Abstract
BACKGROUND
Driver age and blood alcohol concentration are both important factors in predicting driving risk, however little is known regarding the joint import of these factors on neural activity following socially-relevant alcohol doses. We examined age and alcohol effects on brain oscillations during simulated driving, focusing on two region-specific frequency bands implicated in task performance and attention: posterior alpha power (PAP; 8–12 Hz) and frontal theta power (FTP; 4–7 Hz).
METHODS
Participants included 80 younger (25–35 years) and 40 older (55–70 years) community-dwelling, moderate drinkers. Participants consumed placebo, low, or moderate doses of alcohol designed to achieve target peak BrACs of 0, .04 or .065 g/dL, respectively. Electrophysiology was collected during engagement in a simulated driving task involving four scenarios of varied environmental complexity.
RESULTS
A main effect of age was detected in FTP, but neither an alcohol effect nor interactions were observed. For PAP, an age by alcohol interaction was detected. Relative to placebo controls, older and younger participants receiving low dose (.04 g/dL) alcohol evinced divergent PAP alterations, with a pattern of higher power among older participants and lower power among younger. This interaction was noted across the varied environmental contexts.
DISCUSSION
These findings are consistent with the hypothesis that compared to younger individuals, older drivers may be differentially susceptible to alcohol effects. While these age by alcohol interactions in neural activity are provocative, further investigation exploring the mechanisms and behavioral correlates of these effects will be crucial in determining their behavioral impact.
Keywords: Alcohol, Aging, EEG, Driving, Spectral Power
Background
A large literature has examined behavioral risks associated with blood alcohol content of .08 g/dL and above. Less attention has been directed to moderate doses associated with social drinking. However epidemiological data indicate significant increases in crash likelihood at BACs of .04 g/dL (e.g., Borkenstein et al., 1964; Blomberg et al., 2005), and are supported by driving simulation studies (Mets et al., 2011; West et al., 1993). Converging evidence suggests that older age may also increase driving risk (e.g., Cicchino et al., 2015). By 2020, there will be approximately 56 million adults over the age of 65 in the United States (US Census Bureau, 2012), with approximately 48 million active drivers. Current estimates suggest that at least 41% of this older population are current drinkers (SAMHSA, 2014), with few examinations of age by alcohol interactions in driving reported, particularly at doses below the legal limit. Relative to younger individuals, perceptual and cognitive errors are implicated more often in at-fault accidents involving older drivers (McGwin & Brown, 1999). Although neurobehavioral processes in older drivers have received attention (e.g., Dawson et al., 2010; Aksan et al., 2015), these processes are seldom examined under active alcohol conditions (but see Sklar et al., 2014). The current work investigates neural oscillations in older and younger groups of adults following consumption of low-to-moderate doses of alcohol using a simulated driving task.
Electroencephalographic (EEG) power reflects synchronous neuronal discharge. These brain oscillations are the biophysical result of complex neuronal network activity and interactions (Buzsaki & Draguhn, 2004), correlate strongly with functional magnetic resonance imaging measures of brain activation (Laufs et al., 2003; Zumer et al., 2014), and are associated with a range of neurobehavioral processes (e.g., Bashivan et al., 2014). Of relevance to the current study, EEG power in the theta (4–7 Hz) and alpha (8–12 Hz) bands is linked to a diverse set of processes with attentional components. These include visuo-spatial processing, working memory, vigilance, motor control, and performance monitoring. Generally, engagement of these processes is reflected by increases in theta with concomitant decreases in alpha, correlating with the degree of task difficulty/required effort. Although numerous studies have investigated alpha/theta involvement in specific processes (e.g., Missonnier et al., 2011) using neurocognitive tasks (e.g., Stroop), few examine their fluctuation in tasks with high ecological relevance. To this end, simulated driving provides particular utility; its complexity allows examination of neural oscillations during the simultaneous and effortful engagement of numerous neurobehavioral processes, while its face validity allows valuable insight into neural activity during potentially dangerous “real-world” vehicle operation.
The available work examining alterations in EEG power associated with moderate alcohol consumption do not include simulated driving or attention-based laboratory tasks. However, two investigations remain notable. Kovacevic and colleagues (2012) detected reductions in theta following .55–.6 g/kg doses. Boha and colleagues (2009) also noted reductions following a .2 g/kg dose. These studies either did not report alpha band data or failed to detect differences between alcohol and placebo groups during task participation.
Although varying by task and brain region, age differences in electrophysiology measures are often noted (e.g., McEvoy et al., 2001; Missonnier et al., 2011; Gaal et al, 2010; Babiloni et al, 2004). Unfortunately, their examination under acute alcohol conditions is lacking. Our group has begun to interrogate these effects using low-to-moderate alcohol doses. Relative to younger adults, older individuals consuming low-to-moderate doses exhibit psychomotor and set-shifting impairments (Boissoneault et al., 2014; Gilbertson et al., 2009), and employ alternative response strategies on attention tasks (Sklar et al., 2012). We also observed age by alcohol interactions in event-related EEG measures (ERPs) in an attentional task (Lewis et al., 2013); older adults displayed diminished P300 amplitude , relative to placebo. Further, we have detailed age by alcohol interactions in simulated driving behavior, with older adults exhibiting greater impairments in steering rate and speed deviation following low-to-moderate doses (Sklar et al., 2014). Most recently, we investigated alterations in spectral power during working memory maintenance (Boissoneault et al., in press), demonstrating age by alcohol interactions in alpha band power under low dose conditions (.04 g/dl).
In the current work, we examined age and alcohol effects on spectral EEG power during simulated driving. Participants engaged in four driving scenarios with varying environmental complexity and task demands. Based on our previous work, we hypothesized age by alcohol interactions in alpha power during simulated driving. Specifically, due to the attentional components of the driving task, we hypothesized that under acute conditions younger individuals would display lower alpha power, associated with facilitation of attention to external stimuli, while older individuals would fail to engage this suppression, consistent with findings during working memory maintenance (Boissoneault et al., in press). Whether this interaction would persist across driving scenarios and alcohol doses remained an empirical question. Due to conflicting reports in the literature, and our failure to detect theta differences in previous examinations, the degree to which age, alcohol, or their combination might impact theta power also remained an open question.
Methods
Participants
80 younger (25–35 years; 37 women) and 40 older (55–70 years; 15 women) community-dwelling, adult, moderate drinkers, completed the driving simulation protocol. Study recruitment was conducted in north and north-central Florida. Participants were divided into three groups: placebo (n=37, 25 younger, 15 women); .04 g/dL (n=41, 27 younger, 17 women); .065 g/dL (n=42, 28 younger, 20 women). The University of Florida Medical Institutional Review Board approved all procedures.
Screening & Exclusionary Criteria
After completing a brief phone interview, qualifying participants completed a screening session to assess eligibility. Affective measures included inventories for depressive symptoms (Beck Depression Inventory [BDI-II] for younger individuals [Beck et al., 1996]; Geriatric Depression Scale [GDS] for older individuals [Yesavage et al., 1982]) and state anxiety (Anxiety Inventory [AI]; Spielberger, 1983). Demographics, alcohol/substance use histories, and physical/mental health histories were assessed through self-report. Daily alcohol consumption in absolute ounces/day was indexed using a quantity/frequency index (QFI; Cahalan, 1969); for ease of interpretation, these data were converted to “typical drinks/day”. Participants reported their current, routine consumption of over-the-counter and prescription medications. Participants were administered a computerized diagnostic interview (cDIS; Robins et al., 1995) to assess probabilistic psychiatric symptomatology (Axis-I psychiatric diagnoses) consistent with the DSM-IV (American Psychiatric Association, 1994).
Participants were excluded from the study if they a) were not a moderate drinker (USDA/USDHHS, 2010), b) did not have an active driver’s license, c) met cDIS criteria for a probabilistic Axis-I disorder, d) reported a medical condition that might induce cognitive abnormalities (e.g. untreated high blood pressure, head injury with unconsciousness), e) were not yet stabilized (≥3 months) on acceptable medications or were taking medications that contraindicated alcohol consumption, f) were nicotine users, g) tested positive on a pregnancy or drug urine screen (tetrahydrocannabinol, cocaine, benzodiazepines, morphine, and methamphetamine), or h) became nauseated or disoriented during the simulator practice session. Consistent with other reports (Matas et al., 2015), disorientation in the simulator was primarily experienced among older women. 5 younger participants (4 women) and 28 older participants (23 women) experiencing simulator sickness during screening were excluded from the current study.
Medication Use
Medication use in this sample is detailed in previous work (Boissoneault et al., 2014). Briefly, a higher proportion of older than younger adults reported use (61% vs. 25%), with their most commonly reported medications including non-opioid analgesics (18% of older adults) and cholesterol medications (16% of older adults). Participants were instructed to avoid use of sleep aids the night before testing and consumption of sedating allergy medications the day of testing. Compliance was confirmed prior to alcohol administration.
Alcohol Administration
Participants were instructed to fast for at least 4 hours prior to the session. Participants arrived at the laboratory at approximately 9:30 AM, provided consent for laboratory procedures, and were provided with a light breakfast (~220 kcal). To ensure eligibility, participants were administered a urine drug screen, and women of child-bearing potential were administered a pregnancy test. Within age and sex groups, participants were randomly assigned to one of three dose groups. Widmark calculations (Watson et al., 1981; Widmark, 1932) accounting for age, sex, height and weight, were conducted to estimate alcohol volumes required to achieve peak breath alcohol concentrations (BrACs) of 0 g/dL (placebo), .04 g/dL (low), or .065 g/dL (moderate). Alcoholic drinks consisted of 200-proof medical grade alcohol added to 366 ml of chilled, sugar free, caffeine free, citrus soda. Placebo beverages consisted only of the vehicle solution but were sprayed with a small amount of surface alcohol to provide sensory cues, enhance placebo effectiveness and improve control of alcohol expectancy effects. Consistent with our previous work (e.g., Boissoneault et al., in press), beverages were consumed within 5 minutes (2 min/beverage with 1 min in between). Twenty-five minutes after beverage consumption, an additional beverage was administered to all participants. To sustain the target BrAC, participants whose BrACs were <50% of the target (i.e., <.02 or <.0325 g/dL) were administered an active alcohol booster containing half the original alcohol dose. Four participants received a booster beverage: n=2 in the .04 dose group (1 older) and n=2 in the .065 group (1 older). Remaining participants received a placebo booster. To maintain the double-blind nature of the experiment, drink preparation, delivery, and BrAC checks were conducted by study staff who did not participate in testing procedures. BrACs (Intoxilyzer, Model 400; CMI, Inc., Owensboro, KY) and measures of subjective intoxication (1–10 scale, “no intoxication” to “most intoxicated I have ever been”) were gathered throughout the testing session, at 10, 25, 60, 75 and 85 minutes post-consumption. The electronic display on the Intoxilyzer was obscured during breath collection to maintain the blind.
Simulated Driving Device
The STISIM Drive simulator (Systems Technology Inc., Hawthorne, CA) consisted of three monitors placed to provide central and peripheral visual stimuli. The monitors displayed rear/side-view mirrors and a speedometer. Speakers presented vehicle sounds (e.g., acceleration) and voice commands for navigation (e.g., “turn left at the stop sign”). Participants operated the simulator with a steering wheel, a brake pedal, an accelerator pedal, and a turn signal indicator.
Simulated Driving Scenarios
At conclusion of the screening session, participants were given practice in the driving simulator for approximately 20 minutes, including experience with the four scenarios encountered during testing. The order of scenario exposure was varied between sessions to minimize sequencing effects. Participants began the task at 60 minutes following initial beverage consumption, during the descending limb of the BrAC curve. Prior to task initiation participants were instructed to maintain a position in the center of their lane, keep both hands on the steering wheel, signal at turns, and obey all traffic laws (including posted speed limits).
The ‘precision drive’ (PD) scenario included a sparse rural setting on an intersection-free, two lane road. Oncoming traffic that required no action on the part of the participant was infrequently presented. No other vehicles were present in the subject’s lane. The speed limit was 55 mph. The ‘country drive’ (CD) scenario introduced stops, traffic events (e.g., car running stop sign), and intersections. Oncoming traffic, cross-traffic (at intersections), and traffic in the subject’s lane were all included. The off-road landscape remained sparse and rural. The speed limit was 45 mph. In the ‘small-town drive’ (SD) scenario the landscape was suburban, with buildings introduced on either side of the road. The density of cars was increased relative to CD, and pedestrians were introduced. The speed limit was 35 mph. The ‘Metropolis Drive’ (MD) was densely populated with buildings, pedestrians, and bicyclists. Although the speed limit remained 35 mph, traffic flow frequently necessitated lower speeds and more stops than the SD. The driving simulation task was constructed to equate time-to-completion across scenarios. Thus, although scenarios differed by distance, each was completed in approximately 3.5 min. Each scenario was presented only once during testing. Presentation order was counterbalanced between practice and test, but always began with the PD scenario. Including breaks for BrAC collection and transition time between scenarios, total time spent in the driving task was approximately 20 min.
Electrophysiological Procedures
Participants were fitted with an elastic cap containing a 64 electrode array in an expanded international 10/20 system (Electro-Cap International, Inc., Eaton, OH). Linked earlobe electrodes were used as reference with a mid-forehead ground. Two electrodes were placed above and below the outer canthus of the left eye to monitor blinks and eye movements. Impedances were maintained at or below 10 kΩ. NeuroScan 4.4 Acquire (Compumedics USA, Charlotte, NC) was used to record continuous electroencephalography (EEG). The amplifier was set to a gain of 10,000x and an online .15 – 50 Hz band-pass filter. Data were sampled at a rate of 500 Hz.
Spectral Processing
Offline data processing was conducted using the EEGLAB Toolbox (Delorme & Makeig, 2004) and MATLAB 2011B (The MathWorks, Inc., Natick, MA). Data were filtered between .1 and 50 Hz and divided into 2 sec epochs. Epochs containing deflections larger than +/−150 μV were rejected. Consistent with our previous work (Boissoneault et al., in press), individuals were included in these analyses only if their EEG contained at least 20 epochs/scenario, in at least 3 scenarios. Of the 120 individuals meeting criteria for inclusion, an average of 40.1 (SD=10.1) epochs per participant/scenario were collected. Of the 120 individuals included, 6 were missing data from one scenario. Chi square analyses indicated no differences by group or scenario in data exclusion.
Data were subjected to independent component analysis (ICA) (Jung et al., 2001) followed by the automated ADJUST algorithm (Mognon et al., 2011), which identifies and removes artifactual independent components associated with blinks, horizontal and vertical eye movements, and generic discontinuities (i.e., an artifact generated by an impedance fluctuation or device interference). Critically, Mognon et al. (2011) demonstrated that ADJUST has excellent accuracy for detecting these artifacts using manual expert identification as the standard. Data were subjected to Fast Fourier Transformation (FFT) and log transformed (10*log10[μV2]) using EEGLAB’s “pop_spectopo” function. Electrophysiological measures were recorded throughout all driving scenarios. Prior to alcohol administration, five minutes of a baseline EEG were collected, while participants focused on an on-screen fixation point. Preliminary analyses (included below) indicated baseline age differences. Thus, to better control for age and individuals differences unrelated to task, baseline-adjusted power was computed for analysis by subtracting baseline from driving EEG. We limited examination to frontal theta power (FTP), averaging nine frontal electrodes (Fz, F1, F2, F3, F4, F5, F6, F3a, F4a) and parietal alpha power (PAP), averaging eight parietal electrodes (Pz, P1, P2, P3, P4, P1p, Pzp, P2p).
Analysis
Differences in demographics, BrACs, subjective intoxication, and baseline EEG power between younger and older participants were analyzed with t-tests. Mixed model analyses (2 [age groups] x 3 [dose] x 4 [scenario]) were conducted for both theta and alpha power bands. Main and interactive effects were explored using differences of least squares (LS) means. Cohen’s d was used to estimate effect sizes. Bonferroni corrections were applied to secondary analyses exploring relationships that were not hypothesized a priori.
Results
Demographics
Participants primarily self-identified as Caucasian (79.17%; n=95; 40 women). African American (5.00%; n=6; 3 women) and Hispanic (10.83%; n=13; 4 women) individuals were also represented. A small group self-identified as “other” or multiracial (5.00%; n=6; 5 women). T-tests revealed older individuals reported higher age-corrected scores for anxiety symptomatology (M=44.1), relative to younger participants (M=40.1), [t(117)=3.67, p<.001]. Neither group’s anxiety scores indicated significant distress. The age groups did not differ in average daily alcohol consumed. Demographic data are presented in Table 1.
Table 1.
Demographics
| Total M(SD) | Younger M(SD) (n=80) | Older M(SD) (n=40) | Women M(SD) (n=52) | Men M(SD) (n=68) | |
|---|---|---|---|---|---|
| Age (yrs) | 38.33 (15.56) | 27.78 (2.73) | 59.45 (6.13) | 36.68 (14.95) | 39.82 (16.08) |
| Education (yrs) | 16.5 (1.44) | 16.66 (1.23) | 16.17 (1.77) | 16.80 (1.23) | 16.28 (1.57) |
| BDI a/GDS b | n/a | 2.60 (2.93) | 1.39 (1.91) | n/a | n/a |
| AI c | 41.46 (5.94) | 40.14 (4.76) | 44.10 (7.15) | 42.21 (7.21) | 40.78 (4.78) |
| Typical Daily d Drinks | 0.90 (1.58) | 0.97 (1.88) | 0.80 (0.68) | 0.73 (0.62) | 1.05 (2.16) |
Beck Depression Inventory, 2nd ed. (Beck et al., 1996)
Geriatric Depression Scale, (Yesavage et al., 1982)
Anxiety Inventory (Spielberger, 1983); age corrected
Converted from Quantity-Frequency Index (Cahalan, 1969)
BrACs & Subjective Intoxication
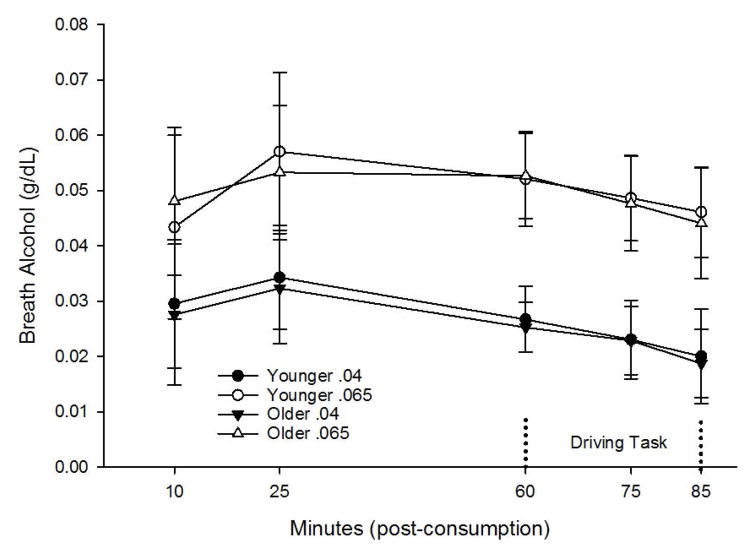
For each active alcohol group and measurement period, t-tests were conducted between age groups. No significant age differences in BrAC were detected at any measurement point during testing (all ps >.366). BrAC values are depicted in Figure 1. BrACs at initiation of the driving task were .025 g/dL for the .04 dose group (Ms=.025 and .026 g/dL for older and younger Ss, respectively), and .053 g/dL for the .065 dose group (Ms=.053 and .053 g/dL for older and younger Ss, respectively).
Fig 1. BrACs Across Dose and Age Group.
No significant differences were observed between age groups at .04 or .065 g/dL doses. Analyses were conducted using data collected immediately preceding (60 min), during (75 min), and following (85 min) driving simulation.
A significant age difference was noted for subjective intoxication at the 85 min measure, such that older participants receiving the .04 g/dL dose reported greater levels of intoxication than the younger .04 g/dL group (Ms=4.08 and 2.93, respectively) [t(38)=2.03, p=.049]. Similar differences were also observed at the 60 min [t(38)=1.90, p=.064] and 75 min [t(38)=1.91, p=.064] measurements, although they did not achieve significance. No age differences were noted among the .065 g/dL group (all ps >.297). No correlations between subjective intoxication measures and spectral power remained significant after Bonferroni correction.
Baseline EEG Power
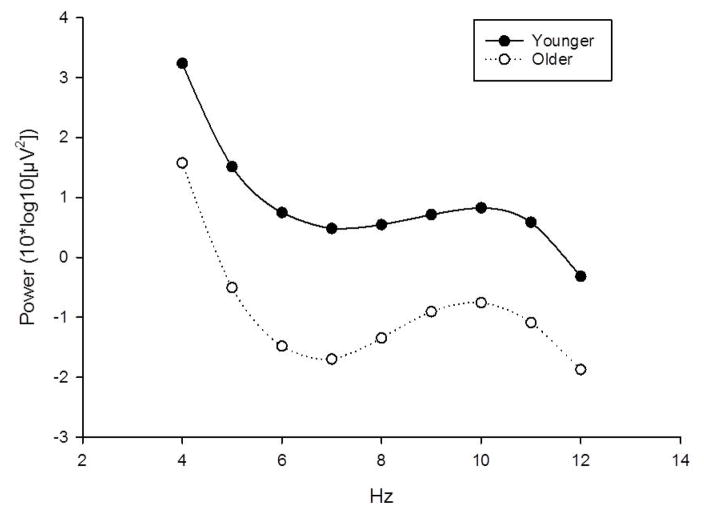
Comparison of EEG power during the pre-alcohol, eyes-open baseline revealed age differences across both frequency bands. Younger individuals displayed greater alpha [t(118)=2.81, p=.006;d=.56] and theta [t(118)=3.88, p<.001; d=.72] power. These data are depicted in Figure 2.
Fig 2. Baseline Alpha and Theta Power Across Age Group.
Younger individuals displayed greater alpha [t(118)=2.81, p=.006] and theta [t(118)=3.88, p<.001] power.
Frontal Theta Power
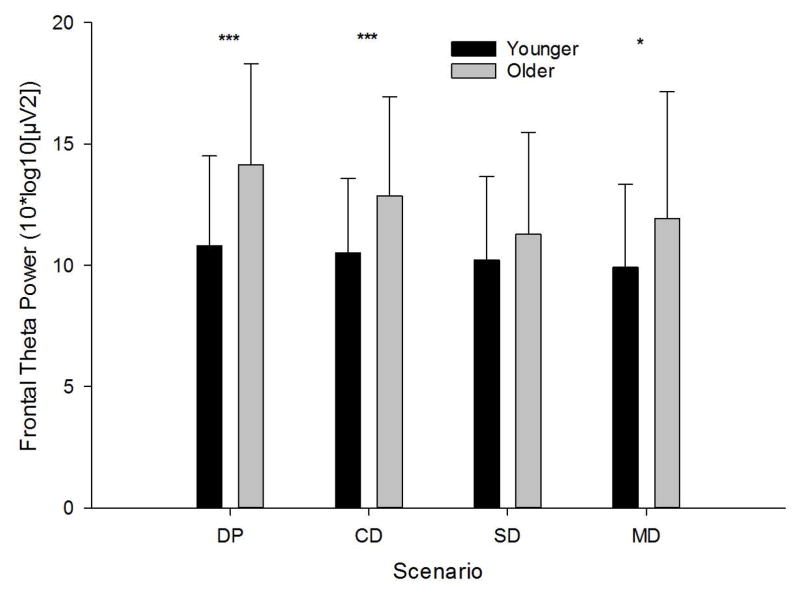
Mixed model analyses of FTP revealed main effects for scenario [F(3,336)=14.04, p<.001] and age [F(1,114)=10.58, p=.001], with older participants displaying greater theta power. No main effect or interaction with dose was noted. A significant interaction between age and scenario [F(3,336)=4.81, p=.003] was detected. Analysis of LS means indicated higher theta power among older individuals, relative to younger, in PD [t(342)=4.32, p<.001;d=.85], CD [t(342)=3.04, p=.003;d=.65], and MD [t(342)=2.69, p=.008;d=.46], after applying Bonferroni corrections (α=.0125). The pattern of means was consistent for SD, but failed to reach significance [p=.168] . Frontal theta power is depicted by age and scenario in Figure 3.
Fig 3. Mean Frontal Theta Power Across Age and Scenario.
Main effects of age were observed in DP, CD, and MD scenarios, such that older individuals displayed elevated theta power (*** p<.001; * p<.05).
Parietal Alpha Power
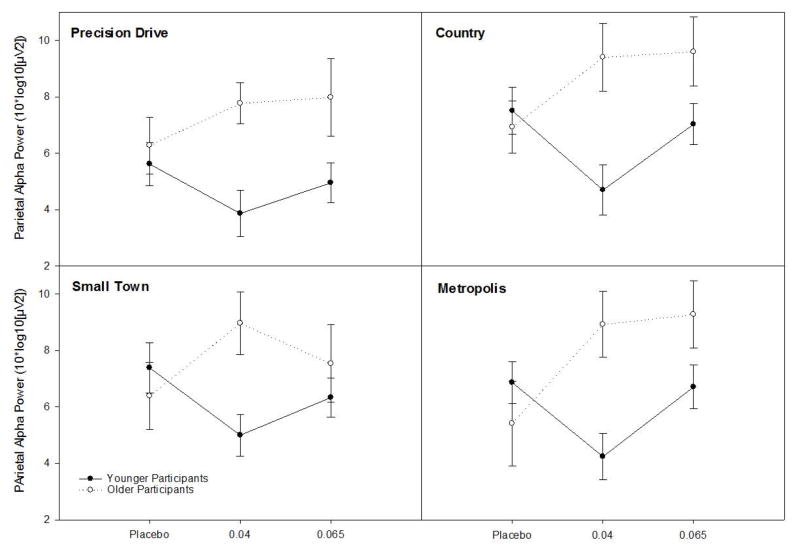
Mixed model analyses of parietal alpha power revealed a significant interaction between age and dose [F(2,114)=3.88, p=.023]. Main effects of age [F(1,114)=7.15, p=.009], with older individuals displaying higher PAP, and scenario [F(3,336)=10.73, p<.001] were also noted.
To better characterize the predicted interaction, LS means were compared between age groups across each level of alcohol, and between placebo and active doses within each age group. Age-dependent effects of low-dose alcohol were observed. Lower PAP was noted among younger individuals at the .04 g/dL dose level, relative to placebo [t(114)=2.26, p=.026;d=.61]. PAP appeared higher among .04 g/dL older adults, relative to placebo, however this was a trend-level difference [t(114)=1.79, p=.075;d=.57]. Significant differences were noted between older and younger .04 g/dL groups [t(114)=3.44, p<.001;d=1.15]. No age difference was noted in the placebo group (p=.567). Comparison at the .065 g/dL dose revealed an age difference approaching significance [t(114)=1.38, p=.058; d=.60]. Neither age group receiving the moderate dose differed from controls (ps > .095). These data are depicted by scenario in Figure 4.
Fig 4. Mean Parietal Alpha Power Across Dose, Age, and Scenario.
Mixed model analyses of parietal alpha power revealed a significant interaction between age and dose [F(2,114)=3.88, p=.023]. Lower PAP was noted among younger individuals at the .04 g/dL dose level, relative to placebo [t(114)=2.26, p=.026;d=.61]. PAP appeared higher among .04 g/dL older adults, relative to placebo, however this was a trend-level difference [t(114)=1.79, p=.075;d=.57]. Significant differences were noted between older and younger .04 g/dL groups [t(114)=3.44, p<.001;d=1.15]. No age difference was noted in the placebo group (p=.567). Comparison at the .065 g/dL dose revealed an age difference approaching significance [t(114)=1.38, p=.058; d=.60]. Neither age group receiving the moderate dose differed from controls (ps > .095).
Scenario Comparison
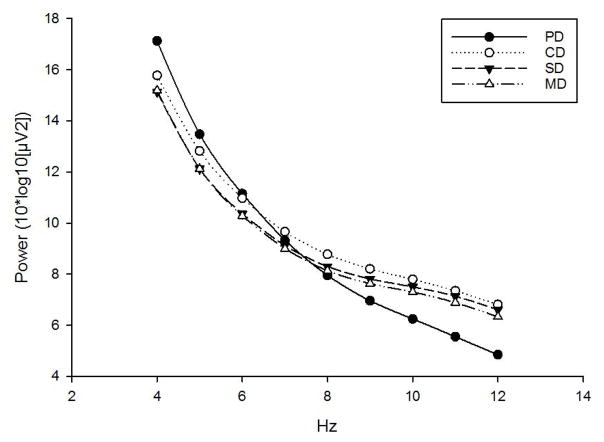
The mixed model analyses indicated significant effects of scenario. Across both PAP and FTP, the MD and SD scenarios displayed equivalent power (ps >.575), and were both lower than that observed in the CD (all ps<.037). EEG power in the PD differed from the other scenarios in a band-dependent manner; power was higher across the theta band (all ps<.007), but lower across the alpha band (all ps>.001). Figure 5 illustrates these relationships.
Fig 5. Spectral Power Across Drive Scenarios.
Across both alpha and theta bands, MD and SD scenarios evoked equivalent power (ps<.560). Both evoked lower PAP and FTP than the CD scenario (ps<.037). PD evoked higher FTP (ps<.007) and lower PAP (ps<.001) than all other scenarios.
Discussion
To our knowledge, the current report provides the first evidence of age-associated differences in neural activity during motor vehicle operation following moderate alcohol consumption. A divergent pattern of age by alcohol effects was observed in alpha power, with increased PAP in the older and decreased in the younger groups receiving. This finding is particularly important given the face validity of the driving task, the consistency of the pattern across varied driving environments, and the socially-relevant BrACs observed at task initiation (.025 g/dL). Approximately 13% of Americans endorse recent (past month) operation of a motor vehicle within two hours of a drinking occasion (National Highway Traffic Safety Administration, 2008). Thus, elucidation of age-specific effects following moderate drinking episodes may have significant implications for traffic safety.
Observation of low-dose alcohol differentially altering neural activity during driving in an age-dependent manner is novel, and indicates underlying differences in neurobehavioral processes. Further investigation designed to interrogate specific processes and their behavioral correlates are required. However, identification of these age differences provides a crucial first step and suggests the need for more pointed analyses of age and alcohol interactions in traffic safety domains.
Construction of a simulated driving task with high face validity relative to “real-world” motor vehicle operation necessarily requires a complex task engaging numerous neurobehavioral processes. Vehicle operation requires engagement of visual-spatial, attentional, working memory, and navigational processes. Engagement of these processes is linked with fluctuation in EEG power (e.g., Klimesch, 1999; Wang et al., 2016; Ehinger et al., 2014). However, these data do not afford clear interpretation of underlying processes or direct comparison with behavioral differences. Several examinations of spectral power utilize event-related paradigms (e.g., Missonnier et al., 2011) wherein EEG power can be compared with discrete behavioral responses. The current study utilized continuous EEG measures, thus patterns represent neural activity across an array of dynamic responses to the environment, including turning, acceleration, braking, stopping, and signaling. Although not conducive to association with specific event-related behaviors (e.g., deceleration rate following red light presentation), these data do afford consideration with the existing literature examining age by alcohol effects, including both neurocognitive and driving studies.
The current examination extends investigations of age-contingent alcohol effects in our work and others’. Given the association between reduction in alpha power and performance improvement across a variety of tasks, these data are consistent with evidence suggesting older individuals display deficits in psychomotor, set-shifting, and attentional functions under acute alcohol conditions (Boissoneault et al., 2014; Gilbertson et al., 2009; Lewis et al., 2013). The observed alpha alterations suggest these deficits may extend to driving; we have previously detailed deficits in driving simulator performance among older individuals following moderate consumption (Sklar et al., 2014; Price et al., 2016). Taken together, these works suggest older individuals may be particularly susceptible to perturbations in neural activity, neurobehavioral processes, and driving abilities following even low/moderate alcohol consumption.
The current data bear comparison with our recent investigation of working memory (Boissoneault et al., in press). In contrast to the driving task, which requires externally-directed attention, we utilized a memory maintenance task requiring inhibition of attention to external stimuli to protect an internal representation of a recently-viewed image. Age-dependent divergence in alpha activity following .04 g/dL consumption was noted, albeit in opposing directions to those noted in the current work (i.e., .04 g/dL older adults displayed decreased alpha during memory maintenance, .04 g/dL younger adults displayed increased alpha). Taken together, these findings suggest that under low-dose conditions alcohol may facilitate advantageous neural activity in younger individuals but contribute to disadvantageous alterations among older individuals, even across tasks with diverse attentional requirements. This interpretation is consistent with observations of alcohol myopia effects (Steele & Josephs, 1990) among younger individuals.
Although our hypotheses and interpretation emphasize the visual attention components of driving, alternative interpretations of these age by alcohol interactions must be considered. Driving requires the integration of both motor control and attentional systems. Adjustment and updating of driving behavior to respond to changing environmental demands requires performance monitoring capabilities which are associated with alterations in alpha and theta power (van Driel et al., 2012). It may be that divergence noted following low dose alcohol reflects differential age-related repertoires in neurocognitive response to low-dose alcohol challenge. For instance, under these conditions younger individuals may enhance visual attention (associated with the observed alpha reductions) while older individuals increase top-down inhibitory processes to suppress responding to task-irrelevant stimuli (associated with the observed alpha increases; [Werkle-Bergner et al., 2012]). While speculative, such alternative interpretations should be considered and tested empirically in further work.
To vary attentional demands throughout the task, stimuli density was manipulated between scenarios. To maintain the face validity of the task, we used environments with varying environmental complexity (e.g., country roads vs. city driving). Based on the varied complexity between scenarios, we speculated scenario differences would emerge. The current data were somewhat counterintuitive, reflecting higher theta and lower alpha power in the PD scenario, which contained the lowest stimuli density. Visual-motor demands may have also contributed to scenario difficulty. The PD scenario was performed at the highest speed (55 mph). Maintenance of lane position and speed, particularly on curved sections, may have been more challenging than the lower speeds required in other scenarios. Future work controlling behavioral demands across scenarios would better characterize these scenario-dependent effects.
This work provides insight into age-related modification of acute ethanol effects, but must be considered with several caveats. Simulated driving remains substantially different than “real-world” driving. For instance, our simulator protocol lacked distractors common in typical driving contexts including passengers, radio, phone calls, and texting. Performance of younger drivers under distractor conditions has been demonstrated to be particularly susceptible to disruption by moderate alcohol doses (Harrison & Fillmore, 2011; Van Dyke & Fillmore, 2015). A simulation containing such distraction might alter the observed patterns of neural alteration. Our between-subjects experimental design also bears consideration. We constructed this protocol to limit nonlinear sequencing effects and differential attrition. In future work, replication of these results using a within-subjects design would strengthen conclusions. The low representation of older women due to simulator sickness precluded meaningful analysis of potential three-way interactions with sex. These analyses must be examined in extensions of this work.
Summary
These data establish provocative age by alcohol interactions in neural oscillations. They illustrate previously unappreciated differences in neurobehavioral processes, and imply potentially increased safety risks in older drivers following low/moderate alcohol consumption. Subsequent work should be directed toward further interrogating the processes underlying these effects, establishing their relevance for behavioral performance, and interrogating their impact on driving risk.
Acknowledgments
Support: Funding was provided by NIAAA R01 AA019802 (SJN, PI) and F31 AA019862 (JB) and the University of Florida Department of Psychiatry. Special thanks to Dr. Mark Fillmore for help with protocol development and study design, and to Dr. Alfredo Sklar for help with data collection and processing.
References
- Aksan N, Anderson SW, Dawson J, Uc E, Rizzo M. Cognitive functioning differentially predicts different dimensions of older drivers' on-road safety. Accid Anal Prev. 2015;75:236–244. doi: 10.1016/j.aap.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, Cappa SF, Cincotti F, Del Percio C, Miniussi C, Vito Moretti D, Rossi S, Sosta K, Rossini PM. Human cortical rhythms during visual delayed choice reaction time tasks. A high-resolution EEG study on normal aging. Behav Brain Res. 2004;153:261–271. doi: 10.1016/j.bbr.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Bashivan P, Bidelman GM, Yeasin M. Spectrotemporal dynamics of the EEG during working memory encoding and maintenance predicts individual behavioral capacity. Eur J Neurosci. 2014;40:3774–3784. doi: 10.1111/ejn.12749. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory. 2. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Blomberg RDPRC, Moskowitz H, Burns M, Fiorentino D. Crash risk of alcohol involved driving: A case-control study. Stamford, Connecticut: Dunlap and Associates, Inc; 2005. [Google Scholar]
- Boha R, Molnar M, Gaal ZA, Czigler B, Rona K, Kass K, Klausz G. The acute effect of low-dose alcohol on working memory during mental arithmetic: I. Behavioral measures and EEG theta band spectral characteristics. Int J Psychophysiol. 2009;73:133–137. doi: 10.1016/j.ijpsycho.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Boissoneault J, Sklar A, Prather R, Nixon SJ. Acute effects of moderate alcohol on psychomotor, set shifting, and working memory function in older and younger social drinkers. J Stud Alcohol Drugs. 2014;75:870–879. doi: 10.15288/jsad.2014.75.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissoneault JFI, Lewis B, Nixon Sj. Effects of age and acute moderate alcohol administration on electrophysiological correlates of working memory maintenance. Alcoholism: Clinical and Experimental Research. doi: 10.1111/acer.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau U.S.C; POPULATION PROJECTIONS PROGRAM, P. D, editor. Projections of the population by selected age groups and sex for the united states: 2015 to 2060. Washington, D.C: 2012. [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cissin L, Crossley H. American drinking practices: A national study of drinking behaviors and attitudes. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. (monograph no. 6) [Google Scholar]
- Cicchino JB, Mccartt AT. Critical older driver errors in a national sample of serious u.S. Crashes. Accid Anal Prev. 2015;80:211–219. doi: 10.1016/j.aap.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Dawson JD, Uc EY, Anderson SW, Johnson AM, Rizzo M. Neuropsychological predictors of driving errors in older adults. J Am Geriatr Soc. 2010;58:1090–1096. doi: 10.1111/j.1532-5415.2010.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ehinger BV, Fischer P, Gert AL, Kaufhold L, Weber F, Pipa G, Konig P. Kinesthetic and vestibular information modulate alpha activity during spatial navigation: A mobile eeg study. Front Hum Neurosci. 2014;8:71. doi: 10.3389/fnhum.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal ZA, Boha R, Stam CJ, Molnar M. Age-dependent features of EEG-reactivity - spectral, complexity, and network characteristics. Neurosci Lett. 2010;479:79–84. doi: 10.1016/j.neulet.2010.05.037. [DOI] [PubMed] [Google Scholar]
- Gilbertson R, Ceballos NA, Prather R, Nixon SJ. Effects of acute alcohol consumption in older and younger adults: Perceived impairment versus psychomotor performance. J Stud Alcohol Drugs. 2009;70:242–252. doi: 10.15288/jsad.2009.70.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Fillmore MT. Alcohol and distraction interact to impair driving performance. Drug and Alcohol Dependence. 2011;117:31–37. doi: 10.1016/j.drugalcdep.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkenstein RF, Dale A. Indiana University. Department of Police Administration. The role of the drinking driver in traffic accidents. Bloomington: 1964. [Google Scholar]
- Jung TP, Makeig S, Mckeown MJ, Bell AJ, Lee TW, Sejnowski TJ. Imaging brain dynamics using independent component analysis. Proc IEEE Inst Electr Electron Eng. 2001;89:1107–1122. doi: 10.1109/5.939827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K. Theta oscillations are sensitive to both early and late conflict processing stages: Effects of alcohol intoxication. PLoS One. 2012;7:e43957. doi: 10.1371/journal.pone.0043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. Neuroimage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Lewis B, Boissoneault J, Gilbertson R, Prather R, Nixon SJ. Neurophysiological correlates of moderate alcohol consumption in older and younger social drinkers. Alcohol Clin Exp Res. 2013;37:941–951. doi: 10.1111/acer.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas NA, Nettelbeck T, Burns NR. Dropout during a driving simulator study: A survival analysis. J Safety Res. 2015;55:159–169. doi: 10.1016/j.jsr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Mcevoy LK, Pellouchoud E, Smith ME, Gevins A. Neurophysiological signals of working memory in normal aging. Brain Res Cogn Brain Res. 2001;11:363–376. doi: 10.1016/s0926-6410(01)00009-x. [DOI] [PubMed] [Google Scholar]
- Mcgwin G, Jr, Brown DB. Characteristics of traffic crashes among young, middle-aged, and older drivers. Accid Anal Prev. 1999;31:181–198. doi: 10.1016/s0001-4575(98)00061-x. [DOI] [PubMed] [Google Scholar]
- Mets MA, Kuipers E, De Senerpont Domis LM, Leenders M, Olivier B, Verster JC. Effects of alcohol on highway driving in the stisim driving simulator. Hum Psychopharmacol. 2011;26:434–439. doi: 10.1002/hup.1226. [DOI] [PubMed] [Google Scholar]
- Missonnier P, Herrmann FR, Rodriguez C, Deiber MP, Millet P, Fazio-Costa L, Gold G, Giannakopoulos P. Age-related differences on event-related potentials and brain rhythm oscillations during working memory activation. J Neural Transm (Vienna) 2011;118:945–955. doi: 10.1007/s00702-011-0600-2. [DOI] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, Buiatti M. Adjust: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48:229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Moulton BE, Peterson A, Haddix D, Drew L. National Survey of Drinking and Driving Attitudes and Behaviors: 2008. Volume II: Findings Report. Washington, DC: National Highway Traffic Safety Administration; 2010. (Report No. DOT HS 811 343) Available at www.nhtsa.gov/staticfiles/nti/pdf/811343.pdf. [Google Scholar]
- Price JL, Reaves SA, Frazier IR, Boissoneault J, Nixon SJ. Alcohol and age effects on the ability to attend to and ignore relevant and irrelevant stimuli in a driving simulator. Alcohol Clin Exp Res. 2016;40:16A–247A. doi: 10.1111/acer.13084. [DOI] [Google Scholar]
- Robins LN, Cottler L, Bucholz KK, Compton W. The diagnostic interview schedule, version iv. St. Louis: Washington University; 1995. [Google Scholar]
- Sklar AL, Boissoneault J, Fillmore MT, Nixon SJ. Interactions between age and moderate alcohol effects on simulated driving performance. Psychopharmacology (Berl) 2014;231:557–566. doi: 10.1007/s00213-013-3269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar AL, Gilbertson R, Boissoneault J, Prather R, Nixon SJ. Differential effects of moderate alcohol consumption on performance among older and younger adults. Alcohol Clin Exp Res. 2012;36:2150–2156. doi: 10.1111/j.1530-0277.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Steele CM, Josephs RA. Alcohol myopia. Its prized and dangerous effects. The American psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- United States. Department of Health and Human Services., United States. Department of Agriculture. & United States. Dietary Guidelines Advisory Committee. Dietary guidelines for americans, 2010. Washington, D.C: G.P.O; 2010. [Google Scholar]
- Van Driel J, Ridderinkhof KR, Cohen MX. Not all errors are alike: Theta and alpha EEG dynamics relate to differences in error-processing dynamics. J Neurosci. 2012;32:16795–16806. doi: 10.1523/JNEUROSCI.0802-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke NA, Fillmore MT. Distraction produces over-additive increases in the degree to which alcohol impairs driving performance. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Rajagovindan R, Han SM, Ding M. Top-down control of visual alpha oscillations: Sources of control signals and their mechanisms of action. Front Hum Neurosci. 2016;10:15. doi: 10.3389/fnhum.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects. Updating the widmark equation. J Stud Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Werkle-Bergner M, Freunberger R, Sander MC, Lindenberger U, Klimesch W. Inter-individual performance differences in younger and older adults differentially relate to amplitude modulations and phase stability of oscillations controlling working memory contents. Neuroimage. 2012;60:71–82. doi: 10.1016/j.neuroimage.2011.11.071. [DOI] [PubMed] [Google Scholar]
- West R, Wilding J, French D, Kemp R, Irving A. Effect of low and moderate doses of alcohol on driving hazard perception latency and driving speed. Addiction. 1993;88:527–532. doi: 10.1111/j.1360-0443.1993.tb02059.x. [DOI] [PubMed] [Google Scholar]
- Widmark E. Die theoretischen grundlagen und die praktische verwendbarkeit der gerichtlich-medizinischen alkohobestimmung. Berlin: Urban & Schwarezenberg; 1932. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zumer JM, Scheeringa R, Schoffelen JM, Norris DG, Jensen O. Occipital alpha activity during stimulus processing gates the information flow to object-selective cortex. PLoS Biol. 2014;12:e1001965. doi: 10.1371/journal.pbio.1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]