Abstract
Background
Physicians have long reported that patients with chronic pain show higher tendencies for alcohol use disorder (AUD), and AUD patients appear to have higher pain sensitivities. The goal of this study was to test 2 hypotheses: (i) binge alcohol consumption increases inflammatory pain and mechanical and cold sensitivities; and (ii) tigecycline is an effective treatment for alcohol‐mediated‐increased pain behaviors and sensitivities. Both female and male mice were used to test the additional hypothesis that important sex differences in the ethanol (EtOH)‐related traits would be seen.
Methods
“Drinking in the Dark” (DID) alcohol consuming and nondrinking control, female and male, adult C57BL/6J mice were evaluated for inflammatory pain behaviors and for the presence of mechanical and cold sensitivities. Inflammatory pain was produced by intraplantar injection of formalin (10 μl, 2.5% in saline). For cold sensation, a 20 μl acetone drop was used. Mechanical withdrawal threshold was measured by an electronic von Frey anesthesiometer. Efficacy of tigecycline (80 mg/kg i.p.) to reduce DID‐related pain responses and sensitivity was tested.
Results
DID EtOH consumption increased inflammatory pain behavior, while it also produced sustained mechanical and cold sensitivities in both females and males. Tigecycline produced antinociceptive effects in males; a pro‐nociceptive effect was seen in females in the formalin test. Likewise, the drug reduced both mechanical and cold sensitivities in males, but females showed an increase in sensitivity in both tests.
Conclusions
Our results demonstrated that binge drinking increases pain, touch, and thermal sensations in both sexes. In addition, we have identified sex‐specific effects of tigecycline on inflammatory pain, as well as mechanical and cold sensitivities. The development of tigecycline as an AUD pharmacotherapy may need consideration of its pro‐nociceptive action in females. Further studies are needed to investigate the mechanism underlying the sex‐specific differences in nociception.
Keywords: Alcohol Use Disorder, Alcohol, Ethanol, Mechanical and Cold Sensitivities, Pain Sensitivity, Tigecycline
Reports of pain patients self‐medicating with alcohol and those with alcohol use disorder (AUD) showing increased pain sensitivity are common (for a review, see Apkarian et al., 2013). An interest in understanding potential interactions of alcohol consumption and inflammatory pain sensitivity has intensified due to overlapping central nervous system circuitry pathways and mechanisms of action (Apkarian et al., 2013). We, and others, have recently shown that treatment with tetracycline analogs reduced alcohol consumption and other alcohol‐mediated responses (Agrawal et al., 2011, 2014; McIver et al., 2012), and tetracycline reduction of pain has been well studied since the first evidence was reported in the 1990s (Bastos et al., 2012). Here, due to our companion studies showing efficacy to reduce binge and dependence drinking as well as alcohol withdrawal symptoms (Bergeson et al., 2016; Martinez et al., 2016; Syapin et al., 2016), we focus on tigecycline (TYGACIL, Pfizer), the first glycylcycline–tetracycline to be approved as an antibiotic by the U.S. Food and Drug Administration (Falagas et al., 2014). Although some studies have demonstrated effective properties of minocycline (second‐generation semisynthetic tetracycline derivative) in the formalin (Cho et al., 2006) and surgically‐induced neuropathic pain models (Padi and Kulkarni, 2008), the novel analgesic properties of tetracycline‐like compounds need to be investigated further, especially if tigecycline, or a derivative, is to become an important AUD treatment. In addition to their antimicrobial actions, tetracyclines have been shown to have anti‐inflammatory actions (Cockeran et al., 2012; Domercq and Matute, 2004), although these effects have not been investigated for tigecycline. However, the drug is known to have excellent tissue penetration and unappreciable metabolism; unchanged drug is found in the feces, with minor secondary glucuronidation and renal elimination (Meagher et al., 2005).
To date, no studies have evaluated the effect of short‐term binge alcohol consumption on pain sensitivity and its treatment. The primary purpose of this study was first to test the hypothesis that acute alcohol consumption increases pain sensitivity and then to test for effects on other somatosensory modalities. Finally, if alcohol‐induced sensitivities were found, we wished to determine whether tigecycline was an effective rescue strategy. As mentioned above, our goal stemmed from the results of our companion reports showing that tigecycline effectively reduced binge and chronic alcohol use and attenuated alcohol withdrawal symptoms (Bergeson et al., 2016; Martinez et al., 2016; Syapin et al., 2016).
The Drinking in the Dark (DID) mouse model of binge drinking was used due to its simplicity and ability to achieve pharmacologically relevant blood alcohol levels under free‐choice conditions (Rhodes et al., 2005). Given the neuroinflammatory nature of the DID procedure (Agrawal et al., 2014), we chose to test the hypothesis that tigecycline, a minocycline analog, would reduce inflammatory pain produced using a standard formalin test (Guindon et al., 2011). Additionally, mechanical and cold sensitivity were measured using electronic von Frey and acetone tests, respectively (Deng et al., 2015). The formalin test is a well‐established model of nociceptive inflammatory pain with a biphasic (early and late) pattern of a transient spike followed by persistent pain behavior (Tjolsen et al., 1992). The early phase is characterized by acute activation of C and Aδ fibers, and the late phase involves an inflammatory reaction in peripheral tissues (Tjolsen et al., 1992), activation of primary afferent nociceptors (Puig and Sorkin, 1996), and the development of central nervous system sensitization (Coderre, 1992; Coderre and Katz, 1997). Both female and male C57BL/6J mice were used due to known sex differences in alcohol responses, as well as known sex differences with tigecycline treatment, which we uncovered (Syapin et al., 2016).
Several important findings have resulted from our studies. Most importantly, strong evidence suggests that limited binge drinking results in changes to inflammatory pain behaviors and mechanical and cold sensitivities, which were reduced by tigecycline in male, but not female mice. Interestingly, tigecycline alone, compared to vehicle controls, was pro‐nociceptive in females and antinociceptive in males. While important sex differences in pain perception and its treatment have been well documented (Chanda and Mogil, 2006; Craft et al., 2004; Mogil and Bailey, 2010; Mogil and Chanda, 2005), further study is necessary to determine how sex influences tigecycline action.
Materials and Methods
Animal Husbandry
Adult (approximately 70 to 180 days of age), female and male, wild‐type C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME) were acclimated to housing under a reverse 12:12 hour light–dark cycle (lights off at 09:00, lights on at 21:00) and provided ad libitum access to standard rodent chow and water. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Texas Tech University Health Sciences Center and conformed to the Guidelines of the National Institutes of Health on the Care and Use of Animals. The animal facility at TTUHSC is AAALAC approved. Each animal was used in only 1 experiment. To avoid influence of scent, females and males were tested on separate days.
Drugs
Tigecycline (#15026) was purchased from Cayman Chemical Company (Ann Arbor, MI), shipped overnight, and immediately stored at −20°C to avoid degradation. Formaldehyde (Fisher Scientific, Pittsburg, PA) was dissolved in saline to obtain a final concentration of 2.5% formalin. The single dose of 80 mg/kg tigecycline was chosen due to efficacy to reduce ethanol (EtOH) consumption and acute alcohol withdrawal seizures (Bergeson et al., 2016; Martinez et al., 2016; Syapin et al., 2016). No other doses were tested. Tigecycline was prepared fresh on the day of the experiment and administered intraperitoneally (i.p.) in a single volume of 0.01 ml/g body weight.
Ethanol Consumption: Drinking in the Dark Paradigm
A modified version of the DID procedure (Rhodes et al., 2005) was performed as previously reported (Agrawal et al., 2014) in which mice had 4 hours of daily access to a 20% v/v EtOH solution for 4 days. Drinking bottles were constructed in‐house from 50‐ml centrifugation tubes (BD Falcon brand; BD Biosciences, Franklin Lakes, NJ) fitted with rubber stoppers and sipper tubes containing double ball bearings and filled with tap water or 20% v/v EtOH. Cage tops were modified to receive an inverted drinking bottle. The day before the start of the experiment, animals were weighed and grouped with balance for weight and cage of origin effects. At 4 pm, on the first day, the water drinking bottles were weighed and placed on the cage. At noon, each water bottle was removed and weighed. A bottle containing 20% EtOH in water was weighed and then placed on the cage. At 4 pm, the EtOH bottles were removed, weighed, and replaced with weighed water bottles. The cycle was repeated daily for 4 days. Mice were given chow ad libitum and weighed again at the end of each experiment.
Formalin Test
The formalin test for inflammatory pain was performed as previously described (Puig and Sorkin, 1996). Briefly, mice were acclimatized to the testing environment (clear plexiglass box 10 × 10 × 10 cm) for at least 15 min or until cessation of exploratory behavior. Mice were subsequently injected i.p. with either tigecycline (80 mg/kg in saline) or saline. Injection time varied based on the experiment performed, with some treated just prior to testing and others the previous evening. Observation of the animal's behavior was performed in consecutive 5‐min periods for 60 min following administration of 2.5% formalin (10 μl) in the right paw. In each 5‐min bin, the total time each animal spent in 3 different behavioral categories was recorded: (0) the injected paw had little or no weight placed on it; (1) the injected paw was raised; and (2) the injected paw was licked, shaken, or bitten. Nociceptive behavior was observed using a mirror angled at 45° below the observation chamber and quantified by the composite pain score–weighted scores technique (CPS–WST 0,1,2) where each pain behavior was weighted by the amount of time spent in each category (0, 1, 2). The area under the curve (AUC), which corresponds to CPS–WST 0,1,2 × time (min), was calculated for the acute phase (0 to 15 min; Phase 1) and the inflammatory phase (15 to 60 min; Phase 2) using the trapezoidal rule (Watson et al., 1997).
Assessment of Mechanical Sensitivity
Mechanical withdrawal thresholds were assessed using a digital electronic von Frey anesthesiometer (IITC Life Sciences, Woodland Hills, CA) equipped with a semi‐flexible tip as described previously (Deng et al., 2015; Guindon et al., 2014). Mice were placed in individual plastic cages (2.75″ W × 4.25″ L × 3.5″ H) on an elevated wire mesh platform, and allowed to habituate to the testing apparatus for at least 30 min until exploratory behavior was no longer observed. Force was applied to the mid‐plantar region of each hind paw by the same experimenter in each study. Stable baseline responses were obtained prior to experimental testing. Mechanical stimulation was terminated upon paw withdrawal; consequently, there was no upper threshold limit set for termination of a testing trial. Paw withdrawal thresholds were assessed in duplicate for each paw. (No significant differences were seen between left and right paws, nor were differences detected over time. The repeated measures were simply completed to increase reliability.) Testing took place on day 0 and daily from days 4 to 10 (i.e., 6 days after 4 days of DID) for all animals.
Assessment of Cold Sensitivity
Cold sensitivity was measured by applying a drop of acetone to the plantar surface of the hind paw as previously described (Deng et al., 2015; Guindon et al., 2014). Mice were placed in individual plastic cages (2.75″ W × 4.25″ L × 3.5″ H) on an elevated open mesh platform and habituated for at least 30 min until exploratory behaviors ceased. Acetone was loaded into a 1‐ml syringe with no needle. Air bubbles were cleared from the syringe prior to acetone application. One drop of acetone (approximately 20 μl) was applied to the paw from the bottom of the elevated cage, through the mesh platform and onto the plantar surface of the hind paw without contact of the syringe barrel. The procedure was performed carefully on nonrestrained animals to ensure contact of the drop only, without touching the syringe to the paw or mesh. Time spent attending to the acetone‐stimulated paw was measured over a 60‐second observation period after acetone application was recorded. Paw withdrawal was sometimes associated with a secondary response, such as rapid flicking of the paw, chattering, biting, and/or licking of the paw. Testing alternated between paws (i.e., right and left) until 5 measurements were taken for each paw, with the averages calculated. An interstimulation interval of approximately 5 min was allowed between testing of right and left paws. (No significant differences were seen between left and right paws, nor were differences detected over time. The repeated measures were simply completed to increase reliability.) Cold sensitivity testing took place on day 0 (the morning after the last DID treatment) and daily from days 4 to 10 (i.e., for 6 days after 4 days of DID) for all animals.
Experimental Procedures
Five experiments were conducted in a blinded manner, and animals were randomly assigned to single experimental condition. Pain testing started on the morning following completion of DID treatment (approximately 16 hours later). Experiment 1 tested the effect of binge drinking and sex differences on inflammatory pain using the formalin test (2.5% intraplantar). The effect of tigecycline (80 mg/kg i.p.) at 4 or 20 hours after its administration was assessed in Experiment 2 using the inflammatory pain model. For Experiment 3, the combined effect of EtOH and tigecycline administration was evaluated in the formalin test to detect potential sex‐specific differences. The effect of 4 days of binge alcohol consumption was evaluated in terms of mechanical and cold sensitivity (Experiment 4). Finally, in Experiment 5, the effects of tigecycline on both mechanical and cold sensitivities following the formalin test (up to 120 min after injection of formalin) were evaluated.
Data Analysis and Statistics
Pain behavior for each treatment group was expressed as mean ± SEM. Paw withdrawal thresholds (mechanical) and duration of attending to the acetone stimulation (cold) were calculated for each paw and averaged. Data were analyzed using analysis of variance (ANOVA) for repeated measures or 1‐way ANOVA as appropriate. The Greenhouse–Geisser correction was applied to all repeated factors; degrees of freedom reported for significant interactions are the uncorrected values. The source of significant interactions was further evaluated by performing 2‐way ANOVAs followed by Bonferroni post hoc tests. The different components of the total variation were settled a priori using multiple regression analysis (Draper, 1998). Analyses were performed using SPSS statistical software (version 22.0; SPSS Incorporated, Chicago, IL) with significance set as p < 0.05.
Results
Binge Ethanol Consumption
DID resulted in representative consumption levels of 4.34 ± 0.3 g/kg/d for female and 4.05 ± 0.2 g/kg/d in male C57BL/6J mice. Overall, these levels are somewhat below our previous reports (Agrawal et al., 2011, 2014) and may reflect a broader age range in this study (previous studies were completed in animals 70 to 80 days of age, and animals were between 70 and 180 days old in this study). The similar intake in the females and males suggests that subsequent behavioral differences were not due to a sex difference in short‐term binge EtOH consumption. Blood EtOH levels were not measured due to the likelihood that handling‐induced stress would complicate our behavior studies.
Binge Ethanol Consumption Showed Facilitating Effects on Nociceptive Behavior in Both Female and Male Mice
DID induced an increase in formalin‐induced pain behaviors, with both females and males showing increased pain thresholds after 4 days of binge drinking, ♀ F(1, 15) = 48.95, p = 0.0001; ♂ F(1, 15) = 38.10, p = 0.0001, in a time‐dependent manner (different phases: Phase 1 = acute, Phase 2 = inflammatory), ♀ F(11, 165) = 57.48, p = 0.0001; ♂ F(11, 165) = 85.34, p = 0.0001 (Fig. 1).
Figure 1.
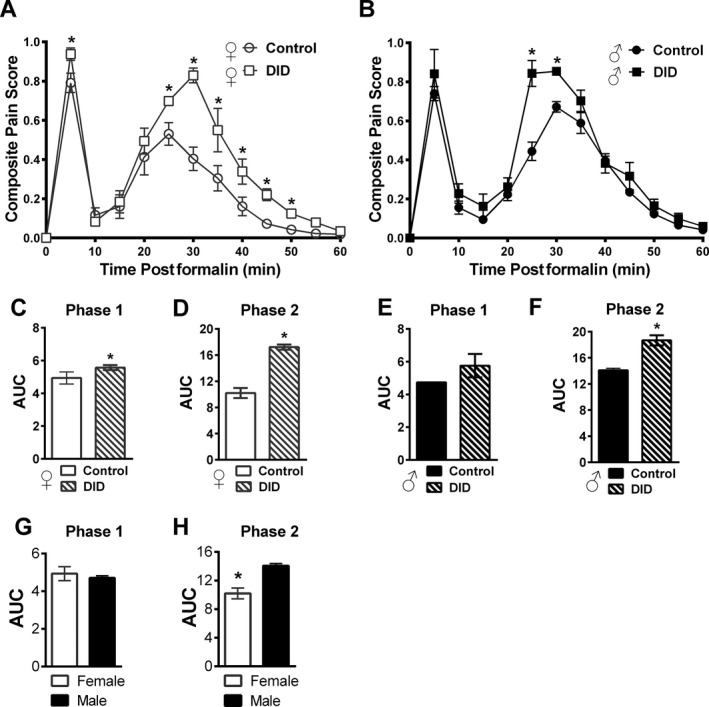
DID ethanol consumption over 4 days promoted increased inflammatory pain sensitivity in male and female mice. DID consumption in female (A) and male (B) mice for 4 consecutive days resulted in higher composite pain scores, relative to the control group. The area under the curve (AUC) is shown for Phase 1 and Phase 2 for female (C, D) and male (E, F) mice, respectively. Consumption increased pain behavior relative to controls for both phases in female (C, D), but only for the inflammatory phase in males (F). Data are expressed as mean ± SEM (n = 6 to 11 per group); *p = 0.049 for DID compared to the control group. ANOVA with Bonferroni post hoc testing was completed. The formalin test showed sex differences in overall intensity. AUC is shown in (G) Phase 1 and (H) Phase 2, with females showing lower AUC values. Data are expressed as mean ± SEM (n = 10 to 11 per group). *p = 0.003 for female versus male mice (ANOVA).
Composite pain scores in females significantly increased in Phase 1 (acute) at 5 min, F(1, 15) = 5.95 p = 0.03, and from 25 to 50 min (inflammatory), F(1, 15) = 5.09 (25 min); 18.89 (30 min); 5.21 (35 min); 6.46 (40 min); 11.68 (45 min); and 16.70 (50 min), all time points p > 0.023 (Fig. 1 A). For males, composite pain scores increased only in the inflammatory phase (Phase 2) at 25 and 30 min, F(1, 15) = 24.82 (25 min) and 21.57 (30 min), p = 0.0001; Fig. 1 B. Figure 1 C,D shows AUC of Phase 1 and Phase 2 for females, while the male AUC for Phase 1 and Phase 2 are shown in Fig. 1 E,F. Females showed a DID‐mediated increase in pain behavior in both Phase 1, F(1, 15) = 4.64, p = 0.049, and Phase 2, F(1, 15) = 28.71, p = 0.0001, while males showed significant increases only in Phase 2, Phase 1 = F(1, 15) = 3.74, p = 0.07; Phase 2 = F(1, 15) = 42.16, p = 0.0001.
Sex differences in nociceptive behavior were detected in the formalin test (see Fig. 1 A,B,G,H). Control female mice showed lower composite pain scores relative to males, F(1, 19) = 22.83, p = 0.0001, in a time‐dependent manner, F(11, 209) = 6.69, p = 0.0001 (male control compared to female control group, curves shown individually, Fig. 1 A,B). Analysis of the AUC revealed a lower female threshold relative to males in the inflammatory phase (Phase 2), F(1, 19) = 23.75, p = 0.0001, but not in the acute phase (Phase 1), F(1, 19) = 0.36, p = 0.55, of the formalin test (Fig. 1 G—Phase 1 and Fig. 1 H—Phase 2).
Tigecycline Treatment Was Pro‐Nociceptive in Female Mice and Antinociceptive in Male Mice
While tigecycline at 80 mg/kg i.p. effectively reduced pain in males, females showed increased pain sensitivity following treatment (Fig. 2 A,B). In female mice, both (4 and 20 hours) time points of systemic (80 mg/kg i.p.) tigecycline administration, F(2, 19) = 14.81, p = 0.0001, increased composite pain scores relative to the control group in a time‐dependent manner, F(22, 209) = 3.15, p = 0.0001 (Fig. 2 A). This increase in pain behavior was observed at 5 and 10 min, F(2, 19) = 7.53 (5 min) and 7.53 (10 min), p = 0.0001, from 25 to 30 min, F(2, 19) = 10.01 (25 min) and 5.57 (30 min), p = 0.012, and from 40 to 45 min, F(2, 19) = 4.36 (40 min) and 8.01 (45 min), p < 0.03; Fig. 2 A, postformalin injection. Analysis of the AUC of pain behavior revealed that in Phase 1 systemic administration of tigecycline (20 hours) increased pain, F(2, 19) = 7.79, p < 0.003; Fig. 2 C. Moreover, in Phase 2 systemic tigecycline (4 and 20 hours) administration increased pain, F(2, 19) = 9.99, p < 0.001, in the formalin test (Fig. 2 D).
Figure 2.

Sex differences with tigecycline treatment in the formalin test were seen without ethanol consumption. In female mice (A), tigecycline (80 mg/kg i.p.) increased composite pain scores relative to the control group whereas, in male mice (B), tigecycline decreased composite pain scores in a time‐dependent manner. Analysis of AUC for pain behavior revealed that tigecycline produced pro‐nociception in female (C, D) and antinociception in males (E, F) relative to vehicle in both Phase 1 and Phase 2 of the formalin test. Data are expressed as mean ± SEM (n = 5 to 11 per group); *p = 0.03 for tigecycline versus control group. ANOVA with Bonferroni post hoc testing was completed.
In male mice, both 4 and 20 hours (p > 0.939) time points of tigecycline systemic (80 mg/kg i.p.) administration, F(2, 16) = 65.70, p < 0.0001, suppressed composite pain scores relative to control group in a time‐dependent manner, F(22, 176) = 6.92, p < 0.0001; Fig. 2 B. This suppression was observed at 5 min, F(2, 16) = 32.09, p < 0.0001, and from 30 to 45 min, F(2, 16) = 50.29 (30 min); 11.67 (35 min); 29.00 (40 min); 6.37 (45 min), all time points p < 0.009 (45 min); Fig. 2 A), postformalin injection. Analysis of the AUC of pain behavior revealed that tigecycline produced antinociception in both phases of the formalin test, F(2, 16) = 50.86 (Phase 1) and 48.66 (Phase 2), for both phases p = 0.0001; Fig. 2 E,F.
Tigecycline Reduced Ethanol‐Mediated Pain in Male, But Not Female Mice
Tigecycline treatment was tested for its ability to reduce EtOH‐mediated pain sensitivity and found to have an unexpected sex difference (see Fig. 3). In female mice, EtOH consumption alone or in combination with systemic administration of tigecycline increased composite pain scores in the formalin test relative to the control group, F(2, 22) = 28.38, p = 0.0001, in a time‐dependent manner, F(22, 242) = 2.45, p = 0.0001; Fig. 3 A. This increase was observed at 5 min, F(2, 22) = 5.35, p = 0.01, and from 25 to 35 min, F(2, 22) = 4.38 (25 min); 26.59 (30 min); and 3.54 (35 min), p = 0.049; Fig. 3 A, postformalin injection. Analysis of the AUC of pain behavior revealed that EtOH consumption (DID) alone or in combination with tigecycline increased pain behavior relative to the control group for both phases, F(2, 22) = 4.78, p = 0.02 (Phase 1); F(2, 22) = 20.60, p = 0.0001 (Phase 2) of the formalin test (Fig. 3 C,D).
Figure 3.

Sex divergent, pro‐nociceptive and antinociceptive, effects were seen with combined ethanol and tigecycline treatment in the formalin test. In female mice (A), DID in combination with tigecycline (80 mg/kg i.p.) increased composite pain scores relative to the control group; whereas, in male mice (B), scores decreased over time. DID + tigecycline increased AUC in females (C, D) and decreased in males (E, F) relative to the control group for both pain phases. Data are expressed as mean ± SEM (n = 6 to 11 per group); *p = 0.049 for DID alone or in combination with tigecycline versus control group; + p = 0.002 for DID + tigecycline versus DID or control groups #p = 0.01 (DID vs. DID + Tig or control groups). Female DID and DID + Tig groups were similar for all time points (p = 0.149). Male control and DID groups did not differ (p = 0.328) across times, except at 25 and 30 min (p = 0.01). ANOVA with Bonferroni post hoc testing was completed.
In male mice, systemic administration of tigecycline in combination with EtOH consumption lowered composite pain scores relative to the DID only or control groups, F(2, 22) = 101.08, p = 0.0001, in a time‐dependent manner, F(22, 242) = 6.98, p = 0.0001; Fig. 3 B, observed at 5 min, F(2, 22) = 13.67, p = 0.0001 and from 30 to 45 min, F(2, 22) = 39.04 (30 min); 20.05 (35 min); 28.15 (40 min); 12.84 (45 min), p = 0.0001; Fig. 3 B, postformalin injection. However, at 25 (p = 0.0001) and 30 (p = 0.01) min postformalin, DID EtOH drinking increased in composite pain scores in comparison to the control group. Analysis of the AUC of pain behavior revealed that EtOH consumption in combination with tigecycline reduced pain behavior relative to the control group for both phases, F(2, 22) = 15.42 (Phase 1) and 103.70 (Phase 2), p < 0.0001, of the formalin test (Fig. 3 E,F). In contrast, DID showed an increase in pain score only in Phase 2 (p = 0.0001 [Phase 2]; p = 0.15 [Phase 1]) relative to control group (Fig. 3 E,F).
Binge Ethanol Consumption Produced Sustained Mechanical and Cold Hypersensitivities
DID consumption increased both mechanical and cold sensitivities. Mechanical sensitivity was measured as a reduction in mechanical paw withdrawal thresholds by DID and showed slow return back to baseline over time, F(7, 70) = 24.67, p = 0.0001, Fig. 4 A. A sex difference was seen as female thresholds were significantly lower overall than in males, F(1, 10) = 58.01, p = 0.0001. The lower mechanical withdrawal thresholds in females were observed on specific post‐DID days, F(1, 10) = 14.77 (day 1); 18.01 (day 3); 6.77 (day 5), p = 0.026. Females returned to baseline on day 3 after EtOH (p = 0.002 for DID, 1 and 2 days after EtOH), whereas males returned to baseline on day 2 after EtOH (p = 0.0001 for DID, 1 day after EtOH) (Fig. 4 A).
Figure 4.
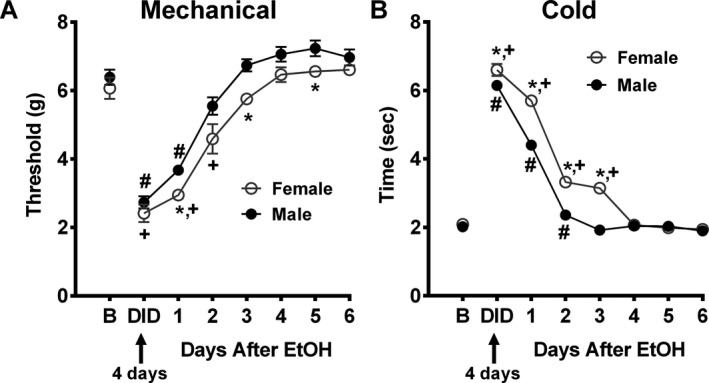
Ethanol consumption promotes mechanical and cold allodynia, with a more robust effect seen in female, compared to male mice. DID resulted in lower mechanical paw withdrawal thresholds (A) and higher cold sensitivity (B) in female, compared to male mice, indicating production of allodynia for both somatosensory modalities. Females returned to baseline on day 3 (mechanical) and on day 4 (cold) after EtOH, whereas males returned to baseline on day 2 (mechanical) and on day 3 (cold) after EtOH (A and B). Data are expressed as mean ± SEM (n = 6 per group); *p = 0.03 for female versus male mice; + p = 0.002 for females at the following time points (DID, 1, 2, and/or 3 after EtOH) versus female baseline group; # p = 0.001 for male at the following time points (DID, 1 and/or 2 after EtOH) versus male baseline group. ANOVA was performed.
Cold sensitization results are shown in Fig. 4 B as increased time spent attending to the acetone cold stimulus and delayed return to baseline, F(7, 70) = 23.69, p < 0.0001. However, female mice spent significantly more time attending to the cold stimulus than males, F(1, 10) = 158.70, p = 0.0001. Differences in the latency were observed after EtOH DID drinking on specific post‐DID days, F(1, 10) = 42.37 (day 1); 42.64 (day 2); 320.99 (day 3); p = 0.0001. Females returned to baseline on day 4 after EtOH (p = 0.0001 for the following time points, DID, 1, 2, and/or 3 days after EtOH), whereas males did so on day 3 after EtOH (p = 0.001 for DID, 1 and/or 2 days after EtOH) (Fig. 4 B).
Tigecycline Had Opposite Effects in Female and Male Mice on Formalin‐Induced Mechanical and Cold Sensitivities
Short‐term studies on mechanical and cold sensitivities were completed to determine whether tigecycline could attenuate the changes produced by the formalin test. Results shown in Fig. 5 indicated that formalin, as expected, produced increased sensitivity and that tigecycline had opposite effects in females and males, similar to the results described in Figs 2 and 3.
Figure 5.

Tigecycline shows sex‐divergent effects on mechanical and cold sensitivities following formalin injection. In female and male mice, mechanical paw withdrawal threshold (C, D) and cold sensitivity (B, C) are not changed at 0 (i.e., baseline) or 30 min after tigecycline (80 mg/kg i.p.) administration. Tigecycline‐treated groups resulted in lower (female, A) or higher (male, B) mechanical paw withdrawal thresholds at 90 and 150 min (60 and 120 min after formalin injection, respectively) relative to control. Male withdrawal thresholds values returned to baseline by the end of the experiment. Tigecycline resulted in higher cold acetone responsiveness in females (C), at 90 and 150 min, while in males (D), higher cold responses were observed at 90 min with lower values at 150 min. Values returning to baseline. Data are expressed as mean ± SEM, (n = 6 per group); *p = 0.04 for tigecycline versus control group was determined by ANOVA.
Mechanical paw withdrawal thresholds (Fig. 5 A,B) were not changed at 0, F(1, 8) = 0.87 (A, female); 0.97 (B, male), p = 0.38, or 30 min, F(1, 8) = 0.02, p = 0.88 (A, female); 0.24 (B, male), p = 0.88, after tigecycline treatment. Tigecycline did not change baseline values. Lower (female) and higher (male) mechanical paw withdrawal thresholds were observed in the tigecycline‐treated groups, F(1, 8) = 22.21 (A, female); 12.18 (B, male), p = 0.008, relative to the control group over time, F(3, 24) = 6.88 (A, female); 7.55 (B, male), p = 0.002. In female mice, the tigecycline group showed a lower mechanical threshold (i.e., increased pain sensitivity) in comparison to the control group at 90 and 150, F(1, 8) = 39.65 (90 min); 44.39 (150 min), p = 0.0001, min after formalin injection, with values not returning to baseline (Fig. 5 A). In contrast, the drug was inhibitory (antinociceptive) in males. Indeed, in males, tigecycline treatment showed higher mechanical thresholds at 90 and at 150, F(1, 8) = 6.22 (90 min); 54.89 (150 min), p = 0.04, min compared to the control group (Fig. 5 B). Unlike the female response, the male values in the tigecycline group returned to baseline by the end of the experiment.
Cold acetone responsiveness (Fig. 5 C,D) showed similar patterns of tigecycline treatment on formalin‐induced effects. No differences in cold sensitivity were seen at 0, F(1, 8) = 2.82 (C, female); 1.52 (D, male), p = 0.25, or 30 min, F(1, 8) = 0.02 (C, female); 0.75 (D, male), p = 0.88, after tigecycline (80 mg/kg) treatment. Higher (female) and lower (male) cold responsiveness was observed in tigecycline‐treated groups, F(1, 8) = 49.81 (C, female); 16.28 (D, male), p = 0.0001, relative to the control group over time, F(3, 24) = 22.37 (C, female); 34.79 (D, male), p = 0.0001. In female mice, the tigecycline group showed higher cold responsiveness in comparison to the control group at 90 and 150, F(1, 8) = 20.14 (90 min); 54.74 (150 min), p = 0.002, min with values not returning to baseline over the duration of the experiment (Fig. 5 C). In contrast, tigecycline reduced cold sensitivity in the male mice. At 90 min, the tigecycline group showed higher, F(1, 8) = 16.22, p = 0.004, cold responsiveness relative to the control group (Fig. 5 D). However, at 150 min, the tigecycline group demonstrated lower, F(1, 8) = 165.78, p = 0.0001, cold responsiveness with values returning to baseline.
Discussion
Alcohol is a well‐known, dose‐dependent analgesic in both humans and animals (Egli et al., 2012). Indeed, as many as 25% of people with chronic pain use alcohol to self‐medicate (Riley and King, 2009). Chronic consumption can result in the development of tolerance to alcohol's analgesic effects as well as peripheral neuropathy leading to an increasing pain alcohol cycle (Egli et al., 2012; Mellion et al., 2011). Brain circuits implicated in addiction, namely the central reward pathway of the nucleus accumbens (NAc), medial prefrontal cortex (mPFC), and circuits between the mPFC and amygdala, also play important roles in pain responses (Apkarian et al., 2013). For example, studies in pain patients showed that the strength of NAc functional connectivity with the mPFC was directly proportional to the amount of pain each patient reported (Kalivas and Volkow, 2005). Basic research employing rodent alcohol pain models are improving our understanding of the interactions of alcohol effect with pain pathways. For example, chronic intermittent drinking paradigms showed development of hyperalgesia using either the Lieber–DeCarli diet (Dina et al., 2006) or voluntary consumption (Fu et al., 2015). The former was shown to have a stress component involving glucocorticoids, the CRF receptor, and PKCε (Dina et al., 2008). While more is known about the longer‐term effects of pain in patients with AUD (for a review, see Apkarian et al., 2013), less is known about the effects of binge alcohol on acute or chronic pain.
Here, our results over 4 days of DID episodes, showed significant EtOH‐mediated increases in inflammatory pain behavior as well as production of both mechanical and cold hypersensitivities. These drinking‐related changes in nociceptive sensitivity may be significant for binge drinkers and individuals with AUD. Given reports of pain reduction by minocycline (Bastos et al., 2013; Kapoor, 2013; Pu et al., 2013), and the results of our companion papers showing that tigecycline reduced binge and dependent EtOH consumption as well as withdrawal symptoms (Bergeson et al., 2016; Martinez et al., 2016; Syapin et al., 2016), we tested the hypothesis that tigecycline would alleviate several negative aspects of high EtOH consumption, while reducing drinking levels. A single medication that possesses the ability to treat several EtOH‐related phenotypes would represent a valuable AUD pharmacotherapeutic agent. In fact, our experiments showed that tigecycline treatment was effective at reducing pain and mechanical and cold hypersensitivities in male mice, but resulted in an exacerbation of alcohol‐mediated nociceptive perception in female mice.
Inflammation and resultant pain are complex process that involves a myriad of different mechanisms. Adding a level of complexity is the differing etiology of this process in males and females. The possibility of an estrogen effect on sex differences could be one explanation, or it could involve mechanisms yet to be associated with our findings. For example, we posit that the sex discrepancy may, in fact, include distinctions in pain sensitivity at the cellular level, as short‐term binge EtOH consumption was similar in the female and male mice and, therefore, cannot explain the sex differences in pain sensitivity observed. Accumulating evidence suggests that inflammatory pain is mediated in males by innate immunity‐related TLR4 microglia activation (Sorge et al., 2011) and increased levels of P2X4 receptors. In females, it appears likely to involve the T cells of the adaptive immune system (Mapplebeck et al., 2016; Sorge et al., 2015). The estrus cycle and/or a dose‐dependent‐related effect could also explain the sex differences. For example, Lenz and colleagues (2013), showed microglial differences in the female and male brain resulting from developmental maturation and, in particular, that microglia were essential to the masculinization of the brain. In addition, minocycline reduced the ED50 for morphine‐induced analgesia in males, but not females (Posillico et al., 2015). However, even taken together, these data do not explain the tigecycline hypersensitivity in females; therefore, additional studies are necessary to assess the female‐specific mechanisms of action.
Tetracycline‐like compounds have many actions, including anti‐apoptotic, anti‐inflammatory, and angiogenic characteristics (Garrido‐Mesa et al., 2013). Tigecycline was FDA approved in 2005 for intravenous use to treat resistant bacterial infections and has not been widely used in research. Minocycline, however, has recently gained attention for its positive effects shown in a variety of experiments involving inflammation and/or neurodegeneration. Minocycline blocks microglia activation and has effects on several biological pathways including poly (ADP‐ribose) polymerase (Alano et al., 2006), matrix metalloproteinases, NF‐κB binding and translocation, PKC, and MAPK signaling (Garrido‐Mesa et al., 2013). Although unproven, it follows that tigecycline may work through similar mechanisms.
To conclude, we have demonstrated that short‐term binge alcohol consumption produced increased pain and both cold and mechanical allodynia. We also showed important sex‐specific, nociceptive properties of tigecycline, which acts on inflammatory pain and mechanical and cold sensitivity thresholds. As mentioned, further studies are needed to investigate the pro‐nociceptive effect in females at mechanistic/pathway levels although it is important to note that hypersensitivity above DID was not seen. Regarding the potential for treatment of AUD with tigecycline, we have now shown in our companion papers that the drug reduced binge and dependent alcohol consumption, withdrawal symptoms, and here, in male mice a reduction in pain and somatosensory sensitization. However, it is important to note that special co‐therapy may be necessary for females to reduce pain as well as cold and touch sensitization.
Acknowledgments
We thank Dr. Deb Finn for assistance with manuscript preparation and editing. We thank NIAAA R21 AA021142 (SEB), the Bryan C. Miller, Jr. and Martha H. Miller Foundation, Inc., The CH Foundation (JG) and Texas Tech University Health Sciences Center School of Medicine 121035 (JG) and the resources/facilities used at the Texas Tech University Health Sciences Center (SEB, JG) for their support. The authors state that no one has a conflict of interest.
References
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE (2011) Minocycline reduces ethanol drinking. Brain Behav Immun 25(Suppl 1):S165–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal RG, Owen JA, Levin PS, Hewetson A, Berman AE, Franklin SR, Hogue RJ, Chen Y, Walz C, Colvard BD, Nguyen J, Velasquez O, Al‐Hasan Y, Blednov YA, Fowler AK, Syapin PJ, Bergeson SE (2014) Bioinformatics analyses reveal age‐specific neuroimmune modulation as a target for treatment of high ethanol drinking. Alcohol Clin Exp Res 38:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano CC, Kauppinen TM, Valls AV, Swanson RA (2006) Minocycline inhibits poly(ADP‐ribose) polymerase‐1 at nanomolar concentrations. Proc Natl Acad Sci U S A 103:9685–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S (2013) Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav 112:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos LF, de Oliveira AC, Watkins LR, Moraes MF, Coelho MM (2012) Tetracyclines and pain. Naunyn Schmiedebergs Arch Pharmacol 385:225–241. [DOI] [PubMed] [Google Scholar]
- Bastos LF, Prazeres JD, Godin AM, Menezes RR, Soares DG, Ferreira WC, Dutra MM, Machado RR, Coelho MM (2013) Sex‐independent suppression of experimental inflammatory pain by minocycline in two mouse strains. Neurosci Lett 553:110–114. [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Nipper MA, Jensen J, Helms ML, Finn DA (2016) Tigecycline reduces ethanol intake in dependent and nondependent male and female C57BL/6J mice. Alcohol Clin Exp Res doi: 10.1111/acer.13251 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda ML, Mogil JS (2006) Sex differences in the effects of amiloride on formalin test nociception in mice. Am J Physiol Regul Integr Comp Physiol 291:R335–R342. [DOI] [PubMed] [Google Scholar]
- Cho IH, Chung YM, Park CK, Park SH, Lee H, Kim D, Piao ZG, Choi SY, Lee SJ, Park K, Kim JS, Jung SJ, Oh SB (2006) Systemic administration of minocycline inhibits formalin‐induced inflammatory pain in rat. Brain Res 1072:208–214. [DOI] [PubMed] [Google Scholar]
- Cockeran R, Mutepe ND, Theron AJ, Tintinger GR, Steel HC, Stivaktas PI, Richards GA, Feldman C, Anderson R (2012) Calcium‐dependent potentiation of the pro‐inflammatory functions of human neutrophils by tigecycline in vitro. J Antimicrob Chemother 67:130–137. [DOI] [PubMed] [Google Scholar]
- Coderre TJ (1992) Contribution of protein kinase C to central sensitization and persistent pain following tissue injury. Neurosci Lett 140:181–184. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Katz J (1997) Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav Brain Sci 20:404–419; discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM (2004) Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain 8:397–411. [DOI] [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG (2015) Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1‐dependent withdrawal. Biol Psychiatry 77:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Alessandri‐Haber N, Green PG, Messing RO, Levine JD (2008) Alcohol‐induced stress in painful alcoholic neuropathy. Eur J Neurosci 27:83–92. [DOI] [PubMed] [Google Scholar]
- Dina OA, Messing RO, Levine JD (2006) Ethanol withdrawal induces hyperalgesia mediated by PKCepsilon. Eur J Neurosci 24:197–204. [DOI] [PubMed] [Google Scholar]
- Domercq M, Matute C (2004) Neuroprotection by tetracyclines. Trends Pharmacol Sci 25:609–612. [DOI] [PubMed] [Google Scholar]
- Draper NR (1998) Applied regression analysis bibliography update 1994‐97. Commun Stat Theory Methods 27:2581–2623. [Google Scholar]
- Egli M, Koob GF, Edwards S (2012) Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 36:2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS (2014) Effectiveness and safety of high‐dose tigecycline‐containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents 44:1–7. [DOI] [PubMed] [Google Scholar]
- Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J (2015) Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague‐Dawley rats. Int J Physiol Pathophysiol Pharmacol 7:136–144. [PMC free article] [PubMed] [Google Scholar]
- Garrido‐Mesa N, Zarzuelo A, Galvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169:337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Deng L, Fan B, Wager‐Miller J, Hohmann AG (2014) Optimization of a cisplatin model of chemotherapy‐induced peripheral neuropathy in mice: use of vitamin C and sodium bicarbonate pretreatments to reduce nephrotoxicity and improve animal health status. Mol Pain 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Guijarro A, Piomelli D, Hohmann AG (2011) Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br J Pharmacol 163:1464–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Kapoor S (2013) Pain‐ameliorating effects of minocycline: an emerging treatment modality. J Neurosci Res 91:1. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM (2013) Microglia are essential to masculinization of brain and behavior. J Neurosci 33:2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapplebeck JC, Beggs S, Salter MW (2016) Sex differences in pain: a tale of two immune cells. Pain 157(Suppl 1):S2–S6. [DOI] [PubMed] [Google Scholar]
- Martinez JM, Groot JA, Curtis DC, Allison CL, Marquardt PC, Holmes AN, Edwards DS, Trotter DRM, Syapin PJ, Finn DA, Bergeson SE (2016) Effective reduction of acute ethanol withdrawal by the tetracycline derivative, tigecycline, in female and male DBA/2J mice. Alcohol Clin Exp Res doi: 10.1111/acer.13259 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SR, Muccigrosso MM, Haydon PG (2012) The effect of doxycycline on alcohol consumption and sensitivity: consideration for inducible transgenic mouse models. Exp Biol Med (Maywood) 237:1129–1133. [DOI] [PubMed] [Google Scholar]
- Meagher AK, Ambrose PG, Grasela TH, Ellis‐Grosse EJ (2005) Pharmacokinetic/pharmacodynamic profile for tigecycline‐a new glycylcycline antimicrobial agent. Diagn Microbiol Infect Dis 52:165–171. [DOI] [PubMed] [Google Scholar]
- Mellion M, Gilchrist JM, de la Monte S (2011) Alcohol‐related peripheral neuropathy: nutritional, toxic, or both? Muscle Nerve 43:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Bailey AL (2010) Sex and gender differences in pain and analgesia. Prog Brain Res 186:141–157. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chanda ML (2005) The case for the inclusion of female subjects in basic science studies of pain. Pain 117:1–5. [DOI] [PubMed] [Google Scholar]
- Padi SS, Kulkarni SK (2008) Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti‐inflammatory and antioxidant mechanisms. Eur J Pharmacol 601:79–87. [DOI] [PubMed] [Google Scholar]
- Posillico CK, Terasaki LS, Bilbo SD, Schwarz JM (2015) Examination of sex and minocycline treatment on acute morphine‐induced analgesia and inflammatory gene expression along the pain pathway in Sprague‐Dawley rats. Biol Sex Differ 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S, Xu Y, Du D, Yang M, Zhang X, Wu J, Jiang W (2013) Minocycline attenuates mechanical allodynia and expression of spinal NMDA receptor 1 subunit in rat neuropathic pain model. J Physiol Biochem 69:349–357. [DOI] [PubMed] [Google Scholar]
- Puig S, Sorkin LS (1996) Formalin‐evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase‐2 activity. Pain 64:345–355. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63. [DOI] [PubMed] [Google Scholar]
- Riley JL 3rd, King C (2009) Self‐report of alcohol use for pain in a multi‐ethnic community sample. J Pain 10:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, LaCroix‐Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS (2011) Spinal cord Toll‐like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 31:15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS (2015) Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syapin PJ, Martinez JM, Curtis DC, Marquardt PC, Allison CL, Groot JA, Baby C, Al‐Hasan YM, Segura‐Ulate I, Scheible MJ, Nicholson KT, Redondo JL, Trotter DR, Edwards DS, Bergeson SE (2016) Effective reduction in high ethanol drinking by semisynthetic tetracycline derivatives. Alcohol Clin Exp Res doi: 10.1111/acer.13253 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992) The formalin test: an evaluation of the method. Pain 51:5–17. [DOI] [PubMed] [Google Scholar]
- Watson GS, Sufka KJ, Coderre TJ (1997) Optimal scoring strategies and weights for the formalin test in rats. Pain 70:53–58. [DOI] [PubMed] [Google Scholar]


