functioning outcomes in schizophrenia. As recently reviewed by Reddy et al. (2015), there are few effective negative symptom treatments and/or interventions in the field. Only a few preliminary studies exist on this topic (reviewed in Elis, Caponigro, & Kring, 2013). For example, a preliminary study by Favrod, Giuliani, Ernst, and Bonsack (2010) found that five individuals with schizophrenia that had received between 10 hours and 25 hours of cognitive/pleasure skills training showed increased self-report anticipatory pleasure and daily activities. In recent work by Velligan et al. (2015), a nine-month motivation-enhancing treatment program using cognitive and behavioral principles showed moderate treatment effects on some negative symptom assessments, though the effect was not sustained through the full nine month period.
Schizophrenia is a heterogeneous and complex illness and not all individuals with schizophrenia show similar clinical presentations (Lang et al., 2015; Tsuang, Lyons, & Faraone, 1990). The current findings add to the body of literature suggesting that there are individual differences in anhedonia/amotivation symptoms in schizophrenia and that these are related to individual differences in DLPFC dysfunction during reward contexts. Such findings highlight the importance of a symptom-specific approach rather than only group-level comparisons. This symptom-specific approach may help to elucidate more homogeneous dimensions of this illness, which in turn can be used to define clearer psychopathologic endophenotypes of schizophrenia.
Overall Limitations
The primary limitation of the current study was that most individuals with schizophrenia were taking dopamine receptor blocking antipsychotic medications, which could affect reward-related neural responses (Abler, Erk, & Walter, 2007; Juckel et al., 2006; Mathews et al., 2012; Nielsen et al., 2012). Despite a potential impact of antipsychotic medication on reward processing, importantly, it is highly unlikely that current findings are purely related to antipsychotic action, as motivational deficits, as evidenced by abnormality of reward-related neural responses, have been observed even in unmedicated patients with first-episode schizophrenia (Esslinger et al., 2012; Schlagenhauf et al., 2009) and individuals at prodromal phase o f the illness (Piskulic et al., 2012; Wotruba et al., 2014; Yung & McGorry, 1996). Importantly, however, our supplemental analysis did not find relationships between neural and behavioral results and antipsychotic medication doses (see Table 5 and Table 6).
Another limitation is that fixed-order task presentations may have impacted current pattern of results: non-incentive baseline conditions were always followed by reward conditions. Thus, there is a possibility that current results might include practice-related effects due to this fixed-order. However, we believe that it is unlikely given empirical evidence of no practice effects from prior work by Chiew and Braver (2013) where participants performed baseline and then reward blocks with reward and no-reward cues randomly intermixed, like our design. When they broke down each block into four epochs, potential practice effects after the first epoch disappeared, while incentive effects were sustained throughout. Additionally, our behavioral data shows general enhancement of rewards on enhancing speed of cognitive performances across groups, but did not show interaction effect of reward and cognitive control different from prior work by Padmala and Pessoa (2011). There is a possibility that task difficulty of current conflict processing was not challenging enough to require cognitive control for participants to yield such interaction effect. Future studies could use task paradigms with increased difficulty for participants. For example, switching between tasks may increase difficulty for participants. Also, it is possible to observe stronger effects for larger amount of rewards in future work. Finally, general negative symptom severity as measured by the BNSS for current participants was somewhat low compared to some other validation studies of the BNSS (Mucci et al., 2015; Strauss, Keller, et al., 2012). Thus, we cannot exclude the possibility that the relatively low level of negative symptom severity for our current participants may have contributed to our not finding diagnostic group differences in reward context effects. It would be useful to include participants with a wider range of negative symptom severity to address this in future studies.
Summary
At a behavioral level, individuals with schizophrenia did not differ significantly from controls in either reward cue or reward context behavioral effects. Further, at the neural level, individuals with schizophrenia, as a group, showed an intact pattern of greater sustained activity during reward context in the bilateral DLPFC like the HC. However, individual difference analyses revealed that greater amotivation symptoms were associated with reduced sustained context-dependent activity in the DLPFC as a function of rewards. Taken together, the current study provided neural evidence suggesting the relevance of patients’ DLPFC function during reward processing to amotivation symptoms of schizophrenia.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health Grant Number R01-MH066031 (grant awardee: Deanna M. Barch, Ph.D). One of co-authors, Deanna M. Barch consults for Pfizer, Amgen Roche and Takeda.
Abbreviations
- BASE
Baseline conditions
- BASE-CUE
Baseline cue cued by “XX” in baseline conditions
- HC
Healthy controls
- REWARD
Reward conditions
- RCTX-CUE
Reward context trials cued by “XX” in reward conditions
- REW-CUE
Reward cue trials cued by “$20” in reward conditions
Footnotes
All authors have no financial or conflicts of interest.
References
- Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology (Berl) 2007;191(3):823–833. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1983b. [Google Scholar]
- Bahlmann J, Aarts E, D’Esposito M. Influence of motivation on control hierarchy in the human frontal cortex. Journal of Neuroscience. 2015;35(7):3207–3217. doi: 10.1523/JNEUROSCI.2389-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry. 2001;58(3):280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal Representations and Motivational Drive in Schizophrenia: The Role of Prefrontal-Striatal Interactions. Schizophrenia Bulletin. 2010 doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends in Cognitive Sciences. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Pagliaccio D, Luking K. Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Current Topics in Behavioral Neurosciences. 2015 doi: 10.1007/7854_2015_376. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Beck LH, Bransome ED, Jr, Mirsky AF, Rosvold HE, Sarason I. A continuous performance test of brain damage. Journal of Consulting Psychology. 1956;20(5):343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience & Biobehavioral Reviews. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. European Journal of Neuroscience. 2012;35(7):1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best MW, Grossman M, Oyewumi LK, Bowie CR. Examination of the Positive and Negative Syndrome Scale factor structure and longitudinal relationships with functioning in early psychosis. Early Intervention in Psychiatry. 2014 doi: 10.1111/eip.12190. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophrenia Bulletin. 2006;32(2):238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annual Review of Psychology. 2015;66:83–113. doi: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biological Psychiatry. 1999;46(3):312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Krug MK, Chiew KS, Kool W, Westbrook JA, Clement NJ, Somerville LH. Mechanisms of motivation-cognition interaction: challenges and opportunities. Cognitive,Affective, & Behavioral Neuroscience. 2014 doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychological Bulletin. 1973;79(6):380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Frontiers in Psychology. 2013;4:15. doi: 10.3389/fpsyg.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychological Review. 1992;99(1):45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology. 1999;108(1):120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophrenia Bulletin. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel DG. Issues in selection of instruments to measure negative symptoms. Schizophrenia Research. 2013;150(2–3):343–345. doi: 10.1016/j.schres.2013.07.005. [DOI] [PubMed] [Google Scholar]
- De Leeuw M, Kahn RS, Vink M. Fronto-striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophrenia Bulletin. 2015;41(1):94–103. doi: 10.1093/schbul/sbu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delawalla Z, Csernansky JG, Barch DM. Prefrontal cortex function in nonpsychotic siblings of individuals with schizophrenia. Biological Psychiatry. 2008;63(5):490–497. doi: 10.1016/j.biopsych.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive,Affective, & Behavioral Neuroscience. 2003;3(1):27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14(9):1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annuals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Christoff K. The lateral prefrontal cortex and complex value-based learning and decision making. Neuroscience & Biobehavioral Reviews. 2014;45c:9–18. doi: 10.1016/j.neubiorev.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One. 2012;7(5):e35622. doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clinical Psychology Review. 2013;33(8):914–928. doi: 10.1016/j.cpr.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Englisch S, Inta D, Rausch F, Schirmbeck F, Mier D, Zink M. Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophrenia Resesarch. 2012;140(1–3):114–121. doi: 10.1016/j.schres.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychological Bulletin. 1999;125(6):777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Favrod J, Giuliani F, Ernst F, Bonsack C. Anticipatory pleasure skills training: a new intervention to reduce anhedonia in schizophrenia. Perspectives in Psychiatric Care. 2010;46(3):171–181. doi: 10.1111/j.1744-6163.2010.00255.x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonances in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2(1):45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. American Journal of Psychiatry. 2010;167(6):686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, Steele JD. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134(Pt 6):1751–1764. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia Bulletin. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Frank MJ. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Archives of General Psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. Frontiers in Neuroscience Neuroanatomy of Reward: A View from the Ventral Striatum. In: Gottfried JA, editor. Neurobiology of Sensation and Reward. Boca Raton (FL): CRC Press.Llc; 2011. [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology. 2007;116(2):268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biological Psychiatry. 2008;64(1):62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Matveeva TM, Gold JM. Imagining the future: degraded representations of future rewards and events in schizophrenia. Journal of Abnormal Psychology. 2011;120(2):483–489. doi: 10.1037/a0021810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia Bulletin. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The brief negative symptom scale: psychometric properties. Schizophrenia Bulletin. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Ronshausen S, Mier D, Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40(5):196–198. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- Koch K, Schachtzabel C, Wagner G, Schikora J, Schultz C, Reichenbach JR, Schlosser RG. Altered activation in association with reward-related trial-and-error learning in patients with schizophrenia. Neuroimage. 2010;50(1):223–232. doi: 10.1016/j.neuroimage.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience. 2009;12(7):939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D’Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Research. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Kring AM, Kerr SL, Smith DA, Neale JM. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. Journal of Abnormal Psychology. 1993;102(4):507–517. doi: 10.1037//0021-843x.102.4.507. [DOI] [PubMed] [Google Scholar]
- Kring AM, Caponigro JM. Emotion in Schizophrenia: Where Feeling Meets Thinking. Current Directions in Psychological Science. 2010;19(4):255–259. doi: 10.1177/0963721410377599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: Neural substrates and behavioral outputs. European Journal of Neuroscience. 2014 doi: 10.1016/j.euroneuro.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang FU, Walther S, Stegmayer K, Anderson-Schmidt H, Schulze TG, Becker T, Jager M. Subtyping schizophrenia: A comparison of positive/negative and system-specific approaches. Comprrehensive Psychiatry. 2015;61:115–121. doi: 10.1016/j.comppsych.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, Carter CS. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage: Clinical. 2013;2:590–599. doi: 10.1016/j.nicl.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cognitive,Affective, & Behavioral Neuroscience. 2008;8(1):99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Goghari VM, Hicks BM, Flory JD, Carter CS, Manuck SB. A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology. 2005;19(6):814–821. doi: 10.1037/0894-4105.19.6.814. [DOI] [PubMed] [Google Scholar]
- Mann CL, Footer O, Chung YS, Driscoll LL, Barch DM. Spared and impaired aspects of motivated cognitive control in schizophrenia. Journal of Abnormal Psychology. 2013;122(3):745–755. doi: 10.1037/a0033069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J, Newcomer JW, Mathews JR, Fales CL, Pierce KJ, Akers BK, Barch DM. Neural correlates of weight gain with olanzapine. Archives of General Psychiatry. 2012;69(12):1226–1237. doi: 10.1001/archgenpsychiatry.2012.934. [DOI] [PubMed] [Google Scholar]
- McClure MM, Barch DM, Flory JD, Harvey PD, Siever LJ. Context processing in schizotypal personality disorder: evidence of specificity of impairment to the schizophrenia spectrum. Journal of Abnormal Psychology. 2008;117(2):342–354. doi: 10.1037/0021-843X.117.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia revisited. Archives of General Psychiatry. 1989;46(10):935–944. doi: 10.1001/archpsyc.1989.01810100077015. [DOI] [PubMed] [Google Scholar]
- Michelon P, Snyder AZ, Buckner RL, McAvoy M, Zacks JM. Neural correlates of incongruous visual information. An event-related fMRI study. Neuroimage. 2003;19(4):1612–1626. doi: 10.1016/s1053-8119(03)00111-3. [DOI] [PubMed] [Google Scholar]
- Morris RW, Vercammen A, Lenroot R, Moore L, Langton JM, Short B, Weickert TW. Disambiguating ventral striatum fMRI-related bold signal during reward prediction in schizophrenia. Molecular Psychiatry. 2012;17(3):280–289. doi: 10.1038/mp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Quail S, Griffiths KR, Green MJ, Balleine BW. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biological Psychiatry. 2015;77(2):187–195. doi: 10.1016/j.biopsych.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Mucci A, Galderisi S, Merlotti E, Rossi A, Rocca P, Bucci P, Maj M. The Brief Negative Symptom Scale (BNSS): Independent validation in a large sample of Italian patients with schizophrenia. European Psychiatry. 2015;30(5):641–647. doi: 10.1016/j.eurpsy.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Broberg BV, Lublin H, Glenthoj B. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Archives of General Psychiatry. 2012;69(12):1195–1204. doi: 10.1001/archgenpsychiatry.2012.847. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Padmala S, Pessoa L. Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. Journal of Cognitive Neuroscience. 2011;23(11):3419–3432. doi: 10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Dubis JW. The mixed block/event-related design. Neuroimage. 2012;62(2):1177–1184. doi: 10.1016/j.neuroimage.2011.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, McGlashan TH. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Research. 2012;196(2–3):220–224. doi: 10.1016/j.psychres.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cerebral Cortex. 1995;5(4):323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Green MF. Motivational Deficits and Negative Symptoms in Schizophrenia: Concepts and Assessments. Current Topics in Behavioral Neurosciences. 2015 doi: 10.1007/7854_2015_379. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264(5581):57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Robertson BR, Prestia D, Twamley EW, Patterson TL, Bowie CR, Harvey PD. Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schizophrenia Research. 2014;160(1–3):136–141. doi: 10.1016/j.schres.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Watanabe M. Integration of cognitive and motivational information in the primate lateral prefrontal cortex. Annuals of the New York Academy of Sciences. 2007;1104:89–107. doi: 10.1196/annals.1390.010. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Koychev I, Correa M, McGuire P. Neurobiological basis of motivational deficits in psychopathology. European Neuropsychopharmacology. 2015;25(8):1225–1238. doi: 10.1016/j.euroneuro.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 2008;196(4):673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, Heinz A. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biological Psychiatry. 2009;65(12):1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Huys QJ, Deserno L, Rapp MA, Beck A, Heinze HJ, Heinz A. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage. 2014;89:171–180. doi: 10.1016/j.neuroimage.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Archives of General Psychiatry. 1996;53(12):1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, Kaiser S. Neural correlates of reward processing in schizophrenia--relationship to apathy and depression. Schizophrenia Research. 2010;118(1–3):154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biological Psychiatry. 2011;69(5):424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Hong LE, Gold JM, Buchanan RW, McMahon RP, Keller WR, Kirkpatrick B. Factor structure of the Brief Negative Symptom Scale. Schizophrenia Research. 2012;142(1–3):96–98. doi: 10.1016/j.schres.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, Kirkpatrick B. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophrenia Research. 2012;142(1–3):88–92. doi: 10.1016/j.schres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophrenia Bulletin. 2014;(40 Suppl 2):S107–S116. doi: 10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowska I, Szymanska O, Marchewka A, Soluch P, Rymarczyk K. Dissociable contributions of the left and right posterior medial orbitofrontal cortex in motivational control of goal-directed behavior. Neurobiology of Learning and Memory. 2011;96(2):385–391. doi: 10.1016/j.nlm.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. NEw York: Thieme; 1988. [Google Scholar]
- Tsuang MT, Lyons MJ, Faraone SV. Heterogeneity of schizophrenia. Conceptual models and analytic strategies. British Journal of Psychiatry. 1990;156:17–26. doi: 10.1192/bjp.156.1.17. [DOI] [PubMed] [Google Scholar]
- Ucok A, Ergul C. Persistent negative symptoms after first episode schizophrenia: A 2-year follow-up study. Schizophrenia Research. 2014;158(1–3):241–246. doi: 10.1016/j.schres.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Roberts D, Mintz J, Maples N, Li X, Medellin E, Brown M. A randomized pilot study of MOtiVation and Enhancement (MOVE) Training for negative symptoms in schizophrenia. Schizophrenia Research. 2015;165(2–3):175–180. doi: 10.1016/j.schres.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Subotnik KL, Gitlin MJ, Gretchen-Doorly D, Ered A, Villa KF, Nuechterlein KH. Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8years later. Schizophrenia Research. 2014 doi: 10.1016/j.schres.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Heckers S, Kassubek J, Erk S, Frasch K, Abler B. Further evidence for aberrant prefrontal salience coding in schizophrenia. Frontiers in Behavavioral Neuroscience. 2010;3:62. doi: 10.3389/neuro.08.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, Stein EA. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34(6):1567–1577. doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Ross TJ, Kurup PK, Salmeron BJ, Rose EJ, Stein EA. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35(12):2427–2439. doi: 10.1038/npp.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, Csernansky JG. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biological Psychiatry. 2008;64(12):1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Sakagami M. Integration of cognitive and motivational context information in the primate prefrontal cortex. Cerebral Cortex. 2007;(17 Suppl 1(1)):101–109. doi: 10.1093/cercor/bhm067. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16(4):620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Wotruba D, Heekeren K, Michels L, Buechler R, Simon JJ, Theodoridou A, Kaiser S. Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Frontiers in Behavavioral Neuroscience. 2014;8:382. doi: 10.3389/fnbeh.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophrenia Bulletin. 1996;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.