Abstract
The field of intracellular organelle targeting using nanoparticles (NPs) is mushrooming rapidly. Thus, the area of nanotechnology-enabled targeting of mitochondrion, the cellular powerhouse, for diseases characterized by mitochondrial dysfunctions such as cancer, diseases of the central nervous system, cardiovascular diseases is also growing at a rapid pace. Optimization of NP’s ability to target the mitochondria requires quantification of the particles in this subcellular organelle and isolation of mitochondria from cells. Conventional gradient centrifugation used in currently available methods may not be appropriate for NP containing mitochondria isolation as these particles undergo Brownian motion under centrifugal forces yielding irreproducible results. There is only one method for centrifugation free mitochondria isolation, however this method requires immune-precipitation. Thus, a reliable centrifugation and immune-precipitation free method is urgently needed to support this growing field of nanotechnology-based mitochondria targeting. Here, we report a mitochondria-targeted magnetic NP, Mito-magneto, to avoid centrifugation and immune precipitation methods for isolation of functional, respiration active pure mitochondria which can be used to analyze and quantify mitochondria targeting properties of various NPs to provide an important tool for the growing field of “mitochondrial nanomedicine”.
Introduction
Mitochondria, the gatekeepers of cellular life and death processes, maintain normal cell and tissue functions; then again mitochondrial dysfunctions play intrinsic roles in various human diseases.1 Early evidence of mitochondrial dysfunctions in human diseases2 and increasing acceptance of such dysfunctions in major diseases3 such as cancer,4 cardiovascular5 and a neurodegenerative diseases6, triggered the scientific community to develop precision therapeutic approaches by accessing mitochondrial targets. However, this dream of mitochondria-targeted precision therapeutics is still far away from the reality due to the barriers which this complex and dynamic organelle imposes to maintain its important cellular functions. As we look ahead for new ways to achieve mitochondria-targeted therapeutics, it is clear that nanotechnology-enabled tools to target mitochondria of different cell types and tissues are undergoing an evolution.
Others7, 8 and we9–15 have reported several types of nanoparticles (NPs) to achieve mitochondria-targeted payload delivery with the aim to provide subcellular target oriented therapeutic interventions where mitochondrial dysfunctions play major roles. Most of the studies in the field are focused on evaluating mitochondria targeting properties of nanomaterials by imaging the mitochondrial network and determining the presence of fluorescent nanomaterials in this extremely dynamic and complex network by qualitative colocalization measurements. We would like to stress that efficacy of mitochondria-targeted therapeutics will require quantitative, not qualitative, determination of nanomaterials in the mitochondrial compartments since different targets are distributed in each of these compartments. Furthermore, understanding the mechanism of action of therapeutic-loaded nanomaterials on fusion-fission active dynamic mitochondria can be extremely difficult to understand without quantitative analysis. This new era of mitochondria-targeted nanomaterials can greatly benefit from the availability of isolated, respiration active pure mitochondria containing these NPs. Isolation of mitochondrial fractions from NP-treated cells/tissues is essential to determine a NP’s mitochondria association properties. Traditional gradient or differential centrifugation based mitochondria isolation protocols16–19 are not suitable for NP containing mitochondria, as particles tend to undergo Brownian motion under centrifugal force20 and settle down depending on the shape, size of the NPs. Thus, use of conventional high centrifugal force based methods for NP-associated mitochondria generates results that are erroneous and difficult to reproduce (Scheme 1A). NPs can simply precipitate in the mitochondrial fraction due to high centrifugal forces used in conventional mitochondria isolation and lead to misinterpretation that NPs are associated with mitochondria.
Scheme 1.
(A) Currently available techniques for isolation of functional mitochondria and their comparison with Mito-magneto with respect to suitability for NP quantification in the mitochondria. (B) A schematic for magnetic isolation of mitochondria using Mito-magneto. (C) An illustration of Mito-magneto and its synthesis.
There are available superparamagnetic microbead based methods for cell sorting21 and isolation of subcellular components such as nuclei22, endosome23, lysosome24, Golgi vessels25, and plasma membranes26. This type of magnetic bead based technique was also adapted for mitochondria isolation by conjugating an anti-mitochondrial membrane translocase of outer membrane (TOM)22 antibody.27 This method was also applied to isolate mitochondria from different tissue types.28 This centrifugation free method requires immune precipitation. Unavailability of a mitochondria isolation technique which is both centrifugation and immune precipitation free and the challanges associated with conventional methods to isolate NP-containing mitochondria made us realize that new technologies are urgently needed to support this growing field of mitochondria targeted nanomaterials. Here, we report an inaugural mitochondria-targeted magnetic NP, Mito-magneto, for efficient isolation of mitochondria using a centrifugation and immune precipitation free protocol (Scheme 1B). A Fe3O4 magnetite based magnetic NP, Mito-magneto, was designed to contain lipophilic, delocalized triphenyl phosphonium (TPP) cations29 on the surface to take advantage of negative mitochondrial membrane potential (Δψm) that exists across the membranes of healthy mitochondrion for mitochondrial association and subsequent isolation of mitochondria by the use of a magnet. Further advantage of iron oxide NPs (IONPs) is the contrast enhancement for magnetic resonance imaging (MRI) and hence an opportunity to image the isolated mitochondrial fraction.
Results and Discussion
Synthesis and Characterization of Mito-magneto
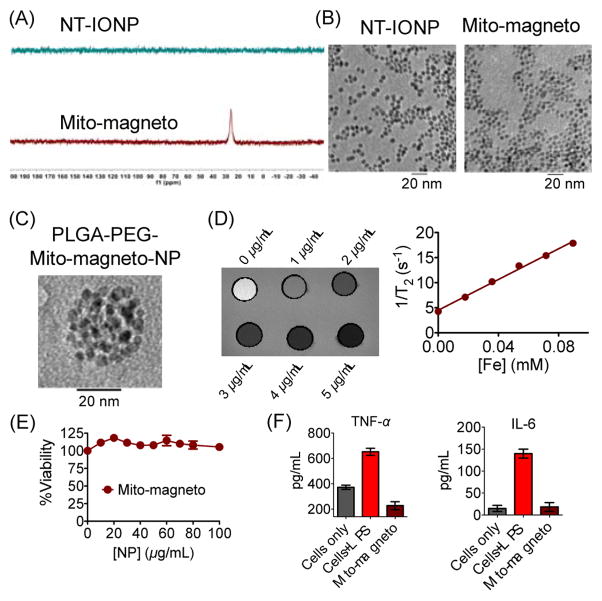
Mito-magneto was synthesized from oleic acid capped IONPs30 (Scheme 1C). Ethanol was used to remove the oleic acid capping and TPP-hexanoic acid (Figure S1) was added to the isolated pellet (Scheme 1). The synthesized Mito-magneto was purified by resuspending in dimethylformamide (DMF) and repeated centrifugation to obtain particles of 20.5±0.1 nm diameter and zeta potential of 23.3±3.1 mV (Figure S2). The small size and high positive surface charge of Mito-magneto indicated that these NPs would be suitable for mitochondrial association. The presence of –TPP moieties on the NP surface was further confirmed by 31P NMR (Figure 1A). Oleic acid capped IONP was used as a control NT-IONP (Figure 1A). Comparison of transmission electron microscopy (TEM) images of commercial IONPs with Mito-magneto indicated that addition of cationic –TPP moieties with a short linker does not cause any aggregation (Figure 1B). This is probably due the fact that the phenyl groups on the surface create a sterically hindered environment and prevents any aggregation. Since Mito-magneto does not have any polyethylene glycol (PEG) coating which is usually used to prevent aggregation of NPs,31 we studied long-term aggregation properties of Mito-magneto by storing the NP solution in DMF at a concentration of 5 mg/mL at 4 °C. This study indicated that the diameter and surface charge did not experience any adverse changes up to 15 days and after that diameter was increased but no significant changes in surface charge was observed (Figure S3). The presence of lipophilic –TPP moieties on the surface makes Mito-magneto soluble in DMF. It is important to note that this particular solubility profile allows further incorporation of Mito-magneto inside other nanomaterials. Further, most commercially available IONPs with solubility in DMF also demonstrate water solubility and hence encapsulation and retention of these NPs in a hydrophobic nanoacapsule environment is challenging. Thus, lipophilic Mito-magneto can be advantageous when there is a requirement of its further encapsulation into other nanomaterials. Mito-magneto has good solubility in DMF, dimethyl sulfoxide (DMSO), acetonitrile, methanol, and it is insoluble in water making this IONP an ideal candidate for further incorporation inside the hydrophobic core of other delivery vehicles. These lipophilic Mito-magneto particles are also advantageous because they can be added to cells in less than 1% DMSO containing media.
Figure 1.
(A) Characterization of Mito-magneto by 31P NMR, (B) Morphology of NPs by TEM, (C) TEM image of Mito-magneto encapsulated PLGA-b-PEG-NPs demonstrating efficient encapsulation of Mito-magneto in the hydrophobic core of other nanomaterials. (D) T2-weighted MRI (TR = 2500 ms, TE = 10.69 ms) of different concentrations of Mito-magneto at 25 °C using a 7T magnet and changes in relaxivity values with varied concentration of Mito-magneto. (E) Cytotoxicity of Mito-magneto in H9C2 cardiomyocytes by the MTT assay. (F) Non immunogenic properties of Mito-magneto by assessing secretion of pro-inflammatory cytokines IL-6 and TNF-α by RAW 264.7 macrophages in presence of 0.05 mg/mL of Mito-magneto for 24 h and comparison with conventional immune stimulator LPS (100 ng/mL).
Mito-magneto can be used to determine mitochondrial localization of other types of nanomaterials for their in vitro and/or in vivo fate in mitochondria targeting. As an example, we encapsulated Mito-magneto in the hydrophobic core of polymeric NPs using poly(lactic-co-glycolic acid) (PLGA)-block (b)-polyethyleneglycol (PEG)-OH (PLGA-b-PEG-OH). TEM analyses demonstrated that multiple Mito-magneto occupy the hydrophobic core of these polymeric NPs (Figure 1C, Figure S4). These NPs had narrow size distribution with a Zaverage value of 56.3±1.3 nm and a negatively charged surface with zeta potential of -11.5±0.1 mV (Figure S4). We would like to stress that this kind of well packed IO-NP loaded polymeric NPs of size <100 nm is difficult to construct using commercially available magnetic NPs which are mostly soluble in water, hexanes, or toluene. Thus, Mito-magneto can be used to isolate mitochondrial compartments by incorporating them in other types of size, charge, and surface functionality varied nanomaterials with abilities to distribute in different mitochondrial compartments.
MRI Phantom Studies with Mito-magneto
A set of Mito-magneto samples with varied Fe concentrations in 0.5% agar was used to calculate magnetic relaxivity of Mito-magneto using a 7 Tesla (T) magnet (Figure 1D). The transverse relaxation times (T2) were measured and the transverse relaxivity (r2) was determined from the slope of the plot of 1/T2 against concentration of Fe in mM. Mito-magneto demonstrated high relaxation enhancement with r2 value of 161.3±11.2 mM–1 s–1 (Figure 1D).
Toxicity and Immunogenicity of Mito-magneto
For the potential utility of Mito-magneto in mitochondria isolation, we first studied cellular toxicity of these NPs. We used rat ventricular H9C2 cardiomyoblasts as these cells are similar to primary cardiomyocytes in energy metabolism.32 A colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-based analysis of H9C2 cells treated with different concentrations of Mito-magneto indicated that these mitochondria targeted NPs is mostly non-toxic (Figure 1E and Figure S5). Immunogenic properties of Mito-magneto were assessed by quantifying pro-inflammatory cytokines, tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6, in RAW 264.7 cells at a concentration of 0.05 mg/mL (Figure 1F). Lipopolysaccharide (LPS) was used as a positive stimulator for macrophages. Mito-magneto did not demonstrate any secretion of these pro-inflammatory cytokines demonstrating its non-immunogenic nature at the concentration used (Figure 1F).
Mito-magneto Based Mitochondria Isolation Using Magnetic Field
First, mitochondrial association property of Mito-magneto was investigated by analyzing the NP treated H9C2 cells by inductively coupled plasma atomic optical emission spectrometry (ICP-OES) for iron analyses of cytosolic and mitochondrial fractions (Figure 2A). A significant portion of Mito-magneto was found to be present in the mitochondrial fraction compared to the cytosol of these treated cells implying that Mito-magneto has strong mitochondrial association properties. For Mito-magneto driven magnetic mitochondria isolation from healthy cells, we first used H9C2 cells. We incubated 5×106 cells with 20 μg/mL Mito-magneto for 12 h. Cells were then collected via trypsinization and counted using trypan blue for any possible cell death. Trypan blue staining of the treated cells indicated high level of viability (Table S1). Cells were then lysed and subjected to a EasySep™ magnet. Simultaneously, mitochondrial and cytosolic fractions from Mito-magneto treated cells were isolated under identical conditions using conventional reagent-based differential centrifugation method. The pellet and supernatant from the magnetic separation and the mitochondrial and cytosolic fractions from conventional isolation of H9C2 cells were subjected to MRI (Figure 2B). Comparison of transverse relaxivities indicated the presence of Mito-magneto in the pellet of magnetic separation and in the mitochondrial fraction of conventional separation. We like to stress that the two mitochondrial fractions containing Mito-magneto isolated by centrifugation and magnetic separation contained same protein concentration and not same amount of IONPs. The difference in MRI signal intensities between reagent and magnetic separation in presence of Mito-magneto could be because of the fact that magnetic isolation leads to the recovery of mitochondria containing IONPs only whereas centrifugal isolation may lead to the recovery of some mitochondria which do not contain IONPs resulting in lesser amount of total Fe concentration in the centrifugally isolated mitochondria. This result was further verified by determination of iron content in various sub-cellular fractions of Mito-magneto treated H9C2 cells using ICP-OES technique (Figure S6, Figure S7 for protein quantification).
Figure 2.

(A) Localization of Mito-magneto in the mitochondrial fraction of H9C2 cells by iron ICP-OES. Cyto: cytosolic and Mito: mitochondrial fractions. (B) T2-weighted MRI (TR = 2500 ms, TE = 10.69 ms) of cytosolic and mitochondrial fractions isolated from untreated or Mito-magneto treated H9C2 cells by reagent-based method or by magnetic isolation. The concentration of Mito-magneto in these studies were maintained at 20 μg/mL and incubation was carried out for 12 h. For magnetic separation, after lysing the cells, Mito-magneto containing mitochondria were isolated using EasySep™ magnet. Cyto: cytosolic fraction; Mito: mitochondrial fraction. (C) Measurement of mitochondrial membrane potential in different cell lines by JC-1 assay. (D) DLS data on isolated mitochondria from J3TBG cells.
Mito-magneto Based Mitochondria Isolation from Different Cell Types
For the potential utility of Mito-magneto in mitochondria isolation, we chose three cell lines of different origins. Ovarian cancer A2780 cells line with human origin, J3TBG glioma cell line from canine origin, and rat ventricular H9C2 cardiomyoblasts were selected. The choice of cell lines is to verify the fact that this technology is capable of isolating mitochondria from cells with different phenotypes, different mitochondrial membrane potentials, and different species. Mitochondrial membrane potential of these three cell lines was determined by tetraethylbenzimidazolylcarbocyanine iodide (JC-1) driven ratiometric assay. This study indicated that the resting Δψm are different in these three cell lines (Figure 2C) and at the end of the study we would like to establish whether mitochondria isolation using Mito-magneto works in various cell lines with different resting Δψm. Mitochondrial and cytosolic fractions from Mito-magneto treated cells under identical conditions were isolated using magnetic separation, conventional reagent-based differential centrifugation method, and TOM22 based magnetic beads. Conventional reagent-based and TOM22 methods were used as controls to compare mitochondrial quality isolated by Mito-magneto driven magnetic separation. Dynamic light scattering measurements on isolated mitochondria showed that these are negatively charged organelles spread over sub-micrometer size range (Figure 2D for J3TBG cells, Figure S8 for H9C2 and A2780 cells, Table 1 for all data).
Table 1.
Characterization of Isolated Mitochondria by DLS
| Cell Line | Isolation Method | Mito-magneto | Diameter (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|---|
| A2780 | Reagent | − | 421.4±33.5 | 0.744±0.198 | −44.1±2.5 |
| A2780 | Reagent | + | 402.6±14.7 | 0.656±0.047 | −43.1±2.1 |
| A2780 | Magnetic | + | 424.6±22.6 | 0.557±0.064 | −45.1±4.3 |
| J3TBG | Reagent | − | 365.6±7.7 | 0.450±0.029 | −34.2±0.7 |
| J3TBG | Reagent | + | 371±8.8 | 0.482±0.046 | −32.5±0.4 |
| J3TBG | Magnetic | + | 377.9±3.6 | 0.457±0.014 | −33.4±0.4 |
| H9C2 | Reagent | − | 381.9±2.6 | 0.647±0.111 | −34.5±1.5 |
| H9C2 | Reagent | + | 354.7±7.6 | 0.447±0.030 | −30.3±6.0 |
| H9C2 | Magnetic | + | 272.3±6.3 | 0.438±0.050 | −31.9±0.6 |
Functional Nature of Isolated Mitochondria
To assess the integrity and functional nature of the isolated mitochondria by Mito-magneto enabled magnetic separation, we conducted cytochrome c oxidase (COX) activity for assessment of function of the electron transport chain (ETC), ATP synthase activity to analyze the ability of these isolated mitochondria to produce ATP, citrate synthase (CS) activity as the marker for Krebs cycle, and ATP production assay on the isolated mitochondrial pellet from H9C2, A2780, and J3TBG cells (Figure 3).
Figure 3.
Mitochondrial fractions were isolated from untreated or Mito-magneto treated H9C2, A2780, and J3TBG cells by reagent-based method or by magnetic isolation or by untreated TOM22 method. The concentration of Mito-magneto in these studies were maintained at 20 μg/mL and incubation was carried out for 12 h. For magnetic separation, after lysing the cells, Mito-magneto containing mitochondria were isolated using EasySep™ magnet. Demonstration of functional nature of respiration active isolated mitochondria by (A) COX activity as ETC marker, (B) ATP synthase activity as a ATP synthesis marker, (C) citrate synthase activity as Krebs cycle marker, and (D) production of ATP by the isolated mitochondria upon supply of ADP substrate.
Comparison of COX activity indicated that the pellet isolated from magnetic isolation using Mito-magneto is the mitochondrial fraction of the treated H9C2 cells. It should be noted that all the mitochondrial fractions with same protein concentration based on the Bicinchoninic acid assay (BCA) were used in these studies. Comparison of COX activity of magnetically isolated mitochondria using Mito-magneto and a positive control for COX confirmed that magnetically isolated mitochondria using Mito-magneto possess functional characteristics of active respiration across all three cell lines (Figure 3A, Figure S9).
We next analyzed ATP synthase activity of the isolated mitochondria using a Complex V analyses kit (Figure 3B, Figure S10). ATP synthase participates in ATP production in the mitochondria (Figure 3B). Analyses of the ATP synthase activity of isolated mitochondria from all three cell lines using Mito-magneto by magnetic separation demonstrated similar activity of this enzyme as shown by Mito-magneto treated mitochondria which were isolated using conventional reagent based method or untreated mitochondria isolated by reagent based method (Figure 3B). Oligomycin A is generally used as an inhibitor of ATP synthase. When the same experiment was performed in presence of oligomycin A, ATP synthase activity in all the samples was inhibited further demonstrating that the mitochondria which were collected using Mito-magneto and a magnet are functionally similar to the mitochondria provided in the kit as a positive control. Thus, these isolated mitochondria are able to perform ATP generation activity using ATP synthase further supporting that they respiration active.
Citrate synthase is a key regulatory enzyme in mitochondrial metabolic pathway where it catalyzes the condensation of acetyl coenzyme A and oxaloacetate (OAA) to produce citrate for the tricarboxylic acid cycle (Figure 3C). Thus, the activity of this enzyme is widely used as a marker for functional nature of mitochondria by assessing oxidative and respiratory capacity. Comparable CS activities in untreated cells isolated by reagent-based method and the mitochondria obtained by Mito-magneto using magnetic isolation indicated that the integrity of the mitochondrial fraction is intact (Figure 3C, Figure S11). The mitochondria isolated by magnetic separation using Mito-magneto demonstrated the same level of CS activity as observed for untreated mitochondria or the fraction isolated by reagent-based method (Figure 3C).
The intactness and functional nature of isolated mitochondria were also determined using luciferase-based bioluminescent quantification of ATP (Figure 3D). In the absence of the substrate, adenosine diphosphate (ADP), there was no significant production of ATP by any of the isolated mitochondrial fractions. However, in the presence of ADP substrate, ATP levels were dramatically increased in the mitochondria that were isolated by magnetic separation using Mito-magneto. The extent of ATP production by magnetic separation was similar to that observed with untreated or Mito-magneto treated mitochondria and isolated by conventional reagent based method (Figure 3D). Thus, all these studies conjointly indicated that the mitochondria isolated by magnetic separation using Mito-magneto have preserved functions of distinct components of the electron transport system.
All the four assays to determine functional nature of isolated mitochondria were performed on the mitochondrial fractions isolated using TOM22 magnetic beads and it was found to be comparable to that isolated either using Mito-magneto or using reagent based methods (Figures 3A–D).
Purity of Isolated Mitochondria
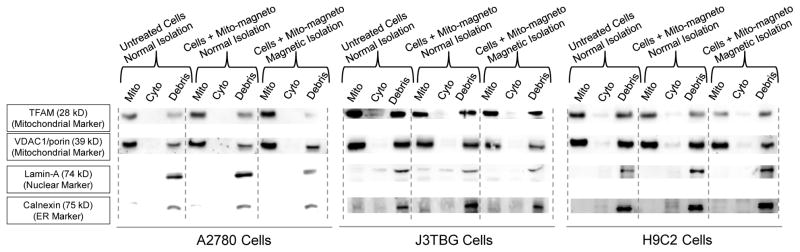
Next, we determined the purity of mitochondria isolated by Mito-magneto enabled magnetic separation and compared the quality of isolated mitochondria with conventional differential centrifugation method. The purity of mitochondrial fraction was analyzed by Western blotting using anti-calnexin as an endoplasmic reticulum (ER) marker, anti-lamin A antibody as a nuclear marker, anti-mitochondrial transcription factor A (TFAM) antibody and anti-voltage dependent anion channels (VDAC1)/porin as mitochondrial markers (Figure 4). Western blot analyses indicated that the mitochondria isolated by magnetic separation using Mito-magneto contained no significant nuclear or ER impurities. Often, centrifugation method, which uses harsh homogenization, although produces high mitochondrial yield, but damage this sophisticated organelle and the isolated mitochondria, contain impurity. In contrast, Mito-magneto based isolation doesn’t need any homogenization and gentle magnetic field based isolation resulted in pure mitochondrial fraction (Figure 4). Western blot analyses indicated that the mitochondria isolated either by magnetic separation using Mito-magneto or centrifugation based methods are highly enriched in mitochondrial proteins and do not contain any significant nuclear or ER impurities across all three cell lines (Figure 4).
Figure 4.
Mitochondria isolated from A2780, J3TBG, and H9C2 cells by reagent method and Mito-magneto treated cells either by reagent method or magnetic isolation were resolved by PAGE followed by Western blot, and the fractions were detected with antibodies against mitochondrial transcription factor A (TFAM) and VDAC1 as mitochondrial markers, lamin A as a nuclear marker, and calnexin for endoplasmic reticulum (ER) to understand the purity of mitochondrial fractions isolated by Mito-magneto.
Morphological Analyses of Magnetically Isolated Mitochondria
Integrity and morphology of mitochondria purified by Mito-magneto with the aid of magnetic field was further studied by TEM (Figure S12). TEM images of isolated mitochondria from untreated or Mito-magneto treated H9C2, J3TBG, or A2780 cells purified by reagent-based isolation or magnetically isolated mitochondria are shown in Figure S12. Morphological investigation of the fractions indicated that most of the isolated mitochondria using Mito-magneto with magnetic field were intact and contained distinct double membrane (Figure S12A). On contrary, when mitochondria were isolated using reagent-based method that requires high centrifugal forces in presence of Mito-magneto, a significant mitochondria population was disrupted and there was rupture of the membrane (Figure S12A). Thus, Mito-magneto coupled-magnetic isolation yields functional and intact mitochondria. We would like to mention that stained regions outside the mitochondria in the TEM images are residual debris.
Effects of Mito-magneto on Mitochondrial Bioenergetics
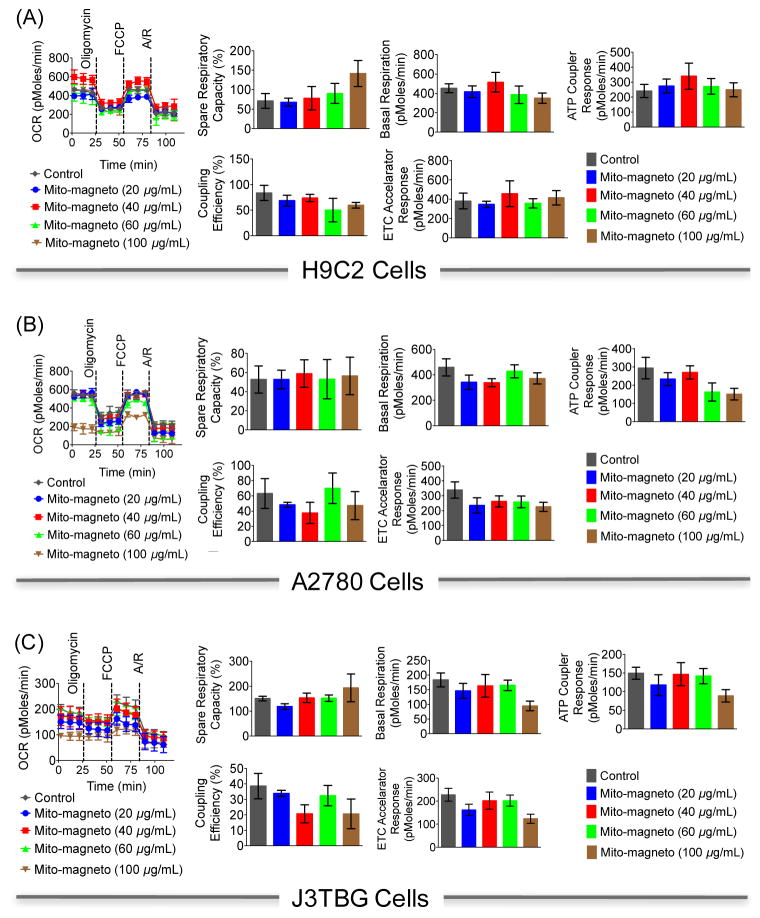
Since the isolated mitochondria by this method contain Mito-magneto, we asked what is the potential toxicity of these NPs on the mitochondria over a given time period. Mitostress analyses on H9C2, A2780, or J3TBG cells by measuring oxygen consumption rates (OCRs) in presence of different concentrations of Mito-magneto ranging from 20 to 100 μg/mL for 24 h and sequential addition of ATP coupler oligomycin, ETC accelerator carbonyl cyanide-p-trifluoromethoxyphenylhydrazone or FCCP, and a combination of mitochondrial complex I inhibitor rotenone and mitochondrial complex III inhibitor antimycin A using Seahorse analyzer suggested that Mito-magneto does not cause any inhibition of mitochondrial functions up to 40 μg/mL, however a concentration of 100 μg/mL demonstrated decreased OCR, basal respiration, and uncoupling of mitochondria (Figure 5). Since the concentration of Mito-magneto used in magnetic separation was 20 μg/mL, which is lower than the concentration that causes disruption of mitochondrial functions, we believe that the mitochondria isolated by this method will have membrane integrity. Thus, although the isolated mitochondria contain the NPs, these NPs do not cause any changes in the mitochondrial functions.
Figure 5.
Effect of different concentrations of Mito-magneto on the respiration of H9C2, A2780, and J3TBG cells. A: Antimycin A and R: Rotenone. The cells were treated with Mito-magneto (20, 40, 60, and 100 μg/mL) for 24 h. The oxygen consumption rate (OCR) was measured using a Seahorse analyzer. Electron transport inhibitor oligomycin (2.0 μM), FCCP, an ionophore (2.0 μM), and a mixture of antimycin-A (1.0 μM; mitochondrial complex III inhibitor) and rotenone (1.0 μM; mitochondrial complex I inhibitor) were used to quantify different parameters of respiration.
Magnetic Isolation Method Eliminates Experimental Artefacts Caused by Centrifugal Isolation of NP Associated Mitochondria
As we have mentioned earlier, the idea behind the development of Mito-magneto as a tool for mitochondria isolation is to provide a centrifugation free method for isolation of the organelle, especially when it is associated with other NPs to support the growing field of “Mitochondrial Nanomedicine”. We, in our lab, had previously designed PLGA-b-PEG-OH based mitochondria targeting soft NPs. In this study, we have treated cells with Quantum dot (QD) loaded mitochondria targeted (surface covered with -TPP moieties) or non-targeted (surface covered with -OH moieties) PLGA-b-PEG NPs (T-QD-NP and NT-QD-NP) and isolated mitochondria from these treated cells using Mito-magneto or conventional centrifugation. Quantification of Cadmium (Cd) in the various sub-cellular fractions show that the targeted PLGA-b-PEG NPs accumulate mainly in the mitochondrial fraction as evident by both magnetic as well as centrifugal isolation techniques (Figure 6 for J3TBG cell line, Figure S13 for H9C2 and A2780 cells). Interestingly, the non-targeted PLGA-b-PEG NPs appear in the mitochondrial fraction of the reagent based isolation but did not appear in the magnetically isolated mitochondria. These results could be indicative of the fact that high centrifugal speed used to isolate mitochondria cause deposition of the large NPs on the bottom. Thus, even the non-targeted NPs appear to accumulate in the mitochondria. But, the use of Mito-magneto based magnetic isolation technique does not require centrifugation and thus help in obtaining true data with minimal aberration. Thus, this preliminary study demonstrates the advantage of Mito-magneto based isolation for other NP-containing mitochondria from healthy cells.
Figure 6.

Quantification of a mitochondria-targeted NP (T-QD-NP) and non targeted NP (NT-QD-NP) through Cd analyses in mitochondrial compartments along with other cellular fractions isolated by conventional reagent based method and Mito-magneto based magnetic separation of J3TBG cells.
Conclusions
In conclusions, Mito-magneto presents a simple and efficient method for isolation of functional, respiration active pure mitochondria. The easiness to synthesize Mito-magneto and ability to trace these NPs by MRI present additional advantages. The purity and integrity of the mitochondria isolated using Mito-magneto were comparable to the conventional differential centrifugation method. Thus, Mito-magneto presents an efficient and reliable tool to the growing field of “mitochondria targeting” with nanomaterials for a number of diseases where targets are located at this complex organelle. In the future, we will investigate the usefulness of this system in isolating mitochondria from different types of cells and tissues to further optimize its use.
Experimental
Materials and Instruments
Details of this experiment can be found in ESI.
Statistics
All data were expressed as mean ± S.D (standard deviation). Statistical analysis were performed using GraphPad Prism® software v. 5.00. Comparisons between two values were performed using an unpaired Student t test. A one-way ANOVA with a post-hoc Tukey test was used to identify significant differences among the groups.
Cell Lines and Cell Culture
H9C2 cardiomyocytes were received as a generous gift from Prof. Mark Anderson, University of Iowa. These cells were cultured at 37 °C in 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 1% L-glutamine, 1% sodium pyruvate, 1% penicillin/streptomycin, and 10% FBS.
Canine J3TBG glioma cells were generously donated from the Peterson Lab at UC David School of Veterinary Medicine. Cells were grown in complete media, consisting of DMEM with 10% FBS and 1% 200 mM L-Glutamine, all purchased from Sigma-Aldrich.
Ovarian cancer A2780 cells were obtained from Prof. Robert Brown, Imperial College London. A2780 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% FBS, 1% penicillin/streptomycin and 2 mM L-glutamine. All the cells were passed every 3 to 4 days and restarted from frozen stocks after 10 passages.
RAW 264.7 cell line was procured from the American type culture collection (ATCC). These macrophages were grown at 37 °C in 5% CO2 in DMEM supplemented with 1% L-glutamine, 1% sodium pyruvate, 1% penicillin/streptomycin, and 10% FBS. All the cells were passed every 3 to 4 days and restarted from frozen stocks after 10 passages.
Synthesis of Mito-magneto
IONPs with oleic acid surface functionalities were synthesized following a literature procedure.30 To 100 μL of the above IONP solution (10 mg/mL) in hexane, 1 mL of ethanol was added and vortexed for a minute. The precipitate obtained was collected via centrifugation at 6000 rpm for 10 min at 4 °C. The precipitation step was repeated 3 times and then to the precipitate, 1 mL of a DMF solution of TPP-hexanoic acid (100 mg/mL) was added and the mixture was sonicated for 1 h. The solution was then centrifuged for 1 h at 14000 rpm (4 °C) and the supernatant obtained was collected in new tubes and were centrifuged further under same conditions for 4 h. The pellet thus obtained was resuspended in DMF (1 mL) and washed for 2 times. The pellet of Mito-magneto finally obtained was resuspended in DMF (100 μL) and stored at 4 °C for further use. NP size (diameter, nm), PDI and surface charge (zeta potential, mV) were obtained from three independent measurements. For TEM studies, 20 μL of NP solution was diluted with 980 μL hexane (for the NT-IONP) or 1% aqueous DMF (for Mito-magneto). The mixture was vortexed for a few seconds and 8 μL of the mixture was dropped into a copper grid and allowed to dry overnight at room temperature. TEM images were recorded on FEI Tecnai20 transmission electron microscope operating at 200 kV. For 31P spectral measurements, 20 μL of NT-IONP or Mito-magneto was lyophilized for overnight and the resultant solid was resuspended in 500 μL of CDCl3 (for NT-IONP) or DMSO-d6 (for Mito-magneto). Iron content in Mito-magneto was determined by ICP-OES.
Mito-magneto Encapsulation in Polymeric NPs
Mito-magneto encapsulated PLGA-PEG-OH-NPs were synthesized by the nanoprecipitation method. Briefly, PLGA-b-PEG-OH (5 mg) and Mito-magneto (25 μg with respect to Fe) were dissolved in 1 mL of acetonitrile and this solution was added dropwise to 10 mL nanopure water with constant stirring. The NPs were allowed to stir for 2 h. Organic solvent was removed by filtering three times through a 100-kDa cutoff Amicon filter. The NPs were resuspended in 1 mL of nanopure water and stored at 4 °C until further use. DLS measurements were performed to determine NP size, PDI, and zeta potential. For TEM characterization, the nanoparticles were diluted 100X in nanopure water and 10 μL of this solution was introduced on a copper grid and allowed to dry overnight.
Cell Viability Studies on H9C2 Cells
Details of this experiment can be found in ESI.
Enzyme-linked Immunosorbent assay (ELISA) for Immunogenic Effect of Mito-magneto on RAW 264.7 Cells
Details of this experiment can be found in ESI.
JC-1 Assay
H9C2, J3TBG, or A2780 cells (1×106 cells /well) were plated in 12-well plates. JC-1 dye was added to each well at a final concentration of 2 μg/mL and incubated for 30 min at 37 °C. The media was then removed from the wells and the cells were washed with 1× PBS (2 times) and fresh PBS (1 mL) was added and fluorescence was read at both 485/528 and 530/590 nm.
Subcellular Fractions by Reagent-based Method
Cells (H9C2, J3TBG or A2780) were seeded in 150 cm2 cell culture flasks and were allowed to grow to near 100% confluency. The cells were then incubated in fresh media for another 12 h. Cells were then trypsinized and collected in centrifuge tubes pre-incubated on ice and sub-cellular fractionation was carried out using reagents from a mitochondria isolation kit for mammalian cells. Reagent A with protease inhibitors (10 mg/mL) was added and the cells were incubated on ice for 2 min. The cells were then lysed by probe sonication; amplitude: 1, 2 pulses of 4s each with a delay time of 5s. Reagent C was then added and was gently mixed. Debris, nuclei and unbroken cells were pelleted down by centrifugation at 1300×g for 5 min. The pellet was suspended in 500 μL of mitochondria assay solution as the debris fraction and stored at −20 °C for further analyses. The supernatant was then subjected to centrifugation at 12000×g for 15 min to obtain pellet for the mitochondrial fraction. This supernatant was stored separately at −20 °C as the cytosolic fraction. The pellet, which is the mitochondrial fraction, was suspended in mitochondria assay solution (200 μL) and stored at −20 °C for further use. The amount of protein in each of the fractions was quantified by Bichinconinic acid (BCA) assay and the amount of iron (Fe) content was quantified by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) and was expressed in micrograms of Fe per microgram of protein.
Subcellular Fractions by Magnetic Isolation Method
Cells (H9C2, J3TBG or A2780) were seeded in 150 cm2 cell culture flasks and were allowed to grow to near 100% confluency. The cells were then incubated with Mito-magneto (20 μg/mL) in 1% DMSO containing media (DMEM for H9C2 and J3TBG cells and RPMI for A2780 cells) for 12 h. Cells were then trypsinized and collected in centrifuge tubes pre-incubated on ice and sub-cellular fractionation was carried out using reagents from a mitochondria isolation kit for mammalian cells. Reagent A with protease inhibitors (10 mg/mL) was added and the cells were incubated on ice for 2 min. The cells were then lysed by probe sonication; amplitude: 1, 2 pulses of 4 s each with a delay time of 5 s. Reagent C was then added and was gently mixed. Debris, nuclei and unbroken cells were pelleted down by centrifugation at 1300×g for 5 min. The pellet was suspended in 500 μL of mitochondria assay solution as the debris fraction and stored at −20 °C for further analyses. The supernatant was then transferred to BD falcon conical tubes for flow cytometry and were placed on an EasySep™ magnet for 15 min after which the supernatant was gently decanted with the magnet around the tube. This supernatant is the cytosolic fraction. The residue in the tube, which is the mitochondrial fraction, was suspended in mitochondria assay solution (200 μL) and stored at −20 °C for further use. The amount of protein in each of the fractions was quantified by BCA assay and the amount of iron (Fe) content was quantified by ICP-OES and was expressed in micrograms of Fe per microgram of protein.
Subcellular Fractions by TOM 22 Method
TOM22 bead-based magnetic isolation was performed with minor modifications of the manufacturer’s protocol. Briefly, the cells were suspended in 1 mL of ice-cold lysis buffer. The cells were then homogenized by probe sonication using the same sequence as described earlier. Debris, nuclei, and unbroken cells were pelleted down by centrifugation at 1300×g for 5 min. To the supernatant, 9 mL of separation buffer and 50 μL of anti-TOM22 microbeads were added and the mixture was allowed to incubate at 4 °C under gentle shaking for 1 h. The mixture was then poured into LS column and subjected to MidiMACS magnetic separation unit. Cytosolic fraction was collected at the bottom of the LS column with the magnet on. The column was then taken off from the magnet and purged forcefully with separation buffer to obtain the mitochondrial fraction.
Transverse Relaxation (r2) Measurements
For the MRI measurements, phantoms containing various concentrations (0, 1, 2, 3, 4 and 5 μg/mL) of mito-magneto or subcellular fractions were made in 0.5% agarose gel inside 200 μL PCR tubes. The phantoms were then embedded in a bed of 0.5% agarose gel. The transverse relaxation time (T2) and the MRI images for the phantoms were then measured using an Agilent (7 Tesla, 200 mm) horizontal bore magnet based MRI instrument. Experimental settings are as follows: Slice width = 1 mm, Repetition Time (TR) = 2500 ms and Echo Time (TE) = 10.69 ms. The transverse relaxivity (r2) was calculated as per the equation r2[Fe] = 1/T2 - 1/T20, where 1/T2 is the relaxation rate in presence of a certain concentration of IONPs, 1/T20 is the relaxation rate of pure water and [Fe] is that particular concentration of Fe.
Citrate Synthase Activity Assay
This assay was performed using a Citrate Synthase activity colorimetric assay kit following manufacturer’s protocol. Briefly, 50 μL of reaction mix containing citrate synthase assay buffer, citrate synthase substrate mix, and citrate synthase developer was added to 10 μL of sample (containing 10 μg of protein), GSH standard or positive control in triplicates on a 96-well plate. Absorbance of the wells were measured at 412 nm immediately after addition of the reaction mix in a kinetic mode. Cirate synthase activity was calculated by considering the absorbance values between two time points in a linear range.
Cytochrome c Oxidase Activity Assay
COX activity colorimetric assay was performed as per manufacturer’s protocol. Briefly, 120 μL of cytochrome C (diluted as per the protocol) was added to 10 μL of sample (containing 10 μg of protein), positive control or blank (assay buffer) in triplicates on a 96-well plate. Absorbance at 550 nm was recorded quickly after addition of the reagent in a kinetic mode for 45–90 min. COX activity was calculated by measuring the rate of change of Optical Density (OD) over a linear range of the absorbance vs. time curve.
Complex V OXPHOS Activity Assay
The mitochondrial ATP synthase activity was assessed by the complex V OXPHOS activity assay kit from Abcam. ATP synthase was exposed to reagents by solubilizing the mitochondria. 40 μL of detergent was mixed with 360 μL isolated mitochondria (5.5 mg/mL) and incubated on ice for 30 min. The supernatant was collected by centrifugation at 12,000xg for 20 min at 4 °C and diluted with 5 mL of 1X Mitochondria Assay Solution (210 mM Mannitol, 70 mM Sucrose, 5 mM Tris-HCl (pH 7.5) and 1 mM EDTA (pH 7.5)). The exposed ATP synthase, 10 μg (with respect to protein), was treated with 200 μL of complex V activity buffer containing ATP, pyruvate kinase (PK), lactate dehydrogenase (LDH), phosphoenolpyruvate, NADH. As controls, oligomycin (200 nM)-treated and non-treated samples were analyzed. The absorbance was measured at 340 nm.
ATP Quantification Assay
ATP quantification was evaluated by CellTiter-Glo® Cell Viability Assay kit. Briefly, 10 μg of isolated mitochondria and 10 μL of ADP solution (1 mM) were added to each well of a 96-well plate and the volume was adjusted to 20 μL by adding mitochondria isolation buffer. Same amount of ADP was added to the blank wells containing MIB only. To this mixture, 100 μL of CellTiter-Glo reagent was added to each of the wells. The plates were equilibrated at 37 °C for 30 min. The luminescence of each well was read with 10 min delay at room temperature.
MitoStress Test, Western Blot Method, and TEM of Isolated Mitochondria
Details of these experiments can be found in ESI.
Mitochondria Isolation from Cells Pretreated with QD loaded Targeted and Non-targeted NPs
To assess how mitochondria isolation method affects the appearance of mitochondria targeted/non-targeted nanoparticles in various cell fractions, Cells (H9C2, J3TBG or A2780) were seeded in 150 cm2 cell culture flasks to grow to near 100% confluency and were treated with 50 μg/mL of QD loaded targeted/non-targeted nanoparticles for 12 h. One set each of T/NT-QD-NP treated cells were subjected to reagent based isolation while another set was subjected to magnetic isolation using Mito-magneto. The cadmium content in each of the cellular fractions was measured using ICP-MS and was expressed in micrograms of Cd per mg of protein.
Supplementary Material
Acknowledgments
This work, in part, was funded by National Heart, Lung, and Blood Institute of National Institutes of Health R56 award (R56HL121392) to S.D. S.D. is funded by the Sylvester Comprehensive Cancer Center. We thank Dr. Khan Hekmatyar for his help with MRI, Nhat Quach for assistance with Western Blotting experiments. We thank Prof. Aaron M. Beedle for Western blot imager, and Dr. Nagesh Kolishetti and David Kolb for helpful discussion. We are thankful to Afoma Umeano for careful reading of this manuscript.
Footnotes
Electronic Supplementary Information (ESI) available: Additional figures and tables. See DOI: 10.1039/b000000x/
S.D. discloses financial interest in Partikula LLC; Partikula did not support the aforementioned work.
Notes and references
- 1.Fulda S, Galluzzi L, Kroemer G. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 2.Luft R, Ikkos D, Palmieri G, Ernster L, Afzelius B. J Clin Invest. 1962;41:1776–1804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace DC. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boland ML, Chourasia AH, Macleod KF. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madamanchi NR, Runge MS. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 6.Lin MT, Beal MF. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 7.Boddapati SV, D’Souza GGM, Erdogan S, Torchilin VP, Weissig V. Nano Lett. 2008;8:2559–2563. doi: 10.1021/nl801908y. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza GGM, Cheng SM, Boddapati SV, Horobin RW, Weissig V. J Drug Target. 2008;16:578–585. doi: 10.1080/10611860802228855. [DOI] [PubMed] [Google Scholar]
- 9.Marrache S, Dhar S. Proc Natl Acad Sci U S A. 2013;110:9445–9450. doi: 10.1073/pnas.1301929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrache S, Dhar S. Proc Natl Acad Sci USA. 2012;109:16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrache S, Dhar S. Chem Sci. 2015;6:1832–1845. doi: 10.1039/c4sc01963f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen R, Banik B, Pathak RK, Kumar A, Kolishetti N, Dhar S. Adv Drug Deliv Rev. 2016;99:52–69. doi: 10.1016/j.addr.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrache S, Pathak RK, Dhar S. Proc Natl Acad Sci U S A. 2014;111:10444–10449. doi: 10.1073/pnas.1405244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalathil AA, Kumar A, Banik B, Ruiter TA, Pathak RK, Dhar S. Chem Commun. 2016;52:140–143. doi: 10.1039/c5cc07316b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen R, Dhar S. Chem Sci. 2016;7:5559–5567. doi: 10.1039/c6sc00481d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sims NR. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 17.Sims NR, Anderson MF. Nat Protoc. 2008;3:1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]
- 18.De Duve C, Pressman BC, Gianetto R, Wattiaux R, Appelmans F. Biochem J. 1955;60:604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frezza C, Cipolat S, Scorrano L. Nat Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 20.Sharma V, Park K, Srinivasarao M. Proc Natl Acad Sci U S A. 2009;106:4981–4985. doi: 10.1073/pnas.0800599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miltenyi S, Muller W, Weichel W, Radbruch A. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 22.Kausch AP, Owen TP, Jr, Narayanswami S, Bruce BD. BioTechniques. 1999;26:336–343. doi: 10.2144/99262rr04. [DOI] [PubMed] [Google Scholar]
- 23.Perrin-Cocon LA, Marche PN, Villiers CL. Biochem J. 1999;338:123–130. [PMC free article] [PubMed] [Google Scholar]
- 24.Diettrich O, Mills K, Johnson AW, Hasilik A, Winchester BG. FEBS Lett. 1998;441:369–372. doi: 10.1016/s0014-5793(98)01578-6. [DOI] [PubMed] [Google Scholar]
- 25.Mura CV, Becker MI, Orellana A, Wolff D. J Immunol Methods. 2002;260:263–271. doi: 10.1016/s0022-1759(01)00546-4. [DOI] [PubMed] [Google Scholar]
- 26.Lawson EL, Clifton JG, Huang F, Li X, Hixson DC, Josic D. Electrophoresis. 2006;27:2747–2758. doi: 10.1002/elps.200600059. [DOI] [PubMed] [Google Scholar]
- 27.Hornig-Do HT, Gunther G, Bust M, Lehnartz P, Bosio A, Wiesner RJ. Anal Biochem. 2009;389:1–5. doi: 10.1016/j.ab.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 28.Franko A, Baris OR, Bergschneider E, von Toerne C, Hauck SM, Aichler M, Walch AK, Wurst W, Wiesner RJ, Johnston ICD, de Angelis MH. PLOS ONE. 2013:8. doi: 10.1371/journal.pone.0082392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith RA, Porteous CM, Gane AM, Murphy MP. Proc Natl Acad Sci USA. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie J, Peng S, Brower N, Pourmand N, Wang SX, Sun SH. Pure Appl Chem. 2006;78:1003–1014. [Google Scholar]
- 31.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanomedicine. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M. Biochim Biophys Acta. 2015;1853:276–284. doi: 10.1016/j.bbamcr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.