Abstract
Background
Reactive aldehydes like acetaldehyde and malondialdehyde generated as a result of alcohol metabolism and cigarette smoke exposure lead to the formation of malondialdehyde-acetaldehyde-adducted proteins (MAA adducts). These aldehydes can adduct to different proteins such as bovine serum album (BSA) and surfactant proteins A or D (SPA, SPD). Macrophages play an important role in innate immunity, but the effect of MAA adducts on macrophage function has not yet been examined. Because macrophage scavenger receptor A (SRA; CD204) mediates the uptake of modified proteins, we hypothesized that the effects of MAA modified proteins on macrophage function are primarily mediated through SRA.
Methods and Results
We tested this hypothesis by exposing SPD-MAA to macrophages and measuring functions. SPD-MAA treatment significantly stimulated pro-inflammatory cytokine TNF-α release in the macrophage cell line, RAW 264.7. A significant reduction in phagocytosis of zymosan particles was also observed. SPD-MAA stimulated a significant dose-dependent increase in TNF-α and IL-6 release from peritoneal macrophages of WT mice. But a significantly less TNF-α and IL-6 were released from peritoneal macrophages of SRA−/− mice. We observed a significant reduction in phagocytosis of zymosan particles in peritoneal macrophages from WT mice treated with SPD-MAA. No further SPD-MAA-induced reduction was seen in peritoneal macrophages form SRA−/− mice. SPD-MAA treatment significantly increased SRA mRNA expression, but had no effect on surface receptor protein expression. Protein kinase C alpha inhibitor and NF-κB inhibitor significantly reduced pro-inflammatory cytokine release in response to SPD-MAA.
Conclusion
In conclusion, our data demonstrate that SRA is important for MAA-adducted protein-mediated effect on macrophage functions.
Keywords: Alcohol, cigarette smoke, adduct, macrophage, scavenger receptor A
INTRODUCTION
Alcohol intoxication compromises host defenses against bacterial infections as it leads to suppression of selected functions of the immune system (Nelson et al., 1989). Individuals with alcohol use disorders (AUDs) are more susceptible to bacterial pneumonia than those without AUDs. (Cook, 1998). In addition, persons with AUDs are two to three times more likely to smoke cigarettes (Bobo and Husten, 2000; Miller and Gold, 1998) leading to more frequent (John et al., 2003) and higher rates of cigarette smoking among AUDs than in the general population (Narahashi et al., 2001).
Macrophages are often called first responder phagocytes, ingesting and killing inhaled bacteria (Yang et al., 2014; Fujiwara and Kobayashi, 2005). They exhibit different anatomical and functional features depending upon their tissue location (Wynn et al., 2013). Macrophages exhibit an inflammatory phenotype and secrete pro-inflammatory mediators such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 and IL-1β, all of which participate in activation of antimicrobial mechanisms (Parihar et al., 2010). Macrophages also produce reactive oxygen and nitrogen intermediates like nitric oxide and superoxide as a defense mechanism against microorganisms (Murray and Wynn, 2011). Because macrophages play an important role in antimicrobial defense as well as to initiate, maintain, and resolve inflammation (Fujiwara and Kobayashi, 2005), any compromise in antimicrobial defense could lead to increased susceptibility to infection and unchecked inflammation resulting in cellular and tissue damage.
Because both acute and chronic alcohol consumption lowers the ability of phagocytes to clear diverse pathogens, alcohol consumption often leads to lower/compromised innate and acquired immune response (Asplund et al., 2013). Short and long-term alcohol intake has also been shown to reduce phagocytic function of macrophages, which might play a role in increasing the risk of infection (Castro et al., 1993; Bagasra et al., 1988). Alcohol consumption, both acute and chronic, alters pro-inflammatory cytokine production. In acute or moderate alcohol consumption, the release of pro-inflammatory cytokines TNF alpha, IL-6 and IL-1 from monocytes is decreased. This could also contribute to compromised immunity, as these cytokines are important for defense against pathogens (Mandrekar et al., 2009). In contrast to this, chronic alcohol consumption leads to increased pro-inflammatory cytokine release, which could result in uncontrolled inflammation (Mandrekar et al., 2009; Szabo, 1997).
Reactive aldehydes such as acetaldehyde and malondialdehyde are formed during alcohol metabolism and oxidative stress in response to excessive alcohol consumption (Tuma and Casey, 2003; Sapkota et al., 2014). These aldehydes, once formed, react together covalently with proteins and generate MAA adducts, which possess immunogenic and pro-inflammatory properties (Tuma, 2002). In addition to alcohol, these reactive aldehydes could also form as a result of cigarette smoking and bind to protein and other molecules (McCaskill et al., 2011). During co-exposure to alcohol and cigarette smoke, high concentrations of acetaldehyde and malondialdehyde are generated resulting in formation of MAA adducts (McCaskill et al., 2011; Wyatt et al., 2001). In lung, MAA covalently binds to surfactant protein D (SPD) secreted by type II epithelial cells to form an adducted protein called SPD-MAA (McCaskill et al., 2011).
Scavenger receptors are pattern recognition receptors expressed on the surface of macrophages (Peiser et al., 2002). Among them, macrophage scavenger receptor A (SRA; CD204) has been the most widely studied (Haworth et al., 1997). SRA has the ability to bind and mediate the cellular uptake of modified lipoproteins (LDL) (Fitzgerald et al., 2000). In addition to modified LDL, this receptor binds to many different ligands such as maleylated bovine serum albumin (BSA), polyribonucleotides, polysaccharides, and aldehyde-modified proteins (de Winther et al., 2000; Duryee et al., 2005; Berger et al., 2014).
MAA-adducted proteins have been shown to be ligands for SRA on liver cells and bronchial epithelial cells (Duryee et al., 2005; Berger et al., 2014). While the effects on hepatic stellate (Kharbanda et al., 2001), liver endothelial and Kupffer cells (Duryee et al., 2004) and airway epithelial cells (Berger et al., 2014) have been reported, the effects of MAA on major macrophage functions and the role of SRA on such effects have not been evaluated. In addition, SPD-MAA, a biologically relevant protein adduct, has been never used in studies of macrophage functions. We, therefore, hypothesized that SPD-MAA, a protein adduct relevant to aldehyde formation in smokers who drink alcohol, modulates macrophage functions primarily via SRA, one of the major receptor for MAA.
MATERIALS AND METHODS
Materials
Protein kinase C (PKC) beta inhibitor LY316976 was purchased from Bio-Techne (Minneapolis, MN), PKC delta inhibitor Rottlerin and PKC alpha inhibitor Gö 6976 were purchased from Millipore (Billerica, MA). PKC zeta inhibitor myristolated PKC zeta inhibitory peptide and PKC epsilon inhibitor Ro 31-8220 were purchased from Enzo life science (Farmingdale, NY). Phorbol myristate acetate (PMA), nitro blue tetrazolium (NBT), fucoidan, fetuin and parthenolide were purchased from Sigma Aldrich (St Louis, MO). Lipopolysaccharide (LPS) was purchased from Fisher Scientific (Pittsburg, PA). Rat Anti-mouse SRA (2F8) was purchased from AbD Serotec (Raleigh, NC) and isotype negative control was purchased from Biolegend (San Diego, CA).
Cell culture and reagents
RAW 264.7 macrophages were purchased from American Type Cell Culture (ATCC, Rockville, MD) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin and maintained at 37°C in a humidified CO2 incubator. Resident peritoneal macrophages were isolated from C57BL/6 wild type mice (Charles River, Wilmington, MA) and SR-A knockout mice bred from homozygous SRA deficient mice (−/−) (B6.Cg-Msr1tm1Csk/J; Jackson Laboratory, Bar Harbor, ME) as described previously (Zhang et al., 2008). All experimental animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. Briefly, peritoneal macrophages (PMs) were isolated by adherence to 24-well, flat-bottom, tissue culture-treated polystyrene plastic plates in which 1 × 106 peritoneal exudate cells were seeded per well and allowed to adhere for 2 h after which any non-adherent cells were washed away. At this point, the cells were 90% macrophages as confirmed by Diff-Quik™ Stain (Siemens Health Care Diagnostic Inc, Newark, DE). Isolated peritoneal macrophages were used for selective experiments.
Malondialdehyde-acetaldehyde synthesis
Human surfactant protein D (SPD) adducted to MAA (SPD-MAA) was prepared as previously reported (Wyatt et al., 2012). Briefly, approximately 1–1.5 mg/mL of SPD was incubated with 1.0 mM acetaldehyde and 1.0 mM MDA in pyrogen-free PBS. The pH was brought to 7.4, and maintained at 37 °C for 72 h. At the end of incubation, the reaction mixture was exhaustively dialyzed against pyrogen-free phosphate buffer solution for 24 h at 4 °C. The endotoxin level in the MAA-SPD was measured by limulus assay and was below the limit of detection.
Pro-inflammatory cytokine release (TNFα and IL-6)
To identify the role of SPD-MAA on pro-inflammatory cytokine release, RAW 264.7 and resident peritoneal macrophages (PMs) were treated with different concentrations of SPD-MAA (10–200 µg/mL) for different times (6–48 h). The supernatant was then collected and stored at -80 °C for later analysis. To block SRA, RAW 264.7 macrophages were pre-incubated with fucoidan (50 µg/mL) for 72 h or anti-SRA (10 µg /mL) for 24 h followed by addition of SPD-MAA for another 6 h.
PKC isoform inhibition
To identify the role of PKC on MAA adduct-mediated pro-inflammatory cytokine release, RAW 264.7 macrophages were pre-incubated with different isoform-specific inhibitors to PKC beta (LY316976), PKC delta (Rottlerin), PKC zeta (myristolated PKC zeta inhibitory peptide), PKC alpha (Gö 6976) and PKC epsilon (Ro 31-8220) at 1 µM concentration for 1 h followed by addition of 200 µg/mL of SPD MAA for another 6 h. Resident peritoneal macrophages (PMs) were incubated with PKC alpha isoform inhibitor, Gö 6976 (1 µM) for 1 h followed by SPD-MAA treatment for 6 h. After 6 h, supernatant was collected and later analyzed for cytokines levels.
NF-κB inhibition
To identify the role of NF-κB in MAA adduct-mediated pro-inflammatory cytokine release, macrophages were pre-incubated with 1 µM parthenolide for 1 h followed by 200 µg/mL SPD-MAA treatment for another 6 h. After 6 h, supernatant was collected and later analyzed for pro-inflammatory cytokines.
ELISA
TNFα and IL-6 levels in the supernatant were measured by ELISA according to the manufacturer’s instructions (R & D Systems, Minneapolis, MN).
Phagocytosis
Phagocytosis of zymosan particles was assayed using a commercially available kit (Cell Biolabs, San Diego, CA). First, the macrophages were treated with 200 µg/mL SPD-MAA for 3 h followed by addition of zymosan particles (5 × 106) for (0–60) min and later analyzed for phagocytosis of the zymosan by measuring the optical density at 430 nm according to manufacturer instructions. For PMs, the macrophage were treated with 200 µg/mL SPD-MAA for 3 h followed by addition of zymosan particles (5 × 106) for 30 min.
Superoxide ion production
PMA stimulated superoxide ion production
Superoxide ion production was assayed using previously established NBT method as previously described (Sim Choi et al., 2006). RAW 264.7 macrophages were pre-treated with 200 µg/mL SPD-MAA for 24 h after which, PMA (300 ng /mL) in NBT solution (1 mg/mL) was added to the macrophages. After 1 h, macrophages were washed with phosphate buffer saline (PBS, pH 7.4) twice and later fixed with 100% methanol. The plate was then allowed to air dry for 10 min. After the complete removal of methanol, 120 µL of 2M potassium hydroxide was added followed by addition of 140 µL of 100 % dimethylsulfoxide and incubated at room temperature for 10 min. After 10 min, 200 µL of the solution was transferred to another 96-well plate and optical density was measured at 630nm (BioTek Instruments, Inc, Winooski, VT).
Opsonized zymosan stimulated superoxide ion production
Opsonized zymosan was prepared using a previously established method (Suhonen et al., 2000). First 10 mg of zymosan A (Sigma Aldrich, St Louis, MO) was opsonized with 1 mL of normal mouse serum (Invitrogen, Frederick, MO) by incubating at 37°C for 30 min followed by washing with PBS (pH 7.4) twice. RAW 246.7 macrophages were pre-treated with 200 µg/mL SPD-MAA for 24 h after which opsonized zymosan (1 mg/mL) in NBT solution (1 mg/mL) was added to the macrophages. After 30 min, macrophages were washed with PBS and superoxide ion production was measured as described above.
Nitrite production
Nitrite production was assayed by measuring nitrite (a stable degradation product of nitric oxide (NO) in the supernatant of cultured RAW 264.7 macrophage using Griess reagent. Briefly, RAW 264.7 macrophages were pretreated with 200 µg/mL of SPD-MAA for 3 h followed by treatment with 0.3 mg/mL zymosan (Sigma Aldrich, St Louis, MO) in Hams F-12 nutrient medium containing 10% FBS. After 24 h, supernatant was collected and nitrite level was measured using a commercial kit (Cayman Chemical, Ann Arbor, MI).
Real-time quantitative RT-PCR
RAW 264.7 macrophages were treated with SPD-MAA (200 µg/mL) for (1–24 h) and later total RNA was isolated from the macrophages using RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The purity of RNA was checked by measurement of optical density (260/280) ratio using a NanoDrop spectrophotometer (Thermoscientific). cDNA was synthesized using 100 ng of template RNA and a TaqMan reverse transcription kit (Applied Biosystems, Austin, TX). Real-time PCR reactions were prepared using TaqMan Master Mix (Applied Biosystems) and primers and probe for SRA (Applied Biosystems; Mm 00446214_m1). Ribosomal (18S) RNA was used as an endogenous control. PCR was performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Threshold values were normalized to the expression of ribosomal RNA. Real-time PCR results are expressed as the fold change increase in comparison to media control.
SRA surface expression
RAW 264.7 macrophages were treated with SPD-MAA (200 µg/mL) for (1–24 h). After treatment, macrophages were washed with ice-cold PBS followed by fixation with 3.7 % paraformaldehyde for 10 min at 4°C. After fixation, macrophages were washed with PBS followed by incubation with rat anti-SRA labeled with FITC and matched isotype control antibody at 4°C for 1 h. After incubation macrophages were washed with PBS three times and the samples were analyzed by flow cytometry. Flow cytometry analysis was performed with FACS Calibur available in the UNMC Flow Cytometry Core (BD Biosciences, San Jose, CA).
Statistics
All data were analyzed using GraphPad Prism (version 5.00 for Windows; GraphPad Software, San Diego CA) and represented as mean ± SE. Data were analyzed for statistical significance using Student’s t-test and both one-way and two-way ANOVA employing Tukey's multiple comparison post-test corrections. A p-value less than 0.05 was regarded as statistically significant.
RESULTS
Cell viability
Cell viability was assessed by trypan blue exclusion method as described previously (Reissis et al., 2013). No cell death was observed for different concentrations of SPD-MAA over a period of 6–48 h as measured by trypan blue exclusion method. Also, the PKC alpha inhibitor, NF-κB inhibitor, fucoidan (a ligand for SRA) and anti-SRA did not affect cell viability (data not shown).
SPD-MAA exposure induces pro-inflammatory cytokine TNFα release
To determine whether exposure to SPD-MAA induces pro-inflammatory cytokine TNF-α release, RAW 264.7 macrophages were exposed to 200 µg/mL of SPD-MAA for different time points (6–48 h). RAW 264.7 macrophages produced TNF-α in response to SPD-MAA in a time-dependent manner. A significant increase (p < 0.0001) in TNF-α release was observed as early as 6 h in comparison to media control (Fig. 1A). Additionally, a dose-dependent increase in TNF-α release was observed in response to SPD-MAA. TNF-α release was significantly increased (p < 0.001) following exposure to 100–200 µg/mL of SPD-MAA at 6 h (Fig. 1B). No significant increase in TNF-α release was observed when exposed to non-adducted lung surfactant protein SPD (Fig. 1B). These data demonstrate that SPD-MAA induces TNF-α release from RAW 264.7 macrophages in both a time- and concentration-dependent manner. Similarly, in peritoneal macrophages (PMs) isolated from WT mice, we observed a significant dose-dependent (p < 0.0001) increase in TNF-α release at 6 h when treated with 200 µg/mL of SPD-MAA (Fig. 1C). No such response was observed in PMs from SRA−/− mice (Fig. 1C). In addition, significantly less TNF-α release was observed at 100 µg/mL (p < 0.05) and 200 µg/mL (p < 0.0001) of SPD-MAA treatment in SRA−/− mice in comparison to WT mice (Fig. 1C). Pretreatment blocking with fucoidan, a ligand for scavenger receptor A, significantly reduced (p < 0.001) SPD-MAA-induced TNF-α release in RAW 264.7 macrophages (Fig. 1D). No reduction in SPD-MAA stimulated TNF-α release was observed when macrophages were treated with fetuin, a glycoprotein negative control for fucoidan (data not shown). Treatment with SRA blocking antibody (2F8) significantly reduced (p < 0.001) SPD-MAA stimulated TNF-α release (Fig. 1E). No reduction in SPD-MAA stimulated TNF-α release was observed when macrophages were treated with isotype-matched negative control, IgG (data not shown).
Figure 1. Effect of SPD-MAA on pro-inflammatory cytokine TNF-α release.
TNFα release from RAW 264.7 macrophages was measured after treatment with 200 µg/mL SPD-MAA for 6, 24 and 48 h (A). After treatment, supernatant was collected and TNF-α level measured by ELISA. SPD-MAA induced TNF-α release from RAW 264.7 (B) and wild type peritoneal macrophages (C) in concentration dependent manner at 6 h. No such effect was seen in SRA−/− mice (C). RAW 264.7 macrophages were pretreated with 50 µg/mL of fucoidan (D) for 72 h or 10 µg /mL of Anti-SRA (E) for 24 h followed by treatment of SPD-MAA for 6 h. Values are the mean ± SEM of three independent experiments.
SPD-MAA exposure induces pro-inflammatory cytokine IL-6 release
To determine whether exposure to SPD-MAA induces pro-inflammatory cytokine IL-6 release, PMs from both WT and SRA−/− mice were exposed to different doses of SPD-MAA for 6 h. A significant increase in IL-6 was detected at the highest concentration of SPD-MAA (200 µg/mL) (p < 0.0001) (Fig. 2). No such increase was observed in PMs from SRA−/− mice. We also observed a significant attenuation of IL-6 release at 200 µg/mL of SPD-MAA (p < 0.0001) treatment in PMs from SRA−/− in comparison to WT mice (Fig. 2). No time or dose-dependent increase in IL-6 was observed when RAW 264.7 macrophages were exposed to SPD-MAA (200 µg/mL) for up to 24 h (data not shown).
Figure 2. Effect of SPD-MAA on pro-inflammatory cytokine IL-6 release.
Peritoneal macrophages (PMs) from wild type (WT) and SRA−/− mice were treated 200 µg/mL SPD-MAA for 6 h. After treatment, supernatant media was collected and IL-6 levels measured by ELISA. SPD-MAA induced IL-6 release from PMs from WT mice at highest concentration at 6 h. No such effect was seen on PMs from SRA−/− mice. Values represent mean ± SEM of three independent experiments.
Effect of SPD-MAA on phagocytosis of zymosan particles
To determine the role of SPD-MAA on phagocytic function, RAW 264.6 macrophages were incubated with 200 µg/mL SPD-MAA for 3 h followed by addition of zymosan particles for 0–60 min. Treatment with SPD-MAA significantly decreased the phagocytosis of zymosan particles at 30 min (p < 0.0001) and 60 min (p < 0.0001) in comparison to their respective media control (Fig. 3A). No reduction was observed when macrophages were treated with non-adducted SPD only (Fig. 3A). A similar result was observed in PMs from WT mice treated with 200 ug/mL SPD-MAA for 3 h. In PMs, a significant increase in phagocytosis of zymosan at 30 min was observed in both media control (p < 0.0001) and SPD (p <0.0001) treatment groups when compared to 0 min (Fig. 3B). When treated with SPD-MAA, a significant decrease in phagocytosis (p < 0.0001) was observed at 30 min when compared to media control and non-adducted SPD treatments (Fig. 3B). The different letters (a,b) are used to indicate the significance from their respective treatment group at 0 min (Fig. 3B).
Figure 3. Effect of SPD-MAA on phagocytosis of zymosan particles.
RAW 264.7 macrophages and peritoneal macrophages (PMs) were treated with 200 µg/mL of SPD-MAA for 3 h followed by addition of zymosan particles for (0–60) min. Phagocytosis of the particles was detected as described in methods. SPD-MAA treated significantly reduced the phagocytosis of particles by RAW 264.7 macrophage (A) and PMs (B) from wild type (WT) mice (white bars). No such effect was seen in PMs from SRA−/− mice (B; black bars). Values represent mean ± SEM of three independent experiments. The different letters (a,b) are used to indicate the significance from their respective treatment group at 0 min.
Effect of SPD-MAA on superoxide ion and nitrite release
To determine the role of SPD-MAA on superoxide ion and nitrite ion release, RAW 264.6 macrophages were pre-incubated with 200 µg/mL SPD-MAA for 24 h followed by addition of PMA (300 ng/mL) for 1 h or opsonized zymosan for 30 min. SPD-MAA significantly reduced superoxide ion release from RAW 264.7 macrophages in response to zymosan (p < 0.001; Fig. 4A). A similar result was observed in response to PMA (p < 0.001; Fig. 4B). Pre-treatment with non-adducted SPD had no such effect (Fig. 4A and 4B). To measure nitrite release, RAW 264.7 macrophages were pre-treated with 200 ug/mL of SPD-MAA for 3 h followed by the addition of zymosan (0.3 mg/mL) for another 24 h. SPD-MAA significantly reduced (p < 0.01) nitrite release in response to zymosan (Fig. 4C). No reduction in nitrite release was observed when exposed to equal concentration of SPD (Fig. 4C).
Figure 4. Effect of SPD-MAA on nitrite and superoxide ion release.
RAW 264.7 macrophages were pretreated with 200 µg/mL SDP-MAA for 24 h followed by treatment with PMA (300 ng/mL) for 1 h (A) or opsonized zymosan (1 mg/mL) for 30 min (B). After treatment time superoxide ion was measured using NBT method. For nitrite release, RAW 264.7 macrophages were pretreated with 200 µg/mL SDP-MAA for 3 h followed by treatment with zymosan (0.3 mg/mL) for another 24 h. After 24 h supernatant was collected and nitrite level measured (C). Values represent mean ± SEM of three independent experiments
Effect of SPD-MAA on SRA expression
To determine the effect of SPD-MAA on SRA message expression, RAW 264.6 macrophages were treated with 200 µg/mL SPD-MAA for 1, 3, 6, and 24 h and later analyzed for SRA mRNA expression. A significant up regulation of SRA mRNA expression was observed at 6 h (p < 0.0278; Fig. 5A). At 24 h, however, SRA mRNA expression returned to baseline. No such effect on SR-A mRNA was seen when RAW 264.6 cells were treated with SPD for 6 h (Fig. 5A).
Figure 5. Effect of SPD-MAA on SR-A expression.
RAW 264.7 macrophages were treated with SPD-MAA for (1–24 h) and later analyzed for SRA message (A) expression by RT-PCR. Results represent fold change in SRA message expression from media control. RAW 264.7 macrophages treated with SPD-MAA (0–24 h) and later analyzed for SRA surface receptor expression by flow cytometry (B). Values represent mean ± SEM of three independent experiments.
To determine the effect of SPD-MAA on surface expression of SRA, RAW 264.6 macrophages were treated with 200 µg/mL SPD-MAA for 0–24 h and analyzed for surface receptor expression by flow cytometry. No significant change in SRA surface expression was detected at 1, 3, 6, or 24 h (Fig. 5B). No rapid transient change in SRA expression was observed at 0–30 min (data not shown).
MAA-adducted protein binds to SRA and causes PKC activation
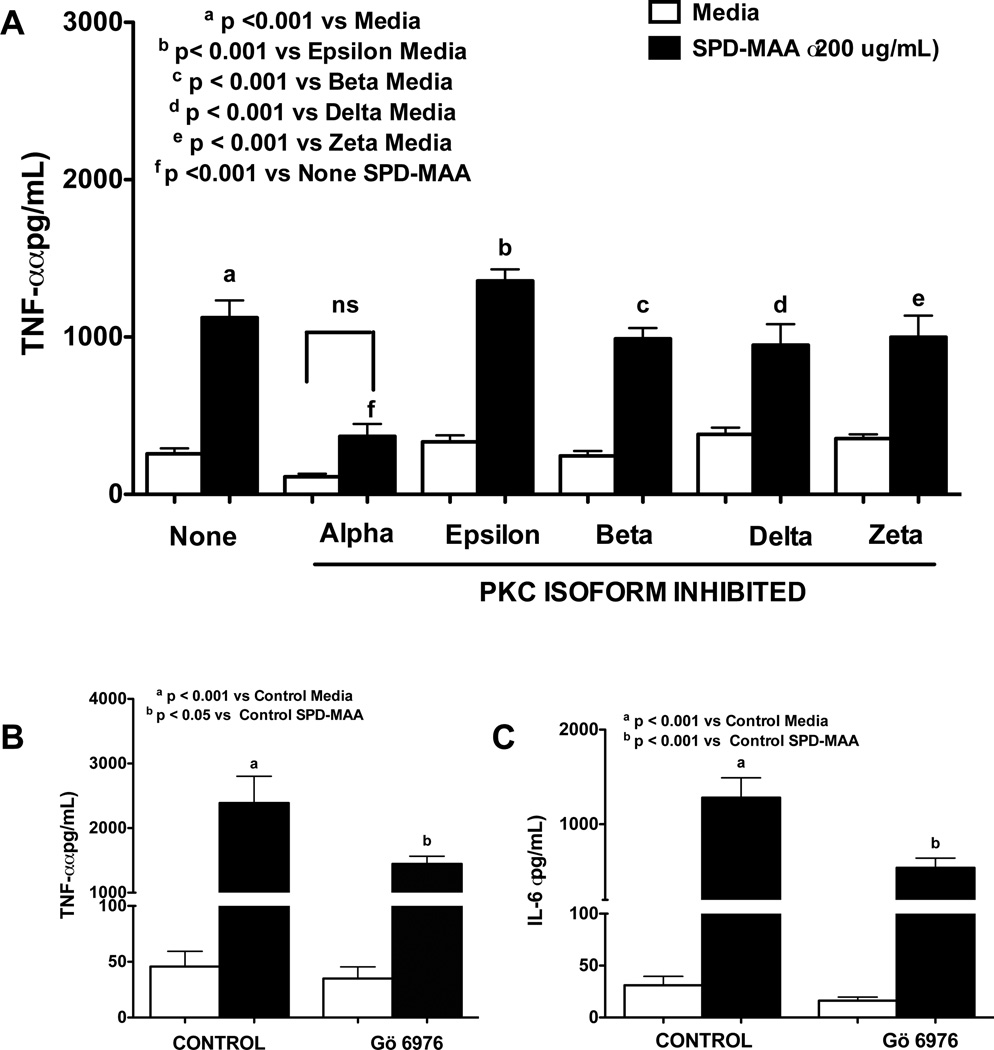
To identify the role of PKC on SPD-MAA induced pro-inflammatory cytokine release, RAW 264.7 macrophages were incubated with different PKC isoform inhibitors at 1 µM concentration (40) for 1 h before addition of 200 µg/mL SPD-MAA for 6 h. PKC alpha inhibitor Gö 6976 pretreatment significantly decreased TNFα release from RAW 264.7 macrophages in response to SPD-MAA (p < 0.001; Fig. 6A). No other PKC inhibitors affected TNF-α release (Fig 6A). A similar result was observed when PMs from WT mice were pretreated with PKC alpha inhibitor Gö 6976 for 1 h (p < 0.05) (Fig. 6B). In addition to TNFα, pretreatment with Gö 6976 also significantly decreased IL-6 release from PMs macrophages from WT mice in response to SPD-MAA at 6 h (p < 0.001) (Fig. 6C).
Figure 6. Effect of PKC isoform inhibitors on SPD-MAA stimulated TNF-α and IL-6 release.
RAW 264.7 macrophages (A) were pretreated with different PKC isoform inhibitors at 1 µM concentration for 1 h before addition of 200 µg/mL SPD-MAA for 6 h (A). PMs from WT mice were pretreated with PKC alpha inhibitor Gö 6976 (1uM) for 1 h and later treated with 200 µg/mL SPD-MAA or SPD for 6 h. After treatment, supernatant was collected and TNFα (A, B) or IL-6 (C) levels were measured by ELISA. Values are the mean ± SEM of three-six independent experiments. Legend same for all 3 figures.
MAA-adducted protein binds to SRA and causes NF-κB activation
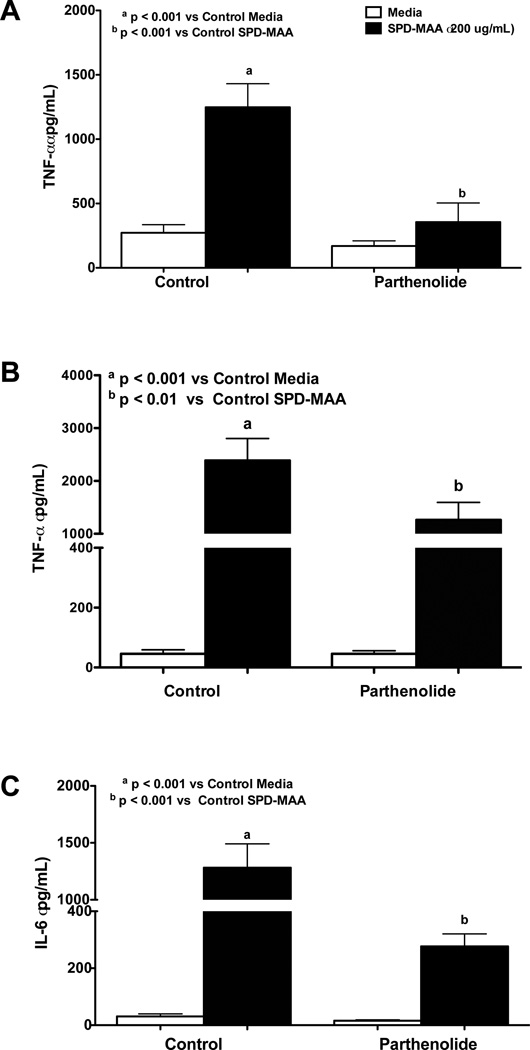
To identify the role of transcription factor NF-κB on SPD-MAA induced pro-inflammatory cytokine release, RAW 264.7 and PMs were incubated with the NF-κB inhibitor parthenolide at 1 µM concentration for 1 h before addition of 200 µg/mL SPD-MAA for 6 h. Parthenolide at the concentration used had no cytotoxic effect and did not induce apoptosis and oxidative stress (Tiuman et al., 2005; Wen et al., 2002). A significant reduction in TNF-α release was observed in both RAW 264.7 macrophages (p < 0.001; Fig. 7A) and PMs (p < 0.01; Fig. 7B). In addition to TNF-α, pretreatment with NF-κB inhibitor, parthenolide, significantly reduced IL-6 release from PMs at 6 h in response to SPD-MAA (p < 0.001; Fig. 7C).
Figure 7. Effect of NF-κB inhibitor on SPD-MAA stimulated TNFα and IL-6 alpha release.
RAW 264.7 macrophages (A) were incubated with NF-κB inhibitor (parthenolide) at 1 µM concentration for 1 h before addition of 200 µg/mL SPD-MAA for 6 h (A). PMs from WT mice were pretreated parthenolide (1uM) for 1 h and later treated with 200 µg/mL SPD-MAA for 6 h SPD (B, C). After treatment, supernatant was collected and TNF-α (A, B) and IL-6 (C) levels were measured by ELISA. Values are the mean ± SEM of three independent experiments. Legend same for all 3 figures.
DISCUSSION
It is well established that macrophages play an important role in immunity. Therefore any alterations in macrophage function can lead to inflammation, injury and infection. Acetaldehyde and malondialdehdye derived MAA adducts have been shown to induce the release of pro-inflammatory cytokines and chemokines by kupffer, endothelial, and stellate cells of the liver (Duryee et al., 2004; Kharbanda et al., 2001). In this study, we demonstrated that MAA adduct effects macrophage functions. Our study shows that SPD-MAA, a MAA adduct to surfactant protein D, activates both RAW 264.7 and peritoneal macrophages to release pro-inflammatory cytokines TNF-α and IL-6. In addition to this, our study also shows that MAA adduct affects phagocytic function as well as superoxide ion and nitrite ion release, which were not studied previously. Our study also showed that PKC alpha and NF-κB may be important for MAA-stimulated TNF-α and IL-6 release.
Our data extend the concept that MAA adducts have a pro-inflammatory effect. MAA adducts have been shown to induce pro-inflammatory cytokine TNF-α in a purified rat heart endothelial cell culture (HEC)(Hill et al., 1998) and stimulate TNF-α, monocyte chemo-attractant protein-1 (MCP-1), and macrophage inflammatory protein-2 (MIP-2) in liver endothelial cells (Thiele et al., 2004). Interestingly, our study showed higher levels of TNF-α release from PMs than from RAW 264.7 macrophages. Unlike TNF-α, only very high concentrations of SPD-MAA stimulated IL-6 release from PMs. Differences in both cytokine expression and response between primary macrophages and immortalized macrophage cell lines could explain this result (Chamberlain et al., 2009).
Our data suggest a role for PKCα, a calcium-dependent classical protein kinase C isoform and transcription factor NF-κB, in pro-inflammatory cytokine release in response to SPD-MAA from RAW 264.7 and PMs. Other PKC isoform-specific inhibitors failed to inhibit TNF-α release. This is in contrast with our previous finding in epithelial cells (Berger et al., 2014) where PKC epsilon was involved in the MAA mediated pro-inflammatory cytokine release. This could be due to difference in cell type involved and cytokine measured (KC). In our study, PKC alpha inhibitor does not totally inhibit TNF alpha and IL-6 release when compared to untreated cells. This may suggest other signaling pathway like p38/JNK as suggested by others (Nikolic et al., 2011; Ben et al., 2013; Hsu et al., 2001) involved in addition to PKC alpha.
In our study, the dose of MAA used is higher than required for airway cells (100 ug/mL) (Berger et al., 2014) and the amount detected (500 ng/mL)(McCaskill et al., 2011) in lung of mice after exposure to alcohol and cigarette smoke for 8 weeks. But, this is the amount detected only after 8 weeks exposure, and people abuse alcohol and smoke cigarette for years. Therefore the amount of adduct formed in their lung could be much higher than detected in the mice.
Our study also reportS that SPD-MAA significantly reduces the phagocytic function of macrophages in RAW 264.7 and peritoneal macrophages. A significant reduction in superoxide ion and nitrite ion release was observed when RAW 264.7 macrophages were exposed to SPD-MAA. Alcohol has been previously reported to reduce phagocytic function, antimicrobial activity, superoxide ion, and nitric oxide release from macrophages (Satapathy and Shrivastava, 2012; Castro et al., 1993; Szabo, 1999; D'Souza et al., 1996; Libon et al., 1993; Andrade et al., 2008). Our findings may suggest MAA adduct formation could be one of the mechanisms through which alcohol may exert such actions.
Our study shows that SPD-MAA mediates effects in both RAW 264.7 and PMs through scavenger receptor A. Only a few previous studies have examined a role for scavenger receptor in MAA mediated effects in mice and bronchial epithelial cells (Thiele et al., 2001). Our study further identified a role for SRA in SPD-MAA mediated pro-inflammatory cytokine release in macrophages. TNF-α and IL-6 release was significantly reduced in SRA−/− PMs in comparison to cells from WT mice. Pretreatment with fucoidan (a ligand that competes with SPD-MAA for scavenger receptor) or SRA blocking antibody significantly reduced SPD-MAA induced TNF-α release from RAW 264.7 macrophages. These results suggest that the SPD-MAA adduct most likely binds to SRA and signals the release of cytokines in both RAW 264.7 and PMs. In addition, MAA-mediated reduction in phagocytic function was also SRA-dependent. Our results from PMs from SRA−/− mice imply that SRA is important for MAA adduct binding resulting in the modulation of zymosan phagocytosis.
We also report that exposure to SPD-MAA induced SRA mRNA expression in RAW 264.7 macrophages. Although SRA mRNA expression is increased, no change in surface expression of SRA at any time point tested was observed. This finding is in contrast with our previous finding in airway cells where a transient decrease in SRA surface expression was observed after short-term exposure to MAA (Berger et al., 2014). This could be due to difference in the cell type involved. Also. SPD-MAA being a ligand for SRA, may induce gene expression and subsequent receptor secretion. But due to rapid binding of MAA to the newly expressed receptors, no change in surface receptor expression could be observed even though new SRA receptors are secreted (Murphy et al., 2005).
A major limitation of our study is that our results are limited to RAW 264.7 and peritoneal macrophages. Because SPD is a lung defense protein shown to be the target of MAA adduction, primary lung macrophages would have been ideal to further confirm SPD-MAA effects observed in RAW 264.7 cells and PMs, but they were difficult to obtain in sufficient viable numbers (500,000 cells per well) to perform the extensive studies reported in the current study. In addition, a large number of mice (30–50) were required to obtain enough cells to conduct a single experiment. It is important to note that MAA adduct formation can involve a wide variety of target proteins and the MAA moiety has itself been shown to be inflammatory and immunogenic (Wyatt et al., 2012). So several previous studies have utilized non-biologically relevant proteins such as BSA for lung and liver studies (Wyatt et al., 2012; Wyatt et al., 2001; Kharbanda et al., 2001; Thiele et al., 2004). Therefore all studies were done in RAW 264.7 and peritoneal macrophages. To overcome the above-mentioned limitation, future in-vivo studies using WT and SRA−/− mice is necessary to further confirm results observed in both macrophages and airway epithelial cells. This will also justify the role of SRA in SPD-MAA mediated lung effects.
To summarize, our current study demonstrates that MAA adducted proteins modulate certain macrophage inflammatory and effector functions in RAW 264.7 and PMs. Such modulations may involve secretion of pro-inflammatory cytokines such as TNF-α and IL-6 as well as compromised phagocytic and superoxide and nitrite ion release. Additionally, our study also emphasizes the functional role of SRA in mediating the effects of SPD-MAA since in the absence of SRA, MAA failed to reduce macrophage function. Our data also suggest that PKCα and NF-κB play roles in MAA adduct-stimulated pro-inflammatory cytokine release by these macrophages.
Figure 8. Proposed model.
Hybrid adduct SPD-MAA in the lung binds to SRA expressed on the macrophage surface and modulates macrophage functions. SPD-MAA exposure decreases phagocytosis, superoxide ion and nitrite release as well as increases pro-inflammatory cytokines TNF-α and IL-6 release. Using SRA competing ligand fucoidan, SRA ligand blocking antibody or knocking out SRA gene diminishes these modulations. PKC alpha inhibitor Gö 6976 and NF-κB inhibitor parthenolide inhibited pro-inflammatory cytokines release from macrophage in response to SPD-MAA.
Acknowledgments
Sources of Support: Department of Veterans Affairs (VA I01BX000728) to TAW and the Central States Center for Agricultural Safety and Health (CS-CASH; U54OH010162) to TAW.
The Authors wish to acknowledge Dr. Geoffrey M. Thiele for critical reading of the manuscript during preparation. The authors would like to thank Philip Hexley, Ph.D, Victoria Smith, and Samantha Wall of the Cell Analysis Facility at the University of Nebraska Medical Center for assistance with flow cytometric measurements.
Footnotes
AUTHORSHIP
M.S., conceived and designed research, performed experiments, analyzed the data, and drafted the manuscript. K.K.K. synthesized MAA-adducted protein and reviewed the manuscript. T.A.W., conceived and designed research; edited, revised and approved the final version of the manuscript.
DISCLOSURE
The authors have no conflicts of interest to disclose.
Reference
- Andrade M, Martins-Filho O, Coelho-Neto J, Mesquita O, Faria A. The Long-term Impaired Macrophages Functions are Already Observed Early after High-dose Ethanol Administration. Scand J Immunol. 2008;68:306–314. doi: 10.1111/j.1365-3083.2008.02142.x. [DOI] [PubMed] [Google Scholar]
- Asplund MB, Coelho C, Cordero RJ, Martinez LR. Alcohol impairs J774. 16 macrophage-like cell antimicrobial functions in Acinetobacter baumannii infection. Virulence. 2013;4:467–472. doi: 10.4161/viru.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Howeedy A, Kajdacsy-Balla A. Macrophage function in chronic experimental alcoholism. I. Modulation of surface receptors and phagocytosis. Immunology. 1988;65:405–409. [PMC free article] [PubMed] [Google Scholar]
- Ben J, Zhang Y, Zhou R, Zhang H, Zhu X, Li X, Zhang H, Li N, Zhou X, Bai H, Yang Q, Li D, Xu Y, Chen Q. Major vault protein regulates class A scavenger receptor-mediated tumor necrosis factor-alpha synthesis and apoptosis in macrophages. J Biol Chem. 2013;288:20076–20084. doi: 10.1074/jbc.M112.449538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JP, Simet SM, DeVasure JM, Boten JA, Sweeter JM, Kharbanda KK, Sisson JH, Wyatt TA. Malondialdehyde-acetaldehyde (MAA) adducted proteins bind to scavenger receptor A in airway epithelial cells. Alcohol. 2014;48:493–500. doi: 10.1016/j.alcohol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo JK, Husten C. Sociocultural influences on smoking and drinking. Alcohol Research and Health. 2000;24:225–232. [PMC free article] [PubMed] [Google Scholar]
- Castro A, Lefkowitz D, Lefkowitz S. The effects of alcohol on murine macrophage function. Life Sci. 1993;52:1585–1593. doi: 10.1016/0024-3205(93)90059-c. [DOI] [PubMed] [Google Scholar]
- Chamberlain LM, Godek ML, Gonzalez-Juarrero M, Grainger DW. Phenotypic non-equivalence of murine (monocyte-) macrophage cells in biomaterial and inflammatory models. Journal of Biomedical Materials Research Part A. 2009;88:858–871. doi: 10.1002/jbm.a.31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcoholism: Clinical and Experimental Research. 1998;22:1927–1942. [PubMed] [Google Scholar]
- de Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: A multifunctional receptor in atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:290–297. doi: 10.1161/01.atv.20.2.290. [DOI] [PubMed] [Google Scholar]
- D'Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol Modulates Alveolar Macrophage Tumor Necrosis Factor-α, Superoxide Anion, and Nitric Oxide Secretion in the Rat. Alcoholism: Clinical and Experimental Research. 1996;20:156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC, Suzuki H, Tuma DJ, Klassen LW, Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol. 2005;68:1423–1430. doi: 10.1124/mol.105.016121. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide Is a Cofactor for Malondialdehyde-Acetaldehyde Adduc Mediated Cytokine/Chemokine Release by Rat Sinusoidal Liver Endothelial and Kupffer Cells. Alcoholism-Clinical and Experimental Research. 2004;28:1931–1938. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Moore KJ, Freeman MW, Reed GL. Lipopolysaccharide induces scavenger receptor A expression in mouse macrophages: a divergent response relative to human THP-1 monocyte/macrophages. J Immunol. 2000;164:2692–2700. doi: 10.4049/jimmunol.164.5.2692. [DOI] [PubMed] [Google Scholar]
- Fujiwara N, Kobayashi K. Macrophages in inflammation. Current Drug Targets-Inflammation & Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GE, Miller JA, Baxter BT, Klassen LW, Duryee MJ, Tuma DJ, Thiele GM. Association of malondialdehyde–acetaldehyde (MAA) adducted proteins with atherosclerotic-induced vascular inflammatory injury. Atherosclerosis. 1998;141:107–116. doi: 10.1016/s0021-9150(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Chiu SL, Wen MH, Chen KY, Hua KF. Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways. J Biol Chem. 2001;276:28719–28730. doi: 10.1074/jbc.M011117200. [DOI] [PubMed] [Google Scholar]
- Jing J, Yang IV, Hui L, Patel JA, Evans CM, Prikeris R, Kobzik L, O'Connor BP, Schwartz DA. Role of macrophage receptor with collagenous structure in innate immune tolerance. J Immunol. 2013;190:6360–6367. doi: 10.4049/jimmunol.1202942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003;38:606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Todero SL, Shubert KA, Sorrell MF, Tuma DJ. Malondialdehyde–acetaldehyde–protein adducts increase secretion of chemokines by rat hepatic stellate cells. Alcohol. 2001;25:123–128. doi: 10.1016/s0741-8329(01)00174-4. [DOI] [PubMed] [Google Scholar]
- Libon C, Forestier F, Cotte-Laffitte J, Labarre C, Quero AM. Effect of acute oral administration of alcohol on superoxide anion production from mouse alveolar macrophages. J Leukoc Biol. 1993;53:93–98. doi: 10.1002/jlb.53.1.93. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill ML, Kharbanda KK, Tuma DJ, Reynolds JD, DeVasure JM, Sisson JH, Wyatt TA. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcoholism: Clinical and Experimental Research. 2011;35:1106–1113. doi: 10.1111/j.1530-0277.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, Gold MS. Comorbid cigarette and alcohol addiction: epidemiology and treatment. Journal of addictive diseases. 1998;17:55–66. doi: 10.1300/J069v17n01_06. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1–15. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Söderpalm B, Ericson M, Olausson P, Engel J, Zhang X, Nordberg A, Marszalec W, Aistrup GL, Schmidt L. Mechanisms of Alcohol-Nicotine Interactions: Alcoholics Versus Smokers. Alcoholism: Clinical and Experimental Research. 2001;25:152S–156S. doi: 10.1097/00000374-200105051-00026. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Nikolic D, Calderon L, Du L, Post SR. SR-A ligand and M-CSF dynamically regulate SR-A expression and function in primary macrophages via p38 MAPK activation. BMC Immunol. 2011;12 doi: 10.1186/1471-2172-12-37. 37-2172-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun. 2010;2:204–215. doi: 10.1159/000296507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- Reissis Y, Garcia-Gareta E, Korda M, Blunn GW, Hua J. The effect of temperature on the viability of human mesenchymal stem cells. Stem Cell Res Ther. 2013;4:139. doi: 10.1186/scrt350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota M, Hottor TK, DeVasure JM, Wyatt TA, McCaskill ML. Protective Role of CYP2E1 Inhibitor Diallyl Disulfide (DADS) on Alcohol-Induced Malondialdehyde-Deoxyguanosine (M1dG) Adduct Formation. Alcoholism: Clinical and Experimental Research. 2014;38:1550–1558. doi: 10.1111/acer.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapathy S, Shrivastava A. Effect of n-Butanol on macrophage functions: An in vitro study. International Journal of Pharma and Bio Sciences. 2012;4:2278–3008. [Google Scholar]
- Sim Choi H, Woo Kim J, Cha Y, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. Journal of Immunoassay and Immunochemistry. 2006;27:31–44. doi: 10.1080/15321810500403722. [DOI] [PubMed] [Google Scholar]
- Suhonen J, Hartiala K, Tuominen-Gustafsson H, Viljanen MK. Borrelia burgdorferi--induced oxidative burst, calcium mobilization, and phagocytosis of human neutrophils are complement dependent. J Infect Dis. 2000;181:195–202. doi: 10.1086/315195. [DOI] [PubMed] [Google Scholar]
- Szabo G. Alcohol's contribution to compromised immunity. Alcohol Health Res World. 1997;21:30–41. [PMC free article] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Thiele GM, Duryee MJ, Willis MS, Sorrell MF, Freeman TL, Tuma DJ, Klassen LW. Malondialdehyde-acetaldehyde (MAA) modified proteins induce pro-inflammatory and pro-fibrotic responses by liver endothelial cells. Comparative Hepatology. 2004;3:1. doi: 10.1186/1476-5926-2-S1-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele GM, Worrall S, Tuma DJ, Klassen LW, Wyatt TA, Nagata N. The Chemistry and Biological Effects of Malondialdehyde-Acetaldehyde Adducts. Alcoholism: Clinical and Experimental Research. 2001;25:218S–224S. doi: 10.1097/00000374-200105051-00035. [DOI] [PubMed] [Google Scholar]
- Tiuman TS, Ueda-Nakamura T, Garcia Cortez DA, Dias Filho BP, Morgado-Diaz JA, de Souza W, Nakamura CV. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob Agents Chemother. 2005;49:176–182. doi: 10.1128/AAC.49.11.176-182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma DJ. Role of malondialdehyde-acetaldehyde adducts in liver injury 1, 2. Free Radical Biology and Medicine. 2002;32:303–308. doi: 10.1016/s0891-5849(01)00742-0. [DOI] [PubMed] [Google Scholar]
- Tuma J, Casey CA. Dangerous byproducts of alcohol breakdown-focus on adducts. Alcohol Research and Health. 2003;27:285–290. [PMC free article] [PubMed] [Google Scholar]
- Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem. 2002;277:38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- Wyatt T, Kharbanda K, McCaskill M, Tuma D, Yanov D, DeVasure J, Sisson J. Malondialdehyde-acetaldehyde (MAA) adducted protein inhalation causes lung injury. Alcohol. 2012;46:51–59. doi: 10.1016/j.alcohol.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Kharbanda KK, Tuma DJ, Sisson JH. Malondialdehyde–acetaldehyde-adducted bovine serum albumin activates protein kinase C and stimulates interleukin-8 release in bovine bronchial epithelial cells. Alcohol. 2001;25:159–166. doi: 10.1016/s0741-8329(01)00177-x. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Huang YT, Koziel H, de Crom R, Ruetten H, Wohlfart P, Thomsen RW, Kahlert JA, Sørensen HT, Jozefowski S. Female resistance to pneumonia identifies lung macrophage nitric oxide synthase-3 as a therapeutic target. eLife. 2014;3:e03711. doi: 10.7554/eLife.03711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Current protocols in immunology. 2008;111:14.1.1–14.1.16. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]