Abstract
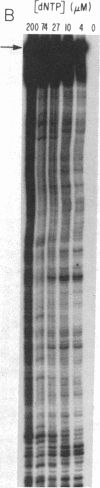
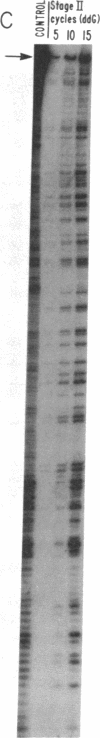
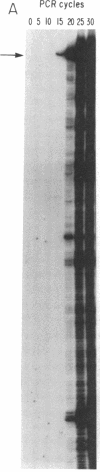
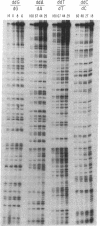
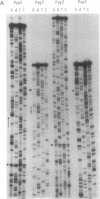
Addition of dideoxyribonucleotides during the exponential phase of the PCR should result in the synthesis of two complementary sequence ladders. We have explored this hypothesis to develop coupled amplification and sequencing of genomic DNA. Coupled amplification and sequencing is a biphasic method for sequencing both strands of template as they are amplified. Stage I selects and amplifies a single target from the genomic DNA sample. Stage II accomplishes the sequencing as well as additional amplification of the target using aliquots from the stage I reaction mixed with end-labeled primer and dideoxynucleotides. We have successfully applied coupled amplification and sequencing to a 300-base-pair fragment 4 kilobases upstream from HOX2B directly from human whole genomic DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carothers A. M., Urlaub G., Mucha J., Grunberger D., Chasin L. A. Point mutation analysis in a mammalian gene: rapid preparation of total RNA, PCR amplification of cDNA, and Taq sequencing by a novel method. Biotechniques. 1989 May;7(5):494-6, 498-9. [PubMed] [Google Scholar]
- Cavalli-Sforza L. L. Opinion: how can one study individual variation for 3 billion nucleotides of the human genome? Am J Hum Genet. 1990 Apr;46(4):649–651. [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Kieffer-Higgins S. Multiplex DNA sequencing. Science. 1988 Apr 8;240(4849):185–188. doi: 10.1126/science.3353714. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Hoener P. A., Collins F. S. Direct sequencing of enzymatically amplified human genomic DNA. Proc Natl Acad Sci U S A. 1988 Jan;85(2):544–548. doi: 10.1073/pnas.85.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., Edwards A., Civitello A. B., Caskey C. T. Multiplex DNA deletion detection and exon sequencing of the hypoxanthine phosphoribosyltransferase gene in Lesch-Nyhan families. Genomics. 1990 Jun;7(2):235–244. doi: 10.1016/0888-7543(90)90545-6. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- Mitchell L. G., Merril C. R. Affinity generation of single-stranded DNA for dideoxy sequencing following the polymerase chain reaction. Anal Biochem. 1989 May 1;178(2):239–242. doi: 10.1016/0003-2697(89)90631-3. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989 Nov 11;17(21):8889–8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988 Nov;120(3):621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen D. B., Eckstein F. Incomplete primer extension during in vitro DNA amplification catalyzed by Taq polymerase; exploitation for DNA sequencing. Nucleic Acids Res. 1989 Dec 11;17(23):9613–9620. doi: 10.1093/nar/17.23.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M., Hood L., Cantor C., Botstein D. A common language for physical mapping of the human genome. Science. 1989 Sep 29;245(4925):1434–1435. doi: 10.1126/science.2781285. [DOI] [PubMed] [Google Scholar]
- Päbo S. Ancient DNA: extraction, characterization, molecular cloning, and enzymatic amplification. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1939–1943. doi: 10.1073/pnas.86.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter B. J., Caskey C. T. Molecular scanning methods of mutation detection. J Biol Chem. 1990 Aug 5;265(22):12753–12756. [PubMed] [Google Scholar]
- Ruano G., Fenton W., Kidd K. K. Biphasic amplification of very dilute DNA samples via 'booster' PCR. Nucleic Acids Res. 1989 Jul 11;17(13):5407–5407. doi: 10.1093/nar/17.13.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano G., Gray M. R., Miki T., Ferguson-Smith A. C., Ruddle F. H., Kidd K. K. Monomorphism in humans and sequence differences among higher primates for a sequence tagged site (STS) in homeo box cluster 2 as assayed by denaturing gradient electrophoresis. Nucleic Acids Res. 1990 Mar 11;18(5):1314–1314. doi: 10.1093/nar/18.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano G., Kidd K. K., Stephens J. C. Haplotype of multiple polymorphisms resolved by enzymatic amplification of single DNA molecules. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6296–6300. doi: 10.1073/pnas.87.16.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Dryja T. P. Detection of DNA sequence polymorphisms by enzymatic amplification and direct genomic sequencing. Am J Hum Genet. 1989 Oct;45(4):547–555. [PMC free article] [PubMed] [Google Scholar]