Abstract
Objective
Antipsychotic medication use has grown rapidly among young children despite the lack of Food and Drug Administration approval for antipsychotics' broad use in this age group. Characteristics of physicians who prescribe antipsychotics to young children were identified, and prescribing patterns were compared for young children and adults.
Methods
Physician-level prescribing data from IMS Health's Xponent™ database linked with American Medical Association Masterfile data were analyzed. Sample included all U.S. psychiatrists plus a 5% random sample of family medicine physicians who wrote at least ten antipsychotic prescriptions per year from 2008-2011 (n=31,713). Logistic and hierarchical binomial regression models were estimated to examine physician prescribing for young children 0-9, and the types and numbers of ingredients used for young children vs. adults 20-64 were examined.
Results
Among antipsychotic prescribers, 42.2% had ≥1 prescriptions for young children. Such prescribing was more likely among younger physicians (e.g., OR=1.70; CI=1.54-1.89 for ≤39 vs. 60+), and those practicing in rural areas (OR=1.11; CI=1.02-1.21), and less likely among males (OR=.93; CI-.88-.98) and those who graduated from a top 25 U.S. medical school (OR=.87; CI=.81-.94). Among physicians who prescribed to both young children and adults, 75.0% of young child prescriptions were for drugs with an FDA-approved indication for this age range (vs. 35.7% of adult prescriptions), and a much smaller number of ingredients were used on average for young child vs. adult patients (median=2 vs. 7).
Conclusions
While prescribing for young children is relatively common across antipsychotic prescribers, prescribing patterns differ for young children relative to adults.
Introduction
The medical community has long recognized that children are not small adults, particularly in terms of medication effectiveness and safety,1 and that psychotropics and other medications with central nervous system effects can be harmful to the developing brain.2 Nevertheless, the practice of treating young children with psychotropics lacking Food and Drug Administration (FDA) approval (“off-label” prescribing) remains common.3–7 A case in point is the use of antipsychotics, namely second-generation antipsychotics, in pre-pubertal children. As of the fall 2015, risperidone and aripiprazole are the only second-generation antipsychotics that have FDA-approved indications for children 0-9, and only for the treatment of irritability associated with autism, a diagnosis that a relatively small proportion of child antipsychotic users have received.5 However, second-generation antipsychotics have been used increasingly by young children for multiple other conditions including attention deficit hyperactivity disorder (ADHD), conduct disorder, psychosis, and mood disorders,3–7 despite caution urged by the American Psychiatric Association (in its Choosing Wisely Recommendations), and the Academy of Child and Adolescent Psychiatry (AACAP).8,9 Their use in preschoolers (0–4 years old) has been received with particular alarm.2,10,11
While the risks of antipsychotic use in adult populations are well established,12–15 the risks in children are still the subject of active research.16, 17 However, there is growing evidence that antipsychotics, including frequently-used second-generation antipsychotics, are associated with serious cardiometabolic side effects, such as excessive weight gain, hypertension, and lipid and glucose abnormalities, when used in children.18,19 The importance of this association is amplified by evidence that cardiometabolic disturbances in childhood predict adult cardiometabolic outcomes.20 Not only is there evidence that children are more vulnerable to these risks than adults,21, 22 but there is also evidence that the youngest of the young are at even higher risk.19, 23 Studies have found that off-label child antipsychotic use is associated with illness severity (psychiatric comorbidity, history of psychiatric hospitalizations) and severely disruptive behaviors,24–27 which suggests that child prescribers may use antipsychotics when psychosocial treatments are not accessible or effective or may prescribe off-label when FDA-approved treatment options have been exhausted.28 Hence, although potentially justifiable, the common and growing practice of off-label antipsychotic prescribing to young children warrants scrutiny given the absence of evidence of efficacy,29 evidence of significant cardiometabolic risks, serious concerns about harmful effects on the developing brain, and the availability (if uneven accessibility) of safer alternative treatments. Also of concern is the documented association between antipsychotic use in young children and geographic region, race, and indicators of social disadvantage such as Medicaid and foster care.25,30,31
While previous studies have identified child characteristics associated with antipsychotic use,3–4,5,6,7, 10, 16, 27, 31–32, 33, 34, 35 our focus is on describing characteristics of physicians who prescribe antipsychotics to young children and examining their prescribing patterns using a unique physician-level, all-payer prescription database. Although evidence suggests that the limited armamentarium of efficacious therapies for children with disruptive behaviors and limited accessibility to evidence-based psychosocial interventions may drive some of the off-label antipsychotic prescribing,36–38 little is known about prescriber factors that influence use in young children or how the types and concentration of prescribing may differ for young children versus adults.
Methods
We obtained monthly physician-level prescribing information from IMS Health's Xponent™ database, which directly captures over 70% of all US prescriptions filled in retail pharmacies and utilizes a patented projection methodology to represent 100% of prescriptions filled in these outlets. IMS Health provided Xponent™ data on all U.S. physicians classified as psychiatrists in the American Medical Association Masterfile (n = 29,857) and a 5% random sample of physicians classified as family medicine physicians (n = 1,856) who wrote at least ten antipsychotic prescriptions per year from 2008-2011. The Xponent™ data includes information on the payer (Medicaid fee-for-service (FFS), Medicare, commercial, or cash) and the age category of the patient (using IMS-provided categories 0-9, 10-19, 20-64, 65+). For the purposes of this study, we define young children as those 0-9. Because the data are obtained from pharmacy transactions and not medical claims they lack patient clinical information (e.g., diagnosis codes). We linked the Masterfile data on physician characteristics (age, sex, geographic location, training institution, and practice setting) to the Xponent™ prescribing data using a prescriber ID. As a result, these data include detailed information on physician characteristics and comprehensive physician-level data on prescribing patterns across all payers for over 31,000 psychiatrists and family medicine physicians who prescribe antipsychotics.
To identify physician characteristics associated with prescribing antipsychotics to young children, we estimated a logistic regression model of whether a physician had any child 0-9 antipsychotic prescriptions over the period 2009-2011 (using 2008 data to measure baseline variables) as a function of: physician sex; age (≤39, 40-49, 50-59, 60+); practice setting (solo/two-person; group practice; other, such as Veterans Administration, military hospitals, U.S. Public Health Service; medical schools, and health maintenance organizations; no classification available); any hospital practice; rural practice; Census region (New England, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain, Pacific, Middle Atlantic); Top 25 medical school according to U.S. News and World Reports in 2011; foreign medical graduate; quartile of total 2008 antipsychotic prescription volume (for adults or children); and share of physician's 2008 total antipsychotic prescriptions paid by a Medicaid FFS program, by Medicare or a commercial payer (including Medicaid managed care plans), or by cash. Because almost 90% of child/adolescent psychiatrists had at least one prescription for a young child, we did not include specialty indicators.
Next, we compared prescribing patterns for young children versus adults 20-64 among the subset of physicians who had at least one prescription for both age groups (n=13,214). We were interested in whether prescribers chose from a different, and possibly narrower, set of antipsychotics given the limited number of products with FDA approval for use in young children. We first compared the share of all antipsychotic prescriptions written for each medication for the patients in the two age groups. We then examined the distribution of the number of different antipsychotic medication ingredients prescribed by physicians for patients in the two groups, both overall, by specialty, and by quartile of 2008 total antipsychotic prescribing volume. Because of the very low use of first-generation antipsychotics (see Appendix for list) among young children, all first-generation antipsychotics were treated as a single ingredient.
We also identified factors associated with prescribing of antipsychotics for which there are no FDA-approved indications for young children. As noted above, only two second-generation antipsychotics, risperidone and aripiprazole, had an approved indication for children 0-9 (see Appendix). Four first-generation drugs – chlorpromazine, haloperidol, prochlorperazine, and trifluoperazine – had approved indications for children 0-9, and prescriptions for these were categorized accordingly, although these drugs were rarely used in young children.
We estimated a hierarchical binomial regression model of the physician's number of young child prescriptions for antipsychotics with no approved indications for children 0-9. We assumed the number of child 0-9 prescriptions for antipsychotics with no approved indications for each physician arises from a binomial distribution characterized by a physician-specific probability, p. We modeled the log-odds of the probability of these prescriptions as a function of the same variables as the logistic regression model described above with two additions -- specialty (child/adolescent psychiatry, other psychiatry, family medicine) plus a physician-specific random effect. The random effect was assumed to be normally distributed with mean 0 and unknown variance τ2; the random effect accounted for clustering of prescriptions written by each physician as well as different physician volumes. We computed odds ratios and corresponding 95% confidence intervals for the physician characteristics. To characterize the degree of between-physician variation in the probability of writing these prescriptions, after adjusting for physician characteristics, we determined the odds a child 0-9 years received such a prescription from a “high” prescriber relative to a “low” prescriber. A high prescriber was operationalized as one who was one standard deviation, τ, above the mean of the physician random effects. To determine whether physicians writing few prescriptions impact our findings, we repeated the binomial regression analysis restricting to physicians with at least 30 prescriptions.
This study was approved by the XXX Institutional Review Board. All analyses used SAS version 9.4.
Results
Of the 31,713 physicians, 13,374 (42.2%) wrote at least one antipsychotic prescription to a patient 0-9 during the three-year period. Physicians with any prescriptions for young children differed along a number of dimensions from prescribers with no prescriptions for this population (Table 1).
Table 1.
Characteristics of 2009–2011 Antipsychotic Prescribers (n=31,713)
| Physicians with no child 0–9 antipsychotic prescriptions (n=18,339) | Physicians with child 0–9 antipsychotic prescriptions (n=13,374) | P-value | |||
|---|---|---|---|---|---|
| Male | 12,046 | 65.7% | 8,584 | 64.2% | .01 |
| Age | |||||
| <=39 | 1,484 | 8.1% | 1,278 | 9.6% | <.001 |
| 40–49 | 3,965 | 21.6% | 3,469 | 25.9% | |
| 50–59 | 5,719 | 31.2% | 4,346 | 32.5% | |
| 60+ | 7,171 | 39.1% | 4,281 | 32.0% | |
| Specialty | |||||
| Child/adolescent psychiatry | 590 | 3.2% | 4,249 | 31.8% | <.001 |
| Other psychiatry | 16,248 | 88.6% | 8,770 | 65.6% | |
| Family medicine | 1,501 | 8.2% | 355 | 2.7% | |
| Practice type | |||||
| Solo/two-person | 6,364 | 34.7% | 3,746 | 28.0% | <.001 |
| Group | 4,647 | 25.3% | 4,109 | 30.7% | |
| Other | 4,117 | 22.5% | 2,923 | 21.9% | |
| No classification | 3,211 | 17.5% | 2,596 | 19.4% | |
| Any hospital practice (full- or part-time) | 6,766 | 36.9% | 4,429 | 33.1% | <.001 |
| Rural practice | 1,500 | 8.2% | 1,412 | 10.6% | <.001 |
| Region | |||||
| New England | 1,926 | 10.5% | 862 | 6.5% | <.001 |
| East North Central | 2,210 | 12.1% | 2,003 | 15.0% | |
| West North Central | 856 | 4.7% | 920 | 6.9% | |
| South Atlantic | 2,926 | 16.0% | 2,679 | 20.0% | |
| East South Central | 671 | 3.7% | 701 | 5.2% | |
| West South Central | 1,037 | 5.7% | 1,395 | 10.4% | |
| Mountain | 1,059 | 5.8% | 733 | 5.5% | |
| Pacific | 3,521 | 19.2% | 1,649 | 12.3% | |
| Middle Atlantic | 4,132 | 22.5% | 2,431 | 18.2% | |
| Top 25 U.S. medical school graduate | 2,843 | 15.5% | 1,318 | 9.9% | <.001 |
| Foreign medical graduate | 4,921 | 26.8% | 4,629 | 34.6% | <.001 |
| Mean (standard deviation) number of total (child and adult) antipsychotic prescriptions, 2008 | 462.3 | 659.5 | 972.9 | 1,111.9 | <.001 |
Source: Xponent™ database, 1/08 – 12/11, IMS Health Incorporated. All Rights Reserved.
Note: Analyses use IMS Health's Xponent™ linked with American Medical Association Masterfile data. The sample includes physicians with ≥10 antipsychotic prescriptions per year over the study period. P-values were obtained using a chi-square test across the two categories of physicians.
Factors Associated with Any Child 0-9 Prescribing
On average, younger physicians were more likely to prescribe antipsychotics to young children (odds ratio [95% confidence interval] for ≤39 years, 40-49, and 50-59 relative to 60+ are: OR=1.70; CI=1.54-1.89, OR=1.40; CI=1.30–1.50, and OR=1.22; 1.14–1.29), and male physicians were less likely to do so than female physicians (OR=.93; CI=.88-.98) (Table 2). Physicians in rural areas were more likely to prescribe to children 0-9 (OR=1.11; CI=1.02-1.21), and those practicing in other types of settings besides group practices were less likely relative to physicians practicing in one- or two-person practices (OR=.86; CI=.80–.92). Graduates of top 25 medical schools were less likely to prescribe to children 0-9 (OR=.87; CI=.81-.94). The likelihood of prescribing to young children increased with total antipsychotic prescribing volume and the share of the physician's prescriptions paid for by Medicaid FFS (OR=9.25; CI=8.28–10.34), decreased with the share paid by cash (OR=.54; CI=.34–.86), and varied by geographic region.
Table 2.
Predictors of the Probability of that a Physician Has Any Antipsychotic Medications for Young Children (0–9 Years)
| Variable | Odds ratio | 95% confidence interval |
|---|---|---|
| Sex | ||
| Male (reference: female) | .93 | .88–.98 |
| Age (reference: 60+) | ||
| <=39 | 1.70 | 1.54–1.89 |
| 40–49 | 1.40 | 1.30–1.50 |
| 50–59 | 1.22 | 1.14–1.29 |
| Practice setting (reference: solo/two-person) | ||
| Group practice | 1.03 | .97–1.10 |
| Other | 0.86 | .80–.92 |
| No classification | 0.99 | .92–1.08 |
| Hospital practice | ||
| Yes (reference: no) | 0.95 | .90–1.00 |
| Rural practice | ||
| Yes (reference: no) | 1.11 | 1.02–1.21 |
| Census Region (reference: Middle Atlantic) | ||
| New England | 0.80 | .72–.89 |
| East North Central | 1.37 | 1.25–1.49 |
| West North Central | 1.53 | 1.36–1.72 |
| South Atlantic | 1.60 | 1.47–1.73 |
| East South Central | 1.51 | 1.33–1.72 |
| West South Central | 1.96 | 1.77–2.18 |
| Mountain | 1.37 | 1.22–1.54 |
| Pacific | 0.76 | .70–.83 |
| Top 25 medical school graduate | ||
| Yes (reference: no) | 0.87 | .81–.94 |
| Foreign medical graduate | ||
| Yes (reference: no) | 1.01 | .95–1.07 |
| Total antipsychotic prescribing volume, 2008 (reference: first quartile) | ||
| Second quartile | 1.82 | 1.69–1.96 |
| Third quartile | 2.88 | 2.67–3.10 |
| Fourth quartile | 5.05 | 4.68–5.46 |
| Share of total antipsychotic prescriptions paid by Medicaid FFS programs, 2008 | 9.25 | 8.28–10.34 |
| Share of total antipsychotic prescriptions paid by cash, 2008 | 0.54 | .34–.86 |
Source: Xponent™ database, 1/08 – 12/11, IMS Health Incorporated. All Rights Reserved.
Notes: Analyses use IMS Health's Xponent™ linked with American Medical Association Masterfile data. Physician specialty variables are not included because most child psychiatrists have at least one child 0–9 antipsychotic prescription over the study period.
Comparison of Prescribing to Children 0-9 vs. Adults 20-64
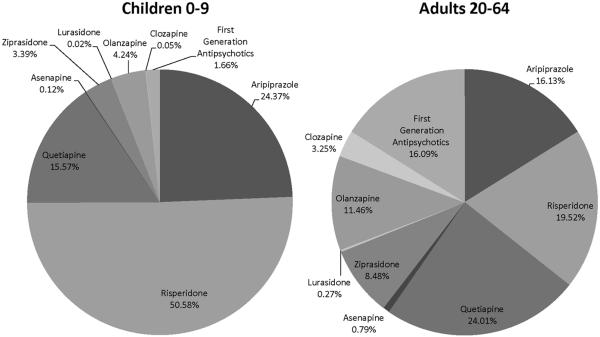
Use of specific medications varied among physicians who prescribed both to young children and adults. Three-quarters of young child prescriptions were for risperidone (50.6%) and aripiprazole (24.4%) (Figure1). In contrast, only 35.7% of their adult prescriptions were for these drugs (risperidone 19.5% and aripiprazole 16.1%). Quetiapine was the most commonly prescribed drug for adults (24.0% of prescriptions). Notably, although more first-generation antipsychotics have FDA approval for this age group and little evidence exists of their cardiometabolic risks, physicians used them rarely for young children (1.7% of prescriptions), and much less often than for adults (16.1%).
Figure 1.
Share of Antipsychotic Prescriptions for Each Ingredient for Children 0-9 Versus Adults 20-64, across Physicians who Prescribed to Both Groups (n=13,214), 2009-2011.
Source: Xponent™ database, 1/08 – 12/11, IMS Health Incorporated. All Rights Reserved.
Note: Analyses use IMS Health's Xponent™ linked with American Medical Association Masterfile data.
Among these physicians with prescriptions both to young children and adults, the median number of different ingredients used across their young child patients was 2 (Interquartile Range (IR)=1,4). Physicians used a much broader set of ingredients for their adult patients, with a median of 7 (IR=6,8) different ingredients (Table 3). The median number of ingredients for young child prescriptions was higher for child/adolescent psychiatrists (3) than for family medicine physicians (1) and other psychiatrists (2). The median number of ingredients also increased with total antipsychotic prescribing volume (median=2 for the first and second volume quartiles and 3 for the third and fourth volume quartiles).
Table 3.
Median and Interquartile Range for Number of Antipsychotic Ingredients Prescribed for Young Children (0–9 Years) and Adults (20–64 Years) across Physicians who Prescribed to Both Groups, 2009–2011
| Median | Interquartile Range | |
|---|---|---|
|
| ||
| All Prescribers: | ||
| Young Children | 2 | 1–4 |
| Adults | 7 | 6–8 |
|
| ||
| Child/Adolescent Psychiatrists: | ||
| Young Children | 3 | 2–4 |
| Adults | 6 | 4–7 |
|
| ||
| Family Medicine Physicians: | ||
| Young Children | 1 | 1-1 |
| Adults | 5 | 4–6 |
|
| ||
| Other Psychiatrists: | ||
| Young Children | 2 | 1–3 |
| Adults | 7 | 6–8 |
|
| ||
| First Quartile of Total Antipsychotic Volume | ||
| Young Children | 2 | 1–3 |
| Adults | 5 | 4–7 |
|
| ||
| Second Quartile of Total Antipsychotic Volume | ||
| Young Children | 2 | 1–3 |
| Adults | 6 | 5–7 |
|
| ||
| Third Quartile of Total Antipsychotic Volume | ||
| Young Children | 3 | 1–4 |
| Adults | 7 | 6–8 |
|
| ||
| Fourth Quartile of Total Antipsychotic Volume | ||
| Young Children | 3 | 1–4 |
| Adults | 8 | 7–9 |
Source: Xponent™ database, 1/08 – 12/11, IMS Health Incorporated. All Rights Reserved.
Notes: Analyses use IMS Health's Xponent™ linked with American Medical Association Masterfile data. Note: All FGAs are viewed as being a single ingredient due to the very low share of first-generation antipsychotics used among children 0–9.
Factors Associated with Number of Child 0-9 Prescriptions for Antipsychotics with No FDA-Approved Indications for that Age Range
Among the 13,374 physicians with at least one antipsychotic prescription for a young child, almost two-thirds (64.0%) had at least one prescription for a medication with no FDA-approved indications for that age range.
On average, physicians age 40-49 and 50-59 were more likely to prescribe antipsychotics with no FDA-approved indication for young children than older physicians (OR=1.18; CI=1.05–1.30 and OR=1.13; CI=1.02–1.24, respectively) (Table 4). Compared to other psychiatrists, child/adolescent psychiatrists and family medicine physicians were less likely (OR=.89; CI=.81–.96) and OR=.55; CI=.39–.72, respectively). Those practicing in other settings than group practices had a higher odds than those in one- or two-person practices (OR=1.15; CI=1.02–1.28). Physicians in rural areas were less likely relative to those in non-rural settings (OR=.87; CI=.76–.98), while foreign medical graduates were less likely relative to US graduates (OR=.88; CI=.80–.95). The odds of prescribing medications with no approved indications for young children increased with total antipsychotic prescribing volume and the share of the physician's prescriptions paid for by Medicaid FFS (OR=1.49; CI=1.27–1.71), and varied by region.
Table 4.
Predictors of the Number of Antipsychotic Prescriptions with No FDA-approved Indication for Young Children among Physicians with At Least One Young Child Antipsychotic Prescription, 2009–2011
| Variable | Odds ratio | 95% confidence interval |
|---|---|---|
| Sex | ||
| Male (reference: female) | 1.03 | 0.94–1.12 |
| Age (reference: 60+) | ||
| <=39 | 1.16 | .98–1.34 |
| 40–49 | 1.18 | 1.05–1.30 |
| 50–59 | 1.13 | 1.02–1.24 |
| Specialty (reference: other psychiatry) | ||
| Child/adolescent psychiatry | 0.89 | .81–0.96 |
| Family medicine | 0.55 | .39–0.72 |
| Practice setting (reference: solo/two-person) | ||
| Group practice | 1.08 | .97–1.19 |
| Other | 1.15 | 1.02–1.28 |
| No classification | 0.92 | .81–1.03 |
| Hospital practice | ||
| Yes (reference: no) | 1.07 | .98–1.17 |
| Rural practice | ||
| Yes (reference: no) | 0.87 | .76–.98 |
| Region (reference: Middle Atlantic) | ||
| New England | 1.17 | .97–1.38 |
| East North Central | 1.19 | 1.03–1.35 |
| West North Central | 1.47 | 1.22–1.72 |
| South Atlantic | .77 | .67–.86 |
| East South Central | .84 | .68–1.00 |
| West South Central | 1.02 | 0.87–1.18 |
| Mountain | 1.46 | 1.19–1.72 |
| Pacific | .90 | .77–1.03 |
| Top 25 medical school graduate | ||
| Yes (reference: no) | 1.09 | .94–1.24 |
| Foreign medical graduate | ||
| Yes (reference: no) | .88 | .80–.95 |
| Total antipsychotic prescribing volume, 2008 (reference: first quartile) | ||
| Second quartile | 1.18 | 1.02–1.35 |
| Third quartile | 1.45 | 1.25–1.65 |
| Fourth quartile | ||
| Share of total antipsychotic prescriptions paid by Medicaid FFS programs, 2008 | 1.49 | 1.27–1.71 |
| Share of total antipsychotic prescriptions paid by cash, 2008 | 4.52 | 0.50–8.53 |
Source: Xponent™ database, 1/08 – 12/11, IMS Health Incorporated. All Rights Reserved.
Note: Analyses use IMS Health's Xponent™ linked with American Medical Association Masterfile data. Young children are defined as those under the age of ten.
Despite adjusting for these characteristics, substantial between-physician variation in prescribing of medications with no approved indication for young children remained. The between-physician variance component, after adjusting for physician characteristics, was 3.65 (s.e.=0.0747). This roughly translates to a range in odds ratio of 45 across all prescribers, implying that the odds of a prescription with no approved indications for children 0-9 for a moderately “high” prescriber of medications with no approved indications is 45 times that when treated by a moderately “low” prescriber. When restricting to physicians writing at least 30 antipsychotic prescriptions (n=7065, 53% of all physicians), our substantive findings generally did not change, although the child/adolescent psychiatrist, prescription share paid by Medicaid, and rural effects were no longer statistically significant. The between-physician odds ratio decreased to 21, still indicating substantial between-physician variation.
Discussion
Using all-payer, physician-level prescription data on psychiatrists and family medicine physicians who prescribed antipsychotic medications over the period 2009-2011, we found that almost half of prescribers had written at least one antipsychotic prescription for a child 0-9. Prescribing to young children was more common among younger physicians, those practicing in rural areas, those with higher total antipsychotic prescribing volume, and those with a higher share of prescriptions paid by Medicaid FFS programs. It was less common among physicians practicing outside of groups, two-person or solo practices; physicians who graduated from a top 25 medical school; and physicians with a high share of antipsychotic prescriptions paid by cash. While three-quarters of prescriptions for young children were for the two drugs with an FDA-approved indication for children in that age range, almost two-thirds of physicians who prescribed to young children had at least one prescription for a medication with no FDA-approved indications for children 0-9. This is in spite of the fact that physicians tended to prescribe from a narrow set of antipsychotics (typically two) for young children.
While previous studies using patient-level claims and survey data have documented rates of antipsychotic use in young children,3–7 little is known about the characteristics of physicians who prescribe these medications to young children or about the patterns of prescribing in this age group. Our results show that physicians treating both adults and children appear to tailor their prescribing to some extent based on age group, adopting what may be a more conservative approach for young children. Physicians are more likely to prescribe an antipsychotic with an FDA-approved indication for this age group to their young child patients (even if the medication may be prescribed for a different condition than the approved indication), and they use a much smaller number of ingredients for those patients relative to their adult patients.
A number of strategies, including prior authorization and step therapy requirements,39–44 tiered formularies,45,46 monitoring and feedback (e.g., computerized alerts, reminders, audit and feedback),47 academic detailing,48,49 and policies restricting detailing efforts by pharmaceutical manufacturers50 have proven effective at influencing physician prescribing behavior in general. Payers and health plans could consider applying these approaches to antipsychotic prescribing to young children in an effort to highlight for prescribers the risk-benefit tradeoffs associated with antipsychotic use in children. That some antipsychotic prescribing among young children may be occurring as a result of limited access to non-pharmacologic evidence-based treatments, particularly when the behaviors are disruptive as is often the case with severely hyperactive children with ADHD,26,36 points to the need for broader interventions focused on the competencies and geographic distribution of the mental health workforce and financing of mental health services.37, 38,51,52 It is significant in this regard that federal and state agencies have already launched initiatives aimed at improving the appropriateness of child psychotropic prescribing, especially for children in foster care.30,53 New HEDIS measures focused on the safe prescribing of antipsychotics for children have also drawn increased attention to this issue from payers, health plans, and provider organizations.
There are several limitations to our analysis. First, our dataset lacks information on the specific indication for which the physician prescribed an antipsychotic as well as information on dosing, duration of medication therapy, and whether other therapies had been tried before an antipsychotic was prescribed. Previous studies of antipsychotic use among young children have relied on claims data (often from a single state Medicaid program or private insurer)3, 4, 6,7, 31 or the National Ambulatory Medical Care Survey (NAMCS), which asks a sample of physicians about patient visits over a one-week period.5,32, 33 While claims data provide information on diagnoses and service use, some claims datasets do not include physician identification numbers or information on physician characteristics. In addition, claims data from an insurer or Medicaid program would not be representative of the universe of a physician's patients, as most physicians treat patients with a variety of payers. While NAMCS provides detailed information on a subset of visits, it is not possible to follow physicians or patients over time. The data used for this study allow us to fully characterize a physician's prescribing to young children over time and to identify physician characteristics associated with different prescribing patterns. Second, the Xponent™ data provide two age categories for children – 0-9 and 10-19 -- so we were obliged to define young children as those 0-9. Third, we were unable to determine whether a given prescription was for an on- vs. off-label indication due to the lack of clinical information. However, we were able to determine whether a physician prescribed to a child 0-9 a medication with no FDA-approved indications for young children. Fourth, while our dataset includes data on all U.S. psychiatrists and a random sample of family medicine physicians who prescribe antipsychotics, we do not have data on pediatricians. However, psychiatrists and family medicine physicians together accounted for three quarters (73.6%) of all child antipsychotic prescriptions filled in 2009, and pediatricians accounted for just 9.5%.54
Conclusions
We found that a large proportion of psychiatrists and family medicine physicians who prescribe antipsychotics write prescriptions to young children, although antipsychotic prescribing patterns appear quite different for this age group relative to adults. Given the dearth of evidence of antipsychotic efficacy in children and the growing evidence of risks associated with child antipsychotic use, payers and provider organizations could consider interventions that are targeted at physicians who prescribe antipsychotics to young children and highlight the clinical tradeoffs associated with the use of these medications in order to ensure the quality, safety, and value of child mental health treatment.
Supplementary Material
Acknowledgements
Grant Support: This research was funded by the National Institute of Mental Health (R01 MH093359 and R01 MH087488).
Footnotes
Disclosures: Drs. _____ have no potential conflicts of interest to disclose. ______provided expert statistical programming. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated (the data vendor) or any of its affiliated or subsidiary entities.
Previous Presentation: Preliminary findings from this paper were presented at the Twelfth Workshop on Costs and Assessment in Psychiatry in Venice, Italy on March 28, 2015.
References
- 1.Bachrach LK. Bare-Bones Fact — Children Are Not Small Adults. New England Journal of Medicine. 2004;351(9):924–926. doi: 10.1056/NEJMe048193. [DOI] [PubMed] [Google Scholar]
- 2.Coyle JT. Psychotropic drug use in very young children. JAMA: The Journal of the American Medical Association. 2000;283(8):1059–1060. doi: 10.1001/jama.283.8.1059. [DOI] [PubMed] [Google Scholar]
- 3.Cooper WO, Hickson GB, Fuchs C, et al. New users of antipsychotic medications among children enrolled in TennCare. Archives of Pediatrics and Adolescent Medicine. 2004;158:753–759. doi: 10.1001/archpedi.158.8.753. [DOI] [PubMed] [Google Scholar]
- 4.Olfson M, Crystal S, Huang C, et al. Trends in antipsychotic drug use by very young, privately insured children. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:13–23. doi: 10.1097/00004583-201001000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Cooper WO, Arbogast PG, Ding H, et al. Trends in prescribing of antipsychotic medications for US children. Ambulatory Pediatrics. 2006;6:79–83. doi: 10.1016/j.ambp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Zito JM, Burcu M, Ibe A, et al. Antipsychotic use by Medicaid-insured youths: impact of eligibility and psychiatric diagnosis across a decade. Psychiatric Services. 2013;64:223–229. doi: 10.1176/appi.ps.201200081. [DOI] [PubMed] [Google Scholar]
- 7.Burcu M, Zito JM, Ibe A, et al. Atypical antipsychotic use among Medicaid-insured children and adolescents: duration, safety, and monitoring implications. Journal of Child and Adolescent Psychopharmacology. 2014;24:1–8. doi: 10.1089/cap.2013.0094. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed February 24, 2015];Choosing Wisely. American Psychiatric Association. Available at: http://www.choosingwisely.org/doctor-patient-lists/americanpsychiatric-association/
- 9.Kealey E, Scholle SH, Byron SC, et al. Quality concerns in antipsychotic prescribing for youth: a review of treatment guidelines. Academic Pediatrics. 2014;14:S68–S75. doi: 10.1016/j.acap.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA: The Journal of the American Medical Association. 2000;283(8):1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]
- 11.Garfield LD, Brown DS, Allaire BT, et al. Psychotropic Drug Use Among Preschool Children in the Medicaid Program From 36 States. American Journal of Public Health. 2015;105(3):524–529. doi: 10.2105/AJPH.2014.302258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novick D, Haro JM, Bertsch J, et al. Incidence of extrapyramidal symptoms and tardive dyskinesia in schizophrenia: thirty-six-month results from the European Schizophrenia Outpatient Health Outcomes Study. Journal of Clinical Psychopharmacology. 2010;30:531–40. doi: 10.1097/JCP.0b013e3181f14098. [DOI] [PubMed] [Google Scholar]
- 13.Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE schizophrenia trial: prospective data from phase 1. Schizophrenia Research. 2008;101:273–86. doi: 10.1016/j.schres.2007.12.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daumit GL, Goff DC, Meyer JM. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophrenia Research. 2008;105:175–87. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods SW, Morgenstern H, Saksa JR, et al. Incidence of tardive dyskinesia with atypical and conventional antipsychotic medications: prospective cohort study. Journal of Clinical Psychiatry. 2010;71:463–74. doi: 10.4088/JCP.07m03890yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry. 2015;72(9):867–74. doi: 10.1001/jamapsychiatry.2015.0500. [DOI] [PubMed] [Google Scholar]
- 17.Lohr WD, Chowning RT, Stevenson MD, et al. Trends in Atypical Antipsychotics Prescribed to Children Six Years of Age or Less on Medicaid in Kentucky. Journal of Child and Adolescent Psychopharmacology. 2015;25(5):440–443. doi: 10.1089/cap.2014.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bobo W, Cooper WO, Stein M, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70:1067–1075. doi: 10.1001/jamapsychiatry.2013.2053. [DOI] [PubMed] [Google Scholar]
- 19.Arango C, Giraldez M, Merchan-Naranjo J, et al. Second-Generation Antipsychotic Use in Children and Adolescents: A Six-Month Prospective Cohort Study in Drug-Naïve Patients. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(11):1179–1190.e1174. doi: 10.1016/j.jaac.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Morrison JA, Friedman LA, Wang P, et al. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. Journal of Pediatrics. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA: The Journal of the American Medical Association. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraguas D, Correll CU, Merchan-Naranjo J, et al. Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: comprehensive review of prospective head-to-head and placebo-controlled comparisons. European Neuropsychopharmacology. 2011;21(8):621–645. doi: 10.1016/j.euroneuro.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Safer DJ. A comparison of risperidone-induced weight gain across the age span. Journal of Clinical Psychopharmacology. 2004;24(4):429–436. doi: 10.1097/01.jcp.0000130558.86125.5b. [DOI] [PubMed] [Google Scholar]
- 24.Kearns M, Hawley K. Predictors of polypharmacy and off-label prescribing of psychotropic medications: a national survey of child psychiatrists. Master's dissertation, University of Missouri, Columbia, Department of Psychology. 2011 Available at gradworks.umi.com/15/21/1521041.html.
- 25.Rawal PH, Lyons JS, MacIntyre JC, 2nd, et al. Regional variation and clinical indicators of antipsychotic use in residential treatment: a four-state comparison. Journal of Behavioral Health Services and Research. 2004;31:178–88. doi: 10.1007/BF02287380. [DOI] [PubMed] [Google Scholar]
- 26.Kamble P, Chen H, Johnson ML, et al. Concurrent use of stimulants and second-generation antipsychotics among children with ADHD enrolled in Medicaid. Psychiatric Services. 2015;66:404–410. doi: 10.1176/appi.ps.201300391. [DOI] [PubMed] [Google Scholar]
- 27.Penfold RB, Stewart C, Hunkeler EM, et al. Use of antipsychotic medications in pediatric populations: what do the data say? Current Psychiatry Reports. 2013;15:426. doi: 10.1007/s11920-013-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rettew DC, Greenblatt J, Kamon J, et al. Antipsychotic medication prescribing in children enrolled in Medicaid. Pediatrics. 2015;135:658–65. doi: 10.1542/peds.2014-2260. [DOI] [PubMed] [Google Scholar]
- 29.Maglione M, Ruelaz Maher A, Hu J, et al. Comparative Effectiveness Reviews, No. 43. Agency for Healthcare Research and Quality; Sep, 2011. Off-label use of atypical antipsychotics: an update. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. [PubMed] [Google Scholar]
- 30.Children's mental health: concerns remain for appropriate services for children in Medicaid and foster care, GAO-13-15. US Government Accountability Office; Washington, DC: Dec 10, 2012. Available at www.gao.gov/assets/660/650716.pdf. [Google Scholar]
- 31.Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Affairs. 2009;28:w770–781. doi: 10.1377/hlthaff.28.5.w770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olfson M, Blanco C, Wang S, et al. National trends in the mental health care of children, adolescents, and adults by office-based physicians. JAMA Psychiatry. 2014;71:81–80. doi: 10.1001/jamapsychiatry.2013.3074. [DOI] [PubMed] [Google Scholar]
- 33.Olfson M, Blanco C, Liu L, et al. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Archives of General Psychiatry. 2006;63:679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 34.Domino ME, Swartz MS. Who are the new users of antipsychotic medications? Psychiatric Services. 2008;59:507–514. doi: 10.1176/appi.ps.59.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison JN, Cluxton-Keller F, Gross D. Antipsychotic medication prescribing trends in children and adolescents. Journal of Pediatric Health Care. 2012;26:139–145. doi: 10.1016/j.pedhc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz A. One drug or 2? Parents see risk but also hope. New York Times; Nov 14, 2014. Available at: http://www.nytimes.com/2014/11/15/us/one-drug-or-2-parents-see-risk-but-also-hope.html?_r=0. [Google Scholar]
- 37.Gellad WF, Stein BD, Ruder T, et al. Geographic variation in receipt of psychotherapy in children receiving attention-deficit/hyperactivity disorder medications. JAMA Pediatrics. 2014;168:1074–6. doi: 10.1001/jamapediatrics.2014.1647. [DOI] [PubMed] [Google Scholar]
- 38.Horvitz-Lennon M. The enduring mismatch between service need and use. Psychiatric Services. 2013;64:1073. doi: 10.1176/appi.ps.641114. [DOI] [PubMed] [Google Scholar]
- 39.Lu CY, Soumerai SB, Ross-Degnan D, et al. Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar illness. Medical Care. 2010;48:4–9. doi: 10.1097/MLR.0b013e3181bd4c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soumerai SB, Zhang F, Ross-Degnan D, et al. Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Affairs. 2008;27:w185–w195. doi: 10.1377/hlthaff.27.3.w185. [DOI] [PubMed] [Google Scholar]
- 41.Farley JF, Cline RR, Schommer JC, et al. Retrospective assessment of Medicaid step-therapy prior authorization policy for atypical antipsychotic medications. Clinical Therapeutics. 2008;30:1524–39. doi: 10.1016/j.clinthera.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Mark TL, Gibson TM, McGuigan K, et al. The effects of antidepressant step therapy protocols on pharmaceutical and medical utilization and expenditures. American Journal of Psychiatry. 2010;167:1202–09. doi: 10.1176/appi.ajp.2010.09060877. [DOI] [PubMed] [Google Scholar]
- 43.Stein BD, Leckman-Westin E, Okeke E, et al. The effects of prior authorization policies on Medicaid-enrolled children's use of antipsychotic medications: evidence from two mid-Atlantic states. Journal of Child and Adolescent Psychopharmacology. 2014;24:374–81. doi: 10.1089/cap.2014.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmid I, Burcu M, Zito JM. Medicaid prior authorization policies for pediatric use of antipsychotic medications. JAMA: The Journal of the American Medical Association. 2015;313(9):966–8. doi: 10.1001/jama.2015.0763. [DOI] [PubMed] [Google Scholar]
- 45.Hodgkin D, Parks Thomas C, Simoni-Wastila L, et al. The effect of a three-tier formulary on antidepressant utilization and expenditures. Journal of Mental Health Policy and Economics. 2008;11:67–77. [PubMed] [Google Scholar]
- 46.Huskamp HA, Deverka PA, Epstein AM, et al. Impact of 3-tier formularies on drug treatment of attention-deficit/hyperactivity disorder in children. Archives of General Psychiatry. 2005;62:435–41. doi: 10.1001/archpsyc.62.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu CY, Ross-Degnan D, Soumerai SB, et al. Interventions designed to improve the quality and efficiency of medication use in managed care: a critical review of the literature 2001–2007. BioMed Central Health Services Research. 2008;8:75. doi: 10.1186/1472-6963-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soumerai SB. Principles and uses of academic detailing to improve the management of psychiatric disorders. International Journal of Psychiatry in Medicine. 1998;28:81–96. doi: 10.2190/BTCA-Q06P-MGCQ-R0L5. [DOI] [PubMed] [Google Scholar]
- 49.O'Brien MA, Rogers S, Jamtvedt G, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews CD000409. 2007 doi: 10.1002/14651858.CD000409.pub2. Available at: http://apps.who.int/rhl/reviews/CD000409.pdf. [DOI] [PMC free article] [PubMed]
- 50.Larkin I, Ang D, Avorn J, et al. Restrictions on pharmaceutical detailing reduced off-label prescribing of antidepressants and antipsychotics in children. Health Affairs. 2014;33:1014–1023. doi: 10.1377/hlthaff.2013.0939. [DOI] [PubMed] [Google Scholar]
- 51.Horvitz-Lennon M, Donohue JM, Domino ME, et al. Improving quality and diffusing best practices: the case of schizophrenia. Health Affairs. 2009;28:701–12. doi: 10.1377/hlthaff.28.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drake R, Skinner J, Goldman HH. What explains the diffusion of treatments for mental illness? American Journal of Psychiatry. 2008;165:1385–92. doi: 10.1176/appi.ajp.2008.08030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran M. Training in psychotropic prescribing helps pediatricians improve care. Psychiatric News. 2015 Jan; Available at: http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2015.1b11.
- 54.Chai G, Mehta H, Moeny D, et al. Atypical antipsychotic drug use in the U.S. outpatient pediatric population. Office of Surveillance and Epidemiology. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM272641.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.